Abstract
Optimization of process parameters for phytase production by Enterobacter sp. ACSS led to a 4.6-fold improvement in submerged fermentation, which was enhanced further in fed-batch fermentation. The purified 62 kDa monomeric phytase was optimally active at pH 2.5 and 60 °C and retained activity over a wide range of temperature (40–80 °C) and pH (2.0–6.0) with a half-life of 11.3 min at 80 °C. The kinetic parameters K m, V max, K cat, and K cat/K m of the pure phytase were 0.21 mM, 131.58 nmol mg−1 s−1, 1.64 × 103 s−1, and 7.81 × 106 M−1 s−1, respectively. The enzyme was fairly stable in the presence of pepsin under physiological conditions. It was stimulated by Ca+2, Mg+2 and Mn+2, but inhibited by Zn+2, Cu+2, Fe+2, Pb+2, Ba+2 and surfactants. The enzyme can be applied in dephytinizing animal feeds, and the baking industry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) is a key mineral element vital for the growth and development of living organisms. It is required for the formation of essential building blocks of life and instrumental in various metabolic pathways including glycolysis, gluconeogenesis and cellular signal transduction [1]. It is primarily stored in the form of phytic acid (PA) or phytate (myo-inositol 1, 2, 3, 4, 5, 6-hexakisphosphate, IP6) in cereals, legumes, oil seeds and nuts, where it constitutes 3–5 % of the dry weight [2] representing 50–80 % of the stored P [3]. PA has been globally recognized as an anti-nutrient due to its poly-anionic nature that leads to complex formation with divalent metal cations, proteins, starch and lipids, and thereby interfering with their absorption in the gastro-intestinal (GI) tract. Furthermore, the undigested PA is discharged into the environment and contributes to phosphorus eutrophication of water bodies [4].
Ruminant animals digest phytic acid with the help of microbial phytases produced by the rumen microflora. Monogastric animals such as pigs, poultry and fishes are deficient in phytases, therefore, poorly digest phytate phosphorus. This is a complex two-pronged problem as phosphate is essential for animal growth and development, which is excreted in the form of undigested phytate that leads to environmental pollution. This problem is effectively solved by the supplementation of feed with phytate-degrading enzymes.
Phytase (myo-inositolhexakisphosphate phosphohydrolase) degrades phytates to release phosphate and other essential ions. It is widespread in nature, found in plants, animals and microorganisms. The use of phytase reduces P excretion in monogastric animals and avoids supplementation of feeds with inorganic phosphate. Animal stomach is the main functional site for phytase-mediated dephosphorylation in the GI tract. Therefore, an ideal commercial phytase should be active and stable under acidic conditions prevailing in the gastric environment, and should be resistant to proteolytic enzymes like pepsin and trypsin. Despite several reports on phytases, the search for an ideal phytase is still continuing that can effectively hydrolyse phytate in the upper digestive tract (acidic) of monogastric animals and could be resilient at higher temperatures in feed pelleting.
The present investigation highlights the optimization of phytase production by Enterobacter sp. ACSS as well as its purification, characteristics and potential applications.
Materials and methods
Isolation and identification of the bacterial strain
Bacterial isolates, retrieved from soil samples from different locations around Durban (South Africa), were screened for phytase production on modified solid phytase screening medium (PSM) [5] [g/l: Na-phytate (Sigma), 1; glucose, 15; NH4NO3, 2; KCl, 0.5; MgSO4·7H2O, 0.5; MnSO4·4H2O, 0.3; FeSO4·7H2O, 0.3 and agar, 15; pH 6.5]. To avoid false positives due to microbial acid production, the plates were also treated with 20 g/l aqueous cobalt chloride solution. The plates were incubated at room temperature for 5 min, decanted, and then flooded with a freshly prepared solution containing equal volumes of a 62.5 g/l aqueous ammonium molybdate solution and 4.2 g/l ammonium vanadate solution. The isolate obtained from the plant rhizosphere soil which showed a larger phytate-hydrolysis zone was selected and identified as Enterobacter sp. ACSS (Fig. 1), based on a polyphasic approach. The 16S rDNA sequence was deposited in the NCBI GenBank nucleotide sequence database under the accession number KP814680. Stock cultures of the bacterium were maintained on refrigerated nutrient agar slants at 4 °C with periodic revival and also preserved as glycerol stocks at −70 °C.
