Abstract
The aims of this study were to evaluate whether balneotherapy with mineral sulphate–bicarbonate–calcium water could determine substantial symptomatic improvement, and to detect any changes in the quality of life (QoL) of patients with symptomatic knee osteoarthritis (OA). This was a prospective randomized, single blind controlled trial. Sixty outpatients with primary bilateral knee OA, according to ACR criteria, were included in the study and randomized to one of two groups: group I (30 patients) was treated with a daily sulphate–bicarbonate–calcium mineral water bath; group II (30 patients), the control group, continued their regular outpatient care routine. At baseline, after 15 days and after 12 weeks, patients were evaluated by Visual Analogue Scale (VAS) for spontaneous pain, Lequesne and Womac Index for gonarthrosis, SF-36, Arthritis Impact Measurement Scale (AIMS) and symptomatic drugs consumption. We observed a significant improvement of all parameters at the end of the cycle of balneotherapy which persisted throughout the follow-up period, whereas in the control group no significant differences were noted. This symptomatic effect was confirmed by the significant reduction of symptomatic drugs consumption. The differences between the two groups were significant for all considered parameters already from the 15th day and persisted during follow-up. Tolerability of balneotherapy seemed to be good, with light and transitory side effects. Our results confirm that the beneficial effects of balneotherapy in patients with knee OA last over time, with positive effects on the painful symptomatology, a significant improvement on functional capacities and QoL. Balneotherapy can represent a useful backup to pharmacological treatment of knee OA or a valid alternative for patients who do not tolerate pharmacological treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) is the most frequently encountered rheumatic condition, and its prevalence is rising due to the increasing life span of the general population (Zhang and Jordan 2010). The knee is the most commonly affected joint and OA of the knee is a major cause of disability (Peat et al. 2001) that is destined to become an ever more important healthcare problem (Guccione et al. 1990; Fautrel et al. 2005). Current treatment of OA includes both non-pharmacologic and pharmacologic therapies (Jordan et al. 2003; Zhang et al 2007). To date, pharmacologic therapy has largely been confined to analgesics, non-steroidal anti-inflammatory drugs (NSAIDs) or selective cyclooxygenase-2 (COX-2) inhibitors (coxibs). However, the use of NSAIDs is limited by their negative side effects on the gastrointestinal tract and on cartilage metabolism (Ofman et al. 2002; Huskisson et al. 1995), while the use of coxibs is associated with an increase in cardiovascular adverse events (AEs) (Bresalier et al. 2005; Kearney et al 2006). Acetaminophen is better tolerated than NSAIDs and coxibs but does not always provide adequate pain relief (Zhang et al. 2004; Pincus et al. 2005). These reasons often prompt recourse to other complementary or alternative therapies (Zochling et al. 2004). Spa therapy is one of the most commonly used non-pharmacological approaches for OA in many European and Middle Eastern countries. However, despite the long history and popularity of spa therapy, its role in modern medicine is still not clear (Verhagen et al. 2007). Various systematic reviews and meta-analyses on balneotherapy for rheumatic diseases have recently been published (Verhagen et al. 2007; Falagas et al. 2009; Harzy et al. 2009). The authors conclude that there is encouraging evidence to suggest that balneotherapy is effective and safe for the treatment of patients with knee OA, although the results of the existing studies are not strong enough to draw firm conclusions.
Materials and methods
Aim and design
The aims of the present study were to evaluate whether balneotherapy with mineral water containing sulphate, bicarbonate, and calcium could determine substantial symptomatic improvement, and to detect any changes in the quality of life (QoL) of patients with symptomatic knee OA.
This was a prospective randomized, single blind controlled trial. The study protocol followed the Principles of the Declaration of Helsinki (1964) and later amendment and was approved by the Ethics Committee of Siena University Hospital (decision no. 30.11.07).
Subjects
Sixty outpatients of both sexes with primary bilateral knee OA, who fulfilled the ACR criteria (Altman et al. 1986) and were aged between 50 and 75 years, were included in the study between 1 February 2008 and 30 June 2008. Patients were recruited by the general practitioners in the rural area within a 30-km radius of the “Buddha Spa” Resort (Siena, Italy), and resided in the area near the spa, allowing them to continue to live at home and carry out their daily routines during the study period. All patients had been symptomatic [visual analogue scale (VAS) >30 mm] for at least 3 months prior to inclusion in the study. Radiological staging was carried out using the Kellgren method (Kellgren and Lawrence 1957); patients with a radiological score of I–III were included in the study.
