Abstract
Background
This study aimed to investigate the short- and long-term outcomes of laparoscopic gastrectomy (LG) in patients with advanced gastric cancer following neoadjuvant chemotherapy (NAC) to determine its safety and feasibility.
Methods
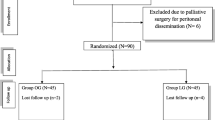
We retrospectively investigated 51 patients who underwent gastrectomy for locally advanced gastric cancer [cT3-4/N1-3 or macroscopic type 3 (> 80 mm) or type 4] following NAC between November 2009 and January 2018. After excluding two patients who underwent palliative surgery due to peritoneal dissemination, 49 patients were ultimately selected for this cohort study. The patients were then divided into the LG group and open gastrectomy (OG) group, after which the clinicopathological characteristics as well as short- and long-term outcomes were examined.
Results
Compared with the OG group, the LG group demonstrated a significantly lower amount of intraoperative blood loss and a shorter hospital stay. The overall complication rates were 10% (2 of 20 patients) and 24% (7 of 29 patients) in the LG and OG groups (P = 0.277), respectively. No significant differences in 5-year disease-free (LG 44.4% vs. OG 53.3%; P = 0.382) or overall survival rates (LG 46.9% vs. OG 54.0%; P = 0.422) were observed between the groups. Multivariate analysis revealed that the surgical procedure (LG vs. OG) was not an independent risk factor for disease-free (P = 0.645) or overall survival (P = 0.489).
Conclusions
LG may be a potential therapeutic option for patients with gastric cancer following NAC considering its high success rates and acceptable short- and long-term outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gastric cancer, the third leading cause of cancer death worldwide, remains a major health problem [1]. Although surgical treatment has been considered the primary therapy, long-term outcomes in advanced cases have still been unsatisfactory. Neoadjuvant chemotherapy (NAC) for advanced gastric cancer has been widely accepted in Western countries based on the results of the MAGIC and FLOT4 trials [2, 3]. In East Asia, however, adjuvant chemotherapy following gastrectomy with D2 lymph node dissection has been the standard treatment for advanced gastric cancer with NAC only being recommended for limited locally advanced cases with specific metastasis.
Surgical approaches for gastric cancer have drastically changed over the past two decades. Since first reported by Kitano et al. [4], laparoscopic gastrectomy (LG), a minimally invasive procedure, has progressed to the point of becoming the established procedure for early gastric cancer and has continued to rapidly spread among developed countries. Despite the limited evidence supporting the use of LG for advanced gastric cancer several years ago, a number of retrospective studies [5,6,7,8] and small-scale randomized controlled trials (RCTs) [9, 10] have confirmed the efficacy and safety of LG for advanced gastric cancer. Moreover, the most recent phase III study, namely, the CLASS-01 trial from China [10], suggested that LG was safe and oncologically comparable to open gastrectomy (OG). In addition, other large-scale RCTs from Japan (JLSSG0901) and Korea (KLASS-02) have been ongoing [11, 12]. However, most of the studies on LG for advanced gastric cancer were from East Asia and excluded patients receiving NAC. Chemotherapy-induced tissue fibrotic changes and edema provide new technical challenges for laparoscopic procedures, while only a few studies have compared the safety and efficacy of OG and LG for patients with advanced gastric cancer following NAC. Therefore, the applicability of LG in such patients remains controversial. This study aimed to investigate the short- and long-term outcomes of LG for patients with locally advanced gastric cancer following NAC to determine its safety and feasibility.
