Abstract
Parathyroid gland injury during thyroid surgery is common and can lead to postoperative hypocalcemia. This study aims to determine the utility of near-infrared autofluorescence (NIRAF) technology for parathyroid gland identification in thyroid surgery. A prospective case series of patients who underwent thyroid surgery between March and June 2021 were examined. Following intra-operative visualisation, parathyroid glands and surrounding tissues were exposed to near-infrared light with a wavelength of approximately 800 nm using the Storz® Near-Infrared Range/Indocyanine Green (NIR/ICG) endoscopic system. Parathyroid glands were expected to show autofluorescence following exposure. Twenty patients who underwent thyroid surgery were included. Eighteen patients (90%) were female, with a median age of 50.0 (IQR 41.0 – 62.5). Surgeries performed include hemithyroidectomy (9 patients; 45.0%), total thyroidectomy (8 patients; 40.0%), completion thyroidectomy (2 patients; 10.0%) and right inferior parathyroidectomy (1 patient; 5.0%). Attempts were made to identify 56 parathyroid glands in this case series. There were 46/56 (82.1%) surgeon-identified parathyroid glands through direct visualisation. Using NIRAF technology, 39/46 (84.8%) were identified as parathyroid glands. There was no inadvertent resection of parathyroid glands or post-operative hypocalcaemia. NIRAF technology has the potential to be a useful tool in confirming the presence of parathyroid glands following direct visualisation intra-operatively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intra-operative identification and preservation of parathyroid glands is an important but challenging aspect of thyroid surgery, as they are reliant on the surgeon’s visual inspection and level of experience [1, 2]. Parathyroid glands can be difficult to distinguish from surrounding fat or lymph nodes and vary widely in their location and number owing to their embryological development [3, 4]. Failure of identification and preservation of parathyroid glands during thyroidectomy may cause devascularisation or inadvertent resection of the gland(s), leading to transient or permanent hypocalcaemia [5, 6]. Transient hypocalcaemia generally responds well to calcium and vitamin D replacement. However, permanent hypoparathyroidism represents a serious complication causing life-long morbidity in 2–5% of patients undergoing thyroid surgery [6, 7].
Several intra-operative localisation techniques have been proposed to facilitate identification of parathyroid glands, including Raman spectroscopy, carbon nanoparticle injection, and indocyanine green (ICG) angiography [8,9,10]. However, there has been an emergence on using near infrared-induced autofluorescence (NIRAF) in recent literature, as parathyroid glands were found to emit a spontaneous autofluorescent signal up to 11 times higher than surrounding tissue when exposed to a near-infrared stimulation [11, 12]. The utilisation of NIRAF technology has been promising, with several studies showing that it could reliably aid in the identification of parathyroid glands in 76.3–100% of cases [13,14,15] and significantly reduce the incidence of post-operative hypocalcaemia in thyroid surgery [16, 17]. Therefore, the aim of this study is to assess the feasibility of NIRAF technology in the identification of parathyroid glands during thyroid surgery.
Methodology
This study involved a prospective case series of patients undergoing thyroid surgery in Chris O’Brien Lifehouse (Sydney, Australia) between March and June 2021. Data on patient demographics, surgical indication, thyroid surgery performed, number of parathyroid glands identified, and corrected calcium and parathyroid hormone (PTH) levels were collected. Local research ethics was obtained for this study, which waived the need for written informed consent.
Imaging System Set-Up and Intraoperative Parathyroid Imaging
Parathyroid autofluorescence was achieved using the Storz® Near-Infrared Range/Indocyanine Green (NIR/ICG) endoscopic imaging system (Karl Storz, Tuttlingen, Germany). The system consists of a 4 K high-definition IMAGE1 S™ RUBINA™ camera (3849 × 2160 pixels), a 10 mm 0˚ HOPKINS® RUBINA™ telescope, and a POWER LED RUBINA™ light source.
The IMAGE1 S™ RUBINA™ camera has two image sensors, with one dedicated to white light detection and another dedicated to NIR/ICG signal. The system enables the camera to present the three different NIR/ICG modes (Overlay, Monochromatic and Intensity Map), and can readily be changed as required in conjunction with the HOPKINS® RUBINA™ telescope. The mode used in this case series is Overlay, where the regular white light image is combined with the NIR/ICG data to generate an overlay image. The NIR/ICG data can be displayed as green or blue overlay. The POWER LED RUBINA™ light source is equipped with dual light-emitting diodes allowing use in both white light as well as fluorescence applications, which can be used individually or simultaneously to support the different NIR/ICG modes. The wider NIR wavelength range provides both visible and NIR excitation light in the area of interest at approximately 800 nm.
