Abstract
Background
Parathyroid gland (PG) identification during thyroid and parathyroid surgery is challenging. Accidental parathyroidectomy increases the rate of postoperative hypocalcaemia. Recently, autofluorescence with near infrared light (NIRL) has been described for PG visualization. The aim of this study is to analyze the increased rate of visualization of PGs with the use of NIRL compared to white light (WL).
Materials and methods
All patients undergoing thyroid and parathyroid surgery were included in this study. PGs were identified with both NIRL and WL by experienced head and neck surgeons. The number of PGs identified with NIRL and WL were compared. The identification of PGs was correlated to age, sex, and histopathological diagnosis.
Results
Seventy-four patients were included in the study. The mean age was 48.4 (SD ±13.5) years old. Mean PG fluorescence intensity (47.60) was significantly higher compared to the thyroid gland (22.32) and background (9.27) (p < 0.0001). The mean number of PGs identified with NIRL and WL were 3.7 and 2.5 PG, respectively (p < 0.001). The difference in the number of PGs identified with NIRL and WL and fluorescence intensity was not related to age, sex, or histopathological diagnosis, with the exception of the diagnosis of thyroiditis, in which there was a significant increase in the number of PGs visualized with NIRL (p = 0.026).
Conclusion
The use of NIRL for PG visualization significantly increased the number of PGs identified during thyroid and parathyroid surgery, and the differences in fluorescent intensity among PGs, thyroid glands, and background were not affected by age, sex, and histopathological diagnosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
More than 80,000 thyroid and parathyroid surgeries for benign or malignant tumors are performed in the United States each year [1]. Intraoperative identification of parathyroid glands (PGs) has been always challenging, even for experienced surgeons [2]. Difficulty with the identification can be related to the small size of the glands, their close proximity to the thyroid gland, the similitude of the glands with the surrounding fat tissue, and the varied anatomical locations (up to 50% ectopic location) [3, 4]. This becomes even more complicated in re-operations due to the presence of significant scar tissue and in extensive central lymph node dissections [5, 6].
Hypocalcemia is one of the most frequent complications of total thyroidectomy. Transient and permanent hypocalcemia are present in 5–60% and 0.5–2% of the patients undergoing total thyroidectomy, respectively [2]. Patients who present with hypocalcemia will need to take oral or even intravenous calcium and vitamin D supplements in some cases for prolonged periods, causing significant discomfort [2]. The most common cause of postoperative hypocalcemia is parathyroid devascularization, followed by accidental resection of parathyroid glands [2]. Unintentional parathyroidectomy during thyroid surgery ranges from 8 to 19% [2, 6].
The correct identification of the glands in parathyroid surgery is important for resolution of the pathology. It can minimize the need for extended neck dissection with its associated morbidity and shorten operative time, and enable minimally invasive approaches [5, 7]. Moreover, it could also avoid recurrences in patients with ectopic or supernumerary glands with secondary hyperparathyroidism (parathyroid hyperplasia) [6]. Ectopic and supernumerary PGs has been reported to present in 16% [8] and 13% of cases, respectively [9].
A variety of techniques have been used to localize PGs [2]. Most of them are not in real time and need to be performed preoperatively: ultrasound, Sestamibi scintigraphy, computed tomography scan, and magnetic resonance imaging [2, 7]. Other techniques are used intraoperatively: intact parathyroid hormone assay, intraoperative methylene blue infusion, and technetium-99 m-Sestamibi localization using a hand-held gamma probe [2]. These methods used to identify PGs are limited in their applicability and sensibility, being inadequate to prevent surgical complications.
The use of near infrared light (NIRL) has been tested for the identification of the PGs but has always been used with a fluorescent dye—either methylene blue, aminolevulinic acid, or indocyanine green (ICG) [5, 7]. These dyes are not innocuous: methylene blue can present postoperative neurologic complications; ICG can produce urticarial and anaphylaxis, and is contraindicated in renal insufficiency; and aminolevulinic acid requires extensive photosensitization therapy [5, 6, 10]. There are few studies on the topic of autofluorescence of PGs; the majority of the literature focuses on autofluorescence spectrometry of PGs [6], but does not provide images of the PGs.
