Abstract
Background
Parathyroid glands (PGs) exhibit autofluorescence (AF) when excited by near-infrared laser. This multicenter study aims to analyze how this imaging could facilitate the detection of PGs during thyroidectomy and parathyroidectomy procedures.
Methods
This was a retrospective Institutional Review Board-approved analysis of prospectively collected data at three centers. Near-infrared fluorescence imaging (NIFI) was used to detect AF from PGs during thyroidectomy and parathyroidectomy procedures. Logistic regression analysis was performed to assess the utility of NIFI to identify PGs and concordance at these centers.
Results
Overall, 210 patients underwent total thyroidectomy (n = 95), thyroid lobectomy (n = 41), and parathyroidectomy (n = 74) (n = 70 per center). Using NIFI, AF was detected from 98% of visually identified PGs. Upon initial exploration, 46% of PGs were not visible to the naked eye due to coverage by soft tissue, but AF from these glands could be detected by NIFI without any further dissection. Overall, a median of one PG per patient was detected by NIFI in this fashion before being identified visually (p = nonsignificant between centers). On logistic regression, smaller PGs were more likely to be missed visually, but localized by AF on NIFI (odds ratio with increasing size, 0.91; p = 0.02).
Conclusions
In our experience, NIFI facilitated PG identification by detecting their AF, before conventional recognition by the surgeon, in 37–67% of the time. Despite the variability in this rate across centers, there was a concordance in detecting AF from 97 to 99% of the PGs using NIFI. We suggest the incorporation of AF on NIFI alongside conventional visual cues to aid identification of PGs during neck operations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
In the US, more than 80,000 patients undergo thyroidectomy and parathyroidectomy procedures every year.1 Although identification of the parathyroid glands (PGs) is a critical part of these procedures, this could be challenging due to small gland size, location, and scarring.2 Furthermore, ectopic and supernumerary PGs may be seen in 16 and 13% of patients, respectively.3 In the literature, the rate of inadvertent PG removal during thyroid surgery has been reported to be in the range of 8–19%.4
Intraoperatively, PGs are typically identified visually, based on their characteristic visual cues. Over the years, several adjunctive methods have been proposed for intraoperative localization of PGs, including intraoperative technetium-99 m-sestamibi localization5 and intravenous injection of methylene blue6 and aminolevulinic acid.7 These techniques were limited in their utility and did not significantly improve postoperative outcomes.2 More recently, the use of indocyanine green (ICG) has been described for intraoperative PG localization; however, this proved to be limited by low sensitivity, the need for an intravenous injection, and the risk of allergic and vasovagal reactions.8
When excited by a diode laser, parathyroid tissue fluoresces in an 800–900 nm near-infrared spectrum. Although this phenomenon was first described by Das and colleagues,9 it was the Vanderbilt University group that first used it intraoperatively and coined the term parathyroid autofluorescence (AF).10 In their largest study to date, the Vanderbilt group was able to correctly identify 97% of 264 PGs using spectroscopy.11 Due to various reasons, this technique was not adopted by other groups. In 2016, Falco and colleagues demonstrated that parathyroid AF could also be detected using commercially available ICG near-infrared fluorescence imaging (NIFI) systems.12 Since then, the use of NIFI for detecting PG AF was adopted by several other groups due to its simplicity, safety, and non-invasiveness.13,14 The aim of this multicenter study was to analyze how this imaging could facilitate the detection of PGs during thyroidectomy and parathyroidectomy procedures.
Materials and Methods
Study Design and Data Collection
From prospective institutional databases of three tertiary-care centers, 210 patients who underwent NIFI during thyroid and parathyroid surgery were identified. Patients were contributed by the Cleveland Clinic Foundation, OH, USA (N = 70); Hôpital Européen, Marseille, France (N = 70); and Instituto Argentino de Diagnostico y Tratameitno (IADT) Argentina (N = 70). All procedures met the ethical standards of institutional and/or national research committees and were in accordance with the 1964 Helsinki Declaration and its subsequent amendments. Following Institutional Review Board approval, data were collected prospectively and independently at each center. Data analysis was performed retrospectively.