Exclusion criteria were severe co-morbidity of the heart, lung, liver, cerebrum or kidney, acute illness, juvenile diabetes, varices, systemic blood disease, neoplasm, pregnancy or nursing. Patients who had undergone thermal treatments, joint lavage, arthroscopy or treatment with intra-articular hyaluronic acid during the previous 6 months, or who had been treated with intra-articular corticosteroids during the past 3 months, were excluded, as were all patients who had been treated with chondroprotective agents less than 6 months prior to the study.
Interventions
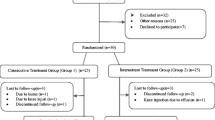
Following confirmation that the patients fulfilled the screening criteria as defined above and having obtained written informed consent, the patients were randomized 1:1 and allocated to one of two groups using a computer-generated table of random numbers: group I (30 patients) was treated with a daily sulphate-bicarbonate-calcium mineral water bath; group II (30 patients), the control group, continued their regular outpatient care routine (Fig. 1).
The block randomization list was kept by individuals who had no contact with the investigators who assigned patients to their randomized treatment, and who did not perform any patient assessment or conducted the statistical .
Patients allocated to the control group were offered spa therapy at the end of the study in order to prevent withdrawal from the study and for ethical reasons.
Group I patients were treated daily at the “Buddha Spa” centre (Siena, Italy) with a sulphate-bicarbonate-calcium (Table 1) mineral water heated at 38°C for 20 min in a bathtub for a total of 12 applications carried out over a period of 2 weeks.
Patients in both groups were advised to continue their established pharmacological and non-pharmacological treatments, with the exception of analgesic drugs (500 mg acetaminophen tablets) and NSAIDs (150 mg Diclofenac tablets, 20 mg Piroxicam tablets, 750 mg Naproxen tablets, 200 mg Aceclofenac), which were to be consumed as required and noted daily in a diary. Furthermore, we asked patients not to have corticosteroid or hyaluronic acid infiltrations, arthroscopic surgery or joint lavage during the study period, and not to begin treatment with new chondroprotective agents. Further spa treatments were not permitted during the follow-up period.
Assessments
All patients underwent general medical evaluation and rheumatologic examination by the same physician (C.G.) before the start of the study.
At the screening visit, blood samples were taken for erythrocyte sedimentation rate (ESR), c-reactive protein (CRP), electrolyte, creatinine and complete blood count analysis; urinalysis was also performed.
All the demographic, patient history and clinical data were collected through identical questionnaires.
Following basal screening, each patient was examined 3 consecutive times; at baseline (T0), after 2 weeks (T1), and after 3 months (T2) from the beginning of the study in the Rheumatology Unit.
All assessments were performed by the same investigator (B.B.) who was blinded to which study arms the patients belonged. The patients were also firmly instructed not to reveal the type of treatment performed by the investigators.
Clinical assessments at each examination included:
-
Spontaneous pain on a 0- to 100-mm visual analogue scale (VAS), with 0 representing the absence of pain;
-
The Lequesne Index of severity for knee OA (Lequesne et al. 1987);
-
The Womac Index for knee OA evaluated patients’ total pain score (W-TPS), total stiffness score (W-TSS) and total physical function score (W-TPFS) (Bellamy et al. 1991; Salaffi et al. 2003). The VAS version of the index was used, with the patient assessing each question on a 0- to 10-mm VAS and the total index score being represented by the sum of the 24 component item scores.
-
Quality of life (QoL) was recorded on two different scales, the Medical Outcomes Study 36-Item Short Form (SF-36) (Ware and Sherbourne 1992; Apolone and Mosconi 1998), which is the most popular generic health status instrument, and the Arthritis Impact Measurement Scales (AIMS), an arthritis-specific instrument (Meenan et al. 1980; Salaffi et al. 1991);
-
Consumption of NSAIDs and analgesics was reported in a daily diary given to each patient and calculated according to the number of tablets taken weekly.
All adverse events, whether reported spontaneously by the patients or observed by the physician at the spa, were reported in a diary, noting the severity and any possible correlations with the treatment. The diary was handed to us at the end of the study, whereas serious adverse events were to be reported immediately to the University of Siena’s Rheumatology Unit, and resulted in the patient’s exclusion from the study.