Postoperative hypoparathyroidism and recurrent hyperparathyroidism could be reduced with use of a safe, accurate, and effective method to identify PGs intraoperatively [2]. The aim of this study is to analyze the increased rate of visualization of PGs with the use of NIRL compared to white light (WL).
Materials and methods
This is a retrospective review of a prospectively maintained database. After Institutional Review Board approval, data were collected from all consecutive patients operated on for thyroid and parathyroid surgery between October 2015 and February 2016. All procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
The following variables were obtained from the patients’ medical records: age; sex; histopathological diagnosis [thyroid cancer (papillary, follicular, not specified), goiter, primary hyperparathyroidism, thyroiditis, hyperthyroidism]; number of PGs identified with NIRL and WL; difference in number of PGs identified with NIRL and WL; number of patients in which the number of PGs identified with NIRL was greater, the same, and lower than with WL; and fluorescence intensity from PGs, thyroid glands (TGs), and background (BG) tissue in the different pathologies. Descriptive statistics were conducted using statistical software R.
Surgical procedure
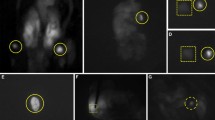
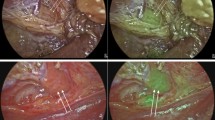
All surgeries were performed by experienced head and neck surgeons. The procedure started with a transverse cervicotomy, then sternohyoid and sternothyroid muscles were retracted, and an inspection of the PGs was performed. Surgeons reported the number of PGs that were visualized with the naked eye using only WL. Then, operative room’s WL was turned off and the surgical field was illuminated with NIRL using a laser system—the class 1 laser provides an irradiance of 5 mW/cm2 at 750 nm. A second inspection with NIRL was performed, and the number of PGs identified was reported. PGs were visualized as intense fluorescent spots with NIRL (Fig. 1). In cases of primary hyperparathyroidism, the highly fluorescent parathyroid adenomas were resected and a histopathological analysis was performed. Videos from all surgeries were recorded, and the fluorescent intensities of the different tissues (PGS, TGs and BG tissue) obtained from the colored images were analyzed using Image J Software®.
If a significant change in the color in a PG was identified after the dissection, a single dose of 0.5 milliliters of ICG was intravenously administered and perfusion of the glands was evaluated with fluorescence. If PGs acquired ICG and a fluorescence image was visualized, the glands were considered well perfused. This procedure was performed in only one patient out of the 74 thyroid and parathyroid surgeries.
Statistical analyses
The numbers of PGs identified with NIRL and WL were compared using the Wilcoxon Signed-Rank for paired samples. It was analyzed if the difference in the number of PGs visualized with each method (NIRL and WL) was affected by any covariant. An ordered logit model was fitted to the difference in the number of PGs visualized between NIRL and WL with fixed effects for age, sex, and the presence or absence of thyroid cancer, goiter, primary hyperparathyroidism, and thyroiditis. The mean fluorescent intensities of PGs, TGs, and BGs were compared using a linear mixed model with fixed effects for the different tissues (PG, TG, and BG), adjusted by age, sex, and presence or absence of thyroid cancer, goiter, primary hyperparathyroidism, and thyroiditis. A random effect was included to account for the within-patient variability, and Tukey’s range test for multiple comparisons was used to compare fluorescent intensities of the different tissues.
Results
Cohort description
The cohort included in this study consists of 74 patients, 68.9% (n = 51) women and 31.1% (n = 23) men (Table 1). The mean age was 48.4 (±13.5) years old. The patients were diagnosed with different thyroid and parathyroid pathologies including: thyroid cancer, goiter, hyperthyroidism, and primary hyperparathyroidism. All patients with diagnosis of primary hyperparathyroidism (n = 17) presented with a single-gland parathyroid adenoma; no PG hyperplasias or double parathyroid adenomas were registered in this study. The indication for surgery in patients with diagnosis of thyroiditis (n = 8) was hyperthyroidism (n = 3), papillary thyroid cancer (n = 2), and goiter (n = 2).
