Abstract
Background
Single-incision laparoscopic cholecystectomy (SILC) has been widely introduced into the clinical practice, but the real clinical benefits for patients still remain a matter of debate. We conducted a systematic review, according to the PRISMA guidelines comparing clinical and peri-operative outcomes of SILC and conventional laparoscopic cholecystectomy (CLC).
Method
A literature search, including only randomised controlled trials (RCTs), was performed via PubMed, Google Scholar, Cochrane Library and Embase database. The reviewers extracted data from the manuscripts of selected articles including patient demographics, operative time, morbidity rate, post-operative length of stay, conversion rate, cost data, pain and satisfaction with cosmetic results.
Result
Seventeen RCTs matching the inclusion criteria were finally selected for the analysis. A total of 1293 patients were involved in the review, including 663 (51.3 %) patients who have undergone SILC and 630 (48.7 %) patients who have undergone CLC. Post-operative pain was significantly worse in SILC patients in four studies, in CLC patients in four studies, while in the remnants seven studies, no differences in pain scores were found. Data on satisfaction for post-operative cosmetics were significantly better for SILC patients in all studies but two. Operating time was significantly longer in SILC group while there is no statistically significant difference in conversion rate. Morbidity rate was similar in both groups, as was the incidence of bile duct injuries. Costs were significantly higher in SILC group. SILC was considered a more challenging procedure in all studies.
Conclusion
The role of SILC is still controversial. Until now, no real significant benefit has been proven: overall satisfaction is the only clear advantage of SILC, and this is mainly related to cosmetic results. Indications to SILC are mainly limited to patients with uncomplicated disease, with BMI ≤ 30 kg/m2, whose surgery is unlikely to be converted to an open or multiport approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Nearly 30 years ago, the introduction of laparoscopic cholecystectomy revolutionized surgery, the main reason for its widespread use being the following: less post-operative pain, faster recovery, better cosmetics and quicker return to full activities, all resulting in the improvement of post-operative quality of life. Furthermore, some of the advantages of laparoscopy are ascribable to reduced abdominal wall trauma, which led both to reduced incidence of surgical site infections and, in the long term, to reduced occurrence of incisional hernia.
Actually, the aforementioned benefits have never been demonstrated in randomized controlled trial for laparoscopic cholecystectomy. Despite this lack of evidence, laparoscopic cholecystectomy has been accepted and is nowadays considered as the gold standard treatment of gallstones disease.
Over the last decade, new efforts have been made to further reduce abdominal wall trauma, introducing innovative minimal-invasive techniques. Among those, natural orifice trans-luminal endoscopic surgery (NOTES) is a challenging technique, still lacks appropriate instrumentation and has the disadvantage of requiring the closure of the access to the peritoneal cavity. Furthermore, NOTES approach to cholecystectomy requires an access through internal viscera or structures that have no direct relations to the targeted organ, thus posing ethical issues and criticisms [1].
Another new technique, single-incision laparoscopic cholecystectomy (SILC), which uses the umbilicus as a natural orifice allowing easy access to peritoneal cavity, easy conversion to standard laparoscopy and its easy closure, has been widely introduced into the clinical practice. As from the results of most RCTs and systematic reviews, the single-access approach to laparoscopic cholecystectomy is mainly indicated in patients with BMI < 30/35 kg/m2, thus excluding severe and morbid obese patients [2]. Further contraindications to SILC are acute cholecystitis and all the so-called difficult cholecystectomies (patients with a Nassar score or an adhesions score III or IV) [3]. Previous surgery on the upper abdomen may be a relative contraindication. In all cases where predictive indexes of difficult cholecystectomy are unclear [4], the procedure may start with a single-access approach to exploration and possibly being converted to standard laparoscopy. Routine or a-la-demande intra-operative cholangiography is not a contraindication to the single-access approach [5].
Much has been reported in the literature on single-site approaches to all most common operations. Most likely, the development of these procedures has been partially supported by strong commercial marketing and publication competition [6], [7], but the real clinical benefits for patients still remain a matter of debate. In the last 3 years, many randomized trials on SILC have been published, trying to answer the question whether such a new approach is worthwhile or not and whether is safe and cost-effective. We conducted a systematic review, according to the PRISMA guidelines comparing clinical and peri-operative outcomes of SILC and conventional laparoscopic cholecystectomy (CLC) with the intent to assess the present evidence and actual indications of the single-incision approach.
