Abstract
Background
Recent studies have reported the feasibility of indocyanine green fluorescence imaging of adrenal tumors to guide dissection. Although the adrenal has been reported to concentrate the dye more than surrounding tissues, the amount of tissue distinction and how this compares with conventional vision has not been quantified before. The aim of this study is to quantify this distinction using color analysis.
Methods
This was an institutional review board-approved retrospective study. By excluding adrenal cortical carcinoma, metastasis and pheochromocytoma, video recordings of 50 patients who underwent robotic transabdominal lateral adrenalectomy with indocyanine green (ICG) imaging for adrenocortical tumors between August 2015 and May 2018 were reviewed. Using a color analysis software, the pixel intensity of adrenal tumors versus adjacent retroperitoneal tissues was calculated for conventional red, green and blue, as well as indocyanine green (ICG) scales. Statistical analysis was performed using ANOVA.
Results
A total of 50 patients underwent unilateral robotic transabdominal lateral adrenalectomy. All procedures were completed robotically without a conversion to laparoscopy or open. Morbidity was 4%. Measured pixel intensity of adrenal tumors was higher than adjacent retroperitoneal tissues for all conventional color and ICG modes (p < 0.0001), with the gradient being more pronounced for ICG green versus conventional red, green and blue modes.
Conclusions
To our knowledge this is the first study attempting to encode tissue planes in robotic adrenalectomy. The results show that the visual contrast distinction observed between adrenal and adjacent retroperitoneal tissues can be quantified using pixel intensity. ICG enabled the distinction of tissue planes with a wider gradient compared to conventional RGB view, quantifying its subjective benefits reported in prior studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Laparoscopy revolutionized the care of patients with adrenal tumors by giving the patients the opportunity to undergo the procedure through small, rather than big open incisions that were associated with a certain morbidity and a prolonged hospital stay [1, 2]. Subsequently, robotic approaches to adrenalectomy using articulated instruments and three-dimensional view have been described [3, 4]. Excellent patient outcomes have been described for both laparoscopic and robotic adrenalectomy (RA) techniques [5, 6].
Indocyanine green is a sterile, anionic and amphiphilic tricarbocyanine dye that fluoresces when excited with near-infrared (NIR) fluorescence at 800 nm. Since the original Food and Drug Administration approval in 1959 [7, 8] it has found multiple applications in abdominal surgery to study tissue perfusion including assessment of anastomoses, identification of the common bile duct and mapping out tumors [9,10,11,12]. The fact that the adrenal ranks as the top third intra-abdominal organ regarding the rate of its blood flow [13] has encouraged the incorporation of ICG imaging into adrenalectomy procedures to guide dissection in both robotic and laparoscopic approaches [14, 15]. We previously reported that in about half of robotic adrenalectomy procedures, ICG view provided a better visual distinction between adrenal tumors and surrounding retroperitoneal tissues [15, 16]. To our knowledge, this subjective superiority in visual distinction has not been objectively quantified. The aim of this study was to quantify the ability of ICG view to distinguish adrenal tumors from surrounding tissues using color analysis. Our hypothesis was that the differences in the color intensity between adrenal tumors and surrounding tissues could be measured and quantified.
Methods
This was an Institutional Review Board (IRB)-approved study of patients undergoing robotic adrenalectomy at a single center between August 2015 and May 2018. Inclusion criteria were (1) robotic transabdominal lateral adrenalectomy, (2) the use of ICG green fluorescence during the procedure and (3) adrenocortical tumors. During this period, 81 patients underwent robotic transabdominal lateral adrenalectomy by one surgeon (EB) at the Department of Endocrine Surgery, Cleveland Clinic, OH. By excluding non-adrenocortical tumors (malignant tumors, pheochromocytomas, schwannomas, lymphangiomas and cysts), 50 consecutive patients who underwent robotic lateral transabdominal adrenalectomy were included in this study. Intra-operative video recordings of these patients were reviewed. The decision for surgery was made based on American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons guidelines [17]. The techniques for RA and ICG imaging have been previously described in detail [15]. Intra-operatively, once the abdomen was entered and liver and spleen were retracted for right and left-sided lesions, respectively, 5 mg of ICG was administered intravenously by the anesthesia team. The surgeon switched back between ICG and regular views intermittently. The ICG injection was repeated based on the discretion of the surgeon during the case. Clinical data were entered into an IRB-approved database.
