Abstract
Recent findings from our laboratory highlight the role of the modulation of the innate immune function and systemic inflammatory response in the effectiveness of balneotherapy in rheumatic diseases, specifically in elderly patients with osteoarthritis. Immune-neuroendocrine and stress mediators are involved in these effects. The ‘bioregulatory effect of balneotherapy’ has also been recently proposed as a mechanism of effectiveness that consists of a reduction in systemic pro-inflammatory mediators together with the achievement of an optimal innate response through stimulation (or at least lack of impairment) of the innate defences against pathogens (i.e. phagocytosis, microbicide activity) mediated by neutrophils, also generating immunophysiological adaptations through an optimal balance between the pro- and the anti-inflammatory responses in which regulatory T cells seem to have a crucial role. In the present paper, we aim to analyse the main conclusions related to how balneotherapy with the use of peloids (pelotherapy) affects the innate and inflammatory responses, constituting an immunophysiological mechanism underlying the proven clinical benefits of this intervention. We also introduce novel results regarding the innate response (phagocytic process) of monocytes in this therapy, an inflammatory cell that has not yet been studied in this context. Increased chemotaxis together with a decline in oxidative burst, without changes in phagocytosis, could be the main response induced by this modality of balneological intervention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) is one of the most common chronic health conditions and the most prevalent arthritic disease, generally affecting elderly people (Glyn-Jones et al. 2015; Musumeci et al. 2015). OA affects synovial joints leading to cartilage destruction, deterioration of the bone, and inflammation of the synovium, thus causing stiffness, swelling of the joint, pain, and mobility impairment (Glyn-Jones et al. 2015; Kapoor 2015). Although it has been traditionally considered a non-inflammatory degenerative arthropathy, it is now largely accepted that OA is a complex condition and that both soluble and cellular mediators of immunity are strongly involved in the pathogenesis and aggravation of this condition (Scanzello and Goldring 2012; Rahmati et al. 2016; Loukov et al. 2018; Saberi Hosnijeh et al. 2019). While the pathophysiological mechanisms of OA are yet to be fully known, several immune and inflammatory processes within the joint and synovium are clearly key mediators in these processes (Scanzello and Goldring 2012; Rahmati et al. 2016; Mathiessen and Conaghan 2017). Apart from inflammatory events taking place locally in joint tissues, low-grade systemic inflammation plays a crucial role in this pathology, due to the fact that it could lead to the onset and progression of OA. In turn, inflammatory mediators produced in local tissues could be reflected systemically and contribute to perpetuating low-grade systemic inflammation (Sohn et al. 2012; Berenbaum 2013; Robinson et al. 2016; Gálvez et al. 2017; Scanzello 2017; Ortega et al. 2018). Patients with OA also present an immune-neuroendocrine dysregulation affecting the negative feedback between the inflammatory and stress responses, aggravating the systemic low-grade inflammation present in this condition (Gálvez et al. 2017). Furthermore, neutrophils from patients with OA have a reduced functional capacity, pointing to a suppression of the innate immune system as well as an altered innate/inflammatory regulation (Gálvez et al. 2017). Moreover, circulating monocytes seem to present an activated state in this pathology (Loukov et al. 2018).
Balneotherapy and some of its modalities, like pelotherapy or mud therapy, are effective, well-tolerated, non-invasive, complementary strategies in the treatment of OA (Harzy et al. 2009; Fioravanti et al. 2012, 2015a; Espejo-Antúnez et al. 2013a; Liu et al. 2013; Forestier et al. 2016; Antonelli et al. 2018; Fraioli et al. 2018). Pelotherapy is a balneological intervention in which peloids or muds are applied externally for therapeutic purposes. Muds used in pelotherapy consist of a maturated mixture of geologic fine-grained materials, mineral-medicinal water, and biological compounds (Gomes et al. 2013).
Together with the proven clinical benefits of mud therapy, recently the physiological mechanisms underlying the amelioration of OA symptoms are being increasingly understood. Several authors have proposed that inflammatory (Bellometti and Galzigna 1998; Fioravanti et al. 2011a, 2015b; Ortega et al. 2017; Gálvez et al. 2018a) and stress mediators (Ortega et al. 2017, 2018) are involved in the physiological effects of mud therapy and thus could take part in the biological basis underlying its clinical benefits. However, the effects of pelotherapy on the cellular immune response have not yet been clearly elucidated. Systemically, only neutrophils and regulatory T cells have been assessed in OA patients after pelotherapy interventions, showing a potential role in the anti-inflammatory and innate/inflammatory bioregulatory effects in this treatment (Gálvez et al. 2018a). Nevertheless, the influence of pelotherapy on circulating monocytes from elderly OA patients is still fully unknown. Given the importance of these cells not only in cytokine production but also in innate immune defensive functions, it is important to examine whether monocyte defensive functions are affected by mud therapy.
Therefore, one objective of the present study was to determine the effect of a cycle of mud-bath therapy on the phagocytic process (chemotaxis, phagocytosis, and respiratory or oxidative burst) of circulating monocytes from elderly patients with knee OA. In addition, the other objective was to discuss recent results regarding innate/inflammatory responses as proposed mechanisms of the effectiveness of pelotherapy in OA.
Materials and methods
Patients and experimental design
Using the same experimental design and group of patients of previous studies from our group (Gálvez et al. 2018a), we evaluated the effect of hyperthermia-induced stress by pelotherapy on the phagocytic process (chemotaxis, phagocytosis, and oxidative burst) of isolated monocytes from elderly patients with knee OA. This prospective study was conducted in the spa centre ‘El Raposo’, Puebla de Sancho Pérez, Badajoz, Spain, in a group of elderly patients with OA participating in the Social Thermalism Programme organised by the Ministry of Health, Social Policy, and Equality of Spain.