A neighbor-joining tree showing the phylogenetic relationships of Enterobacter sp. ACSS, with type strains of Enterobacter species. Yersinia enterocolitica ATCC 9610T (AF366378) was used as an outgroup. Bootstrap values are expressed as percentages of 1000 replications and shown at the branch points
Enzyme preparation and phytase assay
The seed culture was prepared by submerged cultivation of the bacterium in Luria–Bertani broth at 37 °C at 200 rpm for 12 h. Phytase was produced by inoculating 2 % (CFU ≈ 1.5 × 108) of the seed culture in Erlenmeyer flasks (250 ml) containing 50 ml of PSM broth [g/l: Na-phytate (Sigma), 1; glucose, 15; NH4NO3, 2; KCl, 0.5; MgSO4.7H2O, 0.5; MnSO4.4H2O, 0.3 and FeSO4.7H2O, 0.3; pH 6.5]. Unless otherwise indicated, the fermentation was conducted at 37 °C for 48 h at 200 rpm. The cells were harvested by centrifugation at 8800×g for 20 min and the cell-free supernatant was used to assay phytase by determining inorganic phosphate (Pi) according to the method as described by Heinonen and Lahiti [6]. One unit of phytase activity is defined as the amount of enzyme that liberate 1 nmol of Pi per second under standard assay conditions [3, 7]. The protein concentration was determined using Bradford reagent (Bio-Rad) [8] with bovine serum albumin as the standard.
Optimization of process parameters
A variety of carbon and nitrogen sources, inorganic salts, and cultivation parameters like inoculum levels, incubation temperatures, incubation periods, agitation rates, initial pH values and others that may affect the production of phytase were optimized by the conventional ‘one-variable-at-a-time’ approach. The variables that significantly affected phytase production were identified, and these were further optimized by the statistical software package ‘Design Expert’ (Version 6.0, Stat-Ease Inc., Minneapolis, USA).
Plackett Burman methodology
Two-level Plackett-Burman design (PBD) is an efficient technique to select significant process parameters by screening n variables in n + 1 experiments. A total of 20 trials with 17 variables and 2 controls or unassigned variables were screened in the present design (Supplementary Table 1), which included a variety of carbon and nitrogen sources, inorganic salts, and cultivation parameters. The principal effect of each variable was estimated as the difference between the average of the measurements made at the high level (+1) of that factor and the average of the measurements made at the low level (−1) of that factor, which was determined by the following equation:
where E(X i ) is the concentration effect of the tested variable. C i+ and C i− are the phytase activities from the trials where the variable (X i ) under study was present at high and low concentrations, respectively, and N is the number of experiments. The significance of each variable (p value) was determined via Student’s t-test.
Path of steepest ascent
Experiments were carried out along the steep path that initiated from the origin, as the center of the Plackett-Burman design, to move rapidly towards the vicinity of the optimum conditions. Experiments were designed by increasing or decreasing the concentrations of variables based on the PBD results until no further increase in response was observed. The experimental design and results of the steepest ascent method are shown in Supplementary Table 2.
Response surface methodology using central composite design (CCD)
Four significant parameters (wheat bran, peptone, ammonium sulfate and inoculum size), identified by Plackett-Burman design, were chosen as independent variables. Each factor was studied at five different levels (−α, −1, 0, +1, +α) as per CCD, with a total of 30 experiments and the experimental design is shown in Supplementary Table 3a. The behavior of the system was explained by the following second order polynomial equation:
where Y is predicted value of response, β 0 is intercept, β 1, β 2, β 3, β 4 are linear coefficients, β 11, β 22, β 33, β 44 are squared coefficients, β 12, β 13, β 23, β 24, β 34 are interaction coefficients and A, B, C, A 2, B 2, C 2, D 2, AB, AC, BC, BD, CD are independent variables.
The conditions predicted by the statistical model was validated with respect to phytase production in 0.25–2.0 l shake-flasks, and also in l l (Multifors) and 5 l (Minifors) lab fermenters (Infors, Bottmingen-Basel, Switzerland). The fermenters were operated at 40 °C, 200 rpm with 1 vvm of aeration. Samples were drawn at the desired intervals, harvested and cell-free supernatants were used in phytase assays.