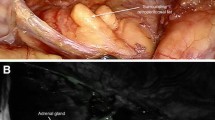
Surgical videos were analyzed retrospectively using ImageJ software (National Institutes of Health, Bethesda, MD). The measurements were made for three different surgical scenes during the procedure and for each scene, measurements were performed from three different regions on the adrenal and adjacent retroperitoneum. The measurements were performed along the edge of the adrenal tumor and adjacent retroperitoneum using a circular region of interest tool with a diameter of 1 cm. The ImageJ software calculated the mean pixel intensity within the region of interest tool. The measurements from the same regions were obtained for both ICG and regular views within red, green and blue spectrums using the “Histogram analysis mode” on the software (Figs. 1, 2). Pixel intensity of adrenal tumors versus retroperitoneum on ICG fluorescence and conventional view red, green and blue modes was compared using Mann–Whitney U with JMP version 13.1 (SAS Institute, Inc. Cary, NC). The difference in pixel intensity between adrenal tumors and retroperitoneum defines the gradient between these tissues. The higher this gradient is, the more distinct is the border between the adrenal tumor and retroperitoneum. Continuous data are expressed as mean ± standard error of mean.
Results
There was a total of 50 patients who underwent robotic transabdominal lateral adrenalectomy and fulfilled the inclusion criteria for the study (Table 1). Diagnosis was cortisol-secreting adrenocortical adenoma (ACA) in 20, non-secreting ACA in 16 patients and aldosterone-secreting ACA in 14 patients. Most (84%) patients were female, with an average tumor size of 3.2 cm. Tumors were located in the left in 28 (56%) and right in 22 (44%). All of the procedures were completed robotically, with no conversion to open. The patients stayed an average of 1.5 days at the hospital. Ninety-day complication rate was 4% (one patient each developed ileus and diarrhea). There were no complications related to the administration of ICG.
Measured pixel intensity of adrenal tumors was higher than adjacent retroperitoneal tissues for all conventional and ICG scales (p < 0.0001) (Fig. 3). The color gradient between adrenal tumor and adjacent retroperitoneal tissues was wider for the ICG green mode versus conventional view red, green and blue modes. Figure 4 shows the values in pixel intensity for adrenal tumors versus retroperitoneum in ICG green mode for all of the measurements made for the study.
Scatter plots showing the pixel intensity of adrenal tumors versus retroperitoneum (RP) on ICG green (A), conventional view red (B), green (C) and blue (D) and RGB (E) modes. Y-axis is the pixel intensity of the signal and the X-axis denotes the 450 paired measurements done for the study (Color figure online)
Discussion
To our knowledge this is the first study to quantify differences in tissue distinction obtained with conventional and ICG views during adrenalectomy procedures. Traditionally, surgeons have relied on the recognition of the classic golden yellow color of the adrenal to distinguish it from the surrounding yellow fat in the retroperitoneum. The historical challenge has been in defining tissue planes when the color of the adrenal was similar to that of the surrounding retroperitoneal fatty tissues and also in patients with abundant retroperitoneal fat. Although ultrasound has been used to identify adrenal tumors and guide dissection [7], it has a low utility in patients with small tumors and requires the interruption of the surgical procedure each time it is used. This study shows that ICG fluorescence imaging is an adjunctive tool which delineates the borders of adrenocortical masses more distinctly compared to conventional visual view. The identification of the borders of adrenal tumors is important in order to get in to the right plane, prevent capsular violation and minimize blood loss during minimally invasive adrenalectomy. It is important to know whether this technique can be generalized to other adrenal surgeons. We believe it is, as the intravenous dosing has been standardized at 5 mg per injection and the imaging technique is reproducible. Other investigators have also reported that adrenal neoplasms can be resected minimally invasively under ICG fluorescence guidance by identifying vascular structures and enhancing the borders of the tumor [14, 18]. These reports highlight the utility of this technology for both practicing surgeons and also for training purposes. The superior tissue distinction created by ICG versus conventional view can also be used to guide the trainees to find the correct the tissue planes during teaching cases.