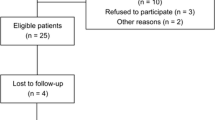
Thirty-six patients from 62 to 80 years in age (mean age ± SEM 70.71 ± 1.05 years) diagnosed with primary knee OA enrolled in the study. At first, 68 patients were assessed for eligibility and were informed of the investigation. The inclusion criteria were to be 60 years old and above, and to be diagnosed with primary knee OA by a rheumatologist following the ACR criteria (Altman et al. 1986). The exclusion criteria were having any infection, any neoplastic, cardiopulmonary, vascular, inflammatory, immune, or other musculoskeletal pathology, having had a total or partial knee replacement, having received any modality of physical therapy or intra-articular injections of corticosteroids or hyaluronic acid in the previous six months, having consumed NSAIDs in the previous three days, or having received corticosteroid or anti-cytokine therapy. Forty-two patients complied with the inclusion and exclusion criteria and were included in the study. Finally, six patients were lost to follow-up, leaving 36 patients for evaluation. All participants gave their written informed consent prior to inclusion in the study.
Blood sampling was performed between 8 and 9 a.m. both in baseline and in post-treatment state. Baseline sampling was carried out before starting the sessions of mud-bath therapy and post-treatment sampling was performed 24 h after the last session of mud therapy, in order to avoid the assessment of the acute effect of the intervention. The study received approval from the Ethical Committee of the University of Extremadura, Spain (reference number 036-15) in accordance with the guidelines of the European Community Council Directives and the Helsinki Declaration.
Balneological intervention
The balneological intervention was the same as reported in previous studies from our group (Ortega et al. 2017; Gálvez et al. 2018a). Briefly, mineral-medicinal water of ‘El Raposo’ spa contains bicarbonate, calcium, chloride, and sodium as predominant ions. The peloid is composed of around 40% mineral-medicinal water and 60% solid content (a mixture of silt, clay, and sand) (Carretero et al. 2010; Pozo et al. 2013). The main microalgae species present in the mud is Monoraphidium pusillum (Gálvez et al. 2018a). Then, it matures naturally for 5–8 months in a tank together with the mineral-medicinal water until the mud presents optimal biological, chemical, and physical properties for therapeutic use.
During 10 consecutive days, the patients received a daily session of pelotherapy consisting in the whole-body application of peloid (40–42 °C) with a drying period of 45–60 min, followed by a mud bath (mineral-medicinal water and peloid at 38–40 °C) for 15 min. Mud remnants were removed with a water jet (38–40 °C) for 2 min. No adverse events were reported.
Whole blood sampling
Blood samples were drawn by antecubital fossa vein puncture and collected in heparinised tubes for the phagocytosis and oxidative burst assay by flow cytometry, and in EDTA tubes for monocyte isolation and chemotaxis assay.
Monocyte isolation
The ‘Monocyte Isolation Kit II’ (Miltenyi Biotec, Bergisch Gladbach, Germany) is an indirect magnetic labelling system for the isolation of untouched monocytes from human peripheral blood mononuclear cells (PBMC), obtaining highly pure unlabelled monocytes by negative selection or depletion of the magnetically labelled cells (non-monocytes). They are labelled using a cocktail of biotin-conjugated monoclonal antibodies against CD3, CD7, CD16, CD19, CD56, CD123, and glycophorin A, as well as anti-biotin monoclonal antibodies conjugated to MicroBeads as secondary labelling reagents. The reason for choosing this isolation technique was that, for the evaluation of chemotaxis, it was considered very important that monocytes did not suffer any direct labelling of surface molecules so that they would not become activated.
Cell pellet from isolated PBMC using Histopaque®-1077 (Sigma-Aldrich, St. Louis, MO, USA) was resuspended in 30 μL of buffer solution, 10 μL of FcR blocking reagent and 10 μL of Biotin-Antibody Cocktail. The suspension was incubated at 4–8 °C for 10 min. Then, 30 μL of buffer solution and 20 μL of Anti-Biotin MicroBeads were added, and the suspension was incubated for 15 min at 4–8 °C. After this, the cells were washed by adding 2 mL of buffer and centrifuging at 300g for 10 min. The supernatant was discarded and the cells were resuspended in 500 μL of the buffer. Then, cell suspension was applied onto an LS MACS® Column (Miltenyi Biotec, Bergisch Gladbach, Germany) in the magnetic field of a MidiMACS™ Separator (Miltenyi Biotec, Bergisch Gladbach, Germany). The magnetically labelled non-monocytes were depleted by retaining them on the magnetic column as they passed through, while the effluent with unlabelled cells was collected, representing the monocyte fraction. Then, the column was washed three times by adding 3 mL of buffer each time to allow total recovery of non-labelled cells, collecting the effluent in the same tube of isolated monocytes. The purity of monocyte suspension was determined by flow cytometry, obtaining monocytes with purity higher than 90%, and cell viability higher than 98% as determined by the trypan blue exclusion test.