Methods
A literature search was performed via PubMed, Google Scholar, Cochrane Library and Embase database. Literature search was completed in December 2014. Inclusion criteria for search include only randomised controlled trials (RCTs). Search terms such as “transumbilical endoscopic surgery”, “embryonic natural orifice transumbilical endoscopic surgery”, “single port laparoscopic surgery”, “single incision laparoscopic surgery”, “laparoendoscopic single site surgery”, “single access laparoscopic surgery”, “one port umbilical surgery”, “natural orifice transumbilical surgery”, “single-port access”, “single-site laparoscopic”, “single-instrument port laparoscopic surgery”, “single-port”, “single-incision”, “single-site”, “one-port”, “single-trocar”, “single-access”, “E-NOTES”, “SPL”, “SILS”, “LESS”, “SALS”, “SPA” and “SILC” were used to identify the eligible studies. The clinical trials of the US National Institutes of Health and Cochrane Database of Systematic Reviews and Controlled Trials Register were also searched to identify the ongoing RCTs and relevant articles. The reviewers extracted data from the manuscripts of selected articles including patient demographics, operative time, morbidity rate, post-operative length of stay, conversion rate, cost data, pain and satisfaction with cosmetic results. The review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [8].
Inclusion and exclusion criteria
The objective of this study was comparing functional and surgical outcomes of patients treated with either conventional laparoscopic cholecystectomy or SILC. Therefore, only studies that provided the comparison between the two aforementioned groups were included. To improve statistical power, only randomized controlled trial were included; prospective non-randomized, retrospective and improperly conducted RCTs were excluded from the analysis. Only English language published studies were considered. Abstract publications from conferences were excluded from this review. When more than two studies were performed by the same institution or authors, they were included only if there was no overlap between the results of the studies. Otherwise, the larger, higher quality study was included in the analysis. Studies with a population aged <18 years were excluded. CLC was defined as three or four port surgery carried out with either French or American position. SILC was defined as laparoscopic surgery done through a single trans-umbilical incision. Conversion to CLC was considered when another incision or trocar outside the umbilicus was needed to complete the procedure.
Technologies and surgical technique: CLC
CLC is carried out through an American position where the patient lies supine and the surgeon is positioned on the patient’s left side or French position where the patient is in low stirrups and the surgeon is between the patient’s legs. Position and size of trocars vary from one institution to another. The standard technique utilizes four trocars. Most authors use an optical trocar of 10–12 mm introduced in the periumbilical region. One operating trocar of 10 mm is usually situated to the left side of the mid-epigastric region. Two operating trocars of 5 mm are placed in the inferior aspect of the right upper quadrant and in the epigastric region. Some surgeons prefer the three trocars technique where the trocar in the epigastric region used to achieve a better traction is omitted. There is a current tendency to reduce the trocars size during laparoscopic procedures. In laparoscopic cholecystectomy, this is possible by using 5-mm scopes and clip appliers.
The gallbladder is grasped and retracted cranially towards the right shoulder. This manoeuvre, combined with a 20° head up tilt of the operating table, facilitates the exposure of Calot’s triangle. The critical view of safety principles, described by Strasberg in 1995, is the most important factor in this phase of surgery [9]. Dissection of the gallbladder is started from the presumed point of the infundibulum-cystic duct junction, incising the serosa from both it’s ventral and dorsal aspects using blunt and energized (usually HF monopolar) dissection. This manoeuvre should continue distally until there is a sufficient length to apply clips and the complete course of the cystic duct along its juncture with the common bile duct is readily apparent. The ductal or vascular structure should not be divided until all of the relevant anatomy has been identified. The completed dissection should reveal two structures entering the gallbladder, and the bottom of the liver bed should be visible. It is not necessary to see the common bile duct [10]. Some surgeons have described the use of a retrograde dissection beginning at the fundus of the gallbladder and a subtotal cholecystectomy. However, such techniques are presently performed by only a limited number of surgeons [11], [12]. Once the cholecystectomy is completed, the gallbladder is extracted trough the umbilical trocar.
Technologies and surgical technique: SILC
SILC may be carried out through disposable devices, reusable devices or a multiple fascia punctures technique (Swiss cheese technique). Among disposable devices, foam devices (i.e. SILS®) require the use of a retrieval bag for gallbladder withdrawal; when 2-component devices with abdominal wall protection sleeves (i.e. Tri-port®, Gel-port®) are used, there is no need for retrieval bags to withdraw the gallbladder. Reusable devices like the Storz X-cone® and Endo-cone® allow the insertion of more instruments from different angles of direction (also necessary to overcome lack of flexibility), require a longer skin and fascia incision, and entail the need for a retrieval bag for gallbladder removal [13].
