Abstract
Background
Laparoscopic technique has been increasingly used in gastrectomy, but the safety and feasibility of the laparoscopic total gastrectomy (LTG) for advanced proximal gastric cancer (PGC) after neoadjuvant chemotherapy (NAC) is unclear.
Methods
A retrospective analysis of 146 patients who received NAC followed by radical total gastrectomy at Fujian Medical University Union Hospital from January 2008 to December 2018 was performed. The primary endpoints were long-term outcomes.
Results
The patients were divided into two groups: 89 were in the LTG group and 57 were in the open total gastrectomy (OTG) group. The LTG group had a significantly shorter operative time (median 173 min vs. 215 min, p < 0.001), less intraoperative bleeding (62 ml vs. 135 ml, p < 0.001), higher total lymph node (LN) dissections (36 vs 31, p = 0.043), and higher total chemotherapy cycle completion rate (≥ 8 cycles) (37.1% vs. 19.7%, p = 0.027) than OTG. The 3-year overall survival (OS) of the LTG group was significantly higher than that of the OTG group (60.7% vs. 35%, p = 0.0013). Survival with inverse probability weighting(IPW) correction for Lauren type, ypTNM stage, NAC schemes and the times at which the surgery was performed showed that there was no significant difference in OS between the two groups (p = 0.463). Postoperative complications (25.8% vs. 33.3%, p = 0.215) and recurrence-free survival (RFS) (p = 0.561) between the LTG and OTG groups were also comparable.
Conclusion
In experienced gastric cancer surgery centers, LTG is recommended as the preferred option for such patients who performed NAC, owing to its long-term survival is not inferior to OTG, and it offers less intraoperative bleeding, better chemotherapy tolerance than conventional open surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
According to the latest global oncology data in 2020, gastric cancer is the fifth most common tumor and third leading cause of cancer-related deaths [1]. Owing to its high morbidity and mortality rates, progress in the treatment of gastric cancer is of great concern. The MAGIC [2] study was the first to demonstrate that neoadjuvant chemotherapy (NAC) can improve the chances of the radical resection of advanced gastric cancer by downstaging the tumor and eliminating possible micro-metastases. The results brought hope to the patients with advanced gastric cancer (AGC) who have lost the chance to undergo surgery. The NCCN guidelines recommended that NAC should be administered to all advanced gastric cancers [3, 4], and it was included in the standard care for the multimodal treatment of gastric cancer in many countries around the world.
Laparoscopic gastrectomy (LG) was first reported by Kitano et al. [5] in 1994, and after 20 years of development, LG for gastric cancer has gradually progressed. The findings of the Japanese (JCOG0912) [6] and Korean (KLASS-01) [7] studies showed that compared with open gastrectomy (OG), LG has the advantages of less pain, better cosmetic results, and less intraoperative bleeding, and while it is widely accepted as the preferred surgical approach for early gastric cancer, it has gradually extended to patients with locally advanced gastric cancer. The results of a randomized controlled trial (CLASS-01) conducted in China showed that LG had similar short-term and long-term outcomes as open surgery in patients with advanced distal gastric cancer [8].
However, the radical resection of gastric cancer after NAC faces technical challenges due to the fibrotic response of vascular tissue and alteration of the normal anatomical plane [9,10,11]. LG has the advantages of a large field of view and good maneuverability. An RCT study conducted by Li Z [9] showed that laparoscopic surgery had better surgical safety and postoperative chemotherapy tolerance than open surgery for distal gastrectomy after NAC. However, there is still a lack of studies on whether LG has better postoperative chemotherapy tolerance and long-term survival benefits in patients with advanced proximal gastric cancer (PGC) after NAC. The only study available by Nicole van der Wielen et al. showed that LTG after NAC was comparable to open surgery in terms of surgical safety, with no significant differences in postoperative recovery and complications. However, the patients included in that study were from a European population and only had a one-year follow-up, which did not provide a reference for long-term survival outcomes [12].
In the Eastern population, there is still a research gap regarding whether LTG after NAC is superior to OTG in patients with advanced PGC. This study aimed to investigate the oncologic efficacy of LTG compared with OTG in the treatment of PGC after NAC, with the aim of providing evidence for the use of minimally invasive surgery in these populations.