The following variables were obtained at all centers: age, sex, body mass index (BMI), histopathological diagnosis (benign thyroid nodule/multinodular goiter, hyperthyroidism, thyroid cancer, primary hyperparathyroidism), type of surgery (total thyroidectomy, thyroid lobectomy, parathyroidectomy), number of PGs identified intraoperatively, anatomical and NIFI details of each identified PG [side (right or left), location (upper or lower), detection by NIFI, and first method of identification (naked-eye exploration by operating surgeon or NIFI of the surgical field)]. Additionally, the Cleveland Clinic provided size measurements for all PGs.
Surgical Procedure
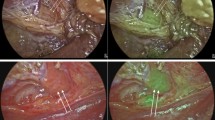
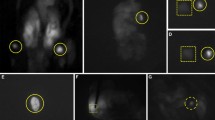
All operations were performed by experienced endocrine surgeons. A transverse cervical incision was made and the sternohyoid and sternothyroid muscles were retracted laterally. After exposure of the central neck on each side, a visual inspection of the surgical field was performed, and size (only at the Cleveland Clinic) and location (at all centers) of PGs identified on NE, without further dissection, were noted. The operating room lights were then turned off for NIFI. The operative field was exposed to near-infrared light, and the resulting fluorescence was captured using the Fluobeam device (Fluoptics, Grenoble, France) (Fig. 1). PGs were seen as hyperfluorescent spots on a hypofluorescent background (Fig. 2). Whether the PGs were first detected with NE or with AF during the procedure was recorded. A candidate soft tissue was considered parathyroid tissue if verified on frozen section, or if it exhibited the following classic visual cues used by endocrine surgeons: (1) yellowish-brown color; (2) a discrete shape (typically ovoid) in contrast to amorphous fat; and (3) distinct vasculature seen along its substance.
Statistical Analysis
Statistical analysis was performed using SAS Studio version 3.6 (SAS Institute, Inc., Cary, NC, USA). Clinical and intraoperative parameters were compared using the Kruskal–Wallis and χ2 tests, and univariate logistic regression was performed to determine the factors associated with the detection of PGs with AF. A p value < 0.05 was considered statistically significant.
Results
Description of the Cohort
The entire study cohort consisted of 210 patients, of which 87% (n = 183) were female (Table 1). Mean age was 53.1 years (± 14.0) and BMI was 26.5 kg/m2 (± 6.5). Indications for surgery included benign thyroid nodule/multinodular goiter (n = 83), hyperthyroidism (n = 10), thyroid cancer (n = 43), and primary hyperparathyroidism (n = 74). Patients underwent total thyroidectomy (n = 95), thyroid lobectomy (n = 41), and parathyroidectomy (n = 74).
The patient cohorts from three centers were similar regarding demographic parameters, except for BMI (21.4 ± 1.8, 27.0 ± 5.4, and 31.3 ± 6.9 kg/m2 at centers 1, 2, and 3, respectively; p < 0.001).
Intraoperative Detection of Parathyroid Glands
A total of 594 PGs were identified at three centers (center 1: 219; center 2: 159; center 3: 216) (Table 2). Due to more thyroid lobectomies and fewer parathyroidectomies, a lower number of PGs was identified at center 2. The overall sensitivity of NIFI for detecting AF from PGs was 98% (584/594), with concordance across centers, ranging between 97 and 99% (p = 0.08).
Of all PGs, 46% (272/594) could not be identified on initial visual exploration of the surgical field with NE due to coverage by soft tissue, but AF from these glands could be detectable by NIFI without any further dissection. The percentage of PGs whose locations were first detected by NIFI, rather than NE in this regard, was different at each center (40, 67, and 37%; p < 0.001).
In 77% (161/210) of patients, at least one PG, which could not be identified on initial NE exploration of the operative field, was detected by AF on NIFI without the need for further dissection (77, 84, and 69% at each center; p = 0.09). Overall, a median of one PG per patient was detected by NIFI in this fashion before being identified visually (1, 2, and 1 at each center; p = 0.052).