When a multiple fascia punctures technique is preferred, at the end of the operation, two of the fascial openings are connected with a small incision to allow the passage of the retrieval bag and gallbladder withdrawal.
SILC may be accomplished by reusable standard straight laparoscopic instruments, reusable pre-shaped curved instruments or disposable bendable instruments. The latter two allow triangulation within the operative field. SILC should be carried out preferably under the guidance of 5-mm 30° scopes connected to a high-definition imaging system. Dedicated scopes, either those longer than the standard ones or the chip-on-the-tip 5-mm EndoEYE® video-endoscope (Olympus), enhance vision during single-access laparoscopic procedures.
The patient lies legs apart in supine position, with the surgeon usually standing between the legs (French position), and the assistant on patient’s left side.
Access to the peritoneal cavity is obtained through a skin incision of about 15–20 mm either right around the upper edge of the umbilicus as in most CLC or dividing longitudinally the umbilicus itself. A 20-mm fascia incision is created to allow the introduction of the single-site-access device. Pneumoperitoneum is then established. Usually, three instruments may be introduced through the single-access device: a 5-mm 30° scope, and two 5-mm working instruments.
The Calot’s triangle is dissected with the left-hand instrument in order to achieve the Strasberg’s critical view of safety visualizing and dissecting free the cystic duct and artery. Tissue dissection during SILC may be accomplished by either HF monopolar or US devices. Ultrasonic dissection provides an almost bloodless field, preventing oozing from tissue division; however, the use of disposable US shears increases costs considerably. The cystic artery is either divided between clips or closed and divided by ultrasonic shears, whereas the duct is preferably secured with titanium or absorbable clips. Use of 5-mm-diameter disposable clip appliers is advised. Closure-division of the cystic duct by ultrasonic shears was never performed within RCTs in order to avoid possible bias, even when the duct was less than 5 mm in diameter and could have been easily treated by US energy, thus reducing need for instrument exchange. Gallbladder dissection from the gallbladder fossa is accomplished in the usual manner, and the specimen is removed with a retrieval bag or through the access device, thus avoiding abdominal wall contamination.
When the fundus-first technique is carried out, gallbladder was dissected preferably by ultrasonically activated shears, thus avoiding oozing due to not performing prior cystic artery ligature. With this technique, once the gallbladder is fully mobilized, traction on the infundibulum is improved, allowing a better visualization of the Calot’s triangle, identification of anatomical structures and an easier dissection of cystic artery and duct. Nevertheless, during fundus-first dissection, maximum care should be taken while approaching the infundibulum, in order to avoid injuries to hidden posterior structures.
Several technical variations for traction and improved exposure have been proposed such as transfixing stay sutures or internal retractors. A monofilament nylon suture with straight needle is passed through the abdominal wall right below the costal arch, passed through the fundus, hence back through the abdominal wall to suspend the gallbladder by anchoring it to the wall. A second stay suture may be passed in a similar fashion through the infundibulum to provide lateral traction, thus achieving a wider opening of Calot’s triangle. The Endograb® is a small 2-component internal retractor featuring springs and hooks, that may be introduced through the access device, used to make traction by anchoring tissues, with ease to be replaced according to surgeon’s needs [13], [14]. One component is used to grab the fundus, and the second component’s hook is used to hang the gallbladder up to the wall [13], [14].
Results
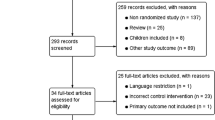
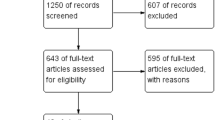
The first search yields 2150 articles. The reviewers excluded in the first step the majority of the manuscripts on the basis of the abstracts and titles. In the second step, 44 articles were screened and 11 were excluded: 4 meta-analyses and 7 reviews. In the last step, 16 articles were excluded from the final list: 13 reporting retrospective or non-randomized controlled studies, 2 reporting studies with partial results included in another article considered for this review and 1 not English written. Finally, 17 RCTs matching the inclusion criteria were selected for the analysis. The flowchart for systematic review is shown in Fig. 1. A total of 1293 patients were involved in the review, including 663 (51.3 %) patients who have undergone SILC and 630 (48.7 %) patients who have undergone standard laparoscopic cholecystectomy. Details of patient study groups, interventions and primary outcomes analysed in the studies included in the systematic review are shown in Table 1.