Materials and methods
Study design and patient selection
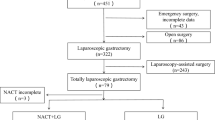
A prospective collection and retrospective analysis were performed using the data of 146 patients diagnosed with gastric adenocarcinoma of the upper middle stomach and treated with NAC followed by total gastrectomy between January 2008 and December 2018 at the Fujian Medical University Union Hospital. All data were obtained from a prospective database at the center. The patients provided informed consent and the surgical approach method was selected according to the patient's intensions. Eligible participants were histologically proven to have gastric adenocarcinoma with clinical stage T2-4aN0/ + M0 after NAC by a preoperative evaluation according to the 8th staging system of the American Joint Committee on Cancer [13]. Patients with distant metastases, palliative resection, gastric stump cancer, or no adjuvant chemotherapy data were excluded. The patient selection process is illustrated in Fig. 1. All patients underwent D2 curative gastrectomy three weeks after NAC. The study was approved by the Ethics Committee of Union Hospital affiliated with the Fujian Medical University.
Perioperative chemotherapy
All patients received at least two cycles of NAC based on 5-Fu and were routinely recommended to receive adjuvant chemotherapy after surgery. The specific information on NAC regimens is as follows: three-week cycles of SOX (S-1 40–60 mg orally twice daily on days 1–14; oxaliplatin 130 mg/m2 intravenously on day 1) [14]. Three-week cycles of XELOX(1000 mg/m2 of capecitabine orally twice daily on days 1 to 14 and 130 mg/m2 of oxaliplatin intravenously on day 1). Three-week cycles of DS (S-1 40–60 mg orally twice daily on days 1–14; docetaxel 40 mg/m2 intravenously on day 1) [15]. Two-week cycles of FOLFOX4 (oxaliplatin 85 mg/m2 intravenously on day 1, leucovorin 200 mg/m2 as a 2-h intravenous infusion followed by bolus fluorouracil 400 mg/m2, and a 22-h intravenous infusion of fluorouracil 600 mg/m2) [16], and others including and apatinib regimens (oxaliplatin plus apatinib). Contrast-enhanced abdominopelvic CT was performed every two cycles, after which the tumor regression was evaluated and graded according to the Response Evaluation Criteria in Solid Tumors (RECIST) [17] guidelines. Treatment management for all patients was based on the Japanese Gastric Cancer Association (JCGC) [18]. All surgical procedures, including the extent of the lymph node (LN) dissection, were performed according to the guidelines of the Japanese Research Society for the Study of Gastric Cancer [19, 20], while staging was performed according to the TNM classification (AJCC, 8th edition) [13]. The pathological response was based on the estimation of the percentage of vital tumor cells in relation to the macroscopically identifiable tumor bed and included the following categories that were quantified using the Becker regression criteria. Toxicities were evaluated according to the National Cancer Institute-Common Terminology Criteria for Adverse Events version 4.0.
Surgical operations
The abdominal cavity was explored to check whether the liver, peritoneum, mesenteric, pelvic, and other metastatic gastric serous membranes had been invaded. All the operations were performed by one experienced team of surgeons who had performed at least 50 open total gastrectomy and 50 laparoscopic total gastrectomy with D2 lymphadenectomy.
Definitions
LN noncompliance rate: when more than one group of LNs was not detected [21, 22].
Blood loss: calculated by the number of gauze pieces and the amount of suction.
Operation time: the time from the start of the skin incision to the end of the abdominal closure.
Postoperative complications: complications occurring within 30 days after surgery that were recorded through hospitalization and discharge summaries; the severity of the complications was graded according to the Clavien-Dindo scoring system [23]. Major complications were defined as Clavien-Dindo 3A or higher.
Textbook outcome: Curative resection was performed, with no perioperative complications, a pathological radical resection (R0) with more than 15 lymph nodes resected, no major complications (Clavien-Dindo 3A or higher), no reinterventions, length of hospital stay less than 21 days, and no mortality or readmission in the first 30 days [24].