Chemotaxis assay in isolated monocytes
To evaluate monocyte chemotaxis, a modification (Ortega et al. 1996) of the original technique described by Boyden (1962) was carried out, using a Boyden chamber. This technique is appropriate for quantifying chemotaxis of isolated inflammatory cells (Ortega et al. 1997). N-Formyl-methionyl-leucyl-phenylalanine peptide (fMLP; 10−8 M, Sigma-Aldrich) was placed in the lower compartment of the Boyden chamber to act as a chemoattractant. A total of 300 μL of monocyte suspension (106 cells/mL in Hank’s medium [Sigma-Aldrich]) were placed into the upper compartment of the Boyden chamber. Between the upper and the lower compartment, there was an isoporepolyvinylpyrrolinone-free polycarbonate filter with a pore diameter of 5 μm (Millipore, Burlington, MA, USA). The cells were allowed to migrate through the filter, from the upper face to the lower face following the fMLP gradient, for 90 min at 37 °C in a 5% CO2 atmosphere. After incubation, the filters were removed from the chambers, fixed in methanol, and stained using the conventional haematoxylin-eosin method. The results were expressed as the chemotaxis index, which represents the number of monocytes counted in 16 fields at random on the lower face of the filter under × 100 phase-contrast microscopy (Ortega et al. 1997).
Study of the monocytes’ phagocytic capacity and oxidative burst in whole blood by flow cytometry
Monocytes’ phagocytic capacity and oxidative burst (production of superoxide anion, O2−) against opsonised bacteria (Staphylococcus epidermidis) were assessed in circulating monocytes using a modification of the Robinson and Carter (1993) technique, via flow cytometry analysis of heparinised whole blood. This quantitative technique allows a very accurate determination of the percentage of active monocytes that have ingested bacteria (phagocytic monocytes) and have produced O2− (O2− producing monocytes), as well as the number of bacteria ingested per cell (which reflects phagocytic activity) and O2− production per cell (oxidative burst activity), as measured by using the mean fluorescence intensity (mfi) of active cells.
Briefly, the bacteria were stained with fluorescein isothiocyanate (FITC) (1 μg/mL) and opsonised with human serum. A total of 200 μL of blood from each patient were incubated (for 1 h at 37 °C in the dark with shaking) with 50 μL of opsonised bacteria, Hoechst 33342 (10 μg/mL), 7-actinomycin-D (7AAD; 1 μg/mL), 250 μL of PBS, and foetal bovine serum (FBS; 2%). For the controls, 100 μL of blood was incubated with 10 μg/mL of Hoechst 33342, 1 μg/mL of 7AAD, 400 μL of PBS, and 2% FBS. Blood samples were then analysed with a flow cytometer (MACSQuant® VYB, Miltenyi Biotec, GmbH, Germany) (Gálvez et al. 2017).
Statistical analysis
The values are expressed as mean ± standard error of the mean (SEM). The normal distribution of the variables was checked using the Kolmogorov-Smirnov normality test. The Student t test for paired samples was used for comparison between groups, taking p < 0.05 as the minimum significance level. Calculations were performed using the IBM® SPSS® Statistics version 22 software package.
Results
We evaluated the effect of the cycle of mud-bath therapy on the main stages of the phagocytic process in peripheral monocytes. In response to the antigenic stimulus fMLP as a chemoattractant, the chemotactic capacity of circulating monocytes dramatically increased (p < 0.001) after the cycle of pelotherapy (Fig. 1). Figures 2 and 3 show the results corresponding to phagocytosis, which constitutes the next stage of the phagocytic process. The percentage of ‘phagocytic monocytes’ as well as their phagocytic activity did not change significantly after the intervention (p > 0.05; Fig. 2 and Fig. 3). Finally, the potential oxygen-dependent microbicidal capacity of monocytes was evaluated through the oxidative burst. Results regarding O2− production are shown in Figs. 4 and 5. Both the percentage of ‘O2− producing monocytes’ (p < 0.001; Fig. 4) and their oxidative burst activity (p < 0.01; Fig. 5) significantly decreased after the treatment.
Percentage of circulating ‘phagocytic monocytes’ (monocytes that have phagocytised at least a bacterium as evaluated by flow cytometry) in OA patients before (pre-treatment) and after (post-treatment) the cycle of pelotherapy. Each column represents the mean ± SEM of independent experiments performed in duplicate for each participant. No statistically significant changes were found
Phagocytic activity of circulating monocytes (mfi, mean fluorescence intensity values as determined by flow cytometry) in OA patients before (pre-treatment) and after (post-treatment) the cycle of pelotherapy. Each column represents the mean ± SEM of independent experiments performed in duplicate for each participant. No statistically significant changes were found
Oxidative burst activity (mfi, mean fluorescence intensity values as determined by flow cytometry) of circulating monocytes from OA patients before (pre-treatment) and after (post-treatment) the cycle of pelotherapy. Each column represents the mean ± SEM of independent experiments performed in duplicate for each participant. **p < 0.01 with respect to basal values
Discussion
Although pelotherapy has been used empirically for millennia, over the last few years, the clinical benefits of mud therapy in OA patients have been reported in high-quality studies (meta-analysis, systematic reviews, and randomised controlled trials) (Espejo-Antúnez et al. 2013a, 2013b; Liu et al. 2013; Fioravanti et al. 2015a; Fraioli et al. 2018). Mud therapy improves several clinical parameters such as analgesic drug consumption, function, stiffness, pain, and quality of life (Espejo-Antúnez et al. 2013a; Fraioli et al. 2018). Because of this, and since it has little to no adverse effects, pelotherapy is an important complementary treatment in the management of OA, particularly in elderly patients, for whom treatment options are usually limited because of the higher risk of adverse events due to the presence of multiple pathologies and prescribed medications (McAlindon et al. 2014). However, the biological mechanisms of effectiveness underlying these clinical benefits have not yet been fully elucidated.
