Abstract
Background
To determine whether laparoscopic surgery can be used in high-risk patients with gastric cancer.
Methods
The clinicopathological data of 3743 patients with primary gastric adenocarcinoma, collected from January 2007 to December 2014, were retrospectively analyzed. Patients who had ≥ 1 of the following conditions were defined as high-risk patients: (1) age ≥ 80 years; (2) BMI ≥ 30 kg/m2; (3) ASA (American Society of Anesthesiologists) grade ≥ 3; or (4) clinical T stage 4 (cT4). Propensity score matching (PSM) was used to reduce confounding bias; then, we compared the short-term and long-term efficacy of laparoscopic gastrectomy (LG) with open gastrectomy (OG) in high-risk patients with gastric cancer.
Results
A total of 1296 patients were included in PSM. After PSM, no significant difference in clinicopathological data was observed between the LG group (n = 341) and the OG group (n = 341). The operative time (181.70 vs. 266.71 min, p < 0.001) and blood loss during the operation (68.11 vs. 225.54 ml, p < 0.001) in the LG group were significantly lower than those in the OG group. In the LG and OG groups, postoperative complications occurred in 39 (11.4%) and 63 (18.5%) patients, respectively, p = 0.010. Multivariate analysis showed that laparoscopic surgery was an independent protective factor against postoperative complications (p = 0.019). The number of risk factors was an independent risk factor for postoperative complications (p = 0.021). The 5-year overall survival rate in the LG group was comparable to that in the OG group (55.0 vs. 52.0%, p = 0.086). Hierarchical analysis further confirmed that the LG and OG groups exhibited comparable survival rates among patients with stages cI, pI, cII, pII, cIII, and pIII (all p > 0.05).
Conclusions
For high-risk patients with gastric cancer, LG not only exhibits better short-term efficacy than OG but also has a comparable 5-year survival rate to OG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gastric cancer is the fourth most common malignancy in humans, and it is the second most common malignancy associated with cancer mortality [1]. Radical gastrectomy is the dominant treatment for patients with resectable gastric cancer. Since the first case of laparoscopic-assisted gastrectomy was performed in 1994 [2], a growing number of studies have confirmed that laparoscopic gastrectomy (LG) has superior short-term efficacy with regard to factors such as intraoperative blood loss, postoperative complications, time to flatus, and length of hospital stay [3,4,5]; additionally, patients undergoing LG can achieve long-term survival comparable to those undergoing open gastrectomy (OG) [6,7,8]. However, it is unknown whether patients with a preoperative ASA (American Society of Anesthesiologists) grade ≥ 3, BMI ≥ 30 kg/m2, age ≥ 80 years, or a clinical T stage of 4 (cT4) can benefit from LG.
Previous studies have shown that an ASA grade ≥ 3, BMI ≥ 30 kg/m2, age ≥ 80 years, and cT4 classification are sensitive indicators of surgical risk assessment in patients with cancer, and patients with these indicators are often defined as high-risk patients [9,10,11,12,13,14,15,16,17,18,19]. With the aging of society and the increasing number of obese people [20, 21], surgeons will face an increasing number of high-risk patients with gastric cancer. Therefore, for the first time, this study compared the short-term and long-term effects of LG with OG in high-risk gastric cancer patients during the same period and explored whether these patients can benefit from LG.