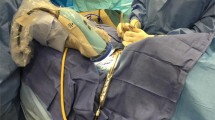
All thyroidectomies were performed by experienced, high-volume thyroid surgeons (CEP and MSE) with routine use of Nerve Integrity Monitoring–Neuro™ 3.0 (Medtronic, Minnesota, United States). Attempts were made to identify two parathyroid glands during hemithyroidectomy, and four parathyroid glands during total thyroidectomy. Parathyroid imaging was carried out following intra-operative visualisation of the parathyroid gland(s). The tip of the laparoscope was held in place approximately 5 cm above the operative site. White light images were collected, and the operating theatre lights were dimmed. The parathyroid gland alongside the surrounding structures, including thyroid tissue, adipose tissue, and lymph nodes, were then exposed to near-infrared light. Both green and blue overlay filters were used. After visual verification of the fluorescent spots provided by the imaging system, near-infrared light images were collected, and the surgery was resumed in a conventional manner (see Fig. 1). Corrected calcium and PTH levels at 4 h were collected post-operatively if the patient underwent a total or completion thyroidectomy. Normal reference levels for corrected calcium and PTH in Australia is between 2.10 – 2.60 mmol/L and 1.0 – 7.0 pmol/L respectively.
Results
Twenty patients who underwent thyroid surgery were included in this case series. Eighteen patients (90%) were female, with a median age of 50.0 (IQR 41.0–62.5) at time of surgery. Indications for surgery include papillary thyroid carcinoma (8 patients; 40.0%), Bethesda 4 thyroid nodule (4 patients; 20.0%), multinodular goiter (6 patients; 30.0%), Graves’ Disease (1 patient; 5.0%) and parathyroid adenoma (1 patient; 5.0%). Type of thyroid surgery performed include hemithyroidectomy (9 patients; 45.0%), total thyroidectomy (8 patients; 40.0%), completion thyroidectomy (2 patients; 10.0%) and right inferior parathyroidectomy (1 patient; 5.0%).
Attempts were made to identify 56 parathyroid glands in this case series (18 glands from 9 hemithyroidectomies, 32 glands from 8 total thyroidectomies, 4 glands from 2 completion thyroidectomies and 2 glands from 1 right inferior parathyroidectomy). There were 46/56 (82.1%) surgeon-identified parathyroid glands through direct visualistion. Using NIRAF, 39/46% were identified as parathyroid glands, which resulted in a sensitivity of 84.8%. The additional time taken for parathyroid autofluorescence imaging and photodocumentation took approximately 5–10 min. Identification of the remaining 10 parathyroid glands were not identified in the surgical bed. No parathyroid glands were seen attached to the specimen.
In 10 patients (50%) who underwent total or completion thyroidectomy, no patients had temporary or permanent hypocalcaemia. The median post-operative corrected calcium and PTH was 2.41 mmol/L (IQR 2.35–2.52, range 2.25–2.60) and 1.90 pmol/L (IQR 0.70 – 2.90, range 0.50–4.20) respectively. No resected parathyroid gland(s) in the thyroid histological specimens were noted for each patient. Clinical characteristics of patients in this case series are summarised in Table 1.
Discussion
Our early experience with the Storz® NIR/ICG imaging system demonstrates that NIRAF technology is a useful adjunct to parathyroid gland identification during thyroid surgery. The sensitivity of the Storz® NIR/ICG imaging system in detecting parathyroid autofluorescence is reasonably high (84.8%) – our results are consistent with the findings of Ladurner et al., who identified 205 parathyroid glands from 117 patients who underwent thyroid surgery, and were able to confirm 87.3% of those parathyroid glands using the Storz® NIR/ICG imaging system [15]. In addition, our results using the Storz® NIR/ICG imaging system is consistent with similar systems such as the Fluobeam® 800 (Fluoptics, Grenoble, France), another commercially available NIRAF imaging system which was able to identify between 76.3 and 100% of parathyroid glands following visual inspection [13, 18,19,20].
To our knowledge, this is the first Australian series to report parathyroid autofluorescence utilising the Storz® NIR/ICG imaging system. The objective is not to replace the critical surgical skill in identifying and preserving parathyroid glands during routine surgery, but to assist the surgeon by confirming the presence of parathyroid glands identified in the operating field and distinguishes parathyroid glands from surrounding tissues such as fat and lymph nodes following visual inspection.