We had previously found that an immune-neuroendocrine dysregulation involving systemic inflammatory and stress mediators and innate immune cell function is present in OA, as was manifested in the elevated concentrations of inflammatory cytokines (IL-1β, TNF-α, IL-8, IL-6, and TGF-β) as well as in the elevated levels of extracellular 72 kDa heat shock protein (eHsp72) and decreased levels of cortisol, together with the depression of the functional capacity of neutrophils (phagocytic and microbicidal activities), compared with healthy age-matched controls (Gálvez et al. 2017). The elevated systemic inflammatory state present in OA also contributes to perpetuate the pro-inflammatory and catabolic events occurring within the joint as well as systemic low-grade inflammation, hence playing an important part in the onset and progression of this pathology (Gálvez et al. 2017; Ortega et al. 2018). An altered stress response and abnormal immune-neuroendocrine regulation could also explain the increased concentrations of inflammatory cytokines. In this context, low concentration of cortisol can be insufficient to inhibit excessive cytokine release (Gálvez et al. 2017), and increased serum concentration of eHsp72 in OA patients might induce inflammatory cells to release more pro-inflammatory cytokines, establishing a positive feedback that could play a pivotal role in the perpetuation of the elevated inflammatory response (Gálvez et al. 2017; Ortega et al. 2018). In addition, the suppression of the neutrophils’ defences against pathogens suggested an altered bioregulation of the innate/inflammatory responses in OA (Gálvez et al. 2017). These novel results profiling the altered systemic inflammatory and immune-neuroendocrine status of elderly OA patients laid the foundation for the evaluation of mechanisms of the effectiveness of mud therapy in this pathology. These biomarkers play a key role in the pathophysiology and symptomatology of OA, and could explain the clinical improvements found after pelotherapy. This is why these biomarkers were identified as potential targets for this intervention.
In this way, our group carried out a comprehensive evaluation of the effect of pelotherapy on the systemic cytokine profile, cortisol and eHsp72 serum concentrations, and neutrophil function in the same OA patients. Clinical improvements caused by mud therapy in OA patients (knee flexion angle, OA-related pain, stiffness, physical function, and health-related quality of life) were mediated, at least partly, by its systemic anti-inflammatory effects, neuroendocrine-immune regulation, and innate/inflammatory bioregulation (Ortega et al. 2017; Gálvez et al. 2018a). The global anti-inflammatory effect of this strategy was manifested in a dramatic reduction in the elevated systemic levels of the inflammatory cytokines IL-1β, TNF-α, IL-8, IL-6, and TGF-β (Ortega et al. 2017), as well as eHsp72 (Ortega et al. 2017, 2018). Besides, the circulating concentrations of IL-8 (linked to joint pain) positively correlated with pain scores, and TGF-β (associated with osteophyte formation and cartilage damage) levels negatively correlated with knee flexion angle, revealing that patients with lower IL-8 concentrations had less pain, and patients with lower TGF-β levels presented better knee mobility and functionality after a cycle of mud therapy (Gálvez et al. 2018a). Thus, the decrease in systemic inflammation caused by the pelotherapy intervention constitutes a mechanism of effectiveness underlying pain and stiffness amelioration, and reduction in impairment. Moreover, the patients presented an increase in their serum concentrations of cortisol, plausibly as a result of the elevated temperature of muds and waters, since thermal stress is able to induce neuroendocrine reactions such as the activation of the hypothalamic-pituitary-adrenal axis (Ortega et al. 2017). These increased physiological levels of cortisol are the reflection of the stabilisation of the negative feedback between the cytokine-mediated inflammatory response and the HPA-axis-mediated stress response. This stabilisation could also play a part in the decline in the inflammatory response, in agreement with the decrease in the systemic concentrations of inflammatory cytokines and eHsp72 (Ortega et al. 2017). The paradoxical decline in serum eHsp72 concentration (probably due to an adaptation at the end of the intervention) strongly indicates a role for eHsp72 in the systemic anti-inflammatory effects of mud therapy (Ortega et al. 2017, 2018), since it could trigger an anti-inflammatory Hsp-cytokine feedback, playing an important part in the reduction of inflammatory cytokines (Ortega et al. 2012; Gálvez et al. 2017; Ortega et al. 2018). Lastly, although there was a global anti-inflammatory effect in this therapy, this was not accompanied by additional suppression of the defences of the innate immune system. Instead, neutrophils’ defensive functions increased considerably, reflecting a greater defence capacity against pathogens and therefore a potential lower susceptibility to infections (Gálvez et al. 2018a). The increase in cortisol levels could be mediating this stimulation, considering that stress-induced physiological concentrations of glucocorticoids stimulate some of the functions of the innate immune system (Forner et al. 1995; Ortega 2003). This improvement in the neutrophil-mediated innate immune response along with the observed dramatic anti-inflammatory effects has been proposed as a ‘bioregulatory effect of balneotherapy’ (Ortega 2016; Gálvez et al. 2018a).
Additionally, in the same patients, we assessed the effect of the same mud therapy protocol on two important immunoregulatory T cell subsets in inflammatory pathologies such as CD8+ and CD4+ regulatory T (Treg) cells. The percentage of CD8+ CD28− Treg cells increased, with a potential role in contributing to reduce the inflammatory status and downregulate excessive inflammatory activity. On the other hand, the percentage of CD4+ CD25+ FOXP3+ Treg cells decreased, possibly indicative of the anti-inflammatory effects of pelotherapy. The decrease in the previously elevated inflammatory state might explain that less CD4+ Treg cells were needed to counterbalance the excessive inflammatory responses in this pathology (Gálvez et al. 2018a). In this way, it was demonstrated that Treg cells contribute, at least partially, to the anti-inflammatory effects of pelotherapy in OA patients.