Number of parathyroid glands visualized with NIRL and WL
Across the entire cohort, the number of PGs visualized ranged between 1 and 4 (Table 2). The mean number of PGs identified was 2.5 (±0.8) and 3.7 (±0.7) with WL and NIRL, respectively (Fig. 2 and Table 2). The number of PGs visualized with NIRL was significantly higher compared to WL (p < 0.0001). In 86.5% (n = 64) of patients, the 4 PGs were identified with NIRL, while in only 12.2% (n = 9) of patients, the 4 PGs were visualized with WL. With the exception of one patient (1.4%), the number of PGs visualized with NIRL was equal or greater than the number of PGs visualized with WL. In fact, in 81.1% (n = 60) of the patients, NIRL was able to visualize more PGs than WL, and in 17.5% (n = 13) of the patients, the same number of PGs was identified with both types of light.
It was further investigated if differences in the number of PGs visualized with each method were affected by any covariant. The number of PGs visualized with NIRL and WL was the same regardless of the age, sex, and histopathological diagnosis (thyroid cancer, goiter, hyperthyroidism, and primary hyperparathyroidism) with the exception of the diagnosis of thyroiditis, in which there was a significant increase in the number of PGs visualized with NIRL (p = 0.026). The chance of detecting a greater difference between NIR and WL visualizations is about three times higher for patients with thyroiditis than for those without thyroiditis.
Differences in light intensities between thyroid, parathyroid, and background
We compared the corrected mean of fluorescence intensity of TGs, PGs, and BG registered in each patient (Figs. 2, 3).
As shown in both Table 3 and Fig. 3, the intensities for TGs, PGs, and BG were all significantly different (p < 0.0001). The mean fluorescence intensity for PGs was 47.6 (±26.9), 25.3 units higher than the intensity for TGs (22.2), which was itself 13.1 units higher than the BG intensity. The fluorescence intensity was not dependent on the gender, age, or pathological diagnosis of the patient, but was only dependent on the tissue exposed to NIRL. Out of the 74 thyroid and parathyroid surgeries, only in five cases a PG presented significant changes in color with WL. In these cases, ICG was intravenously administrated to assess perfusion showing no deficiencies. No parathyroid autotransplantations were performed in this series.
All patients were discharged on the first postoperative day. No permanent hypocalcemia were reported. All patients with diagnosis of primary hyperparathyroidism had a successful operation with normal postoperative serum calcemias at six months follow-up. No mortalities were registered.
Discussion
This study proves the utility of NIRL in the identification of autofluorescent PGs during thyroid and parathyroid surgery. Visualization of the PGs was increased with NIRL compared with WL. The mean visualization rate of PGs was 2.5 (±0.8) with WL. When NIRL was used, surgeons were able to identify a mean of 3.7 (±0.7) PGs, increasing the visualization rate. These results suggest that this method can be useful to guide surgeons during dissection in thyroid and parathyroid surgeries. PGs are very small and when not recognized, accidental resection and devascularization may occur, increasing the rate of morbidity after thyroid surgery [11, 12]. Sasson and colleagues [13] have reported a rate of incidental parathyroidectomy of 19%, while biochemical hypocalcemia may increase up to 83% when a PG is accidentally resected [13–15].
The use of NIRL to visualize PGs has been previously studied. Paras and colleagues [16] studied the fluorescent behavior of PGs during spectroscopy, concluding that NIRL can potentially be used to localize the glands. Autofluorescent PG visualization with NIRL was previously reported by McWade and colleagues [6]. They reported 100% sensitivity in PG identification with the use of spectroscopy. They found that the signal of parathyroid tissue was between 1 and 25 times higher compared to TGs and BG [1].
The reason why PGs are autofluorescent when exposed to NIRL is still unknown. There are different theories reported in the literature. One theory relates it to the presence of the increased number of calcium receptors in the PGs and could also explain the increased fluorescence intensity of the parathyroid adenomas. However, this theory does not explain autofluorescence in TG and other tissues. The second theory is related to the differences in PG cellular density and the different proportions of the adipose tissue, chief cells, and oxyphil cells. Our group is currently studying the presence of a pseudo-colloid in the PGs and TGs that could be implicated in the autofluorescence signaling.