Materials and methods
Patients
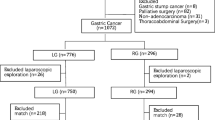
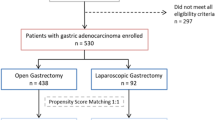
In this retrospective analysis, data were collected from 3743 patients diagnosed with primary gastric adenocarcinoma at Fujian Medical University Union Hospital (FMUUH) Department of Gastric Surgery from January 2007 to December 2014. According to previous studies, patients with ≥ 1 of the following conditions were defined as high-risk patients: (1) age ≥ 80 years; (2) BMI ≥ 30 kg/m2; (3) ASA grade ≥ 3; and (4) cT4 stage [22]. A total of 1601 patients were included. The exclusion criteria were as follows: (1) gastric stump cancer (n = 53); (2) intraperitoneal or distant metastasis confirmed during or after the operation (n = 67); (3) neoadjuvant chemotherapy (n = 43); (4) conversion to open laparotomy (n = 2); or (5) incomplete pathological data (n = 140). A total of 305 patients were excluded. The remaining 1296 patients undergoing radical gastrectomy were entered into the statistical analysis, of whom 378 patients underwent OG, and 918 underwent LG (see flowchart in Fig. 1). Tracheal intubation was given, and combined intravenous anesthesia was performed [23]. Preoperative clinical tumor-node-metastasis (cTNM) staging and postoperative pathological tumor-node-metastasis (pTNM) staging were based on the 7th American Joint Committee on Cancer (AJCC) staging system [24]. Two attending physicians staged the tumor before the operation according to gastroscopy, abdominal CT, total abdominal ultrasonography, and other examination results [25]. All patients were informed of the advantages and disadvantages of LG and OG before surgery and the procedure was selected by the patients themselves; all surgeries were performed by the same experienced surgical team. All patients signed an informed consent form. This study was approved by the FMUUH Ethics Committee.
Definitions
High-risk patients: Patients with ≥ 1 of the following conditions: (1) age ≥ 80 years; (2) BMI ≥ 30 kg/m2; (3) ASA grade ≥ 3; or (4) cT4 stage were considered high-risk patients [22].
Surgical complications
Complications developing within the scope of surgery, such as wounds or intra-abdominal cavity, and complications associated with surgery, such as wound infections and abdominal infections, were considered surgical complications. Non-surgical complications: Complications unrelated to the surgical field, such as pneumonia, were considered non-surgical complications [26]. Serious complications: Postoperative complications were graded according to the Clavien-Dindo classification system; complications greater than grade III were defined as serious complications [27].
Overall survival time
Survival time was defined as the period from the date of surgery to the date of death or final follow-up. All patients were monitored until death or September 2017, and the median follow-up time was 42 months (range 2–125 months).
Statistical analysis
A logistic regression model was chosen to calculate the propensity scores, and the following covariates were included: age, sex, ASA grade, BMI, tumor location, adjuvant chemotherapy, and nodal status. Continuous variables are reported as the means ± SD. Categorical and continuous variables were compared using a χ2 test or Fisher’s exact test and a t test, respectively. The cumulative survival rate was calculated using the Kaplan–Meier method and a log-rank test. Logistic regression analysis was used to identify independent risk factors associated with complications. A Cox proportional hazards regression model was used to determine independent prognostic factors associated with survival. All statistical analyses were performed using SPSS v. 18.0 for Windows (SPSS Inc., Chicago, IL, USA). Values of p lower than 0.05 were considered statistically significant.
Results
Clinicopathological characteristics of patients
Tables 1 and 2 show the demographic data of all the patients (n = 1296) and the propensity score-matched patients (n = 682). After propensity score matching (PSM), no significant differences between OG and LG patients were observed in clinicopathological characteristics, such as age, sex, BMI, ASA grade, clinical stage, and pTNM stage. The relationships between the number of risk factors and the pathological stage of patients after PSM are shown in Supplementary Tables 1 and 2.
Intraoperative outcomes
The operative time (181.70 vs. 266.71 min, p < 0.001) and blood loss during surgery (68.11 vs. 225.54 ml, p < 0.001) were significantly lower in the LG group than in the OG group. The number of harvested lymph nodes (35.00 vs. 31.24, p < 0.001) in the LG group was higher than that in the OG group. The difference between two groups in the digestive tract reconstruction and gastrectomy extent was not significant (all p > 0.05, Table 3).