Indeed, recently there have been a few other studies evaluating different inflammatory and cartilage degradation mediators in OA patients undergoing pelotherapy, but analysed separately in different studies and patients. Fioravanti and co-workers (Fioravanti et al. 2011a, 2015b) have reported that circulating concentrations of the adipokines adiponectin and resistin, important mediators of inflammation and cartilage metabolism, decrease after pelotherapy in patients with OA. Moreover, their research group has also found mud therapy to modulate the expression of microRNA related to cartilage degradation (Giannitti et al. 2017). Matrix metalloproteinases, which are also involved in cartilage turnover, contribute to extracellular matrix integrity after mud therapy, as shown in the reduction in MMP-3 serum levels after the intervention in OA patients (Bellometti et al. 2005). A reduction in prostaglandin E2 and leukotriene B4 systemic levels after pelotherapy has also been reported in OA patients (Bellometti and Galzigna 1998).
As discussed above, physiological mechanisms of the effectiveness of mud therapy are beginning to be increasingly understood but, what gives rise to these biological effects? It has been proposed that the mechanism of action of mud therapy is complex and multifactorial (Fioravanti et al. 2011b; Morer et al. 2017). Although temperature plays a central role in this thermotherapeutic treatment due to the fact that mineral-medicinal water and mud are very good in the transference of heat (Gálvez et al. 2018a, 2018b), the peculiarity of mud therapy is that, in addition to their physical properties, the chemical and biological composition of mineral-medicinal water and mud contribute to the achievement of the beneficial effects of this therapy (Flusser et al. 2002; Beer et al. 2003; Odabasi et al. 2008; Sarsan et al. 2012; Gomes et al. 2013). Hormesis has been proposed as a potential phenomenon by which adaptive beneficial effects occur in pelotherapy, and it is related to heat and/or biochemical elements such as hydrogen sulphide (H2S) and radon (Gálvez et al. 2018b). In any case, even though some of these mechanisms could be involved to a lesser or larger degree in the physiological responses induced by balneotherapy, these responses consist mainly of neuroendocrine and inflammatory/immune effects that have been investigated fundamentally in OA.
It is noteworthy that even though the main physiological effects of pelotherapy in OA seem to be anti-inflammatory effects, very few studies have assessed the influence of this intervention on innate immune cells in this context (Ortega et al. 2017; Gálvez et al. 2018a). Particularly, there are no studies on the role of monocytes in mud therapy in OA patients. Phagocytic cells such as monocytes carry out their non-specific defence function through the phagocytic process, which can be divided into different stages, from the time point when the phagocyte leaves the blood vessel to when the pathogen is destroyed. These stages, particularly modulated under physiological stress, are adherence, chemotaxis, attachment, ingestion, and killing of the foreign agent (Ortega-Rincón 1994; Ortega 2003). In the present work, we also present original results regarding monocyte function, specifically the phagocytic process, focusing on chemotaxis, phagocytosis, and oxidative or respiratory burst as an indirect measurement of the oxygen-dependent microbicidal capacity but also indicative of dysregulated oxidative stress.
Chemotaxis is a necessary event of monocytes/macrophages for the beginning of the phagocytic process and eventually reaching the focus of infection. In fact, chemotaxis is accepted as a good index of the activation status of macrophages in the context of innate immune function (Doherty et al. 1987), and a good chemotactic capacity is crucial in avoiding infectious diseases, particularly in aged individuals and under stress situations (Forner et al. 1994; Ortega et al. 1997). The chemotactic capacity of circulating monocytes dramatically increased after the cycle of mud-bath therapy, indicating that the balneological intervention stimulated the function of monocytes allowing them to reach damaged tissue or a focus of infection in response to a gradient of a chemotactic agent (in our case fMLP). This effect can be mediated by the increased circulating cortisol concentration after the hyperthermia-induced stress during the pelotherapy intervention (Ortega et al. 2017), since it has been clearly demonstrated that physiologically increased glucocorticoid concentrations under stress situations mediate the increased chemotaxis of macrophages (Ortega et al. 1997). Considering that high pharmacological concentrations of glucocorticoids can inhibit the immune response, including chemotaxis (Rinehart et al. 1974), but physiological concentrations of glucocorticoids can stimulate it (Ortega et al. 1992, 1997), it is plausible to speculate that cortisol could be involved in the proposed potential hormetic and bioregulatory effects on the innate/inflammatory immune response mediated by hyperthermia-induced stress in balneological interventions (Ortega et al. 2017; Gálvez et al. 2018a, 2018b).