Recurrence: confirmed by imaging or biopsy of the suspected lesion. Recurrence-free survival (RFS) was defined as the period from the first day after surgery until recurrence was detected. Early recurrence was recurrence occurring within one year after surgery [25]. For RFS, the last follow-up records of patients who died without tumor recurrence were reviewed. The pattern of recurrence was classified as local recurrence (LR) (anastomosis, remnant stomach, and perigastric lymph nodes), peritoneal metastases (PM) (peritoneal implants, pelvic implants, and cancerous ascites), and distant metastases (DM) (brain, liver, lung, bone, and extra-regional lymph nodes).
Statistical analysis
SPSS version 26 (IBM, Armonk, NY, USA) and R version 4.0.3 (http://www.r-project.org) were used to perform all the analyses. Categorical variables were analyzed using the chi-square test or Fisher's exact test and are presented as frequencies and percentages. Continuous variables were analyzed using Student's t-tests and are expressed as mean ± standard deviation. The Meier method, adjusted survival curves with inverse probability weights(IPW) [26], and differences between curves were analyzed using the log-rank test. Statistical significance was set at p < 0.05.
Results
Clinical characteristics
A total of 146 patients (89 in the LTG group and 57 in the OTG group) were included, and the clinicopathological data of the two groups are shown in Table 1. The two groups were comparable in age, sex, BMI, comorbidity index, ECOG score, cT and cN stages before NAC, tumor size, TRG grading, and ypTNM stage, except for the Lauren type (p > 0.05).
Operative outcomes and oncological outcomes
We compared the short-term surgical outcomes of the two groups, as the Table 2 results showed that the LTG group had a shorter operative time, with a median operative time of 173 min (IQR 105–241 min), while the OTG group had a median operative time of 215 min (IQR 147–283 min) (p < 0.001). Intraoperative blood loss was significantly lower in the LTG group (mean, 62 ml) than that in the OTG group (mean, 135 ml) (p < 0.001). The postoperative recovery was similar in both groups, with the mean time to first off-bed being 2.5 days in the LTG group and 2.8 days in the OTG group, and the mean time to first liquid diet and half-liquid diet being 5.1 days and 7.3 days in the LTG group, respectively, compared with 5.3 days and 7.6 days in the OTG group, respectively. The time to extubation was 9.4 days in the LTG group and 9.5 days in the OTG group (p > 0.05). We also found that the discharge time in the LTG group and the OTG group was 10.90 ± 4.60 days vs 12.67 ± 5.17 days respectively (p = 0.033).
We further analyzed the oncological outcomes in both groups. All patients underwent radical TG. We evaluated the oncological quality of safe resection by total LN dissection and textbook outcomes. The average number of total LN dissections was significantly higher in the LTG group (37 ± 13) than that in the OTG group (31 ± 10) (p = 0.043) (Table 2). The average number of peri-gastric LN (No.1–7) dissections was significantly higher in the LTG group than in the OTG group (28 ± 11 vs. 24 ± 9, p = 0.036), and there was no significant difference in the number of LN dissections of extraperigastric regions and each station (Table 3). A subgroup analysis of the relationship between the degree of LN dissection and the radiological response after NAC (RECIST standard) and ypTNM stage was performed. There was no significant difference in the average number of LN dissections between the LTG and OTG groups, including the group with good tumor regression (PR/CR) (Supplementary eFigure 2B) and the group with ypTNM stage I (Supplementary eFigure 2D). However, in the group with poor tumor regression (PD/SD) (Supplementary eFigure 2C), the number of peri-gastric and No.11p LN dissections was significantly higher in the LTG group than in the OTG group. Regarding ypTNM II/III, the total average number of LN dissections was significantly higher in the LTG group than in the OTG group (36 ± 14 vs. 30 ± 10, p = 0.015, Fig. 2), and there were significantly higher peri-gastric LN dissections, while No. 3 and 7 LNs were dissected more often (Supplementary eFigure 2E) (specific LN dissection numbers are shown in Supplementary material eTables 1, 2, and 3). The percentage of LN dissection numbers [≥ 27, the LN dissection number cut-off point of 27 by unrestricted cubic spline (as shown in Supplementary material eFigure 1)] in the LTG group was significantly higher than that in the OTG group (78.7% vs. 61.45%, p = 0.037). The textbook outcomes were similar in both groups (LTG vs. OTG: 60.7% vs. 57.9%), with no statistically significant difference in LN noncompliance rates (61.8% vs. 71.9%, p = 0.218; Fig. 3).