Inclusion and exclusion criteria
In all studies but one [15], acute cholecystitis within 3 months and severe pancreatitis were considered as exclusion criteria. Obesity has always been considered an important limit of SILC. Body mass index ≥30 kg/m2 was an exclusion criteria in five studies [1, 16–19], ≥35 kg/m2 in two [20], [21], ≥40 kg/m2 in three [22–24], ≥45 kg/m2 in one [25]. In five studies, a high BMI value was not considered an exclusion criteria, but despite this, the mean BMI of those studies never exceed 30 kg/m2 [15] [26–29]. Other exclusion criteria were considered previous abdominal surgery in six studies [17, 19, 24, 25, 27, 29], bleeding disorders, American Society of Anaesthesiologist (ASA) > III and chronic disease associated with pain were other exclusion criteria considered in the studies mentioned above.
Post-operative pain
Pain score was assessed by a visual analogue scale (VAS) post-operatively with a 10-cm horizontal score ranging from 0 (no pain) to 10 (unbearable pain). Pain scores were reported at different time intervals in 15 out of 17 studies, pain scores were also measured at rest and on movement in two studies [21] [29], (Table 2). Post-operative pain was significantly worse in SILC patients in four studies [1], [23], [25], [27] and in CLC patients in four studies [16], [19], [26], [28], while in the remnant seven studies, no differences in pain scores were found [17] [18], [20–22], [24], [29], The study from Ostlie et al. [30] did not measure pain scores, but the total dose of required analgesics. Authors found a trend towards a more frequent (even though not significant) administration of pain-relieving medications in SILC group [30]. In the study by Chang et al. [29], pain was measured at 4 and 24 h post-procedure. In this study, severity of pain was also measured at rest and on movement at umbilical and extra-umbilical sites. SILC was found less painful only at the 24 h post-procedure assessment, both at rest and on movement, but only at the extra-umbilical sites. No difference was found between SILC and CLC during all other pain assessments at any site, both at rest and on movement [29]. In the study by Jorgensen et al., no significant differences were seen between the two groups neither regarding pain score nor regarding opioids requirement [21].
Cosmetic results and quality of life
Data on satisfaction for post-operative cosmetics resulted significantly better in SILC patients in all studies but two [23, 29] (Table 3). However, it is not clear whether such an advantage may lead to better quality of life (QoL). In fact, among the five studies reporting data of QoL assessment, post-operative quality of life resulted significantly better in the SILC patients group in four [1, 17, 24, 26], whereas was worst in one [25].
Operating time and length of stay
In 12 out of 16 studies, operating time was significantly longer in the study group. SILC lasted an average 20 min more than CLC. Post-operative length of stay did not differ significantly in both patient groups (Table 4).
Conversion
As shown in Table 4, there is no statistically significant difference in conversion rate between SILC and control group. There were a total of 24 patients (6.5 %) in the SILC group converting to multiport and/or open cholecystectomy. In the SILC group, the main reasons for conversion to conventional laparoscopic cholecystectomy were limited visualization of Calot’s triangle due to adhesions, arterial bleeding and difficult dissections at the junction of the cystic duct and common bile duct. Conversion to a conventional laparoscopic procedure was associated with longer operative time [15, 22, 25, 29].
Complications
Intra-operative and post-operative complications reported in the studies are retained bile duct stones, bile leakage, bile duct damage, urinary and pulmonary infection, wound complications such as seroma and haematoma, and post-operative incisional hernia. Morbidity rate, as shown in Table 4, was similar in both groups, as was the incidence of bile duct injuries. Factors associated with wound complications were: higher body mass index, longer skin incision, Swiss cheese (multi-puncture) technique [31]. In Marks et al.’s [25] study, a statistically significant difference in post-operative hernia rates is also reported: 8.4 % in SILC patients versus 1.2 % in CLC patients (p = 0.03).
Cost data
Data on hospital charges are reported in three studies. In the paper written by Leung et al. [15], hospital charges were found to be significantly different between SILC and CLC groups (CLC $15,717 ± $14,231 vs SILC $17,817 ± $5358; p < 0.0001). Furthermore, also the following subcharges were found to be significant: operating room charges (CLC $4445 ± $1078 vs SILC $5358 ± 893; p < 0.0001); medical/surgical supplies (CLC $3312 ± $6526 vs SILC $5102 ± $1529; p < 0.0001) and anaesthesia costs (CLC $579 ± $7616 vs SILC $820 ± $23,957; p < 0.0001). Ostlie et al. [30] reported more doses of analgesics used and greater hospital charges in the single-site group that trended towards significance (29.7 K ± 27.3 for SILC and 20.6 K ± 6.9 K for CLC, p = 0.08). In the paper of Bucher [26], operative cost were higher for SILC (p < 0.001) although median time to return to work was shorter in comparison with CLC (p = 0.003).