Statistical analysis
Power analysis (α = 0.05; β = 0.80) determined that a sample size of 30 patients in each group was needed to detect a decrease in the VAS score ≥15, with an SD of 20, at week 12 of the study.
All data are expressed as mean and standard deviation (SD) or as median and interquartile range (IQR).
The differences in variables between the two groups were calculated using a proportion test, Mann–Whitney test or t test. For the time comparisons during the follow-up, the Friedman test for repeated measures was applied, followed by the Dunn post-hoc test, if necessary.
Drug consumption was assessed using the Friedman test and the Dunn post-hoc test or the Mann–Whitney test. Where the Dunn test was significant, a one-sided Wilcoxon test was applied to identify the direction of differences. For VAS pain and W-TPFS, the effect size is equal to the mean change in score from baseline to 3 months, divided by the standard deviation of the baseline score.
Response to treatment was analysed for all patients who entered the randomized trial (Intention To Treat Analysis) using the “last-observation-carried-forward” method. For all tests, a p value of less than 0.05 was considered as statistically significant. The computations were executed using GraphPad Prism software, v. 5.0.
Results
Baseline comparison of the two groups showed no statistically significant differences in demographic and clinical characteristics or in radiological features (Table 2). The objective examination and the clinical data obtained at the basal visit showed the presence of some pathologies associated with gonarthrosis. In particular, in the group treated with balneotherapy, one patient had prostatic hypertrophy, five presented arterial hypertension (well managed with antihypertensive drugs), two patients had scars at gastric ulcer sites, three had non-insulin-dependent diabetes (controlled with oral antidiabetic agents) and two patients suffered from migraine. In the control group, two patients presented arterial hypertension (well managed with pharmacological treatment), two had non-insulin-dependent diabetes and two had prostatic hypertrophy. All patients selected concluded the follow-up period.
The median parameters over time measured throughout the follow-up (T0–T3) are summarized in Table 3. Concerning the VAS pain scale, in group I we observed a statistically significant (p < 0.001) reduction of pain at the end of treatment (T2), with a further improvement at T3 (p < 0.001). In the control group (group II) there were no significant differences during the follow-up period (p = 0.40). The Lequesne index score for knee OA decreased significantly (p < 0.001) after 2 weeks in group I, with a further improvement at the end of the follow-up period. The control group showed no significant differences between the basal time and all other times. The differences between the two groups were already significant (p < 0.001) at day 15 (T2) and persisted during the follow-up period (Table 3).
Detailed assessment of pain (W-TPS), stiffness (W-TSS) and physical function (W-TPFS) showed significant improvements in group I, persisting throughout the follow-up (p < 0.001), whereas the control group showed no significant difference from baseline (p = 0.33 in W-TPS, p = 0.85 in W-TSS, p = 0.75 in W-TPFS, respectively). The differences between the two groups were significant already from the 15th day and persisted during follow-up (Table 3).
The effect size for VAS pain and W-TPFS is shown in Table 4.
The results obtained from the SF-36 survey showed a significant improvement (p < 0.001) in both physical and mental components, persisting throughout the follow-up period (T0–T3) in group I. No significant modifications in either components of the SF-36 survey were found in the control group during the study period.
Concerning the AIMS score, there was a significant reduction at the end of treatment (T2) in group I (p < 0.001), which lasted throughout the follow up period (p < 0.001). In contrast, the control group presented no significant differences during the follow-up period (p = 0.87).
Regarding the use of symptomatic drugs, there was a significant (p < 0.001) reduction of NSAID and acetaminophen consumption in group I in T2–T3 period. In the control group, there were no significant changes in drugs consumption at any point in the follow-up (Fig. 2).
Concerning tolerability, in the group treated with balneotherapy, one patient had a febrile episode and suspended the therapy for 2 days, and another had an episode of mild hypotension, which however did not make it necessary to interrupt the therapy. In the control group, 10% of patients presented side effects, which were prevalently gastrointestinal, such as epigastralgia and gastric pyrosis. This was probably correlated to the higher use of symptomatic drugs compared to the patients in group I.