Postoperative complication
There was no significant difference in the postoperative complication rate between the two groups (LTG vs. OTG: 25.8% vs. 33.3%; p = 0.215). The most common postoperative complication in the LTG group was pulmonary infection (eight cases, one of which had a serious complication), followed by anastomotic fistula (six cases). The most common postoperative complication in the OTG group was anastomotic fistula (n = 8), followed by pulmonary infection (n = 6) (Supplementary material eTable 4).
Perioperative chemotherapy
The perioperative chemotherapy characteristics are shown in Table 4, with the mean number of cycles completed for NAC in the LTG and OTG groups being 3.2 and 2.6 cycles, respectively, while the mean number of cycles completed for adjuvant chemotherapy was 4.4 and 3.8 cycles, respectively. Compared with the OTG group, the LTG group had significantly higher rates of adjuvant chemotherapy completion of four cycles (65.1% vs. 45%, p = 0.045), five cycles (52.45% vs 27.5%, p = 0.013), and six cycles (41.3% vs. 22.5%, p = 0.050). We further analyzed the total perioperative chemotherapy completion rate (≥ 8) and postoperative chemotherapy cycles in relation to the surgical approaches. The results showed that the mean total perioperative chemotherapy cycle completion in the LTG group was significantly higher than that in the OTG group (6.64 vs 5.14, p = 0.003), and the perioperative chemotherapy completion rate (≥ 8cycles) was also significantly higher (37.1% vs. 19.3%, p = 0.027).
Survival outcomes
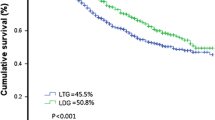
In the K–M survival curve analysis, the3-year OS of the LTG group was significantly better than that of the OTG group (35% vs. 60.7%, p = 0.0013) (Fig. 4A), with a median OS of 38 months in the LTG group and 30 months in the OTG group. Given the difference in the Lauren type between the two groups (p = 0.019, Table 1) and the significant independent effect of ypTNM staging on long-term survival, an inverse probability weighted (IPW) corrected survival curve, adjusted for the Lauren type, ypTNM stage, NAC schemes and the times at which the surgery was performed (Fig. 4B) showed that the 3-year OS of the LTG group is not inferior to that of the OTG group, p = 0.463. There was also no significant difference in the RFS between the two groups (Fig. 4C–D). Further analysis of the differences in the recurrence patterns between the two groups (Supplementary material Table 2) showed that the OTG group had a higher overall recurrence rate than the LTG group, but this difference was not statistically significant (61.4% vs. 46.1%, p = 0.07). The patients in the LTG and OTG groups did not differ significantly in the type of recurrence, including early recurrence (17 vs. 14, p = 0.431), local recurrence (7 vs. 10, p = 0.075), peritoneal metastasis (16 vs. 13, p = 0.476), and distant metastasis (19 vs. 18, p = 0.166) (Table 5).
Survival outcome. Comparison of survival curves between laparoscopic total gastrectomy (LTG) group and open total gastrectomy (OTG) group according to inverse probability weighting(IPW). A Overall Survival curve before IPW.B Overall Survival curve after IPW. C Recurrence-Free Survival curve before IPW. D Recurrence-Free Survival curve after IPW
Discussion
In this study, the early postoperative recovery indices and postoperative complications were similar between LTG and OTG for AGC after NAC. However, LTG showed an equivalent OS with shorter operative times, less intraoperative bleeding, higher numbers of LN dissections, and higher perioperative chemotherapy completion rates. This is the first study to evaluate the oncological prognosis of the neoadjuvant treatment of PGC with LTG and OTG in an eastern population. The results suggested that LTG may be not inferior in terms of oncological efficacy and surgical safety.