Discussion
Each new surgical technique introduced into the clinical practice must be compared to the gold standard technique before acceptation and consequent widespread diffusion. When laparoscopic surgery was introduced in the early 1990s, the scientific community raised many concerns on feasibility and safety, and, despite the advantage for patients were “self-evident”, this scepticism brought to a slow acceptance and worldwide diffusion of laparoscopy.
Each technique has its own specific complication profile that must be accepted as a part of innovation [32]: analysis of results of the single-port approach to LC should be done bearing in mind that SLC is nowadays a very well standardized technique, safe, often performed as an outpatient or one-day surgery procedure, and that very little room for improvements is left. Furthermore, most general surgeons can perform CLC proficiently, whereas performing SILC requires specific additional training [33].
Promoters of SILC stress that the expected major benefits of this procedure are reduced pain and better cosmetics. Nevertheless, less post-operative pain is not yet confirmed by randomized trials.
In spite of our natural tendency to believe that one modest incision would hurt less than four standard laparoscopic incisions, the converse may be probably true [34]. Blinmann et al. [35] as also reported by Garg [32] showed that total tension—and hence pain—across a wound rises nonlinearly by increasing wound’s length. Tension rises in proportion to the square of wound’s length, instead. Therefore, total tension across multiple incisions may be less than total tension across a single incision, the length of which is equal to or greater than the sum of 3–4 standard laparoscopic incisions. In Blinmann’s study, the calculated total tension across two 10-mm and two 5-mm port CLC wounds and that across one SILC wound was found to be similar [32, 35].
Luna et al. [20] analysed inflammatory response between interventions and control group measuring interleukin (IL-6) and C-reactive protein (CPR) level just before anaesthetic induction and post-operatively. The systemic inflammatory response to surgery is considered to be a consequence of tissue trauma, and this, in turn, influences surgical outcome, especially less pain and faster recovery. The laparoscopic approach has been shown to attenuate the inflammatory response when compared to open surgery. In their paper, the authors found that the inflammatory response was similar in both single-port and conventional laparoscopic group reflecting no significant differences in terms of reported pain and quantity of on-demand analgesics delivered [20].
Satisfaction with the cosmetic results has been demonstrated to be superior in SILC patients than in CLC patients in all randomized studies but two and all meta-analyses [36–38]. In 2012, Hey et al. [39] published data on possible candidates to SILC: Post-operative images taken after SILC or CLC were shown to all patients awaiting elective cholecystectomy, and patients were asked which procedure they would have preferred based on these pictures. The same question was asked after completion of a questionnaire constructed using published objective data comparing reported outcomes of SILC and CLC. Only 16 % of subjects opted for CLC before questionnaire completion, this percentage increased up to 88 % after knowing outcomes data of both procedures (p < 0.001). These data show that the risk for complications has a higher influence than cosmetic results in determining the choice of procedure [39]. The bias of this study is that data of a well established procedure were compared to early data of a procedure that may be still in its learning curve phase. Furthermore, in a “willingness to pay” survey conducted within the Marks et al. [25] study, only a little more than 50 % of patients stated that they would have accepted to pay more for undergoing SILC instead of CLC.
Although not significant, a trend towards higher complication rates in SILC patients has been recognized, and this aspect must be further investigated: The doubt that a larger number of patients enrolled in future studies as well as longer follow-ups may lead to significant differences does exist. Possible explanation of this raised risk of complications is the demonstrated significantly impaired exposure of the operating field in SILC compared to CLC [1] and the overall higher difficulty of SILC, as subjectively assessed by surgeons [30]. Wound complication rates were found higher in SILC group probably caused by heavier trauma to the umbilical site [38], 11.7 vs 4.9 % [25]. Despite these data, the largest consecutive series of patients undergoing single-access laparoscopic procedures published in 2013 showed that the incidence of wound complications in these patients is acceptably low and is further reduced once the learning curve is over [31]. With the end of their learning curve, surgeons will become more and more confident with this new approach, and this will affect positively on post-operative results. There is a general agreement among authors that SILC is more difficult than CLC, opinion confirmed by the operating time that remains longer even after the learning curve is completed.