Batch and fed-batch production
Batch and fed-batch fermentations were conducted in a 5 l glass fermenter (Minifors, Infors HT, Switzerland) containing 2.5 l of optimized medium with 4.14 % (v/v) inoculum at 37 °C. Dissolved oxygen (DO) in the fermenter was maintained at 30 % air saturation using aeration in cascade mode controlling the agitation speed (maximum, 700 rpm) and airflow. Batch fermentation was carried out for 36 h, while fed-batch fermentation continued up to 96 h. Feeding was done at every 12 h with concentrated (10×) optimized medium to maintain the total reducing sugar concentration under 20 g/l. However, in order to reduce the clogging of feed-pipe due to wheat bran, its concentration was kept at 16.2 g/l in the feed.
Purification, PAGE and zymography
The crude extract (2 l) was subjected to 55–85 % ammonium sulfate precipitation and the precipitate was suspended in 0.1 M sodium acetate buffer (pH 5.5). It was desalted through Hi-Prep™ 26/10 column (GE Healthcare, Sweden) against 20 mM sodium acetate buffer (pH 5.5) using an AKTA purifier system (GE Healthcare, Sweden). The concentrated and desalted sample was loaded onto a HiTrap™ DEAE FF (GE Healthcare, Sweden) anion-exchange column previously equilibrated with 20 mM Tris–HCl buffer (pH 8.0). The column was eluted with a linear gradient of 0–1 M NaCl in 20 mM Tris–HCl buffer (pH 8.0) at a flow rate of 1 ml/min. Active fractions were pooled and applied to Superdex™ 200 increase 10/300 column (GE Healthcare, Sweden), and eluted with 50 mM sodium acetate buffer (pH 5.5) at 0.75 ml/min. Elution of the protein was monitored at 280 nm. The active fractions were then pooled and concentrated using Biomax Mr. 10,000 cut-off Millipore membrane (Millipore, USA).
SDS–PAGE was carried out on a Mini PROTEAN gel electrophoresis unit (BioRad Laboratories, USA) according to Laemmli [9] using 12 % cross-linked polyacrylamide gel. For zymography, the samples were electrophoresed on 12 % native-PAGE and activity stained according to the method of Bae et al. [10]. A clear zone of hydrolysis confirmed phytase activity.
Enzyme characterization
Effect of pH on activity and stability
The effect of pH on phytase activity was studied over a pH range between 1.0 and 11.0 in different buffer systems [HCl-KCl buffer (1.0–2.0), Citrate buffer (pH 3.0), Na-acetate buffer (pH 4.0–5.0), Citrate buffer (pH 6.0) Tris–HCl buffer (pH 7.0–8.0) and glycine-NaOH (9.0–11.0]. The pH stability of the enzyme was determined by pre-incubation of phytase with buffers of different pH and the residual enzyme activity was determined at an interval of 30 min up to a period of 6 h under standard assay conditions.
Effect of temperature on activity and stability
The effect of temperature on phytase activity was assessed over a temperature range of 30–90 °C. The thermostability of the enzyme was determined by pre-incubation at different temperatures and determining the residual enzyme activity at 30-min intervals up to a period of 6 h.
Substrate specificity and kinetic parameters
The substrate specificity of purified phytase was tested against Na-phytate, p-nitrophenyl phosphate, sodium pyrophosphate, glucose-1-phosphate, glucose-6-phosphate and ATP by incubating the enzyme separately with the substrates (5 mM) and measuring the residual phytase activity. Enzyme without any agent acted as control while activity with Na-phytate was regarded as 100 %. The K m and V max of phytase was determined using Lineweaver–Burk plot and by applying the Michealis–Menten equation (Eq. 1) using different concentrations (0.125–2 mM) of substrates. The catalytic constant (K cat) was determined from the V max recorded for each substrate by applying Eq. (2) while catalytic efficiency was calculated by applying Eq. (3).
where V 0 is the initial velocity, V max is the maximum velocity, K m is the Michaelis–Menten constant and [E] T is the total enzyme concentration.