LTG group has a shorter operative time, less intraoperative blood loss, and a higher number of LN dissections than OTG. Studies by Fujisaki et al. [9, 27] showed that compared with conventional open surgery, laparoscopic surgery required a longer operative time, which seemed to contradict our results. However, the quality of laparoscopic surgery is considered to be related to the annual volume of laparoscopic surgeries performed at this center. In our center, we perform over 1000 laparoscopic gastrectomy operations annually. So far, we have completed 12,000 laparoscopic radical gastrectomy. After continuous exploration and improvement in the skill of laparoscopic surgery, we have summarized and formed a standardized laparoscopic technique. For example, we developed a novel procedure for laparoscopic supra-pancreatic lymph node dissection [1]. In addition, in this study, we performed laparoscopic surgery for patients after NAC, which was on the basis of the increasing maturity of laparoscopic technology. The above factors may be the reasons why the operative time shortened in the laparoscopic group. And previous studies at our center [28] showed a significantly shorter operative time for laparoscopic radical gastric cancer than for open radical gastric cancer, which is consistent with the results of this study. After NAC, the tumor and tumor surroundings, including LN, were altered with varying degrees of fibrosis, causing changes in the anatomical planes of blood vessels and nerves, making surgery more difficult. A complete resection in the correct anatomical plane is the key to achieve a "bloodless" operation and a higher number of LN dissections. The magnification effect of laparoscopy can overcome the limited visual field of open surgery because of the human eye, facilitating access to the correct anatomical level and allowing the completion of more delicate surgical operations, thus dissecting more LN, reducing intraoperative blood loss, and reducing the "leakage" of cancer cells into the intra-abdominal surgical field, with better recent efficacy and surgical safety.
Our results showed no significant difference in early postoperative recovery and postoperative complications between the two groups. The Class-02 study [29] confirmed that LTG by an experienced surgeon for early gastric cancer is comparable in safety to open surgery. Previous studies on performing LG after neoadjuvant therapy mainly focused on distal gastrectomy. For example, a prospective study by Ziyu et al. [9] showed no significant difference between laparoscopic distal gastrectomy and open surgery after neoadjuvant therapy for the first liquid and defecation after surgery, which was consistent with our results. They concluded that postoperative complications were fewer in laparoscopic distal gastrectomy than in open surgery, and our results showed a slightly higher postoperative complication rate in the OTG group than in the LTG group. As the sample size was too small, no significant difference was observed between the two groups, and future large-scale multicenter data are needed to further explore the potential advantages of laparoscopic total gastrectomy after neoadjuvant therapy in terms of postoperative complications.
Our study explored the long-term survival outcomes of LTG and OTG after NAC and showed that the 3-year overall survival rate was significantly higher in the LTG group than in the OTG group. While after an IPW survival correction for the Lauren type, ypTNM stage, NAC schemes and the times at which the surgery was performed, we found that the 3-year OS of the LTG group is not inferior to that of the OTG group, p = 0.463. In addition, we found better compliance with perioperative chemotherapy in the LTG group, which had a significantly higher completion rate of 8 cycles of perioperative chemotherapy than the OTG group, and complete perioperative chemotherapy has been shown to be important for improving long-term survival [30]. These factors may be potential reasons for the potential higher long-term survival outcomes in patients with LTG than in those with OTG.
This study has the following limitations: (1) It was a single-center study, and due to the low acceptance of NAC in Chinese, more patients preferred surgery only than surgery after NAC. Therefore, the sample size in this study was relatively small, there is a possible bias due to not entirely consistent chemotherapy regimens. (2) We did not assess the possible impact of chemotherapy toxicity. (3) We did not assess postoperative functional status; therefore, we cannot comment on whether performing laparoscopic surgery after NAC improved patients' quality of life. (4) In the early stage of this study, there was a lack of consensus on the use of peritoneal cytology examination and we did not routinely perform this examination. The lack of information on peritoneal cytology may lead to the possibility of p Stage IV patients, which could have an impact on the results of this study. Nevertheless, this study is the first to describe the oncologic outcomes of LTG versus OTG for locally advanced upper middle gastric cancer after NAC in an Eastern population.