Discussion
The present randomized single blind controlled trial indicates that a cycle of sulphate–bicarbonate–calcium mineral baths has significant beneficial effects on pain, functional capacity and quality of life in patients with knee OA. All evaluation parameters were significantly reduced at the end of the balneotherapy cycle and remained stable for 12 weeks. In contrast, there were no significant modifications of the parameters measured throughout the follow-up period in the group treated with conventional therapies alone. The differences between the two groups were significant for all considered parameters already from the 15th day and persisted during follow-up. Our results showed actually high values of effect sizes in the balneotherapy group for W-TPFS and VAS pain and they are very different from the results reported by other authors (Forestier et al. 2010). This discordance may be related to the different profiles of the patients at baseline and to the different length of the follow-up. Furthermore, our results demonstrated higher values of effect sizes compared to those for other treatments of knee OA, including hyaluronic acid, paracetamol and NSAIDs (Arrich et al. 2005; Neame et al. 2004; Bjordal et al. 2004). In our opinion, this difference may be due to the favorable psychological impact of “spa therapy”
The reduction in symptomatic drug consumption shown in the group treated with balneotherapy appeared to be particularly evident until week 12 of the follow–up period. Conversely, in the control group, there was no significant change in the consumption of symptomatic drugs. The reduction in the consumption of symptomatic drugs (acetaminophen and NSAIDs) induced by balneotherapy is particularly important, above all for the elderly, considering the toxicity of NSAIDs (Ofman et al. 2002; Huskisson et al. 1995; Bresalier et al. 2005; Kearney et al. 2006), as well as their cost, given that their use is often coupled with gastro-protective therapies.
The results of our trial confirm those of studies previously reported in the literature on OA. Nguyen et al. (1997) reported that spa therapy of 3 weeks duration had a beneficial symptomatic carryover effect (6 months) in patients with lumbar spine, knee and hip OA. In a large multicentre randomized prospective clinical trial in patients with knee OA, Forestier et al. (2010) reported that a 3-week course of spa therapy, together with home exercises and the usual pharmacological treatments, offer benefits after 6 months. Our previous trial (Fioravanti et al. 2010a) demonstrated that the beneficial effects of spa therapy in patients with knee OA last over time (9 months) and are related to positive effects on the painful symptomatology and a significant improvement in functional capacities.
Verhagen et al (2007) emphasized the importance of quality of life indexes as outcome measurements in spa studies. Although there have been a number of spa studies on knee OA, few of them have included any type of quality of life assessment (Nguyen et al. 1997; Yilmaz et al. 2004; Evcik et al. 2007; Gaál et al. 2008; Fioravanti et al. 2010a). In this study, we used two different QoL scales, the SF-36 (Ware and Sherbourne 1992), which is the most popular generic health status instrument, and the AIMS, an arthritis-specific instrument (Meenan et al. 1980), both in their validated Italian versions (Apolone and Mosconi 1998; Salaffi et al. 1991). The statistically significant improvement observed in both the QoL indexes assessed in the group treated with balneotherapy, both at the end of treatment and at the 3-month follow-up, is an evident demonstration of the efficacy of this therapy on the various aspects that characterize OA. Furthermore, our results regarding SF-36 score are in line with those obtained by Ylmaz et al. (Yilmaz et al. 2004).
The mechanism by which spa therapy improves the symptoms of OA is still not fully understood (Sukenik et al. 1999; Fioravanti et al. 2010c).
The net benefit is probably the result of a combination of factors, among which the mechanical, thermal and chemical effects are most prominent.
Buoyancy, immersion, resistance and temperature all play important roles. According to the gate theory, pain relief may be due to the pressure and temperature of the water on skin; hot stimuli may also influence muscle tone and pain intensity, helping to reduce muscle spasm and to increase the pain threshold (Melzack and Wall 1965). However, seeing that experimental evidence available in this field is scarce (Shani et al. 1985), it is impossible to hypothesise whether the clinical effects observed were actually caused by the minerals present in the waters used.
Mud-bath therapy increases plasma β-endorphin levels and the secretion of corticotrophin, cortisol, growth hormone and prolactin (Kuczera and Kokot 1996; Cozzi et al. 1995).
It has recently been demonstrated that thermal mud-pack therapy induces a reduction in circulating levels of Prostaglandin E2 (PGE2), Leukotriene B4 (LTB4), Interleukin-1β (IL-1β), Tumour Necrosis Factor-α (TNF-α), Matrix Metalloproteinases-3 (MMP-3) and Adiponectin, which are important mediators of inflammation and cartilage degradation in OA (Bellometti and Galzigna 1998; Ardiç et al. 2007; Bellometti et al. 2002, 2005; Fioravanti et al. 2010b).