Materials and methods
Patients
This was a retrospective analysis of a prospectively maintained gastric cancer database in the Department of Surgery at four affiliated hospitals from the Jikei University School of Medicine, Tokyo, Japan and the Department of Surgery at Machida Municipal Hospital, a regional referral hospital in Tokyo, Japan. Between November 2009 and January 2018, 51 patients had been diagnosed with advanced gastric cancer and underwent NAC followed by gastrectomy. The eligibility criteria for NAC were cT3-4/N1-3 or macroscopic type 3 (> 80 mm) or type 4 advanced gastric cancer. Routine NAC regimens consisted of TS-1 + cisplatin (SP) or TS-1 + oxaliplatin (SOX). Patients with human epidermal growth factor 2 (HER2)-positive gastric cancer received trastuzumab + TS-1 + oxaliplatin (tmab + SOX) or trastuzumab + capecitabine + oxaliplatin (tmab + CAPOX). Surgery was performed 4 to 6 weeks after the completion of chemotherapy. Patients who underwent palliative gastrectomy (n = 1) and staging laparoscopy (n = 1) for macroscopic peritoneal dissemination were excluded from the present study. Ultimately, 49 patients were selected and subsequently divided into two cohorts: the LG group and the OG group. All patients underwent gastrectomy according to the treatment guidelines of the Japanese Gastric Cancer Association [13].
All surgeries were performed by board-certified surgeons from the Japanese Society of Gastroenterological Surgery. In addition, all LG procedures were performed by qualified surgeons certified by the Endoscopic Surgical Skill Qualification System of the Japan Society for Endoscopic Surgery. This study was approved by our Institutional Review Board and included prospective data collection and retrospective analysis of data obtained from patients undergoing gastrectomy. All patients and their families were informed about the innovative nature of the study, and written informed consent was obtained before surgery.
Operative technique
All patients underwent radical gastrectomy with D2 lymph node dissection according to the Japanese classification of gastric carcinoma by the Japanese Gastric Cancer Association [14]. Total or distal gastrectomy was performed depending on the location of the primary tumor. During total gastrectomy, the spleen was removed when the tumor invaded the upper one-third of the greater curvature. The reconstruction method used in both groups comprised either Billroth I/II or Roux-en-Y procedures depending on the surgeon’s preference. The selection of either a laparoscopic or an open surgical approach was based on the attending surgeon’s discretion or on the patient’s preference.
In the LG group, reconstruction was achieved through intracorporeal anastomosis. During intracorporeal reconstruction, Roux-en-Y gastrojejunostomy, Billroth II anastomosis with a functional side-to-side anastomosis, or delta-shaped Billroth I anastomosis was performed for patients with distal gastrectomy [15]. Esophagojejunostomy was performed using a functional end-to-end anastomosis or overlap anastomosis among patients with total gastrectomy [16].
In the OG group, Billroth I anastomosis was performed using a circular stapler or hand suturing. Billroth II or Rou-en-Y gastrojejunostomy was conducted using a functional side-to-side anastomosis. In cases that required total gastrectomy, Rou-en-Y esophagojejunostomy was performed using a circular stapler.
Data collection and perioperative management
Adverse events from NAC were evaluated using the Common Terminology Criteria for Adverse Events version 4.0, and only those with grade 3 or higher were recorded. The radiological responses in all patients were assessed using the Response Evaluation Criteria In Solid Tumors (RECIST, version 1.1) based on enhanced computed tomography conducted before and after NAC [17]. All patients were managed using a standardized clinical pathway protocol during the perioperative period. Oral feeding was started after the passage of flatus. Patients were discharged once they were free from complications. Clinicopathological parameters, such as age, body mass index, American Society of Anesthesiologists score, and Carlson comorbidity index [18], as well as perioperative data, such as operative time, intraoperative blood loss, presence or absence of postoperative complications, length of postoperative hospital stay, clinicopathological TNM stage, and tumor regression grade, were evaluated according to the Japanese classification of gastric carcinoma by the Japanese Gastric Cancer Association [14]. Anastomotic stricture was defined as a condition that required endoscopic dilation. Anastomotic leakage was radiologically evaluated using water-soluble contrast material on the third postoperative day. Postoperative complications were defined according to the Clavien–Dindo classification system, and only those with grade 2 or higher were recorded [19]. Hospital mortality was defined as death during hospitalization or postoperative death of any cause within 30 days. Some patients underwent adjuvant chemotherapy with TS-1 alone or TS-1 plus cisplatin for 1 year based on the pathological stage and patients’ preference. Patients were followed via outpatient clinic visitations every 3 months for the first 2 years after surgery, every 6 months for the next 3 years, and annually thereafter. All patients were followed from the date of surgery until death or until the end of the follow-up period (September 2019).