Effect of metal ions, surfactants and reducing agents
The effect of metal ions and surfactants was studied by pre-incubating the enzyme for 30 min at 37 °C, 100 rpm with 1 and 5 mM concentration of various metal ions including BaCl2·2H2O, CaCl2·2H2O, CoCl2·2H2O, CuSO4·5H2O, FeSO4·7H2O, MgSO4·7H2O, MnCl2·4H2O, PbCl2 and ZnCl2, and 0.1–1.0 mM concentration of non-ionic (triton X-100, tween-20, tween-80), anionic (SDS) and cationic (CTAB) surfactants. In addition, the effect of 1 and 5 mM concentration of EDTA, and DTT was also investigated. Enzyme without any agent served as the control. The residual enzyme activities were thereafter determined.
Applications of phytase
Phosphate liberation from animal feed and simulated gastric fluid (SGF)
Finley milled wheat bran, chicken feed and fish feed were autoclaved (121 psi, 15 min) and 2 g (dry weight) of each was suspended in 50 ml of 0.1 M acetate buffer (pH 5.5). Phytase (50 U) was added to each suspension and incubated at 37 and 50 °C. Aliquots (1 ml) were removed at desired time intervals and centrifuged at 10,620×g for 2 min at 4 °C. The supernatants were collected for estimating Pi liberated as described earlier [7]. Pi released from animal feed was determined by adding 1 g of feedstock (wheat bran, chicken feed and fish feed) to 9 ml simulated gastric fluid (SGF) (250 mM Glycine–HCl containing 2.0 mg/ml NaCl and 3.2 mg/ml pepsin). The pH was adjusted with HCl or NaOH to 1.5, 2.0, 2.5, 3.5, 5.5, or 6.5 as required. Phytase (10 U) was added to pre-incubated solutions (37 °C, 30 min) and incubated with agitation at 37 °C for 60 min. The amount of released phosphorus was determined using standard phytase assay [11].
Hydrolysis of insoluble metal-phytates
Hydrolysis of insoluble metal-phytates was studied according to the method as described by Sapna and Singh [12]. Briefly, stock solutions of metals ions (Ca2+, Co2+, Cu2+, Fe2+, Mg2+, Mn2+ and Zn2+, 100 mM each) were prepared by dissolving CaCl2·2H2O, CoCl2·2H2O, CuSO4·5H2O, FeSO4·7H2O, MgSO4·7H2O, MnCl2·4H2O and ZnCl2·7H2O in Milli-Q water. Equal volumes of 100 mM salt solution and 10 mM sodium phytate were mixed and incubated overnight at 4 °C for precipitation. The precipitated salts were separated next day by centrifugation at 425×g at 4 °C for 1 min. The pellets were washed thrice with Milli-Q water followed by re-suspension in 0.5 ml of 0.1 M Na-acetate buffer (pH 5.5). The metal-phytates were hydrolysed by incubating with 10 U of phytase at 50 °C for 12 h and aliquots were taken every 3 h to determine the amount of Pi in the supernatant after centrifugation. The substrate and enzyme controls were also run simultaneously and values were deducted from the test value [12].
Application in improvement of bread making performance
Basic bread dough was prepared by mechanical mixing of brown bread flour (400 g) with dry yeast (8 g), sugar (15 g), ascorbic acid (3.75 mg), water (275 ml), fat (10 g) and NaCl (5 g) for 5 min. Phytase (0.2, 0.6 and 1.0 U/g) was added prior to mixing of separate bread doughs, while the controlled experiment lacked this enzyme. It was left for proofing for 45 min, followed by baking at 25 °C for 25 min. The breads were then assessed for soluble Pi. Shelf life of the bread was estimated by Weibull hazard method [13].
Results and discussion
Plant rhizosphere is a highly dynamic environment that harbors diverse microorganisms actively involved in nutrient cycling. Isolation of a high phytate-hydrolysing bacterium in this study, from the rhizosphere of sugarcane indicates its possible involvement in mobilization of P from recalcitrant phytate molecules in soil. The bacterium is Gram-negative, rod-shaped, non-spore forming facultative anaerobe that grows optimally at 37 °C in Luria–Bertani broth. The carbohydrate utilization profile on Omnilog-Biolog identification system (Biolog, Inc, CA) indicated it to be a species of Enterobacter which was further confirmed by 16S rDNA sequence analysis. Interestingly, though the 16S rDNA sequence of Enterobacter sp. ACSS displayed high identity with E. cloacae and E. ludwigii strains, it formed outgroup in the phylogenetic tree (Fig. 1) indicating its unique characteristics.