Some of the drawbacks of SILC, such as instrument triangulation, ergonomics and surgical exposure, might be solved by robotic surgery accomplished with a novel platform dedicated to procedures performed through a single laparoscopic access. Results from a prospective longitudinal observational study conducted on 100 consecutive da Vinci single-access cholecystectomies [40], with feasibility without conversion and safety as primary end points, showed that the robotic approach is safe and allows a quicker overcoming of the learning curve phase. Conversion rate was minimal with mean total operating time 72 min and console time 32 min. Nevertheless, operating time does not decrease by increasing surgeon’s experience. After subjective evaluation through a questionnaire collecting surgeon’s opinions, single-access robotic cholecystectomy was judged more complex than CLC, but easier than manual SILC; at present objective data and evidence of the benefit of robotic single-incision cholecystectomy are still lacking, whereas there are many concerns on the increasing overall costs.
In terms of cost analysis, several authors have shown that SILC is more expensive compared to CLC [15, 26, 30]. However, Joseph et al. [41] reported that no significant differences in cost between SILC and CLC instead of a shorter hospital stay (p = 006) for SILC. Same results are reported by Love et al. [42] in their cost comparison. The authors concluded that there was not any significant difference in cost when standard materials and equipment were used and the duration of the procedure considered. This is said to be due to the products required in the operation being under development [30] and that these costs cannot be compared to those costs of an operation being done routinely. Hence, with increased usage of the SILC procedure, the costs might reduce.
The studies included in this review mainly identify the contraindications to SILC, while specific indications are lacking. In a study not included in this review, Beninato et al. [43] tried to expand the indications to SILC to all patients with biliary disease without exclusion criteria. The authors found that patients with acute cholecystitis and gallstone pancreatitis had longer operative times and a higher conversion to 4-port cholecystectomy than patients with biliary colic. Similarly, relationships with longer operative time and conversion rate were found in obese patients compared to non-obese patients, while no difference in post-operative complication rate was found in these groups. The authors concluded that SILC can be offered to patients with a wide spectrum of biliary disease with the awareness that this may result in an increased operative time and a higher likelihood of conversion to multiport laparoscopy.
In conclusion, the role of SILC is still controversial. Published data suggest that SILC, compared to CLC, involves same post-operative pain and same length of hospital stay, but requires longer operating time with higher in-hospital costs especially when disposable instruments are used. Data on post-operative complications are not significantly worse even though a trend towards a higher morbidity rate does exist. What is of utmost relevance is that in all studies SILC was considered more challenging than CLC, being marked by a poorer quality of exposure of the operating field. Overall satisfaction is the only clear advantage of SILC, and this is mainly related to cosmetic results. However, it is still not clear whether this may turn in better post-operative quality of life. What may be presumed from this review is that indication to SILC should be mainly limited to patients with uncomplicated disease, with BMI ≤ 30 kg/m2, whose surgery is unlikely to be converted to an open or multiport approach, thus limiting the actual role of such a procedure and most likely preventing its widespread diffusion.
References
Lirici MM, Califano AD, Angelini P, Corcione F (2011) Laparo-endoscopic single site cholecystectomy versus standard laparoscopic cholecystectomy: results of a pilot randomized trial. Am J Surg 202:45–52
Connor S (2009) Single-port-access cholecystectomy: history should not be allowed to repeat. World J Surg 33:1020–1021
Nassar AHM, Ashkar KA, Mohamed AY, Hafiz AA (1995) Is laparoscopic cholecystectomy possible without video technology? Minim Invasive Ther Allied Technol 4:63–65