In our series, all the PGs recognized with WL were also autofluorescent when exposed to NIRL. Also, in the 17 cases of primary hyperparathyroidism, the autofluorescent images of parathyroid adenomas and normal parathyroid glands biopsied were confirmed with histopathological analysis. We compared the intensities between the PGs, TGs, and BG with different pathologies, and PGs were significantly more fluorescent than the other tissues (p value <0.0001). In our study, fluorescent intensity of PGs was not affected by age or sex. This method was especially useful in cases of thyroiditis, in which the distorted anatomy and subacute inflammation can represent a surgical challenge and identification of PGs is even more difficult.
One of the most important advantages and safety aspects of this method is that no drug or dye is needed to visualize the autofluorescent PGs. Early detection of PGs with the use of fluorescence may decrease the chance of PG devascularization and accidental parathyroidectomy, potentially reducing the rate of postoperative hypocalcemia. Moreover, considering that the PGs are a sentinel to find the recurrent laryngeal nerve, this technique could potentially aid in the localization of the nerve, reducing operative time. More prospective randomized studies are needed to support this hypothesis.
This technique is safe and easily reproducible. A hand-held camera is used to illuminate the surgical field, and images can be visualized and recorded on a computer in real time. The NIRL used to visualize PGs is in a similar spectrum to the operating room (OR) lights, which guarantees the safety of this technique and no potential harm for the patient or staff. In this study, NIRL was used to identify PGs during thyroid and parathyroid surgeries, but this technique was not used to differentiate normal and pathologic parathyroid tissue in cases of primary hyperparathyroidism.
Early localization of PGs allows the surgeon to protect the vascularization of glands, reduce unnecessary dissection, and avoid re-implantation of the glands in the muscle when accidentally resected. When PG vitality is uncertain, a single dose of intravenous ICG can be administered to confirm the perfusion of the glands. Fortunity and colleagues [17] have described the use of ICG to evaluate perfusion of the PGs in 36 patients. They found that two patients had non-well-perfused PGs and developed transient hypocalcemia [17]. Chakedis and colleagues [18] reported that the use of ICG was useful to guide the localization of parathyroid adenomas. In our experience, the use of ICG helped to confirm the perfusion of the glands in five cases. Recently Zaidi and colleagues [19] studied the use of fluorescence to determine perfusion of PGs in 27 patients. The authors reported a correlation between hypocalcemia and PG perfusion [19].
The main limitation of this study is the lack of histopathological confirmation in all the autofluorescent PGs. Diagnosis was only confirmed when a biopsy or resection of the PG was performed as part of a treatment (primary hyperparathyroidism). In the remaining cases, the number of glands identified with WL relied on the subjective impression of experienced head and neck surgeons. Due to institutional review board (IRB) restrictions, we were not able to biopsy normal glands in patients that were operated on for thyroidectomies and this practice was not part of the standard of care.
Finally, a further limitation of the study was that we were not able to determine the existence of false positives of this technique with the NIRL and WL due to the same ethical restrictions to biopsy PGs in patients with no indication of biopsy as part of the standard of care, due to the risk of postoperative PG devascularization and postoperative hypocalcemia. Future prospective randomized studies are necessary to evaluate the impact of this technology on the decreased rate of hypocalcemia after thyroidectomies.
Conclusion
Visualization of PGs with NIRL significantly increased the number of PGs identified during thyroid and parathyroid surgeries. The difference in fluorescent intensity between PGs, TGs, and BG are not affected by age, sex, or histopathological diagnosis. Prospective randomized trials should be performed to evaluate the real impact of this technology on postoperative outcomes.
References
McWade MA, Paras C, White LM, Phay JE, Mahadevan-Jansen A, Broome JT (2013) A novel optical approach to intraoperative detection of parathyroid glands. Surgery 154:1371–1377
Gu J, Wang J, Nie X, Wang W, Shang J (2015) Potential role for carbon nanoparticles identification and preservation in situ of parathyroid glands during total thyroidectomy and central compartment node dissection. Int J Clin Exp Med 8:9640–9648
Okada M, Tominaga Y, Yamamoto T, Hiramitsu T, Narumi S, Watarai Y (2016) Location frequency of missed parathyroid glands after parathyroidectomy in patients with persistent or recurrent secondary hyperparathyroidism. World J Surg 40:595–599
Dumitras M, Strambu V, Radu PA, Radu PA, Iorga C, Bengulescu I, Popa F (2015) Technical factors involved in parathyroid surgery. Chirurgia 110: 425.