Postoperative outcomes
In the LG and OG groups, postoperative complications occurred in 39 (11.4%) and 63 (18.5%) patients, respectively, p = 0.010. Twenty-one (6.2%) and 34 (10.0%) patients had surgical complications in the LG and OG groups, respectively, p = 0.068. The incidence of postoperative bleeding and wound infection was significantly lower in the LG group than in the OG group (1 vs. 8, p = 0.038; 1 vs. 8, p = 0.038). Twenty (5.9%) and 38 (11.1%) patients had non-surgical complications in the LG and OG groups, respectively, p = 0.013. The incidence of pneumonia in the LG group was significantly lower than that in the OG group [17 (5.0%) vs. 36 (10.6%), p = 0.007]. In addition, the incidence of serious complications in the LG group was significantly lower than that in the OG group [5 (1.5%) vs. 15 (4.4%), p = 0.023]. In the LG group, the postoperative hospital stay (12.63 vs. 18.03), time to flatus (3.79 vs. 4.13), and time to food intake (5.03 vs. 5.37) were significantly shorter than those in the OG group (all p < 0.05, Table 4).
Univariate and multivariate analyses of factors associated with complications
In the univariate analysis, age, BMI, the number of risk factors, Charlson Comorbidity Index (CCI), and surgical procedure were closely related to postoperative complications (p < 0.05). Furthermore, the multivariate analysis showed that LG was an independent protective factor against postoperative complications, p = 0.019, and the number of risk factors was an independent risk factor for postoperative complications, p = 0.021 (Table 5).
Univariate and multivariate analyses of factors associated with overall survival
The univariate analysis showed that ASA grade, tumor diameter, tumor location, pathological type, pTNM stage, cTNM stage, operative time, gastrectomy extent, and gastrointestinal reconstruction were closely related to the 5-year overall survival rate after the operation (p < 0.05). In the multivariate analysis, tumor diameter and pTNM stage were independent predictors of long-term survival (all p < 0.05, Table 6).
Survival after surgery
Figure 2 shows that postoperative 5-year overall survival in the LG group was comparable to that in the OG group (55.0 vs. 52.0%, p = 0.086) for high-risk patients with gastric cancer. According to the 7th AJCC-TNM staging system, the two groups of patients were stratified into p stages I–III and c stages I–III, corresponding to A–F in Fig. 3. Hierarchical analysis showed that the overall survival rate of each subgroup of the LG group was comparable to that of the corresponding OG subgroup, all p > 0.05.
Discussion
In recent years, laparoscopic radical gastrectomy has been popular because it has the advantage of being minimally invasive. The Korean Laparoendoscopic Gastrointestinal Surgery Study (Klass-01) showed that the postoperative short-term efficacy of LG was superior to that of OG in early gastric cancer patients, especially regarding wound-related complications (LG vs. OG, 3.6 vs. 7.0%, p = 0.005) [28]. Seigo’s findings suggest that the long-term survival after LG in early gastric cancer is comparable to that after OG [8]. In addition, a multicenter prospective study from China showed no significant difference in the incidence of postoperative complications between LG and OG in patients with advanced gastric cancer (LG vs. OG, 15.2 vs. 12.9%, p = 0.285), indicating that surgeons can safely perform D2 lymph node dissection on advanced gastric cancer patients [5]. LG has also been reported to achieve long-term effects for advanced gastric cancer similar to those of OG [29]. However, at present, the vast majority of studies focus on typical gastric cancer patients, while less attention is given to high-risk patients.
By 2050, approximately 23% of people will be over 65 years old; by 2030, 57.8% of people will meet obesity standards [20, 21]. The aging population and obesity have become increasingly prominent issues. Surgical exposure is difficult in obese patients. Older patients or those with poor basic conditions cannot tolerate surgery well. Advanced tumors and other factors largely affect the success of the surgery. All of the above have been identified as surgical risk factors [22]. Therefore, the question of whether this subset of high-risk patients (elderly, high BMI, high ASA grade, and cT4) can also benefit from laparoscopic surgery has attracted increasing attention from researchers.