Statistical analysis
The chi-square test was used to compare percentages of events between dichotomous groups, while Fisher’s exact test was used when cells had an expected frequency of less than 5. The Mann–Whitney U test was used to compare continuous variables between the groups. Survival rates were calculated using the Kaplan–Meier method, and statistical analysis was performed using the log-rank test. Uni- and multivariate analyses using Cox proportional hazards regression were performed to determine the risk factors for survival. A cutoff point for each continuous variable associated with survival was identified using the sample median. Thereafter, all variables with P values less than 0.2 on univariate analysis, including pathological stage and surgical procedure (LG vs. OG), were entered into multivariate analysis. A P value of less than 0.05 was considered statistically significant. All statistical analyses were performed using Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA, USA).
Results
The patient characteristics are summarized in Table 1. The median ages of the patients in the LG and OG groups were 71.5 and 67.0 years, respectively. Most of the variables were comparable between the two groups. Although the tumor diameter tended to be larger in the LG group than in the OG group (75 vs. 50 mm), the difference was not statistically significant (P = 0.106). The most frequent NAC regimen was SP in both groups. The clinical response rates were 60% (12 of 20 patients) and 62% (18 of 29 patients) in the LG and OG groups (P = 0.935), respectively. Among all included patients, only one in the OG group achieved a pathological complete response.
The adverse events that occurred during NAC are presented in Table 2. Grade 3 or 4 toxic events occurred in 20% (4 of 20 patients) and 17% (5 of 29 patients) of the patients in the LG and OG groups (P = 1.000), respectively. The most frequent event was neutropenia, followed by leukopenia and anemia. No chemotherapy-related deaths were noted.
Table 3 presents the surgical data of the two groups. No significant differences in the type of gastrectomy, presence or absence of splenectomy, or number of dissected lymph nodes were observed between the groups. Despite having a longer operative time (362 vs. 314 min; P = 0.082), the LG group exhibited a significantly lower amount of intraoperative blood loss than the OG group (56.5 vs. 501 mL; P < 0.001). One patient in the LG group underwent R1 resection due to positive peritoneal lavage cytology. No patients in the LG group required conversion to open surgery.
The postoperative variables are presented in Table 4. The overall rates of complications were 10% (2 of 20 patients) and 24% (7 of 29 patients) in the LG and OG groups (P = 0.277), respectively. The most common surgical complications were pancreatic fistula and abdominal abscess (n = 2 for each, 4%). Among all included patients, three required reoperation due to abdominal bleeding (n = 2) and anastomotic leakage (n = 1). The LG group demonstrated a significantly shorter time to postoperative oral feeding and duration of postoperative hospital stay compared to the OG group (P = 0.001 and 0.005, respectively). No hospital deaths were observed in either group.
Disease-free survival (DFS) and overall survival (OS) were then analyzed in the two groups. The median follow-up period was 38 (range 3–115) months, and no patients were lost to follow-up. A total of 23 patients died during the follow-up period, of them 20 died because of cancer and 3 because of other diseases. The calculated 5-year DFS rates were 44.4% and 53.2% in the LG and OG groups (P = 0.382; Fig. 1a), respectively. Nine patients in the LG group developed disease recurrence, with 6 (30%), 2 (10%), and 2 (10%) occurring in the peritoneum, liver, and lymph nodes, respectively. On the other hand, 12 patients in the OG group developed disease recurrence, with 6 (21%), 4 (14%), and 3 (10%) occurring in the peritoneum, liver, and lymph nodes, respectively. Recurrence patterns were similar between the groups. The calculated 5-year OS rates were 46.9% and 56.0% in the LG and OG groups, respectively, with no significant difference between the groups (P = 0.422; Fig. 1b).