Sound S, Okoh A, Yigitbas H, Yazici P, Berber E (2015) Utility of indocyanine green fluorescence imaging for intraoperative localization in reoperative parathyroid surgery. Surg Innov. doi:10.1177/1553350615613450
McWade MA, Sanders ME, Broome JT, Solórzano CC, Mahadevan-Jansen A (2016) Establishing the clinical utility of autofluorescence spectroscopy for parathyroid detection. Surgery 159:193–203
Tummers QR, Schepers A, Hamming JF, Hamming JF, Kievit J, Frangioni JV, vn de Velde CJ, Vahrmeijer AL (2015) Intraoperative guidance in parathyroid surgery using near-infrared fluorescence imaging and low-dose methylene blue. Surgery 158:1323–1330
Phitayakorn R, McHenry CR (2006) Incidence and location of ectopic abnormal parathyroid glands. Am J Surg 191:418–423
Pattou FN, Pellissier LC, Noël C, Wambergue F, Huglo DG, Proye CA (2000) Supernumerary parathyroid glands: frequency and surgical significance in treatment of renal hyperparathyroidism. World J Surg 24:1330–1334
Alander JT, Kaartinen I, Laakso A, Pätilä T, Spillmann T, Tuchin VV, Venermo M, Välisuo P (2012) A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging 22:2021. doi:10.1155/2012/940585
McHenry CR, Speroff T, Wentworth D, Murphy T (1994) Risk factors for postthyroidectomy hypocalcemia. Surgery 116:641–648
Pattou F, Combemale F, Fabre S, Carnaille B, Decoulx M, Wemeau JL, Racadot A, Proye C (1998) Hypocalcemia following thyroid surgery: incidence and prediction of outcome. World J Surg 22:718–724
Sasson AR, Pingpank JF Jr, Wetherington W, Hanlon AL, Ridge JA (2001) Incidental parathyroidectomy during thyroid surgery does not cause transient symptomatic hypocalcemia. Arch Otolaryngol Head Neck Surg 127:304–308
Wingert DJ, Friesen SR, Iliopoulos JI, Pierce GE, Thomas JH, Hermreck AS (1986) Post-thyroidectomy hypocalcemia. Incidence and risk factors. Am J Surg 152:606–610
Demeester-Mirkine N, Hooghe L, Van Geertruyden J, De Maertelaer V (1992) Hypocalcemia after thyroidectomy. Arch Surg 127:854–858
Paras C, Keller M, White L, Phay J, Mahadevan-Jasen A (2011) Near-infrared autofluorescence for the detection of parathyroid glands. J Biomed Opt 16:067012
Vidal Fortuny J, Belfontali V, Sadowski SM, Karenovics W, Guigard S, Triponez F (2016) Parathyroid gland angiography with indocyanine green fluorescence to predict parathyroid function after thyroid surgery. Br J Surg 103:537–543
Chakedis JM, Maser C, Brumund KT, Bouvet M (2015) Indocyanine green fluorescence-guided redo parathyroidectomy. BMJ Case Rep. doi:10.1136/bcr-2015-211778
Zaidi N, Bucak E, Yazici P, Soundararajan S, Okoh A, Yigitbas H, Dural C, Berber E (2016) The feasibility of indocyanine green fluorescence imaging for identifying and assessing the perfusion of parathyroid glands during total thyroidectomy. J Surg Oncol 113:775–778
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Jorge Falco, Fernando Dip, Pablo Quadri, Martin de la Fuente, Marcos Prunello, and Raul J. Rosenthal have no conflicts of interest or financial ties to disclose. The equipment was provided by Fluoptics® France.
Rights and permissions
About this article
Cite this article
Falco, J., Dip, F., Quadri, P. et al. Increased identification of parathyroid glands using near infrared light during thyroid and parathyroid surgery. Surg Endosc 31, 3737–3742 (2017). https://doi.org/10.1007/s00464-017-5424-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-017-5424-1