Indeed, it is generally believed that in high-risk patients with colorectal cancer, traditional laparotomy, rather than laparoscopic surgery, is always recommended due to their preoperative high-risk status [30], and pneumoperitoneum, established by carbon dioxide, may cause a number of adverse pathophysiological reactions, including hypercapnia, reduced venous return, increased peak airway pressure, and decreased lung compliance [31]. However, many studies have recently shown that even in high-risk patients with colorectal cancer, laparoscopic surgery is still safe and feasible, and it also has satisfactory clinical efficacy compared to open surgery. Jensen et al. concluded that laparoscopic colorectal resections in high-risk patients resulted in fewer hospitalization days, less intraoperative blood loss, and fewer cardiovascular complications than open surgery, and long-term survival was comparable to that in patients who underwent open surgery [32]. Feroci et al. found that patients with high-risk factors who underwent laparoscopic colorectal resection had lower rates of systemic complications and 30-day postoperative mortality than patients undergoing open surgery [33]. However, studies on whether the minimally invasive advantages of LG can reduce the surgical stress in high-risk patients with gastric cancer and improve the short-term and long-term efficacy of surgery have not been reported.
The results of this study showed that among high-risk patients, the LG group had significantly better postoperative recovery than the OG group with regard to factors such as time to flatus and food intake. The numbers of overall complications, non-surgical complications, and serious complications were significantly lower than those in the OG group, especially for bleeding, wound infection, and pneumonia; these complications may benefit from reductions in the bleeding during the operation, surgical invasiveness, and inflammatory response of the body associated with the LG procedure [3, 16, 18]. Although age ≥ 80 years, BMI ≥ 30 kg/m2, ASA grade ≥ 3, and cT4 stage were not independent risk factors for postoperative complications, the number of risk factors was an independent risk factor for postoperative complications. These results suggest that in high-risk patients, the body’s tolerance to surgical stress is affected by the synergistic effect of these four factors rather than by a single factor. High-risk patients with ≥ 1 risk factors should be given more attention after surgery to reduce postoperative complications. Additionally, laparoscopic surgery is an independent protective factor against postoperative complications. Therefore, in the context of postoperative complications, high-risk patients are more likely to benefit from LG. Moreover, our long-term postoperative survival analysis found that the 5-year overall survival rate in the LG group was comparable to that in the OG group and in all subgroups in the stratified analysis. Several studies have shown that CCI was associated with both the short-term and long-term efficacy of cancer-related surgery [34, 35]. Considering the population of patients assessed, we also evaluated the impact of CCI on these patients and found that CCI was not associated with postoperative complications and overall survival. As mentioned above, the results of this study indicate that LG is safe and feasible in high-risk patients and has significantly better short-term efficacy and comparable long-term efficacy to OG.
It has been reported that patients with metastasis before or after surgery and those undergoing neoadjuvant chemotherapy have significant differences in their short-term and long-term postoperative outcomes compared with those without metastasis or neoadjuvant chemotherapy [36,37,38]. In addition, previous studies comparing the long-term and short-term efficacy of laparotomy and laparoscopic surgery have excluded the above types of patients [5, 39]. Therefore, to reduce the baseline bias of cases and objectively assess the long-term and short-term efficacy of laparoscopic surgery for high-risk patients, we also excluded the patients with metastasis or patients treated with neoadjuvant chemotherapy, in this study.
Recently, several studies have confirmed that the fast-track surgery (FTS) protocol was feasible for gastric cancer patients who underwent open or laparoscopic surgery [40,41,42]. However, research focused on the application of FTS for high-risk patients has not been reported. For the high-risk patients included in this study, we did not apply FTS. Therefore, we did not analyze whether these patients could benefit from FTS.
This study has the following limitations. First, this was a single-center study lacking external validation, and its results should, therefore, be confirmed by a multicenter prospective study. Second, this was a retrospective study that may have some bias. Third, this study did not analyze details about chemotherapy such as the number of chemotherapy program cycles and its impact on long-term survival. Nevertheless, this study used PSM to report a comparison of laparoscopic and open procedures in high-risk patients with gastric cancer for the first time and confirmed that the short-term efficacy of LG was better than that of OG and the long-term efficacy was comparable.