The univariate analysis results of the risk factors for long-term outcomes are summarized in Table 5. An age of 70 years or older was significantly associated with OS (P = 0.009) and tended to be associated with DFS albeit not significantly (P = 0.063). Multivariate analysis incorporating the type of surgical procedure (LG vs. OG) revealed that an age of 70 years or older (P = 0.009) and postoperative complications (P = 0.027) were independently associated with OS, whereas no factors were associated with DFS (Table 6).
Discussion
To date, only a few studies have compared short-term outcomes between laparoscopic distal gastrectomy and open surgery in patients with gastric cancer following NAC [20, 21]. Moreover, the long-term safety and feasibility of LG, including total gastrectomy, for patients with advanced gastric cancer following NAC have remained unclear. This study was designed to investigate the surgical outcomes and survival rates of LG following NAC and to compare them to those of OG. To the best of our knowledge, this is the first cohort study investigating the long-term outcomes of LG in patients with gastric cancer following NAC. The present study showed that laparoscopic gastrectomy following NAC was safe and feasible given its good short- and long-term outcomes.
While the main NAC regimen used was SP in the current study, several useful NAC regimens have been reported for advanced gastric cancer including epirubicin + cisplatin + fluorouracil [2], docetaxel + oxaliplatin + fluorouracil + leucovorin (FLOT) [3], oxaliplatin + fluorouracil + leucovorin (FOLFOX) [22], SP [23], SOX [24], and CAPOX [25]. Among the aforementioned regimens, SP has been one of the most utilized regimens in Japan, with previous reports showing a clinical response rate of 65.0–69.7% and grade 3 or 4 adverse event rates of 15.4–19.0% [23, 26]. Although our study included a few other regimens, their efficacy and toxicity appeared to be acceptable. On the other hand, the most effective NAC regimen for advanced gastric cancer remains unknown. According to the treatment guidelines of the Japanese Gastric Cancer Association [13], SP is the standard first-line regimen for unresectable/recurrent gastric cancer and was mostly adopted in this study. However, there is less evidence of SP use for NAC regimen [27]. The efficacy of several NAC regimens (i.e., FLOT, FOLFOX, or CAPOX) have recently been demonstrated [3, 22, 25], and this issue requires to be further evaluated.
The LG group demonstrated a significantly lower amount of intraoperative blood loss and better postoperative recovery (i.e., a shorter time to first oral intake and a shorter hospital stay) compared with the OG group, with previous studies reporting similar findings for advanced gastric cancer patients without NAC [7, 28]. Thus, the current study suggests that the benefits of laparoscopic procedures remain the same for patients with advanced gastric cancer who undergo NAC. In particular, intraoperative blood loss has been reported to be associated with the prognosis of many malignant tumors, including gastric cancer [29,30,31]. Therefore, careful operative techniques should be utilized to minimize intraoperative blood loss.
No significant differences in postoperative complication rates were observed between the LG (2 patients, 10%) and OG groups (7 patients, 24%) (P = 0.277), a finding consistent with the morbidity rates (10.5–30.7%) reported in previous studies on patients who underwent open D2 gastrectomy following NAC [32,33,34,35]. Moreover, among the two cases of postoperative complications that developed in the LG group, one was due to heart failure, a nonsurgical complication classified as a patient-related factor. Hence, the incidence of surgical complications in the LG group can be considered quite low (1 of 20, 5%). A previous study on patients with NAC showed that LG reduced postoperative complication rates by half [21]. Moreover, the study suggested that the advantages of LG over OG for postoperative complications might be more substantial among those receiving NAC for the following reasons. Although chemotherapy-induced tissue fibrotic changes can make surgery more difficult and perhaps increase postoperative complication rates, such issues may be mitigated by laparoscopy given that it allows for visual magnification, better exposure, and more delicate maneuvers of organs, vessels, and nerves. While we agree with the aforementioned reasons, our study may have a certain bias. As previously mentioned, all laparoscopic procedures in the current study were performed by qualified surgeons certified by the Endoscopic Surgical Skill Qualification System of the Japan Society for Endoscopic Surgery. These surgeons performed more than 50 laparoscopic surgeries for gastric cancer annually and had more surgical experience than some of the surgeons performing OG in the current study. With several studies indicating an association between surgical experience and postoperative complications [36, 37], generalizing our findings among surgeons with various levels of expertise is rather difficult.