Conclusion
The results of this study suggested that LTG can be safely performed by experienced surgeons for patients with locally advanced upper gastric cancer receiving NAC and the perioperative chemotherapy is better tolerated with an equivalent 3-year long-term survival.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249
Wilke H, Preusser P, Fink U et al (1989) Preoperative chemotherapy in locally advanced and nonresectable gastric cancer: a Phase II study with etoposide, doxorubicin, and cisplatin. J Clin Oncol 7(9):1318–1326. https://doi.org/10.1200/JCO.1989.7.9.1318
Tokunaga M, Sato Y, Nakagawa M, Aburatani T, Matsuyama T, Nakajima Y et al (2020) Perioperative chemotherapy for locally advanced gastric cancer in Japan: current and future perspectives. Surg Today 50:30–7
Ajani Jaffer A, D’Amico Thomas A, Bentrem David J (2022) Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 20(2):167–192
Kitano S, Iso Y, Moriyama M, Sugimachi K (1994) Laparoscopy assisted Billroth I gastrectomy. Surg Laparosc Endosc 4:146–148
Katai H, Mizusawa J, Katayama H et al (2020) Survival outcomes after laparoscopy-assisted distal gastrectomy versus open distal gastrectomy with nodal dissection for clinical stage IA or IB gastric cancer (JCOG0912): a multicentre, non-inferiority, phase 3 randomised controlled trial. Lancet Gastroenterol Hepatol 5(2):142–151
Kim HH, Han SU, Kim MC, Korean Laparoendoscopic Gastrointestinal Surgery Study (KLASS) Group et al (2019) Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with stage I gastric cancer: the KLASS-01 randomized clinical trial. JAMA Oncol 5(4):506–513. https://doi.org/10.1001/jamaoncol.2018.6727
Huang CM, Liu H, YFHu et al (2022) Laparoscopic vs open distal gastrectomy for locally advanced gastric cancer: five-year outcomes from the CLASS-01 randomized clinical trial. JAMA Surg 157(1):9–17
Li Z, Shan F, Ying X et al (2019) Assessment of laparoscopic distal gastrectomy after neoadjuvant chemotherapy for locally advanced gastric cancer: a randomized clinical trial. JAMA Surg 154(12):1093–101
An JY, Kim KM, Kim YM, Cheong JH, Hyung WJ, Noh SH (2012) Surgical complications in gastric cancer patients preoperatively treated with chemotherapy: their risk factors and clinical relevance. Ann Surg Oncol 19(8):2452–8
Fujisaki M, Mitsumori N, Shinohara T et al (2020) Short- and long-term outcomes of laparoscopic versus open gastrectomy for locally advanced gastric cancer following neoadjuvant chemotherapy. Surg Endosc 35:1682–1690
van der Wielen N, Straatman J, Daams F (2021) Open versus minimally invasive total gastrectomy after neoadjuvant chemotherapy: results of a European randomized trial. Gastric Cancer 24(1):258–271
Amin MB, Edge S, Greene F et al (eds) (2017) 8th edn. Springer, New York
Koizumi W, Takiuchi H, Yamada Y, Boku N, Fuse N, Muro K et al (2010) Phase II study of oxaliplatin plus S-1 as first-line treatment for advanced gastric cancer(G-SOX study). Ann Oncol 21:1001–1005
Koizumi W, Kim YH, Fujii M, Kim HK, Imamura H, Lee KH et al (2014) Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START). J Cancer Res Clin Oncol 140:319–328
De Vita F, Orditura M, Matano E, Bianco R, Carlomagno C, Infusino S et al (2005) A phase II study of biweekly oxaliplatin plus infusional 5-fluorouracil and folinic acid (FOLFOX-4) as first-line treatment of advanced gastric cancer patients. Br J Cancer 92:1644–9
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–47
Japanese Gastric Cancer Association (2011) Japanese classification of gastric carcinoma 3rd English edition. Gastric Cancer 14:101–12
Japanese Gastric Cancer Association (2011) Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 14(2):113–123. https://doi.org/10.1007/s10120-011-0042-4
JGC Association (2017) Japanese gastric cancer treatment guidelines 2014 (ver 4). Gastric Cancer 20(1):1–19
De Steur WO et al (2015) Quality control of LN dissection in the Dutch gastric cancer trial. Br J Surg 102:1388–1393