In addition to the high sensitivity rates, we have noted several advantages of using the Storz® NIR/ICG imaging system. When autofluorescence is detected, parathyroid tissue is well differentiated from surrounding tissue, which aids in meticulous dissection to minimise the risk of parathyroid gland devascularisation. This technology omits the need for intravenous administration of indocyanine green (ICG) as a contrast agent to visualise the parathyroid glands through ICG angiography, which may result in adverse effects such as urticaria, tachycardia and anaphylaxis [21]. In addition, it has an easy learning curve without significantly increasing operative time, which may be helpful for low-volume thyroid surgeons.
Furthermore, NIRAF technology can also be used to screen the thyroid gland surface after its resection to look for inadvertent parathyroid gland(s) adhered to the capsule, as well as to inspect the resected central neck dissection tissue to look for unidentified parathyroid glands. This is particularly useful for the latter, as it can be challenging to visually distinguish parathyroid glands from fat or lymph nodes in patients with central compartment lymphadenopathy secondary to malignancy or lymphocytic thyroiditis, and/or significant adiposity in the central neck [22, 23] (see Fig. 2).
The main disadvantages of the Storz® NIR/ICG imaging system include its initial cost to set up the system, inability to localise parathyroid glands covered deeply by other tissues, and false positives from brown fat, metastatic lymph nodes, colloid nodules and/or blood stains in the operative field. Furthermore, theatre lights need to be dimmed prior to usage, which may disrupt the usual workflow of the operating theatre and subsequently increase operative time. Furthermore, the Storz® NIR/ICG imaging system does not enable assessment of parathyroid vascularisation, compared to near-infrared imaging with indocyanine green dye injection [24].
The Storz® NIR/ICG imaging system is simple, safe and easy to use. Outside of thyroid surgery, the Storz® NIR/ICG imaging system is cost-effective due to its versatility – for instance, this system can also be used in laparoscopic surgery to identify biliary tree structures with the aid of ICG injection and ensure safe dissection during cholecystectomy [25].
This study’s weakness lies in its relatively small sample size; therefore, the results may not be generalisable to other centers. The early adoption of our institution in using NIRAF technology and its associated learning curve may also explain our slightly lower sensitivity rate (84.8%) compared to other studies reporting sensitivities of 87.3% or higher [13,14,15, 20, 26]. In addition, this study does not demonstrate evidence of NIRAF technology in reducing rates of hypoparathyroidism. Therefore, future studies should be designed as a randomised controlled trial to assess the real-life impact of NIRAF technology in the incidence of post-operative hypocalcaemia and inadvertent resection of parathyroid gland(s) in thyroid surgery.
Conclusion
NIRAF technology has the potential to be a useful tool in confirming the presence of parathyroid glands following direct visualisation intra-operatively. Future prospective studies and randomised controlled trials are required to confirm these findings and obtain new insights to improve thyroid surgery outcomes.
References
Rudin AV, Thompson G (2018) Revision parathyroidectomy. In: Parameswaran R, Agarwal A (eds) Evidence-Based Endocrine Surgery. Springer, pp 293–305
Chen H, Wang TS, Yen TW et al (2010) Operative failures after parathyroidectomy for hyperparathyroidism: the influence of surgical volume. Ann Surg 252(4):691–695
Thompson N, Eckhauser F, Harness J (1982) The anatomy of primary hyperparathyroidism. Surgery 92(5):814–821
Akerström G, Malmaeus J, Bergström R (1984) Surgical anatomy of human parathyroid glands. Surgery 95(1):14–21
Edafe O, Antakia R, Laskar N, Uttley L, Balasubramanian S (2014) Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcaemia. Br J Surg 101(4):307–320
Lorente-Poch L, Sancho JJ, Ruiz S, Sitges-Serra A (2015) Importance of in situ preservation of parathyroid glands during total thyroidectomy. Br J Surg 102(4):359–367