The results of long-term outcomes showed that the LG and OG groups had 5-year OS rates of 46.9% and 56.0%, respectively. Previous clinical trials on NAC for locally advanced gastric cancer, such as large type 3, type 4, cT3-4, or N + , showed 5-year OS rates of 47.0%–57.7% [22, 35, 38]. Directly comparing such results with those of the current study is difficult because of the differences in patient background and study design. However, the patient population included in the current study does overlap with those in previous studies, suggesting that long-term outcomes may not be disappointing. In addition, multivariate analysis was performed to determine the risk factors for long-term outcomes in gastric cancer patients with NAC. The current study indicated that the surgical procedure was not an independent risk factor for OS and DFS. One of the major concerns of using laparoscopic procedures for advanced cancer is the possibility of intraoperative peritoneal seeding of malignant cells. However, the study by Shoup et al. indicated that port-site recurrence for advanced disease was relatively rare [39]. Furthermore, the LOC-A study, one of the largest studies in Japan that focused on long-term outcomes for advanced gastric cancer, showed that laparoscopic procedures did not cause any specific recurrence [8]. In the present study, the pattern of recurrence was similar between both groups, while no incidence of port-site recurrence was noted in the LG group. Although we believe that LG following NAC does not affect recurrence patterns, larger-scale studies are required to establish oncological safety.
Previous reports have shown that the incidence of postoperative complications is a negative prognostic factor that affects not only OS but also RFS in patients with gastric cancer [40, 41]. One possible reason for this association is that patients with postoperative complications may have some factors that promote decreased host immunity against cancer cells [42]. Our findings also indicated that postoperative complications were independently associated with OS. However, this factor was not included in the multivariate analysis for DFS given that it had a P value > 0.2 in univariate analysis. Accordingly, after entering this variable into multivariate analysis and repeating our analysis, we found that postoperative complications were not independently associated with DFS (P = 0.066, data not shown). Other reasons for the correlation between postoperative complications and long-term outcome must therefore be considered. If frail patients with potentially poor prognosis easily developed postoperative complications, the incidence of these complications might not be an independent negative prognostic factor [43]. Accordingly, given that the current study included many elderly patients, our results might have been affected by the frailty of some patients.
One clear limitation of the present study was the lack of randomization in the two treatment arms. Another limitation was the retrospective design and limited sample size. Moreover, type II error probably existed in our study due to the small sample size. Further large-scale randomized prospective studies are thus required to clearly establish the safety and efficacy of LG for gastric cancer following NAC.