Response of the PBD (Supplementary Table 1a) was observed with high variability in phytase production (0.41–29.81 U/ml), indicating the importance of optimization by statistical approaches. Five variables (wheat bran, peptone, tween-20, ammonium sulfate and inoculum size) were identified as the most significant factors (p < 0.05) out of 17 nutritional and cultural variables (Supplementary Table 1b). However, we continued with wheat bran, peptone, ammonium sulfate and inoculum size for further optimization. Although tween-20 has a marked effect on phytase production but it was omitted as it shows interference during purification steps. The production of phytase is reported to be inducible or constitutive [14]. In the present study, the positive signal of wheat bran indicated that phytase production by Enterobacter sp. ACSS was induced in the presence of phytate containing substrates. Wheat bran has a high phytate content [15, 16]; therefore, it may act as an inducer for enhanced production of phytase by the bacterium. The enzyme production was further improved to 55.68 ± 3.06 U/ml using path of steepest ascent/descent (Supplementary Table 2).
RSM using CCD was applied to determine the optimal levels of the four selected variables and their interaction was studied for enhanced phytase production. The results of CCD experiments are presented along with the mean predicted and observed responses (Supplementary Table 3a). It was analyzed by standard ANOVA (Supplementary Table 3b).
The model F values of 1550.42 and ‘Prob > F value’ of <0.0001 implied the model was highly significant. There was only a 0.01 % chance that the model F value could occur due to noise. All four linear coefficients (A, B, C and D) and the quadric terms (A 2, B 2, C 2 and D 2) were significant for phytase production. The interaction of A with C and D, and D with B and C were significant model terms.
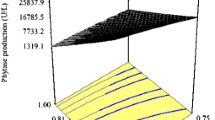
Response surfaces with contour plots (at the base) were generated to represent the interaction of two variables while keeping another variable fixed at ‘0’ level. Figure 2 presents the three dimensional response surface curves established for phytase production in this study. Figure 2a shows increase in phytase production with increase in the wheat bran and ammonium sulfate concentration up to 18.3 and 2.6 g/l, respectively. Any further increase in these two variables repressed the enzyme production. Similarly, increase in peptone concentration up to 15.5 g/l had a favorable impact on phytase production (Fig. 2b), and thus, 15.5 g/l peptone and inoculum size of 4.14 % were optimal for maximum phytase production. Peptone was also shown to be a critical factor for the production of biohydrogen using Enterobacter sp. MTCC 7104 [17].
The experimental model and its predictions were validated for all four variables within the design space by five random sets of experimental combinations as suggested by Design Expert software. The predicted response for phytase production was in good agreement with the actual response, confirming the suitability of the model. Use of statistical models to improve enzyme production is a routine practice and it has been used to enhance the production of phytase from several microorganisms [3, 4, 11].
There was a slight decrease in the batch production levels (79.2 U/ml) in the fermenter as compared to shake flasks (83.2 U/ml) but the fermentation time was reduced by 4 h. Phytase production was growth-dependent and after 24 h phytase as well as biomass production declined during batch cultivation (Fig. 3a). Fed-batch fermentation was carried out to achieve higher biomass for improved and sustained production of phytase. Time course profiles of phytase production, biomass and total residual sugar during fed-batch fermentation are shown in Fig. 3b. The total reducing sugar was utilized fast and 51 % was consumed during the first 12 h (exponential phase). Its concentration at the time of converting batch to fed-batch was 10.3 g/l, and at the end of fed-batch fermentation 5.8 g/l of reducing sugar was left in the medium. Based on optimization of feeding time (data not shown) the culture was fed after every 12 h, which maintained the bacterium in its logarithmic phase until 64 h and the biomass improved by 2.6-fold with a concomitant 1.9-fold improvement in overall phytase production (153.2 U/ml) as compared to submerged production in batch mode. Several studies have confirmed enhancement in enzyme production due to fed-batch cultivations; however, most of the fed-batch studies pertaining to the use of Enterobacter family of bacteria have been focused on exopolysaccharides [18], polyglucosamine [19] and 2, 3-butanediol [20] though Enterobacter sp. are known to be potential producers of several industrial enzymes. Kleist et al. [21] attained high phytase levels (120 U/ml) using rapid glucose controlling system for fed-batch cultivation of E. coli, while Verma and Satyanarayana [22] achieved 47.33 and 36.91 U/ml cell-bound phytase from Pichia anomala using cyclic fed-batch and fixed volume fed-batch strategies. The production of phytase by Aspergillus ficuum was enhanced by 11 and 40 % due to the addition of feed containing glucose and Na-phytate, respectively [23]. To the best of our knowledge, this is the first report on fed-batch production of an industrially significant enzyme by any Enterobacter sp.