Lirici MM, Califano A (2010) Management of complicated gallstones: results of an alternative approach to difficult cholecystectomies. Minim Invasive Ther 19:304–315
Bagloo MB, Dakin GF, Mormino LP, Pomp A (2011) Single-access laparoscopic cholecystectomy with routine intraoperative cholangiogram. Surg Endosc 25(5):1683–1688
Allemann P, Schafer M, Demartines N (2010) Critical appraisal of single port access cholecystectomy. Br J Surg 97:1476–1480
Rodhes M (2010) Commentary on critical appraisal of single port access cholecystectomy. Br J Surg 97:1481
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) The PRISMA group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097. doi:10.1371/journal.pmed1000097
Strasberg SM, Hertl M, Soper NJ (1995) An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am college Surg 18:279–285
Yamashita Y, Kimura T, Matsumoto S (2010) A safe laparoscopic cholecystectomy depends upon the establishment of a critical view of safety. Surg Today 40:507–513
Michalowski K, Borman PC, Krige JEJ, Gallagher PJ, Terblanche J (1998) Laparoscopic subtotal cholecystectomy in patients with complicated acute cholecystitis or fibrosis. Br J Surg 85:904–906
Tian Y, Wu SD, Su Y, Kong J, Yu H, Fan Y (2009) Laparoscopic subtotal cholecystectomy as an alternative procedure designed to prevent bile duct injury: experience of a hospital in northern china. Surg Today 39:510–513
Kia MA, Lee C, Martinez JM, Zundel N (2011) Single port cholecystectomy: the pathway back to a standard technique. Surg Laparosc Endosc Percutan Tech 21:314–317
Schlager A, Khalaileh A, Shussman N, Elazary R, Keidar A, Pikarsky AJ, Ben-Shushan A, Shibolet O, Horgan S, Talamini M, Zamir G, Rivkind AI, Mintz Y (2010) Providing more through less: current methods of retraction in SIMIS and NOTES cholecystectomy. Surg Endosc 24:1542–1546
Leung D, Yetasook AK, Carbray J, Butt Z, Hoeger Y, Denham W, Barrera E, Ujiki MB (2012) Single-incision surgery has higher cost with equivalent pain and quality-of-life scores compared with multiple-incision laparoscopic cholecystectomy: a prospective randomized blinded comparison. J Am Coll Surg 215:702–708
Zheng M, Qin M, Zhao H (2012) Laparoendoscopic single-site cholecystectomy: a randomized controlled study. Minim Invasive Ther Allied Technol 21:113–117
Aprea G, Coppola Bottazzi E, Guida F, Masone S, Persico G (2011) Laparoendoscopic single site (LESS) versus classic video-laparoscopic cholecystectomy: a randomized prospective study. J Surg Res 166:e109–e112
Cao ZG, Cai W, Qin MF, Zhao HZ, Yue P, Li Y (2011) Randomized clinical trial of single-incision versus conventional laparoscopic cholecystectomy: short-term operative outcomes. Surg Laparosc Endosc Percutan Tech 21:311–313
Tsimoyannis EC, Tsimogiannis KE, Pappas-Gogos G, Farantos C, Benetatos N, Mavridou P, Manataki A (2010) Different pain scores in single transumbilical incision laparoscopic cholecystectomy versus classic laparoscopic cholecystectomy: a randomized controlled trial. Surg Endosc 24:1842–1848
Luna RA, Nogueira DB, Varela PS, Rodrigues Neto E, Norton MJR, Ribeiro LB, Peixoto AM, Mendonca YL, Bendet I, Fiorelli RA, Dolan JP (2013) A prospective, randomized comparison of pain, inflammatory response, and short-term outcomes between single port and laparoscopic cholecystectomy. Surg Endosc 27:1254–1259
Jorgensen LN, Rosenberg J, Al-Tayar H, Assaadzadeh S, Helgstrand F, Bisgaard T (2014) Randomized clinical trial of single-versus multi-incision laparoscopic cholecystectomy. Br J Surg 101:347–355
Sinan H, Demirbas S, Ozer MT, Sucullu I, Akyol M (2012) Single incision laparoscopic cholecystectomy versus laparoscopic cholecystectomy: a prospective randomized study. Surg Laparosc Endosc Percutan Tech 22:12–16
Ma J, Cassera MA, Spaun GO, Hammil CW, Hansen PD, Aliabadi-Wahle S (2011) Randomized controlled trial comparing single-port laparoscopic cholecystectomy and four-port laparoscopic cholecystectomy. Ann Surg 254:22–27
Lee PC, Lo C, Lai PS, Chang JJ, Haung SJ, Lin MT, Lee PH (2010) Randomized clinical trial of single-incision laparoscopic cholecystectomy versus minilaparoscopic cholecystectomy. Br J Surg 97:1007–1012