In conclusion, this preliminary retrospective study showed that LG and OG for advanced gastric cancer following NAC had comparable short- and long-term outcomes. Moreover, the present study suggests that LG may be a therapeutic option for patients with gastric cancer following NAC.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65:87–108
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, Trial Participants MAGIC (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355:11–20
Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, Kopp HG, Mayer F, Haag GM, Luley K, Lindig U, Schmiegel W, Pohl M, Stoehlmacher J, Folprecht G, Probst S, Prasnikar N, Fischbach W, Mahlberg R, Trojan J, Koenigsmann M, Martens UM, Thuss-Patience P, Egger M, Block A, Heinemann V, Illerhaus G, Moehler M, Schenk M, Kullmann F, Behringer DM, Heike M, Pink D, Teschendorf C, Löhr C, Bernhard H, Schuch G, Rethwisch V, von Weikersthal LF, Hartmann JT, Kneba M, Daum S, Schulmann K, Weniger J, Belle S, Gaiser T, Oduncu FS, Güntner M, Hozaeel W, Reichart A, Jäger E, Kraus T, Mönig S, ßBechstein WO, Schuler M, Schmalenberg H, Hofheinz RD, FLOT4-AIO Investigators (2019) Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 393:1948–1957
Kitano S, Iso Y, Moriyama M, Sugimachi K (1994) Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 4:146–148
Hu Y, Ying M, Huang C, Wei H, Jiang Z, Peng X, Hu J, Du X, Wang B, Lin F, Xu J, Dong G, Mou T, Li G, Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group (2014) Oncologic outcomes of laparoscopy-assisted gastrectomy for advanced gastric cancer: a large-scale multicenter retrospective cohort study from China. Surg Endsoc 28:2048–2056
Lee JH, Lee CM, Son SY, Ahn SH, Park DJ, Kim HH (2014) Laparoscopic versus open gastrectomy for gastric cancer: long-term oncologic results. Surgery 155:154–164
Shinohara T, Satoh S, Kanaya S, Ishida Y, Taniguchi K, Isogaki J, Inaba K, Yanaga K, Uyama I (2013) Laparoscopic versus open D2 gastrectomy for advanced gastric cancer: a retrospective cohort study. Surg Endosc 27:286–294
Kinoshita T, Uyama I, Terashima M, Noshiro H, Nagai E, Obama K, Tamamori Y, Nabae T, Honda M, Abe T, LOC-A Study Group (2019) Long-term outcomes of laparoscopic versus open surgery for clinical Stage II/III gastric cancer: A multicenter cohort study in Japan (LOC-A study). Ann Surg 269:887–894
Cai J, Wei D, Gao CF, Zhang CS, Zhang H, Zhao T (2011) A prospective randomized study comparing open versus laparoscopy-assisted D2 radical gastrectomy in advanced gastric cancer. Dig Surg 28:331–337
Park YK, Yoon HM, Kim YW, Park JY, Ryu KW, Lee YJ, Jeong O, Yoon KY, Lee JH, Lee SE, Yu W, Jeong SH, Kim T, Kim S, Nam BH, COACT group, (2018) Laparoscopy-assisted versus open D2 distal gastrectomy for advanced gastric cancer: results from a randomized phase II multicenter clinical trial (COACT 1001). Ann Surg 267:638–645
Inaki N, Etoh T, Ohyama T, Uchiyama K, Katada N, Koeda K, Yoshida K, Takagane A, Kojima K, Sakuramoto S, Shiraishi N, Kitano S (2015) A multi-institutional, prospective, Phase II feasibility study of laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for locally advanced gastric cancer (JLSSG0901). World J Surg 39:2734–2741
Hur H, Lee HY, Lee HJ, Kim MC, Hyung WJ, Park YK, Kim W, Han SU (2015) Efficacy of laparoscopic subtotal gastrectomy with D2 lymphadenectomy for locally advanced gastric cancer: the protocol of the KLASS-02 multicenter randomized controlled clinical trial. BMC Cancer 15:355
Japanese Gastric Cancer Association (2011) Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 14:113–123
Japanese Gastric Cancer Association (2011) Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14:101–112