Phytase from Enterobacter sp. was purified to homogeneity, with an overall purification of 14.22-fold and a yield of 3.79 % (Table 1). The purified enzyme (Fig. 4) has an apparent molecular mass of 62 kDa on SDS-PAGE gel (Fig. 4b) and appears to migrate as a monomer during Superdex™ gel filtration chromatography (data not shown). The enzyme is active over a broad range of acidic pH and temperature, and exhibited remarkable stability. The enzyme was optimally active at pH 2.5 (Fig. 5a) and fairly stable at pH 2 (Fig. 5b), retaining complete activity for 120 min. At pH 3 and pH 4 it lost only 10 % of activity after 120 min. Half-life of the enzyme at pH 2.0, 3.0, 4.0, 5.0 and 6.0 was 577.5, 433.1, 770.0, 247.5 and 91.2 min, respectively. As the pH approached neutrality, stability decreased at a faster rate. A loss of 60 % of activity was observed at pH 6 and a complete loss of activity was observed after 240 min. Thermo-acido-philic enzymes are of major commercial interest. Although members of the family Enterobacteriaceae prefer to grow in neutral pH, they are known to survive the low-pH stress during passage through the stomach and in the fermented and acidified fecal material [24]. The role of phosphate transport systems in acidic habitats [25] and survival of Escherichia coli in seawater [26] has been already established. Acid-stable phytase (phyC) from Citrobacter freundii showed two distinct pH optima at 2.5 and 4.5 with an optimal temperature at 50 °C [27]. The phytase from Enterobacter sp. ACSS was active over a temperature range of 30–80 °C, with optimal activity at 60 °C (Fig. 5c). The relative activities at 60 and 70 °C were 73 and 39 %, respectively (Fig. 5d). Half-life of the enzyme at 50, 60, 70 and 80 °C was 866.2, 693.0, 37.8 and 11.3 min, respectively. The activity was lost completely at 80 °C after 55 min incubation. E. cloacae produced a thermostable β-d-galactosidase which was optimally active at 50 °C [28], while recombinant sucrose isomerase from Enterobacter sp. FMB1 and lipase from Enterobacter sp. Bn12 showed optimal activity at 50° and 60 °C, respectively [29, 30].
Purification of phytase from Enterobacter sp. ACSS. a Native-PAGE and zymogram analysis of the phytase, M1 native protein molecular weight marker, lane 1 crude extract, lane 2 the sample after HiTrap DEAE anion-exchange column chromatography, lane 3 the sample after Superdex™ gel filtration chromatography, lane 4 zymogram analysis and b SDS-PAGE of the purified phytase, M2 protein molecular weight marker, lane 1 purified phytase
Enterobacter sp. ACSS phytase acted on sodium phytate as the most preferable substrate with high catalytic efficiency as compared to other tested phosphorylated compounds (Table 2). This observation is consistent with reports on several other phytases including K. pneumoniae 9-3B phytase [2] and Burkholderia sp. strain a13 phytase [31]. However, phytases may display narrow [32] to strict [33] substrate specificity. The enzyme in present investigation showed a highest catalytic efficiency (K cat/K m) of 7.81 × 106 M−1 s−1 with sodium phytate with K m and V max values of 0.21 mM and 131.58 nmol mg−1 s−1, respectively. E.coli phytase had a K m of 0.43 mM [34] while the calculated K m and V max values for recombinant Bacillus subtilis (BSPhyARRMK33) phytase were 0.95 mM and 15.3 μmol l−1 [35].