Marks JM, Phillips MS, Tacchino R, Roberts K, Onders R, DeNoto G, Gecelter G, Rubach E, Rivas H, Islam A, Soper N, Paraskeva P, Rosemurgy A, Ross S, Shah S (2013) Single-incision laparoscopic cholecystectomy is associated with improved cosmesis scoring at the cost of significantly higher hernia rates: 1-year results of a prospective randomized, multicenter, single-blinded trial of traditional multiport laparoscopic cholecystectomy vs single-incision laparoscopic cholecystectomy. J Am Coll Surg 216:1037–1047
Bucher P, Pugin F, Buchs NC, Ostermann S, Morel P (2011) Randomized clinical trial of laparoendoscopic single-site versus conventional laparoscopic cholecystectomy. Br J Surg 98:1695–1702
Lai ECH, Yang GPC, Tang CN, Yih PCL, Chan OCY, Li MKW (2011) Prospective randomized comparative study of single incision laparoscopic cholecystectomy versus conventional four-port laparoscopic cholecystectomy. Am J Surg 202:254–258
Asakuma M, Hayashi M, Komeda K, Shimizu T, Hirokawa F, Miyamoto Y, Okuda J, Tanigawa N (2011) Impact of single-port cholecystectomy on postoperative pain. Br J Surg 98:991–995
Chang SKY, Wang YL, Shen L, Iyer SG, Madhavan K (2014) A randomized controlled trial comparing post-operative pain in single-incision laparoscopic cholecystectomy versus conventional laparoscopic cholecystectomy. World J Surg 39:897–904
Ostlie DJ, Jaung AD, Iqbal CW, Sharp SW, Snyder CL, Andrews WS, Sharp RJ, Holcomb GW III, St Peter SD (2013) Single incision versus standard 4-port laparoscopic cholecystectomy: a prospective randomize trial. J Pediatr Surg 48:209–214
Weiss HG, Brunner W, Biebl MO, Schirnhofer J, Pimpl K, Mittermair C, Obrist C, Brunner E, Hell T (2014) Wound complications in 1145 consecutive transumbilical single-incision laparoscopic procedures. Ann Surg 259:89–95
Garg P, Thakur JD, Garg M, Menon GR (2012) Single-incision laparoscopic cholecystectomy vs. conventional laparoscopic cholecystectomy: a meta-analysis of randomized controlled trials. J Gastrointest Surg 16:1618–1628
Hao L, Liu M, Zhu H, Li Z (2012) Single-incision versus conventional laparoscopic cholecystectomy in patients with uncomplicated gallbladder disease: a meta-analysis. Surg Laparosc Endosc Percutan Tech 22:487–497
Rattner DW (2013) Beauty is in the eye of the beholder. J Am Coll Surg 216:1048–1077
Blinman T (2010) Incision do not simply sum. Surg Endosc 24:1746–1751
Zehetner J, Pelipad D, Darehzereshki A, Mason R, Lipham JC, Katkhouda N (2013) Single access laparoscopic cholecystectomy versus classic laparoscopic cholecystectomy: a systematic review and meta-analysis of randomized controlled trials. Surg Laparosc Endosc Percutan Tech 23:235–243
Pisanu A, Reccia I, Porceddu G, Uccheddu A (2012) Meta-analysis of prospective randomized studies comparing single-incision laparoscopic cholecystectomy (SILC) and conventional multiport laparoscopic cholecystectomy (CMLC). J Gastrointest Surg 16:1790–1801
Wang D, Wang Y, Ji ZL (2012) Laparoendoscopic single-site cholecystectomy versus conventional laparoscopic cholecystectomy: a systematic review of randomized controlled trials. ANZ J Surg 82:303–310
Hey J, Roberts KJ, Morris-Stiff GJ, Toogood GJ (2012) Patients views through the keyhole: new perspectives on single-incision vs. multiport laparoscopic cholecystectomy. HPB 14:242–246
Pietrabissa A, Sbrana F, Morelli L, Badessi F, Pugliese L, Vinci A, Klersy C, Spinoglio G (2012) Overcoming the challenges of single-incision cholecystectomy with robotic single-site technology. Arch Surg 147:709–714
Joseph S, Moore BT, Sorensen GB, Earley JW, Tang F, Jones P, Brown KM (2011) Single-incision laparoscopic cholecysteomy: a comparison with the gold standard. Surg Endosc 25:3008–3015
Love KM, Durham CA, Meara MP, Mays AC, Bower CE (2011) Single incision laparoscopic cholecystectomy: a cost comparison. Surg Endosc 25:1153–1158
Beninato T, Kleiman DA, Soni A, Nissan DA, Filicori F, Servais EL, Fahey TJ III, Zarnegar R (2015) Expanding the indications for single-incision laparoscopic cholecystectomy to all patients with biliary disease: is it safe? Surg Laparosc Endosc Percutan Tech 25:10–14
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Marco Maria Lirici, Simone Maria Tierno and Cecilia Ponzano have no conflict of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Lirici, M.M., Tierno, S.M. & Ponzano, C. Single-incision laparoscopic cholecystectomy: does it work? A systematic review. Surg Endosc 30, 4389–4399 (2016). https://doi.org/10.1007/s00464-016-4757-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-016-4757-5