In conclusion, for high-risk patients with gastric cancer, LG not only has better short-term outcomes than OG but also results in comparable long-term survival. This study may help clinicians choose a reasonable surgical procedure for high-risk patients with gastric cancer.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65:87–108
Kitano S, Iso Y, Moriyama M, Sugimachi K (1994) Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 4:146–148
Kawamura H, Okada K, Isizu H, Masuko H, Yamagami H, Honma S, Ueki S, Noguchi K, Kondo Y (2008) Laparoscopic gastrectomy for early gastric cancer targeting as a less invasive procedure. Surg Endosc 22:81–85
Katai H, Mizusawa J, Katayama H, Takagi M, Yoshikawa T, Fukagawa T, Terashima M, Misawa K, Teshima S, Koeda K, Nunobe S, Fukushima N, Yasuda T, Asao Y, Fujiwara Y, Sasako M (2016) Short-term surgical outcomes from a phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer: Japan Clinical Oncology Group Study JCOG0912. Gastric Cancer 20:699–708
Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J, Xue Y, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Chen P, Liu H, Zheng C, Liu F, Yu J, Li Z, Zhao G, Chen X, Wang K, Li P, Xing J, Li G (2016) Morbidity and mortality of laparoscopic versus open D2 distal gastrectomy for advanced gastric cancer: a randomized controlled trial. J Clin Oncol 34:1350–1357
Lee JH, Han HS, Lee JH (2005) A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc 19:168–173
Hayashi H, Ochiai T, Shimada H, Gunji Y (2005) Prospective randomized study of open versus laparoscopy-assisted distal gastrectomy with extraperigastric lymph node dissection for early gastric cancer. Surg Endosc 19:1172–1176
Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N, Japanese Laparoscopic Surgery Study G (2007) A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg 245:68–72
Kim MC, Kim W, Kim HH, Ryu SW, Ryu SY, Song KY, Lee HJ, Cho GS, Han SU, Hyung WJ, Korean Laparoscopic Gastrointestinal Surgery Study G (2008) Risk factors associated with complication following laparoscopy-assisted gastrectomy for gastric cancer: a large-scale korean multicenter study. Ann Surg Oncol 15:2692–2700
Takeshita H, Ichikawa D, Komatsu S, Kubota T, Okamoto K, Shiozaki A, Fujiwara H, Otsuji E (2013) Surgical outcomes of gastrectomy for elderly patients with gastric cancer. World J Surg 37:2891–2898
Gretschel S, Estevez-Schwarz L, Hunerbein M, Schneider U, Schlag PM (2006) Gastric cancer surgery in elderly patients. World J Surg 30:1468–1474
Topal B, Leys E, Ectors N, Aerts R, Penninckx F (2007) Determinants of complications and adequacy of surgical resection in laparoscopic versus open total gastrectomy for adenocarcinoma. Surg Endosc 22:980–984
Kunisaki C, Makino H, Takagawa R, Oshima T, Nagano Y, Ono HA, Akiyama H, Shimada H (2009) Efficacy of laparoscopy-assisted distal gastrectomy for gastric cancer in the elderly. Surg Endosc 23:377–383
Pikarsky AJ, Saida Y, Yamaguchi T, Martinez S, Chen W, Weiss EG, Nogueras JJ, Wexner SD (2002) Is obesity a high-risk factor for laparoscopic colorectal surgery? Surg Endosc 16:855–858
Bittner R, Butters M, Ulrich M, Uppenbrink S, Beger HG (1996) Total gastrectomy. Updated operative mortality and long-term survival with particular reference to patients older than 70 years of age. Ann Surg 224:37–42
Huang CM, Tu RH, Lin JX, Zheng CH, Li P, Xie JW, Wang JB, Lu J, Chen QY, Cao LL, Lin M (2015) A scoring system to predict the risk of postoperative complications after laparoscopic gastrectomy for gastric cancer based on a large-scale retrospective study. Medicine 94:e812