Spa therapy has been found to cause an increase in Insulin-like Growth Factor-1 (IGF-1) (Bellometti et al. 1997), which stimulates cartilage metabolism, and an increase in Transforming Growth Factor-β (TGF-β), a very potent immunomodulating and anti-inflammatory cytokine (Shehata et al. 2006).
Mud-packs and thermal baths have also been shown to exert positive effects on the oxidant/antioxidant system, resulting in a reduced release of reactive oxygen (ROS) and nitrogen (RNS) (Ekmekcioglu et al. 2002; Bender et al. 2007; Braga et al. 2008; Benedetti et al. 2010).
Many other non-specific factors may also contribute to the effects observed after spa therapy, including changes in the environment, the pleasant scenery and the absence of work duties (Sukenik et al. 1999; Fioravanti et al. 2010c). In our study, however, in order to temper these factors, all the patients selected lived in the area surrounding the spa, continued working and did not modify their lifestyles. Another aspect that often contributes to amplifying the effects of spa therapy is its frequent association with physio-kinesiotherapy. These treatments were excluded from the protocol of this study if they had not already begun and were not already established.
Finally, the tolerability of spa therapy seemed to be good, with light and transitory side effects even in the patients who presented pathologies associated with gonarthrosis.
Some aspects of our study are criticizable and could constitute a potential bias. The main limitations of this study are the small number of the patients enrolled, the short term of the follow-up and the lack of a double-blind-placebo design. However, the persistence of a positive clinical effect at 3 months from baseline suggests that an additional benefit over placebo was obtained.
Conclusions
In conclusion, albeit within the above mentioned limitations, our results show the beneficial effects of a cycle of sulphate–bicarbonate–calcium mineral water baths on the pain management, functional capacity and quality of life parameters in patients with knee OA. This therapy also proved to have long-lasting effects during the follow-up period (3 months). Our results confirm that balneotherapy may therefore be a useful aid, alongside the usual pharmacological and physio-kinesiotherapies, and may represent an alternative treatment in patients with OA with a high risk of drug-related side effects.
References
Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M et al (1986) Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 29:1039–1049
Apolone G, Mosconi P (1998) The Italian SF-36 Health Survey: translation, validation and norming. J Clin Epidemiol 51:1025–1036
Ardiç F, Őzgen M, Aybek H, Rota S, Cubukçu D, Gökgöz A (2007) Effects of balneotherapy on serum IL-1, PGE2 and LTB4 levels in fibromyalgia patients. Rheumatol Int 27:441–446
Arrich J, Piribauer F, Mad P, Schmid D, Klaushofer K, Müllner M (2005) Intra-articular hyaluronic acid for the treatment of osteoarthritis of the knee: systematic review and meta-analysis. CMAJ 172:1039–1043
Bellamy N, Goldsmith CH, Buchanan WW, Campbell J, Duku E (1991) Prior score availability: observations using the WOMAC osteoarthritis index. Br J Rheumatol 30:150–151
Bellometti S, Galzigna L (1998) Serum levels of a prostaglandin and a leukotriene after termal mud pack therapy. J Investig Med 46:140–145
Bellometti S, Cecchettin M, Galzigna L (1997) Mud-pack therapy in osteoarthrosis. Changes in serum levels of chondrocyte markers. Clin Chim Acta 268:101–106
Bellometti S, Galzigna L, Richelmi P, Gregotti C, Berté F (2002) Both serum receptors of tumor necrosis factor are influenced by mud pack treatment in osteoarthrosic patients. Int J Tissue React 24:57–64
Bellometti S, Richelmi P, Tassoni T, Bertè F (2005) Production of matrix metalloproteinases and their inhibitors in osteoarthritic patients undergoing mud bath therapy. Int J Clin Pharmacol Res 25:77–94
Bender T, Bariska J, Vághy R, Gomez R, Kovács I (2007) Effect of balneotherapy on the antioxidant system – a controlled pilot study. Arch Med Res 38:86–89