Kanaya S, Gomi T, Momoi H, Tamaki N, Isobe H, Katayama T, Wada Y, Ohtoshi M (2002) Delta-shaped anastomosis in totally laparoscopic Billroth I gastrectomy: new technique of intraab- dominal gastroduodenostomy. J Am Coll Surg 195:284–287
Inaba K, Satoh S, Ishida Y, Taniguchi K, Isogaki J, Kanaya S, Uyama I (2010) Overlap method: novel intracorporeal esophagojejunostomy after laparoscopic total gastrectomy. J Am Coll Surg 211:e25–e29
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Li Z, Shan F, Wang Y, Li S, Jia Y, Zhang L, Yin D, Ji J (2016) Laparoscopic versus open distal gastrectomy for locally advanced gastric cancer after neoadjuvant chemotherapy: safety and short-term oncologic results. Surg Endosc 30:4265–4278
Li Z, Shan F, Ying X, Zhang Y, Wang Y, Ren H, Su X, Ji J (2019) Assessment of laparoscopic distal gastrectomy after neoadjuvant chemotherapy for locally advanced gastric cancer: a randomized clinical trial. JAMA. https://doi.org/10.1001/jamasurg.2019.3473
Wang X, Zhao L, Liu H, Zhong D, Liu W, Shan G, Dong F, Gao W, Bai C, Li X (2016) A phase II study of a modified FOLFOX6 regimen as neoadjuvant chemotherapy for locally advanced gastric cancer. Br J Cancer 114:1326–1333
Tsuburaya A, Mizusawa J, Tanaka Y, Fukushima N, Nashimoto A, Sasako M, Stomach Cancer Study Group of the Japan Clinical Oncology Group (2014) Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg 101:653–660
Feng D, Leong M, Li T, Chen L, Li T (2015) Surgical outcomes in patients with locally advanced gastric cancer treated with S-1 and oxaliplatin as neoadjuvant chemotherapy. World J Surg Oncol 13:11
Terazawa T, Matsuyama J, Goto M, Kawabata R, Endo S, Imano M, Fujita S, Akamura Y, Taniguchi H, Tatsumi M, Lee SW, Kurisu Y, Kawakami H, Kurokawa Y, Shimokawa T, Sakai D, Kato T, Fujitani K, Satoh T (2020) A phase II study of perioperative capecitabine plus oxaliplatin therapy for clinical SS/SE N1–3 M0 gastric cancer (OGSG1601). Oncologist 25:119–e208
Kochi M, Fujii M, Kanamori N, Mihara Y, Funada T, Tamegai H, Watanabe M, Takayama Y, Suda H, Takayama T (2017) Phase II study of neoadjuvant chemotherapy with S-1 and CDDP in patients with lymph node metastatic Stage II or III gastric cancer. Am J Clin Oncol 40:17–21
Tokunaga M, Sato Y, Nakagawa M, Aburatani T, Matsuyama T, Nakajima Y, Kinugasa Y (2020) Perioperative chemotherapy for locally advanced gastric cancer in Japan: current and future perspectives. Surg Today 50:30–37
Wang Z, Xing J, Cai J, Zhang Z, Li F, Zhang N, Wu J, Cui M, Liu Y, Chen L, Yang H, Zheng Z, WangX GC, Wang Z, Fan Q, Zhu Y, Ren S, Zhang C, Liu M, Ji J, Su X (2019) Short-term surgical outcomes of laparoscopy-assisted versus open D2 distal gastrectomy for locally advanced gastric cancer in North China: a multicenter randomized controlled trial. Surg Endosc 33:33–45
Mörner ME, Gunnarsson U, Jestin P, Svanfeldt M (2012) The importance of blood loss during colon cancer surgery for long- term survival: an epidemiological study based on a population based register. Ann Surg 255:1126–1128
Nagai S, Fujii T, Kodera Y, Kanda M, Sahin TT, Kanzaki A, Yamada S, Sugimoto H, Nomoto S, Takeda S, Morita S, Nakao A (2011) Impact of operative blood loss on survival in invasive ductal adenocarcinoma of the pancreas. Pancreas 40:3–9
Liang YX, Guo HH, Deng JY, Wang BG, Ding XW, Wang XN, Zhang L, Liang H (2013) Impact of intraoperative blood loss on survival after curative resection for gastric cancer. World J Gastroenterol 19:5542–5550
Ahn HS, Jeong SH, Son YG, Lee HJ, Im SA, Bang YJ, Kim HH, Yang HK (2014) Effect of neoadjuvant chemotherapy on postoperative morbidity and mortality in patients with locally advanced gastric cancer. Br J Surg 101:1560–1565