Wu XS, Wu WG, Li ML, Yang JH, Ding QC, Zhang L, Mu JS, Gu J, Dong P, Lu JH, Liu YB (2013) Impact of being overweight on the surgical outcomes of patients with gastric cancer: a meta-analysis. World journal of gastroenterology 19:4596–4606
Fujisaki M, Shinohara T, Hanyu N, Kawano S, Tanaka Y, Watanabe A, Yanaga K (2016) Laparoscopic gastrectomy for gastric cancer in the elderly patients. Surg Endosc 30:1380–1387
Tu R-H, Huang C-M, Lin J-X, Chen Q-Y, Zheng C-H, Li P, Xie J-W, Wang J-B, Lu J, Cao L-L, Lin M (2015) A scoring system to predict the risk of organ/space surgical site infections after laparoscopic gastrectomy for gastric cancer based on a large-scale retrospective study. Surg Endosc 30:3026–3034
Frasson M, Braga M, Vignali A, Zuliani W, Di Carlo V (2008) Benefits of laparoscopic colorectal resection are more pronounced in elderly patients. Dis Colon Rectum 51:296–300
Kelly T, Yang W, Chen CS, Reynolds K, He J (2008) Global burden of obesity in 2005 and projections to 2030. Int J Obes 32:1431–1437
Hemandas AK, Abdelrahman T, Flashman KG, Skull AJ, Senapati A, O’Leary DP, Parvaiz A (2010) Laparoscopic colorectal surgery produces better outcomes for high risk cancer patients compared to open surgery. Ann Surg 252:84–89
Wang J, Zhang L, Huang Q, Wu G, Weng X, Lai Z, Lin P (2017) Monitoring the end-tidal concentration of sevoflurane for preventing awareness during anesthesia (MEETS-PANDA): a prospective clinical trial. Int J Surg 41:44–49
Washington K (2010) 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol 17:3077–3079
Lin JX, Huang CM, Zheng CH, Li P, Xie JW, Wang JB, Jun L, Chen QY, Cao LL, Lin M (2016) Is all advanced gastric cancer suitable for laparoscopy-assisted gastrectomy with extended lymphadenectomy? A case-control study using a propensity score method. Ann Surg Oncol 23:1252–1260
Wang JB, Zheng CH, Li P, Xie JW, Lin JX, Lu J, Chen QY, Cao LL, Lin M, Huang CM (2017) Effect of comorbidities on postoperative complications in patients with gastric cancer after laparoscopy-assisted total gastrectomy: results from an 8-year experience at a large-scale single center. Surg Endosc 31:2651–2660
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of surgery 240:205–213
Kim W, Kim HH, Han SU, Kim MC, Hyung WJ, Ryu SW, Cho GS, Kim CY, Yang HK, Park DJ, Song KY, Lee SI, Ryu SY, Lee JH, Lee HJ (2016) Decreased morbidity of laparoscopic distal gastrectomy compared with open distal gastrectomy for stage I gastric cancer: short-term outcomes from a multicenter randomized controlled trial (KLASS-01). Ann Surg 263:28–35
Shinohara T, Satoh S, Kanaya S, Ishida Y, Taniguchi K, Isogaki J, Inaba K, Yanaga K, Uyama I (2013) Laparoscopic versus open D2 gastrectomy for advanced gastric cancer: a retrospective cohort study. Surg Endosc 27:286–294
Tan PY, Stephens JH, Rieger NA, Hewett PJ (2008) Laparoscopically assisted colectomy: a study of risk factors and predictors of open conversion. Surg Endosc 22:1708–1714
Gerges FJ, Kanazi GE, Jabbour-Khoury SI (2006) Anesthesia for laparoscopy: a review. J Clin Anesth 18:67–78
Poon JT, Law WL, Chow LC, Fan JK, Lo SH (2011) Outcome of laparoscopic resection for colorectal cancer in patients with high operative risk. Ann Surg Oncol 18:1884–1890
Feroci F, Baraghini M, Lenzi E, Garzi A, Vannucchi A, Cantafio S, Scatizzi M (2012) Laparoscopic surgery improves postoperative outcomes in high-risk patients with colorectal cancer. Surg Endosc 27:1130–1137