Benedetti S, Canino C, Tonti G, Medda V, Calcaterra P, Nappi G, Salaffi F, Canestrari F (2010) Biomarkers of oxidation, inflammation and cartilage degradation in osteoarthritis patients undergoing sulfur-based spa terapies. Clin Biochem 43:973–978
Bjordal JM, Ljunggren AE, Klovning A, Slørdal L (2004) Non-steroidal anti-inflammatory drugs, including cyclo-oxygenase-2 inhibitors, in osteoarthritic knee pain: meta-analysis of randomised placebo controlled trials. BMJ 329:1317
Braga PC, Sambataro G, Dal Sasso M, Culici M, Alfieri M, Nappi G (2008) Antioxidant effect of sulphurous thermal water on human neutrophil bursts: chemiluminescence evaluation. Respiration 75:193–201
Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, Lines C, Riddell R, Morton D, Lanas A, Konstam MA, Baron JA, Adenomatous Polyp Prevention on Vioxx (APPROVe) Trial investigators (2005) N Engl J Med 352:1092–1102
Cozzi F, Lazzarin P, Todesco S, Cima L (1995) Hypothalamic-pituitary-adrenal axis dysregulation in healthy subjects undergoing mud-bath applications. Arthritis Rheum 38:724–726
Ekmekcioglu C, Strauss-Blasche G, Holzer F, Marktl W (2002) Effect of sulfur baths on antioxidative defense systems, peroxide concentrations and lipid levels in patients with degenerative osteoarthritis. Forsch Komplementarmed Klass Naturheilkd 9:216–220
Evcik D, Kavuncu V, Yeter A, Yigit I (2007) The efficacy of balneotherapy and mud-pack therapy in patients with knee osteoarthritis. Joint Bone Spine 74:60–65
Falagas ME, Zarkadoulia E, Rafailidis PI (2009) The therapeutic effect of balneotherapy: evaluation of the evidence from randomised controlled trials. Int J Clin Pract 63:1068–1084
Fautrel B, Hilliquin P, Rozenberg S, Allaert FA, Coste P, Leclerc A, Rossignol M (2005) Impact of osteoarthritis: results of nationwide survey of 10,000 patients consulting for OA. Joint Bone Spine 72:235–240
Fioravanti A, Iacoponi F, Bellisai B, Cantarini L, Galeazzi M (2010a) Short- and long-term effects of spa therapy in knee osteoarthritis. Am J Phys Med Rehabil 89:125–132
Fioravanti A, Cantarini L, Bacarelli MR, de Lalla A, Ceccatelli L, Blardi P (2010b) Effects of Spa therapy on serum leptin and adiponectin levels in patients with knee osteoarthritis. Rheumatol Int (in press)
Fioravanti A,Cantarini L, Guidelli GM, Galeazzi M (2010c) Mechanisms of action of spa therapies in rheumatic diseases: what scientific evidence is there? Rheumatol Int (in press)
Forestier R, Desfour H, Tessier JM, Francon A, Foote AM, Genty C, Rolland C, Roques CF, Bosson JL (2010) Spa therapy in the treatment of knee osteoarthritis: a large randomised multicentre trial. Ann Rheum Dis 69:660–665
Gaál J, Varga J, Szekanecz Z, Kurkó J, Ficzere A, Bodolay E, Bender T (2008) Balneotherapy in elderly patients: effect on pain from degenerative knee and spine conditions and on quality of life. Isr Med Assoc J 10:365–369
Guccione AA, Felson DT, Anderson JJ (1990) Defining arthritis and measuring functional status in elders: methodological issues in the study of disease and physical disability. Am J Public Health 80:945–949
Harzy T, Ghani N, Akasbi N, Bono W, Nejjari C (2009) Short-and long-term therapeutic effects of thermal mineral waters in knee osteoarthritis: a systematic review of randomized controlled trials. Clin Rheumatol 28:501–507
Huskisson EC, Berry H, Gishen P, Jubb RW, Whitehead J (1995) Effects of antiinflammatory drugs on the progression of osteoarthritis of the knee. LINK Study Group. Longitudinal investigation of nonsteroidal antiinflammatory drugs in knee osteoarthritis. J Rheumatol 22:1941–1946
Jordan KM, Arden NK, Doherty M, Bannwarth B, Bijlsma JW, Dieppe P et al (2003) EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 62:1145–1155
Kearney PM, Baigent C, Godwin J, Halls H, Emberson JR, Patrono C (2006) Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ 332:1302–1308
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16:494–502
Kuczera M, Kokot F (1996) Effect of spa therapy on the endocrine system. I. Stress reaction hormones. Pol Arch Med Wewn 95:11–20