However, differently to the results previously observed in circulating neutrophils (Gálvez et al. 2018a), neither the percentage of ‘phagocytic monocytes’ nor their phagocytic activity changed significantly after the intervention. The reason can be that neutrophils, but not monocytes, are the main innate immune cells involved in phagocytosis; being macrophages the ‘professional phagocytes’ once differentiated in the tissues from circulating monocytes. Results regarding O2− production also showed a different response of monocytes with respect to our previous studies on neutrophils after the balneological intervention (Gálvez et al. 2018a). Both the percentage of ‘O2− producing monocytes’ and their oxidative burst activity significantly decreased after the treatment. In this context, the microbicidal activity, especially when evaluated by using indirect techniques, such as reactive oxygen species (ROS) production, is the most controversial stage of the phagocytic process when interpreting the effects of physiological stressful situations. For example, it has been reported that sometimes no changes or a decrease in the O2− production of macrophages (which could be interpreted as an index of the oxygen-dependent microbicidal capacity of these cells) do not correspond with the increased global microbicidal activity measured by direct candidacidal techniques (Ortega et al. 2006). It is also important to notice that apart from having a microbicidal function, ROS released during the oxidative burst are also greatly toxic to tissues, being able to indiscriminately damage the target as well as the surrounding cells and other biomolecules. Indeed, all of this results in the induction of local inflammatory and immune responses, and contributes to extending tissue injury (Braga et al. 2008). Then, although one cannot obviate a potential pelotherapy-induced decrease in the oxygen-dependent microbicidal activity mediated by monocytes, it is possible to speculate that the balneological intervention evaluated in this study provokes the induction of a higher anti-inflammatory and patrolling profile of monocytes, with an elevated chemotactic capacity and decreased production of oxygen reactive species, versus a pro-inflammatory profile, then contributing to repair tissue damage. In this way, further studies evaluating changes in the CD14+CD16++ (patrolling monocytes) and CD14++CD16− and CD14++CD16+ (phagocytic and pro-inflammatory monocytes) monocyte populations seem to be necessary (Yang et al. 2014; Ziegler-Heitbrock 2015).
Conclusion
A review of the current literature highlights that mud therapy affects several immune, inflammatory, and stress mediators in elderly OA patients. According to our previous studies, clinical benefits are, at least partially, mediated by systemic anti-inflammatory effects involving inflammatory cytokines, cortisol, eHsp72, and regulatory T cells. Neuroendocrine-immune regulation of the inflammatory/stress feedback response, involving pro-inflammatory cytokines and glucocorticoids, plays a key role in these effects. Neutrophil-mediated innate immune response improves after mud therapy, reflecting a greater defence capacity against pathogens. We have also presented original results regarding monocyte function, suggesting that pelotherapy could induce a switch to an anti-inflammatory patrolling monocytes profile.
In future studies, these mechanisms of effectiveness could be analysed in the context of long-term efficacy, assessing the potential persistence of these biological effects in relation to the presence of clinical benefits. All of this, together with studies elucidating the role of temperature and physico-chemical and biological composition of waters and peloids in the mechanism of action of these therapies, will help to further understand the scientific basis for the clinical improvement in several pathologies, thus allowing to provide more precise therapeutic indications.
References
Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M, Howell D, Kaplan D, Koopman W, Longley S, Mankin H, McShane DJ, Medsger T, Meenan R, Mikkelsen W, Moskowitz R, Murphy W, Rothschild B, Segal M, Sokoloff L, Wolfe F (1986) Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 29(8):1039–1049
Antonelli M, Donelli D, Fioravanti A (2018) Effects of balneotherapy and spa therapy on quality of life of patients with knee osteoarthritis: a systematic review and meta-analysis. Rheumatol Int 38(10):1807–1824
Beer AM, Junginger HE, Lukanov J, Sagorchev P (2003) Evaluation of the permeation of peat substances through human skin in vitro. Int J Pharm 253(1–2):169–175
Bellometti S, Galzigna L (1998) Serum levels of a prostaglandin and a leukotriene after thermal mud pack therapy. J Investig Med 46(4):140–145
Bellometti S, Richelmi P, Tassoni T, Bertè F (2005) Production of matrix metalloproteinases and their inhibitors in osteoarthritic patients undergoing mud bath therapy. Int J Clin Pharmacol Res 25:77–94
Berenbaum F (2013) Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr Cartil 21(1):16–21
Boyden S (1962) The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med 115:453–466
Braga PC, Sambataro G, Dal Sasso M, Culici M, Alfieri M, Nappi G (2008) Antioxidant effect of sulphurous thermal water on human neutrophil bursts: chemiluminescence evaluation. Respiration 75(2):193–201
Carretero MI, Pozo M, Martín-Rubí JA, Pozo E, Maraver F (2010) Mobility of elements in interaction between artificial sweat and peloids used in Spanish spas. Appl Clay Sci 48(3):506–515