Indocyanine green imaging demonstrates tissue perfusion. Since endocrine organs are quite vascular, our group started prospectively evaluating the utility of ICG imaging in endocrine surgeries in 2014 [15, 19, 20]. In the initial feasibility study [7], we reported that a 5-mg dose was most ideal, with adrenal taking the dye at 1 min after injection and the distinction between adrenal and retroperitoneal tissues becoming most obvious at 5 min. We also reported that the fluorescence of adrenal lasted up to 20 min. Subsequently, we reported that the ICG view was superior to the robotic view in half of the patients [15]. In 2018, we determined that different adrenal tumors demonstrated different ICG fluorescence patterns based on histologic origin [16]. The best utility was for adrenocortical tumors operated through a lateral transabdominal approach. In this study, the ICG view was thought to provide better contrast distinction between the adrenal tumor and retroperitoneum compared to non-fluoresced view in 41% of tumors. The current study demonstrates that this subjective impression is related to the fact that ICG imaging creates a more pronounced color gradient in terms of pixel intensity compared to the conventional RGB color spectrums. The relevance of this superiority lies in the fact that this leads to a more accurate identification of the dissection plane between the adrenal tumor and surrounding retroperitoneum. To our knowledge, this has not been quantified before. Although a robotic platform was used in the current study, many laparoscopic platforms also exist and have been described [14]. However, since no tactile feedback is provided by robotic systems, ICG fluorescence is a useful adjunct during RA to accurately define the dissection planes.
Laparoscopic ultrasound is an essential part of our adrenalectomy procedures and we routinely perform before starting dissection. Nevertheless, with the technique presented in the current study, we rarely felt the need to repeat the ultrasound subsequently during dissection, as in most cases we were satisfied with the information obtained with ICG imaging regarding tissue planes.
The results of the current study apply to the removal of ACAs only. We previously showed that most pheochromocytomas and malignant tumors do not show fluorescence upon injection of ICG [16]. Hence this is a drawback of the study.
Another utility of ICG imaging during adrenalectomy is to guide cortical-sparing adrenalectomy by delineating the border between the pheochromocytoma and adrenocortical tissue and confirming the perfusion of the remnant [21,22,23]. This utility has not been analyzed in the current study.
Pixel intensity analysis is a useful method to quantify degrees of fluorescence observed from tissues. We have previously applied this method to quantify the fluorescence pattern of parathyroid glands [24] as well and recommend its adoption by the surgical community to objectively describe fluorescence findings described in various surgical procedures.
An important question is whether the use of ICG imaging during adrenalectomy can decrease complications. Although the current study was not designed to answer this question, the data showed that there was better distinction between adrenocortical tumors and retroperitoneum. By theoretically decreasing the chances of getting into the wrong plane and hence increasing the chances of bleeding and capsular violation, ICG guidance may improve the quality and speed of dissection during minimally invasive adrenalectomy. It may also alert the surgeon about the adrenal vein, as the adrenal vein will remain hypo-fluorescent (unless the ICG injection is repeated while in view) in contrast to the fluorescent adrenal tissue during dissection.
The incidence of a severe allergic reaction with ICG injection was reported to be 0.05% in the literature [16] and is associated with much higher doses (0.5 mg/kg) than were used in this study.
In conclusion, we report the first study in an attempt to encode tissue planes in robotic adrenalectomy. The results show that the visual contrast distinction observed between adrenal and adjacent retroperitoneal tissues can be quantified using pixel intensity. ICG enabled the distinction of tissue planes with a wider gradient compared to conventional RGB view, quantifying its subjective benefits reported in prior studies. Future studies could focus on comparing outcomes of patients undergoing minimally invasive adrenalectomy with or without ICG fluorescence guidance.
References
Mercan S, Seven R, Ozarmagan S, Tezelman S (1995) Endoscopic retroperitoneal adrenalectomy. Surgery 118(6):1071–1076
Gagner M, Pomp A, Heniford BT, Pharand D, Lacroix A (1997) Laparoscopic adrenalectomy: lessons learned from 100 consecutive procedures. Ann Surg 226:238–246
Taskin HE, Berber E (2012) Robotic adrenalectomy. J Surg Oncol 106(5):622–625