Okabe H, Hata H, Ueda S, Zaima M, Tokuka A, Yoshimura T, Ota S, Kinjo Y, Yoshimura K, Sakai Y, Kyoto University Surgical Oncology Group (KUSOG) (2016) A phase II study of neoadjuvant chemotherapy with S-1 and cisplatin for stage III gastric cancer: KUGC03. J Surg Oncol 113:36–41
Wu L, Ge L, Qin Y, Huang M, Chen J, Yang Y, Zhong J (2019) Postoperative morbidity and mortality after neoadjuvant chemotherapy versus upfront surgery for locally advanced gastric cancer: a propensity score matching analysis. Cancer Manag Res 11:6011–6018
Kosaka T, Akiyama H, Miyamoto H, Sato S, Tanaka Y, Sato K, Kunisaki C, Endo I (2019) Outcomes of preoperative S-1 and docetaxel combination chemotherapy in patients with locally advanced gastric cancer. Cancer Chemother Pharmacol 83:1047–1055
Kim MC, Kim W, Kim HH, Ryu SW, Ryu SY, Song KY, Lee HJ, Cho GS, Han SU, Hyung WJ, Korean Laparoscopic Gastrointestinal Surgery Study (KLASS) Group (2008) Risk factors associated with complication following laparoscopy-assisted gastrectomy for gastric cancer: a large-scale korean multicenter study. Ann Surg Oncol 15:2692–2700
Ryu KW, Kim YW, Lee JH, Nam BH, Kook MC, Choi IJ, Bae JM (2008) Surgical complications and the risk factors of laparoscopy-assisted distal gastrectomy in early gastric cancer. Ann Surg Oncol 15:1625–1631
Fazio N, Biffi R, Maibach R, Hayoz S, Thierstein S, Brauchli P, Bernhard J, Stupp R, Andreoni B, Renne G, Crosta C, Morant R, Chiappa A, Luca F, Zampino MG, Huber O, Goldhirsch A, de Braud F, Roth AD, Swiss Group for Clinical Cancer Research SAKK, European Institute of Oncology, Milan, Italy (2016) Preoperative versus postoperative docetaxel-cisplatin-fluorouracil (TCF) chemotherapy in locally advanced resectable gastric carcinoma: 10-year follow-up of the SAKK 43/99 phase III trial. Ann Oncol 27:668–673
Shoup M, Brennan MF, Karpeh MS, Gillern SM, McMahon RL, Conlon KC (2002) Port site metastasis after diagnostic laparoscopy for upper gastrointestinal tract malignancies: an uncommon entity. Ann Surg Oncol 9:632–636
Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Terashima M (2013) Poor survival rate in patients with postoperative intra-abdominal infectious complicationsfollowing curative gastrectomy for gastric cancer. Ann Surg Oncol 20:1575–1583
Kubota T, Hiki N, Sano T, Nomura S, Nunobe S, Kumagai K, Aikou S, Watanabe R, Kosuga T, Yamaguchi T (2014) Prognostic significance of complications after curative surgery for gastric cancer. Ann Surg Oncol 21:891–898
Lagarde SM, Reitsma JB, Ten Kate FJ, Busch OR, Obertop H, Zwinderman AH, Moons J, van Lanschot JJ, Lerut T (2008) Predicting individual survival after potentially curative esophagectomy for adenocarcinoma of the esophagus or gastroesophageal junction. Ann Surg 248:1006–1013
Shimada H, Fukagawa T, Haga Y, Oba K (2017) Does postoperative morbidity worsen the oncological outcome after radical surgery for gastrointestinal cancers? A systematic review of the literature. Ann Gastroenterol Surg 1:11–23
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Muneharu Fujisaki, Norio Mitsumori, Toshihiko Shinohara, Naoto Takahashi, Hiroaki Aoki, Yuya Nyumura, Seizo Kitazawa, and Katsuhiko Yanaga have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fujisaki, M., Mitsumori, N., Shinohara, T. et al. Short- and long-term outcomes of laparoscopic versus open gastrectomy for locally advanced gastric cancer following neoadjuvant chemotherapy. Surg Endosc 35, 1682–1690 (2021). https://doi.org/10.1007/s00464-020-07552-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-07552-1