Doherty DE, Haslett C, Tonnesen MG, Henson PM (1987) Human monocyte adherence: a primary effect of chemotactic factors on the monocyte to stimulate adherence to human endothelium. J Immunol 138(6):1762–1771
Espejo-Antúnez L, Cardero-Durán MA, Garrido-Ardila EM, Torres-Piles S, Caro-Puértolas B (2013a) Clinical effectiveness of mud pack therapy in knee osteoarthritis. Rheumatology (Oxford) 52(4):659–668
Espejo-Antúnez L, Caro-Puértolas B, Ibáñez-Burgos B, Porto-Payán JM, Torres-Piles ST (2013b) Effects of mud therapy on perceived pain and quality of life related to health in patients with knee osteoarthritis. Reumatol Clin 9(3):156–160
Fioravanti A, Cantarini L, Bacarelli MR, de Lalla A, Ceccatelli L, Blardi P (2011a) Effects of spa therapy on serum leptin and adiponectin levels in patients with knee osteoarthritis. Rheumatol Int 31(7):879–882
Fioravanti A, Cantarini L, Guidelli GM, Galeazzi M (2011b) Mechanisms of action of spa therapies in rheumatic diseases: what scientific evidence is there? Rheumatol Int 31(1):1–8
Fioravanti A, Giannitti C, Bellisai B, Iacoponi F, Galeazzi M (2012) Efficacy of balneotherapy on pain, function and quality of life in patients with osteoarthritis of the knee. Int J Biometeorol 56(4):583–590
Fioravanti A, Bacaro G, Giannitti C, Tenti S, Cheleschi S, Gui Delli GM, Pascarelli NA, Galeazzi M (2015a) One-year follow-up of mud-bath therapy in patients with bilateral knee osteoarthritis: a randomized, single-blind controlled trial. Int J Biometeorol 59(9):1333–1343
Fioravanti A, Giannitti C, Cheleschi S, Simpatico A, Pascarelli NA, Galeazzi M (2015b) Circulating levels of adiponectin, resistin, and visfatin after mud-bath therapy in patients with bilateral knee osteoarthritis. Int J Biometeorol 59(11):1691–1700
Flusser D, Abu-Shakra M, Friger M, Codish S, Sukenik S (2002) Therapy with mud compresses for knee osteoarthritis: comparison of natural mud preparations with mineral-depleted mud. J Clin Rheumatol 8(4):197–203
Forestier R, Erol Forestier FB, Francon A (2016) Spa therapy and knee osteoarthritis: a systematic review. Ann Phys Rehabil Med 59(3):216–226
Forner MA, Collazos ME, Barriga C, De la Fuente M, Rodriguez AB, Ortega E (1994) Effect of age on adherence and chemotaxis capacities of peritoneal macrophages. Influence of physical activity stress. Mech Ageing Dev 75(3):179–189
Forner MA, Barriga C, Rodriguez AB, Ortega E (1995) A study of the role of corticosterone as a mediator in exercise-induced stimulation of murine macrophage phagocytosis. J Physiol 488(3):789–794
Fraioli A, Mennuni G, Fontana M, Nocchi S, Ceccarelli F, Perricone C, Serio A (2018) Efficacy of spa therapy, mud-pack therapy, balneotherapy, and mud-bath therapy in the management of knee osteoarthritis. A systematic review. Biomed Res Int 2018:1042576
Gálvez I, Torres-Piles S, Hinchado MD, Alvarez-Barrientos A, Torralbo-Jimenez P, Guerrero J, Martín-Cordero L, Ortega E (2017) Immune-neuroendocrine dysregulation in patients with osteoarthritis: a revision and a pilot study. Endocr Metab Immune Disord Drug Targets 17(1):78–85
Gálvez I, Torres-Piles S, Ortega E (2018a) Innate/inflammatory bioregulation and clinical effectiveness of whole-body hyperthermia (balneotherapy) in elderly patients with osteoarthritis. Int J Hyperth 35(1):340–347
Gálvez I, Torres-Piles S, Ortega-Rincón E (2018b) Balneotherapy, immune system, and stress response: a hormetic strategy? Int J Mol Sci 19(6):1687
Giannitti C, De Palma A, Pascarelli NA, Cheleschi S, Giordano N, Galeazzi M, Fioravanti A (2017) Can balneotherapy modify microRNA expression levels in osteoarthritis? A comparative study in patients with knee osteoarthritis. Int J Biometeorol 61(12):2153–2158
Glyn-Jones S, Palmer AJR, Agricola R, Price AJ, Vincent TL, Weinans H, Carr AJ (2015) Osteoarthritis. Lancet 386(9991):376–387
Gomes C, Carretero MI, Pozo M, Maraver F, Cantista P, Armijo F, Legido JL, Teixeira F, Rautureau M, Delgado R (2013) Peloids and pelotherapy: historical evolution, classification and glossary. Appl Clay Sci 75-76:28–38
Harzy T, Ghani N, Akasbi N, Bono W, Nejjari C (2009) Short- and long-term therapeutic effects of thermal mineral waters in knee osteoarthritis: a systematic review of randomized controlled trials. Clin Rheumatol 28(5):501–507
Kapoor M (2015) Pathogenesis of osteoarthritis. In: Kapoor M, Mahomed NN (eds) Osteoarthritis: pathogenesis, diagnosis, available treatments, drug safety, regenerative and precision medicine. Springer, Cham, pp 1–28
Liu H, Zeng C, Gao SG, Yang T, Luo W, Li YS, Xiong YL, Sun JP, Lei GH (2013) The effect of mud therapy on pain relief in patients with knee osteoarthritis: a meta-analysis of randomized controlled trials. J Int Med Res 41(5):1418–1425
Loukov D, Karampatos S, Maly MR, Bowdish DME (2018) Monocyte activation is elevated in women with knee-osteoarthritis and associated with inflammation, BMI and pain. Osteoarthr Cartil 26(2):255–263
Mathiessen A, Conaghan PG (2017) Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther 19(1):18
McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, Hawker GA, Henrotin Y, Hunter DJ, Kawaguchi H, Kwoh K, Lohmander S, Rannou F, Roos EM, Underwood M (2014) OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr Cartil 22(3):363–388
Morer C, Roques CF, Françon A, Forestier R, Maraver F (2017) The role of mineral elements and other chemical compounds used in balneology: data from double-blind randomized clinical trials. Int J Biometeorol 61:2159–2173