Parameters Affecting the Exhibition of Autofluorescence
There was no association between age, sex, BMI, PG size, and PG location (upper vs. lower) and exhibition of AF. Smaller PGs were more likely to be unrecognized on initial visual exploration, but localized with AF on NIFI (odds ratio of detection with increasing size 0.91, 95% confidence interval 0.84–0.98; p = 0.02) (Table 3).
Discussion
This study demonstrates that NIFI could be an adjunct to detect PGs during thyroid and parathyroid surgeries. With a total of 594 PGs studied, it represents the largest experience to date in the literature. At all centers, sensitivity of NIFI in detecting PG AF was high, and ranged between 97 and 99%. The location of 46% of all PGs was first hinted at by AF on NIFI. These glands exhibited bright AF upon initial NIFI of the operative field, and, following dissection of the covering tissues, were visually or pathologically confirmed to be PGs. In line with this finding, in 77% of patients at least one PG, which was not apparent visually on initial exposure of the central neck, was detected with this technology due to its AF on NIFI. Notably, there was variability in the percentage of PGs that were first discovered by NIFI in this fashion at different centers, which, to our knowledge, is being reported for the first time. The variability may result from patient- and/or surgeon-related factors. Regardless of the variability in results at all centers, between 37 and 67% of PGs were first identified by NIFI. Even at the lower end, this corresponds to more than one-third of all PGs, which underscores a potential benefit from NIFI systems in thyroid and parathyroid surgery.
One potential weakness of this study is the difference between patient cohorts at individual centers in terms of the underlying pathology and surgical management. Center 2 had more thyroid pathologies, which led to more thyroid surgeries than the other two centers. This was a result of the independent data collection at each center. BMI difference, on the other hand, is likely a reflection of the demographic differences between countries. Despite patient heterogeneity, this study shows that NIFI could reliably identify PGs with AF across various disease states and by different surgeons.
Despite the initial discovery of parathyroid AF using spectroscopy,10 this technique was not adopted by other groups due to various challenges. In essence, the spectrometer needed to be in direct contact with the tissue during measurements. Moreover, individual measurements from the same gland could vary, and, to overcome this, multiple measurements from each gland were necessary.11 In 2016, Falco and colleagues demonstrated parathyroid AF using an NIFI camera, and reported that the mean fluorescent intensity of PGs (40.6 ± 26.5) was greater than the thyroid gland (31.8 ± 22.3) and background tissue (16.6 ± 15.4).12 The same group later showed that a mean of 3.7 PGs could be intraoperatively identified using NIFI as opposed to 2.5 PGs with NE exploration during thyroid and parathyroid surgeries. However, no comment was made on what percentage of PGs not visible to NE on initial exposure could be detected by exhibition of AF on NIFI before further dissection.3 The current study fills this gap in the literature.
NIFI visualization of parathyroid AF is safe and feasible. It can be performed using a handheld external camera, and does not interfere with the standard surgical technique. Imaging of each gland takes approximately 1 min to perform, adding a few minutes to the total operating time for all four PGs. Unlike other NIFI techniques, NIFI of parathyroid AF does not require contrast injection, therefore there is no risk of injection-related complications.2 In thyroid operations, the AF characteristics of the PGs do not change after the removal of the thyroid gland. Likewise, parathyroid AF appears to be independent of PG perfusion. Therefore, NIFI of parathyroid AF does not provide information about gland viability.
The effect of the utilization of this new imaging technique on postoperative calcium and parathyroid hormone levels was beyond the scope of this study; however, in a recent study, Benmiloud et al. reported that postoperative hypocalcemia rates after total thyroidectomy decreased from 20.9 to 5.2% after incorporation of NIFI of parathyroid AF.15
Conclusions
NIFI facilitated identification of PGs by detecting their AF before being conventionally recognized by the surgeon in 37–67% of the time in this study. Although there was variability in this rate between centers, there was a concordance in detecting AF from 97 to 99% of the PGs using NIFI. To our knowledge, this is the first study analyzing the concordance of parathyroid AF imaging results at different centers. We suggest the incorporation of AF with NIFI alongside conventional visual cues to aid identification of PGs during neck operations.