Lequesne MG, Mery C, Samson M, Gerard P (1987) Indexes of severity for osteoarthritis of the hip and knee. Validation–value in comparison with other assessment tests. Scand J Rheumatol Suppl 65:85–89
Meenan RF, Gertman PM, Mason JH (1980) Measuring health status in arthritis. The arthritis impact measurement scales. Arthritis Rheum 23:146–152
Melzack R, Wall PD (1965) Pain mechanisms: a new theory. Science 150:971–979
Neame R, Zhang W, Doherty M (2004) A historic issue of the Annals: three papers examine paracetamol in osteoarthritis. Ann Rheum Dis 63:897–900
Nguyen M, Revel M, Dougados M (1997) Prolonged effects of 3 week therapy in a spa resort on lumbar spine, knee and hip osteoarthritis: follow-up after 6 months. A randomized controlled trial. Br J Rheumatol 36:77–81
Ofman JJ, MacLean CH, Straus WL, Morton SC, Berger ML, Roth EA, Shekelle P (2002) A metaanalysis of severe upper gastrointestinal complications of nonsteroidal antiinflammatory drugs. J Rheumatol 29:804–812
Peat G, McCarney R, Croft P (2001) Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis 60:91–97
Pincus T, Wang X, Chung C, Sokka T, Koch GG (2005) Patient preference in a crossover clinical trial of patients with osteoarthritis of the knee or hip: face validity of self-report questionnaire ratings. J Rheumatol 32:533–539
Salaffi F, Cavalieri F, Nolli M, Ferraccioli G (1991) Analysis of disability in knee osteoarthritis. Relationship with age and psychological variables but not with the radiographic score. J Rheumatol 18:1581–1586
Salaffi F, Leardini G, Canesi B, Mannoni A, Fioravanti A, Caporali R, Lapadula G, Punzi L, GOnorthrosis and Quality of Life Assessment (GOQOLA) (2003) Reliability and validity of the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index in Italian patients with osteoarthritis of the knee. Osteoarthritis Cartilage 11:551–560
Shani J, Barak S, Levi D, Ram M, Schachner ER, Schlesinger T, Robberecht H, Van Grieken R, Avrach WW (1985) Skin penetration of minerals in psoriatics and guinea pigs bathing in hypertonic salt solutions. Pharmacol Res 17:501–506
Shehata M, Schwarzmeier JD, Hilgarth M, Demirtas D, Richter D, Hubmann R, Boeck P, Leiner G, Falkenbach A (2006) Effect of combined spa-exercise therapy on circulating TGF-beta1 levels in patients with ankylosing spondylitis. Wien Klin Wochenschr 118:266–272
Sukenik S, Flusser D, Abu-Shakra M (1999) The role of spa therapy in various rheumatic diseases. Rheum Dis Clin North Am 25:883–897
Verhagen AP, Bierma-Zeinstra SM, Boers M, Cardoso JR, Lambeck J, de Bie RA, de Vet HC (2007) Balneotherapy for osteoarthritis. Cochrane Database Syst Rev:CD006864
Ware JE Jr, Sherbourne CD (1992) The MOS 36- item short- form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30:473–483
Yilmaz B, Goktepe AS, Alaca R, Mohur H, Kayar AH (2004) Comparison of a generic and a disease specific quality of life scale to assess a comprehensive spa therapy program for knee osteoarthritis. Joint Bone Spine 71:563–566
Zhang Y, Jordan JM (2010) Epidemiology of osteoarthritis. Clin Geriatr Med 26:355–369
Zhang W, Jones A, Doherty M (2004) Does paracetamol (acetaminophen) reduce the pain of osteoarthritis? A meta-analysis of randomised controlled trials. Ann Rheum Dis 63:901–907
Zhang W, Doherty M, Leeb BF, Alekseeva L, Arden NK, Bijlsma JW et al (2007) EULAR evidence based recommendations for the management of hand osteoarthritis: report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 66:377–388
Zochling J, March L, Lapsley H, Cross M, Tribe K, Brooks P (2004) Use of complementary medicines for osteoarthritis - a prospective study. Ann Rheum Dis 63:549–554
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fioravanti, A., Giannitti, C., Bellisai, B. et al. Efficacy of balneotherapy on pain, function and quality of life in patients with osteoarthritis of the knee. Int J Biometeorol 56, 583–590 (2012). https://doi.org/10.1007/s00484-011-0447-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-011-0447-0