Chen QY et al (2019) Laparoscopic total gastrectomy for upper-middle advanced gastric cancer: analysis based on LN noncompliance. Gastric Cancer 23:184–194
Dindo D et al (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205
Busweiler LA, Schouwenburg MG, van Berge Henegouwen MI, Kolfschoten NE, de Jong PC, Rozema T et al (2017) Textbook outcome as a composite measure in oesophagogastric cancer surgery. Br J Surg 104(6):742–750
Bin-Bin Xu, Jun Lu, Zheng Zhi-Fang (2019) The predictive value of the preoperative C-reactive protein-albumin ratio for early recurrence and chemotherapy benefit in patients with gastric cancer after radical gastrectomy: using randomized phase III trial data. Gastric Cancer 22(5):1016–1028
Cole R Stephen, Hernán Miguel A (2004) Adjusted survival curves with inverse probability weights. Comput Methods Progr Biomed 75(1):45–49
Fujisaki M, Mitsumori N (2021) Toshihiko, ShinoharaShort-and long-term outcomes of laparoscopic versus open gastrectomy for locally advanced gastric cancer following neoadjuvant chemotherapy. Surg Endosc 35(4):1682–1690
Bin-Bin Xu, Jun Lu, Zheng Zhi-Fang (2019) Comparison of short-term and long-term efficacy of laparoscopic and open gastrectomy in high-risk patients with gastric cancer: a propensity score-matching analysis. Surg Endosc 33(1):58–70
Liu F-L, Huang C-M, Xu Ze-kuan (2020) Morbidity and mortality of laparoscopic vs open total gastrectomy for clinical stage I gastric cancer: The CLASS02 multicenter randomized clinical trial. JAMA Oncol 6(10):1590–1597
Al-Batran SE, Homann N, Pauligk C et al (2019) Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 393:1948–1957
Funding
Fujian Province Medical "Creating Double High" Construction Funding Support (Fujian Health Medical Administration NO.[2021]76). Funder: Huang Chang-ming.
Author information
Authors and Affiliations
Contributions
LS, HLZ and BX contributed equally to this work and should be considered first coauthors. CHZ had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: LS, CHZ. Acquisition, analysis, or interpretation of data: JL, LS, QYC, ZX, BX, PL, JL. Drafting of the manuscript: LS, HLZ, BX. Critical revision of the manuscript for important intellectual content: HLZ, CHZ, LS, JWX, CMH. Statistical analysis: LS. Administrative, technical, or material support: CHZ. Supervision: CHZ.
Corresponding author
Ethics declarations
Disclosures
Drs. Hua-Long Zheng, Li–li Shen, Bin-bin Xu, Qi-Yue Chen, Jun Lu, Zhen Xue, Jia-Lin, Jian-Wei Xie, Ping Li, Chang-Ming Huang, Chao-Hui Zheng have no conflicts of interest to disclose.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for being included in the study.
Consent for publication
This article doesn’t report an individual participant's data in any form.
Validity and feasibility of the statistics
The validity and feasibility of statistics have been evaluated by a biostatistician, Wu FQ.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.


464_2023_10084_MOESM3_ESM.jpg
Supplementary file3 (JPG 1194 KB)—eFig. 2 Comparison of LN dissections between laparoscopic total gastrectomy (LTG) group and open total gastrectomy (OTG) group. A Comparison of mean No. of lymph node dissection in each station between the two groups. B, C Comparison of mean No. of lymph node dissection in each station between the two groups according to RECIST standard. B CR/PR subgroup. C PD/SD subgroup. D, E Comparison of mean No. of lymph node dissection in each station between the two groups according to ypTNM stage. D stage I. E stage II/III
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, HL., Shen, Ll., Xu, Bb. et al. Oncological outcomes of laparoscopic versus open radical total gastrectomy for upper-middle gastric cancer after neoadjuvant chemotherapy: a study of real-world data. Surg Endosc 37, 6288–6297 (2023). https://doi.org/10.1007/s00464-023-10084-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-10084-z