References
McWade MA, Paras C, White LM, Phay JE, Mahadevan-Jansen A, Broome JT. A novel optical approach to intraoperative detection of parathyroid glands. Surgery. 2013;154(6):1371–7; discussion 1377.
Kahramangil B, Berber E. The use of near-infrared fluorescence imaging in endocrine surgical procedures. J Surg Oncol. 2017;115(7):848–855.
Falco J, Dip F, Quadri P, de la Fuente M, Prunello M, Rosenthal RJ. Increased identification of parathyroid glands using near infrared light during thyroid and parathyroid surgery. Surg Endosc. 2017;31(9):3737–3742.
Gourgiotis S, Moustafellos P, Dimopoulos N, Papaxoinis G, Baratsis S, Hadjiyannakis E. Inadvertent parathyroidectomy during thyroid surgery: the incidence of a complication of thyroidectomy. Langenbecks Arch Surg. 2006;391(6):557–560.
Grubbs EG, Mittendorf EA, Perrier ND, Lee JE. Gamma probe identification of normal parathyroid glands during central neck surgery can facilitate parathyroid preservation. Am J Surg. 2008;196(6):931–5; discussion 935–6.
Dudley NE. Methylene blue for rapid identification of the parathyroids. Br Med J. 1971;3(5776):680–681.
Prosst RL, Gahlen J, Schnuelle P, Post S, Willeke F. Fluorescence-guided minimally invasive parathyroidectomy: a novel surgical therapy for secondary hyperparathyroidism. Am J Kidney Dis. 2006;48(2):327–331.
Zaidi N, Bucak E, Okoh A, Yazici P, Yigitbas H, Berber E. The utility of indocyanine green near infrared fluorescent imaging in the identification of parathyroid glands during surgery for primary hyperparathyroidism. J Surg Oncol. 2016;113(7):771–774.
Das K, Stone N, Kendall C, Fowler C, Christie-Brown J. Raman spectroscopy of parathyroid tissue pathology. Lasers Med Sci. 2006;21(4):192–197.
Paras C, Keller M, White L, Phay J, Mahadevan-Jansen A. Near-infrared autofluorescence for the detection of parathyroid glands. J Biomed Opt. 2011;16(6):067012.
McWade MA, Sanders ME, Broome JT, Solorzano CC, Mahadevan-Jansen A. Establishing the clinical utility of autofluorescence spectroscopy for parathyroid detection. Surgery. 2016;159(1):193–202.
Falco J, Dip F, Quadri P, de la Fuente M, Rosenthal R. Cutting edge in thyroid surgery: autofluorescence of parathyroid glands. J Am Coll Surg. 2016;223(2):374–380.
De Leeuw F, Breuskin I, Abbaci M, et al. Intraoperative near-infrared imaging for parathyroid gland identification by auto-fluorescence: a feasibility study. World J Surg. 2016;40(9):2131–2138.
Ladurner R, Sommerey S, Arabi NA, Hallfeldt KKJ, Stepp H, Gallwas JKS. Intraoperative near-infrared autofluorescence imaging of parathyroid glands. Surg Endosc. 2017;31(8):3140–3145.
Benmiloud F, Rebaudet S, Varoquaux A, Penaranda G, Bannier M, Denizot A. Impact of autofluorescence-based identification of parathyroids during total thyroidectomy on postoperative hypocalcemia: a before and after controlled study. Surgery. 2018;163(1):23–30.
Disclosure
Bora Kahramangil, Fernando Dip, Fares Benmiloud, Jorge Falco, Martin de La Fuente, Silvina Verna, Raul Rosenthal, and Eren Berber have no conflicts of interest or financial support to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kahramangil, B., Dip, F., Benmiloud, F. et al. Detection of Parathyroid Autofluorescence Using Near-Infrared Imaging: A Multicenter Analysis of Concordance Between Different Surgeons. Ann Surg Oncol 25, 957–962 (2018). https://doi.org/10.1245/s10434-018-6364-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-018-6364-2