Agcaoglu O, Aliyev S, Karabulut K, Mitchell J, Siperstein A, Berber E (2012) Robotic versus laparoscopic resection of large adrenal tumors. Ann Surg Oncol 19:2288–2294
Kebebew E, Siperstein AE, Duh QY (2001) Laparoscopic adrenalectomy: the optimal surgical approach. J Laparoendosc Adv Surg Tech A 11(6):409–413
Kahramangil B, Berber E (2018) Comparison of posterior retroperitoneal and transabdominal lateral approaches in robotic adrenalectomy: an analysis of 200 cases. Surg Endosc 32(4):1984–1989
Sound S, Okoh AK, Bucak E, Yigitbas H, Dural C, Berber E (2016) Intraoperative tumor localization and tissue distinction during robotic adrenalectomy using indocyanine green fluorescence imaging: a feasibility study. Surg Endosc 30(2):657–662
Reinhart MB, Huntington CR, Blair LJ, Heniford BT, Augenstein VA (2016) Indocyanine green: historical context, current applications, and future considerations. Surg Innov 23(2):166–175
Dip F, Roy M, Lo Menzo E, Simpfendorfer C, Szomstein S, Rosenthal RJ (2015) Routine use of fluorescent incisionless cholangiography as a new imaging modality during laparoscopic cholecystectomy. Surg Endosc 29(6):1621–1626
Kose E, Kahramangil B, Aydin H, Donmez M, Takahashi H, Acevedo-Moreno LA, Sasaki K, Aucejo F, Berber E (2020) A comparison of indocyanine green fluorescence and laparoscopic ultrasound for detection of liver tumors. HPB 22(5):764–769
Kalmar CL, Reed CM, Peery CL, Salzberg AD (2020) Intraluminal indocyanine green for intraoperative staple line leak testing in bariatric surgery. Surg Endosc. https://doi.org/10.1007/s00464-020-07606-4,May8,2020
Mangano A, Masrur MA, Bustos R, Chen LL, Fernandes E, Giulianotti PC (2018) Near-infrared indocyanine green-enhanced fluorescence and minimally invasive colorectal surgery: review of the literature. Surg Technol Int 33:77–83
Caldwell CB, Ricotta JJ (1987) Changes in visceral blood flow with elevated intraabdominal pressure. J Surg Res 43(1):14–20
DeLong JC, Chakedis JM, Hosseini A, Kelly KJ, Horgan S, Bouvet M (2015) Indocyanine green (ICG) fluorescence-guided laparoscopic adrenalectomy. J Surg Oncol 112(6):650–653
Colvin J, Zaidi N, Berber E (2016) The utility of indocyanine green fluorescence imaging during robotic adrenalectomy. J Surg Oncol 114(2):153–156
Kahramangil B, Kose E, Berber E (2018) Characterization of fluorescence patterns exhibited by different adrenal tumors: determining the indications for indocyanine green use in adrenalectomy. Surgery 164(5):972–977
Zeiger MA, Thompson GB, Duh QY, Hamrahian A, Angelos P, Elaraj D, Fishman E, Kharlip J (2009) American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons Medical Guidelines for the Management of Adrenal Incidentalomas: executive summary of recommendations. Endocr Pract 15(5):450–453
Arora E, Bhandarwar A, Wagh A, Gandhi S, Patel C, Gupta S, Talwar G, Agarwal J, Rathore J, Chatnalkar S (2018) Role of indo-cyanine green (ICG) fluorescence in laparoscopic adrenalectomy: a retrospective review of 55 cases. Surg Endosc 32(11):4649–4657
Kahramangil B, Berber E (2017) Comparison of indocyanine green fluorescence and parathyroid autofluorescence imaging in the identification of parathyroid glands during thyroidectomy. Gland Surg 6(6):644–648
Zaidi N, Bucak E, Yazici P, Soundararajan S, Okoh A, Yigitbas H, Dural C, Berber E (2016) The feasibility of indocyanine green fluorescence imaging for identifying and assessing the perfusion of parathyroid glands during total thyroidectomy. J Surg Oncol 113(7):775–778
Kahramangil B, Berber E (2017) The use of near-infrared fluorescence imaging in endocrine surgical procedures. J Surg Oncol 115(7):848–855
Dip FD, Roy M, Perrins S, Ganga RR, Lo Menzo E, Szomstein S, Rosenthal R (2015) Technical description and feasibility of laparoscopic adrenal contouring using fluorescence imaging. Surg Endosc 29:569–574
Manny TB, Pompeo AS, Hemal AK (2013) Robotic partial adrenalectomy using indocyanine green dye with near-infrared imaging: the initial clinical experience. Urology 82:738–742
Kose E, Kahramangil B, Aydin H, Donmez M, Berber E (2019) Heterogeneous and low-intensity parathyroid autofluorescence: patterns suggesting hyperfunction at parathyroid exploration. Surgery 165(2):431–437
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Husnu Aydin, Mustafa Donmez, Bora Kahramangil, Emin Kose, Ozgun Erten, Serkan Akbulut, Mehmet Gokceimam have no conflicts of interest or financial ties to disclose. Dr. Eren Berber has unrelated consulting agreements with Ethicon, Medtronic, Aesculap and Integra. He has received honoraria for consulting work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aydin, H., Donmez, M., Kahramangil, B. et al. A visual quantification of tissue distinction in robotic transabdominal lateral adrenalectomy: comparison of indocyanine green and conventional views. Surg Endosc 36, 607–613 (2022). https://doi.org/10.1007/s00464-021-08326-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-021-08326-z