Stack BC Jr, Bimston DN, Bodenner DL et al (2015) American association of clinical endocrinologists and American college of endocrinology disease state clinical review: postoperative hypoparathyroidism-definitions and management. Endocr Pract 21(6):674–685
Karipineni F, Sahli Z, Somervell H et al (2018) Are preoperative sestamibi scans useful for identifying ectopic parathyroid glands in patients with expected multigland parathyroid disease? Surgery 163(1):35–41
Hillary SL, Guillermet S, Brown NJ, Balasubramanian SP (2018) Use of methylene blue and near-infrared fluorescence in thyroid and parathyroid surgery. Langenbecks Arch Surg 403(1):111–118
Elbassiouny S, Fadel M, Elwakil T, Elbasiouny MS (2018) Photodynamic diagnosis of parathyroid glands with nano-stealth aminolevulinic acid liposomes. Photodiagn Photodyn Ther 21:71–78
Paras C, Keller M, White L, Phay J, Mahadevan-Jansen A (2011) Near-infrared autofluorescence for the detection of parathyroid glands. J Biomed Opt 16(6):067012
McWade MA, Paras C, White LM et al (2014) Label-free intraoperative parathyroid localization with near-infrared autofluorescence imaging. J Clin Endocrinol Metab 99(12):4574–4580
De Leeuw F, Breuskin I, Abbaci M et al (2016) Intraoperative near-infrared imaging for parathyroid gland identification by auto-fluorescence: a feasibility study. World J Surg 40(9):2131–2138
Falco J, Dip F, Quadri P, de la Fuente M, Rosenthal R (2016) Cutting edge in thyroid surgery: autofluorescence of parathyroid glands. J Am Coll Surg 223(2):374–380
Ladurner R, Lerchenberger M, Al Arabi N, Gallwas JKS, Stepp H, Hallfeldt KKJ (2019) Parathyroid autofluorescence-how does it affect parathyroid and thyroid surgery? a 5 year experience. Molecules (Basel) 24(14):14
Kim SW, Song SH, Lee HS et al (2016) Intraoperative real-time localization of normal parathyroid glands with autofluorescence imaging. J Clin Endocrinol Metab 101(12):4646–4652
Dip F, Falco J, Verna S et al (2019) Randomized controlled trial comparing white light with near-infrared autofluorescence for parathyroid gland identification during total thyroidectomy. J Am Coll Surg 228(5):744–751
Falco J, Dip F, Quadri P, de la Fuente M, Prunello M, Rosenthal RJ (2017) Increased identification of parathyroid glands using near infrared light during thyroid and parathyroid surgery. Surg Endosc 31(9):3737–3742
Kahramangil B, Dip F, Benmiloud F et al (2018) Detection of parathyroid autofluorescence using near-infrared imaging: a multicenter analysis of concordance between different surgeons. Ann Surg Oncol 25(4):957–962
Benmiloud F, Rebaudet S, Varoquaux A, Penaranda G, Bannier M, Denizot A (2018) Impact of autofluorescence-based identification of parathyroids during total thyroidectomy on postoperative hypocalcemia: a before and after controlled study. Surgery 163(1):23–30
Hope-Ross M, Yannuzzi LA, Gragoudas ES et al (1994) Adverse reactions due to indocyanine green. Ophthalmology 101(3):529–533
Kim DH, Kim SW, Kang P et al (2021) Near-infrared autofluorescence imaging may reduce temporary hypoparathyroidism in patients undergoing total thyroidectomy and central neck dissection. Thyroid 31(9):1400–1408
Kose E, Rudin AV, Kahramangil B et al (2020) Autofluorescence imaging of parathyroid glands: an assessment of potential indications. Surgery 167(1):173–179
van den Bos J, van Kooten L, Engelen SME, Lubbers T, Stassen LPS, Bouvy ND (2019) Feasibility of indocyanine green fluorescence imaging for intraoperative identification of parathyroid glands during thyroid surgery. Head Neck 41(2):340–348
Ishizawa T, Bandai Y, Ijichi M, Kaneko J, Hasegawa K, Kokudo N (2010) Fluorescent cholangiography illuminating the biliary tree during laparoscopic cholecystectomy. Br J Surg 97(9):1369–1377
McWade MA, Sanders ME, Broome JT, Solorzano CC, Mahadevan-Jansen A (2016) Establishing the clinical utility of autofluorescence spectroscopy for parathyroid detection. Surgery 159(1):193–202
Funding
No funding received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tjahjono, R., Phung, D., Elliott, M.S. et al. The Utility of Near-Infrared Autofluorescence for Parathyroid Gland Identification During Thyroid Surgery: A Single-Center Experience. Indian J Otolaryngol Head Neck Surg 75, 121–125 (2023). https://doi.org/10.1007/s12070-022-03309-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-022-03309-5