Musumeci G, Aiello FC, Szychlinska MA, Di Rosa M, Castrogiovanni P, Mobasheri A (2015) Osteoarthritis in the XXIst century: risk factors and behaviours that influence disease onset and progression. Int J Mol Sci 16(3):6093–6112
Odabasi E, Turan M, Erdem H, Tekbas F (2008) Does mud pack treatment have any chemical effect? A randomized controlled clinical study. J Altern Complement Med 14(5):559–565
Ortega E (2003) Neuroendocrine mediators in the modulation of phagocytosis by exercise: physiological implications. Exerc Immunol Rev 9:70–93
Ortega E (2016) The “bioregulatory effect of exercise” on the innate/inflammatory responses. J Physiol Biochem 72:361–369
Ortega E, Collazos ME, Barriga C, de la Fuente M (1992) Stimulation of the phagocytic function in guinea pig peritoneal macrophages by physical activity stress. Eur J Appl Physiol 64:323–327
Ortega E, Forner MA, Barriga C (1996) Effect of β-endorphin on adherence, chemotaxis and phagocytosis of Candida albicans by peritoneal macrophages. Comp Immunol Microbiol Infect Dis 19:267–274
Ortega E, Forner MA, Barriga C (1997) Exercise-induced stimulation of murine macrophage chemotaxis: role of corticosterone and prolactin as mediators. J Physiol 498(3):729–734
Ortega E, Giraldo E, Hinchado MD, Martínez M, Ibáñez S, Cidoncha A, Collazos ME, García JJ (2006) Role of Hsp72 and norepinephrine in the moderate exercise-induced stimulation of neutrophils’ microbicide capacity. Eur J Appl Physiol 98(3):250–255
Ortega E, Bote ME, Besedovsky HO, del Rey A (2012) Hsp72, inflammation, and aging: causes, consequences, and perspectives. Ann N Y Acad Sci 1261:64–71
Ortega E, Gálvez I, Hinchado MD, Guerrero J, Martín-Cordero L, Torres-Piles S (2017) Anti-inflammatory effect as a mechanism of effectiveness underlying the clinical benefits of pelotherapy in osteoarthritis patients: regulation of the altered inflammatory and stress feedback response. Int J Biometeorol 61(10):1777–1785
Ortega E, Gálvez I, Martín-Cordero L (2018) Extracellular Hsp70 and low-grade inflammation- and stress-related pathologies. In: Asea A, Kaur P (eds) Heat shock proteins and stress. Heat Shock Proteins, vol 15. Springer, Cham, pp 13–38
Ortega-Rincón E (1994) Physiology and biochemistry: influence of exercise on phagocytosis. Int J Sports Med 15(3):S172–S178
Pozo M, Carretero MI, Maraver F, Pozo E, Gómez I, Armijo F, Martín-Rubí JA (2013) Composition and physico-chemical properties of peloids used in Spanish spas: a comparative study. Appl Clay Sci 83(84):270–279
Rahmati M, Mobasheri A, Mozafari M (2016) Inflammatory mediators in osteoarthritis: a critical review of the state-of-the-art, current prospects, and future challenges. Bone 8:81–90
Rinehart JJ, Balcerzak SP, Sagone AL, LoBuglio AF (1974) Effects of corticosteroids on human monocyte function. J Clin Invest 54(6):1337–1343
Robinson JP, Carter WO (1993) Flow cytometric analysis of granulocytes. In: Bauer KD et al (eds) Clinical flow cytometry, principles and applications. Williams and Wilkins, Baltimore, pp 405–433
Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, Sokolove J (2016) Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol 12(10):580–592
Saberi Hosnijeh F, Bierma-Zeinstra SM, Bay-Jensen AC (2019) Osteoarthritis year in review 2018: biomarkers (biochemical markers). Osteoarthr Cartil 27(3):412–423
Sarsan A, Akkaya N, Ozgen M, Yildiz N, Atalay NS, Ardic F (2012) Comparing the efficacy of mature mud pack and hot pack treatments for knee osteoarthritis. J Back Musculoskelet Rehabil 25:193–199
Scanzello CR (2017) Role of low-grade inflammation in osteoarthritis. Curr Opin Rheumatol 29(1):79–85
Scanzello C, Goldring S (2012) The role of synovitis in osteoarthritis pathogenesis. Bone 51:249–257
Sohn D, Sokolove J, Sharpe O, Erhart J, Chandra P, Lahey L, Lindstrom TM, Hwang I, Boyer KA, Andriacchi TP, Robinson WH (2012) Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via toll-like receptor 4. Arthritis Res Ther 14(1):R7
Yang J, Zhang L, Yu C, Yang XF, Wang H (2014) Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res 2(1):1
Ziegler-Heitbrock L (2015) Blood monocytes and their subsets: established features and open questions. Front Immunol 6:423
Acknowledgements
We are grateful to the Facility of Bioscience Applied Techniques (STAB, University of Extremadura, Spain) and the spa centre ‘El Raposo’ for technical and human support.
Funding
This work was partially supported by the Gobierno de Extremadura-Fondo Europeo de Desarrollo Regional (EE-14-0082-4, GR15041, GR18009, IB18011). Isabel Gálvez is recipient of a ‘Formación del Profesorado Universitario (FPU)’ pre-doctoral contract (FPU15/02395) from the Ministerio de Ciencia, Innovación y Universidades, Spain.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Disclaimer
Funding sources had no role in the study design, collection, analysis, and interpretation of the data or the decision to submit the manuscript for publication.
Rights and permissions
About this article
Cite this article
Gálvez, I., Torres-Piles, S. & Ortega, E. Effect of mud-bath therapy on the innate/inflammatory responses in elderly patients with osteoarthritis: a discussion of recent results and a pilot study on the role of the innate function of monocytes. Int J Biometeorol 64, 927–935 (2020). https://doi.org/10.1007/s00484-019-01748-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-019-01748-4