Ening G, Osterheld F, Capper D, Schmieder K, Brenke C (2015) Charlson comorbidity index: an additional prognostic parameter for preoperative glioblastoma patient stratification. J Cancer Res Clin Oncol 141:1131–1137
Dell’Oglio P, Tian Z, Leyh-Bannurah S, Trudeau V, Larcher A, Moschini M, Di Trapani E, Capitanio U, Briganti A, Montorsi F, Saad F, Karakiewicz P (2017) Short-form Charlson Comorbidity index for assessment of perioperative mortality after radical cystectomy. J Natl Compr Cancer Netw 15:327–333
Musri FY, Mutlu H, Karaagac M, Eryilmaz MK, Gunduz S, Artac M (2016) Primary tumor resection and survival in patients with stage IV gastric cancer. J Gastric Cancer 16:78–84
Claassen YHM, Hartgrink HH, Dikken JL, de Steur WO, van Sandick JW, van Grieken NCT, Cats A, Trip AK, Jansen EPM, Kranenbarg WMMK, Braak J, Putter H, van Berge Henegouwen MI, Verheij M, van de Velde CJH (2018) Surgical morbidity and mortality after neoadjuvant chemotherapy in the CRITICS gastric cancer trial. Eur J Surg Oncol 44:613–619
Jiang L, Yang KH, Guan QL, Chen Y, Zhao P, Tian JH (2015) Survival benefit of neoadjuvant chemotherapy for resectable cancer of the gastric and gastroesophageal junction: a meta-analysis. J Clin Gastroenterol 49:387–394
Hu Y, Ying M, Huang C, Wei H, Jiang Z, Peng X, Hu J, Du X, Wang B, Lin F, Xu J, Dong G, Mou T, Li G, Chinese Laparoscopic Gastrointestinal Surgery Study G (2014) Oncologic outcomes of laparoscopy-assisted gastrectomy for advanced gastric cancer: a large-scale multicenter retrospective cohort study from China. Surg Endosc 28:2048–2056
Wang D, Kong Y, Zhong B, Zhou X, Zhou Y (2010) Fast-track surgery improves postoperative recovery in patients with gastric cancer: a randomized comparison with conventional postoperative care. J Gastrointest Surg 14:620–627
Li Y-j, Huo T-t, Xing J, An J-z, Han Z-y, Liu X-n, Zhao Q-c (2014) Meta-analysis of efficacy and safety of fast-track surgery in gastrectomy for gastric cancer. World J Surg 38:3142–3151
Kim JW, Kim WS, Cheong JH, Hyung WJ, Choi SH, Noh SH (2012) Safety and efficacy of fast-track surgery in laparoscopic distal gastrectomy for gastric cancer: a randomized clinical trial. World J Surg 36:2879–2887
Acknowledgements
This work was supported by the Scientific and technological innovation joint capital projects of Fujian Province (2016Y9031). Construction Project of Fujian Province Minimally Invasive Medical Center (No. [2017]171). The second batch of special support funds for Fujian Province innovation and entrepreneurship talents (2016B013). Startup Fund for scientific research, Fujian Medical University (Grant Number: 2016QH024). Fujian province medical innovation project (2015-CXB-16). Fujian provincial health and family planning commission joint project (WKJ2016-2-27). Chinese physicians association young physician respiratory research fund.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosures
Drs. Bin-bin Xu, Jun Lu, Zhi-fang Zheng, Jian-wei Xie, Jia-bin Wang, Jian-xian Lin, Qi-yue Chen, Long–long Cao, Mi Lin, Ru-hong Tu, Ze-ning Huang, Ju-li Lin, Chao-hui Zheng, Ping Li, and Chang-ming Huang have no conflicts of interest or financial ties to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, Bb., Lu, J., Zheng, Zf. et al. Comparison of short-term and long-term efficacy of laparoscopic and open gastrectomy in high-risk patients with gastric cancer: a propensity score-matching analysis. Surg Endosc 33, 58–70 (2019). https://doi.org/10.1007/s00464-018-6268-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-018-6268-z