Addition of 1 mM Ca2+ to the reaction mixture resulted in a marked increase (143 %) of enzyme activity. The activity also increased due to the addition of 1 mM Mg2+ (120 %) and Mn2+ (110 %). Ca2+ and several divalent metal-ions form positively charged metal–phytate complexes and reduce the negative charge around the active site cleft, thereby activating the enzyme. However, when present in excess, the metal-ions may act as competitive inhibitors and can also precipitate phytates as insoluble metal salts [36] as was observed in this investigation when relatively lower activity was noted with higher concentration (5 mM) of metal-ions. Most of the transition metal-ions including Co2+, Fe2+, Cu2+ and Zn2+ inhibited the activity of purified phytase (Table 3). Inhibition of phytase due to different metal ions is also dependent on the nature and source of enzyme which demonstrates wide variability among microorganisms [37]. Interestingly, phytase activity from Lactobacillus plantarum was significantly activated by Fe2+ while it was reduced due to addition of Cu2+, Mn2+, Co2+, Zn2+ and Ca2+ [38]. All reducing agents and surfactants tested in this investigation were inhibitory. The anionic surfactant SDS showed a maximum of 82 % inhibition in enzyme activity within 30 min.
While investigating the effect of proteolytic cleavage on enzyme resilience under physiological conditions, it was found to be fairly stable with pepsin and it lost only 10.64 % of the initial activity after 120 min of incubation (Supplementary Table 4). However, lesser stability with trypsin corroborated with the earlier findings of phytase from Aspergillus oryzae [12]. Variation in phytase stability to different proteolytic enzymes may be attributed to the difference in protein–protein interactions under different physiological conditions.
Phytase from Enterobacter sp. ACSS was successfully used for liberating Pi from animal feeds (Fig. S1a) and insoluble metal-phytates (Fig. S1b). The amount of Pi released was higher in chicken feed due to a higher phytate content (8.2 mg/g) in the bran-soya-corn based broiler feed as compared to the soya-corn based fish feed (5.1 mg/g). Highest Pi release at pH 2.5 and its applicability in a wide pH range (1.5–6.5) is in agreement with the optimum activity and promising acid-stable characteristics of this enzyme. The amount of Pi released is sufficient to overcome the dietary phosphorus requirement of broilers that varies from 4.8 to 5.7 mg/g [39] and most aquaculture-relevant fish species that varies from 4.06 to 6.62 mg/g of diet [40]. Phytase action on Ca-phytate complex released a maximum of 219.17 µg/ml of Pi, which corroborates with its Ca+2 mediated activation profile. A plausible reason for the Ca+2 mediated activation of phytases may be the stabilization of the active site cleft by Ca+2, as reported previously during studies on alkaline phytase from Bacillus sp. MD2 [41] and acidic phytase from C. freundii [27]. A marked decline in phytase activity due to Fe+2 and Zn+2 and lower hydrolysis of the respective metal-phytate complexes can be attributed to the versatile redox properties of Fe+2 and to the orientation problems in Zn+2 and phosphate group in the enzyme cleavage site [42].
Phytase supplementation to the dough increased the liberation of Pi, and improved reducing sugar and soluble protein in the bread (Table 4). The bread was softer with higher moisture content and had an improved shelf-life than the bread without phytase. This investigation also indicates a significant reduction in proofing time from 45 to 35 and 30 min using 0.25 and 0.5 U/g of phytase, respectively, which is in accordance with earlier report [41].
Conclusions
A robust phytase producing bacterium was isolated from the rhizosphere of sugarcane plant and identified as Enterobacter sp. ACSS based on Biolog phenotypic characteristics and 16S rDNA sequence comparison. The purified enzyme is active over a wide range of pH and temperatures. In addition, the enzyme is fairly stable in the presence of pepsin at an acidic pH. This is the first report on the use of statistical optimization and fed-batch cultivation for phytase production by an Enterobacter sp., which has an immense potential for application in food and feed industries. Further work is underway on cloning and over expression of the enzyme for cost effective production and understanding its structural characteristics.
References
Koppelaar RH, Weikard HP (2013) Assessing phosphate rock depletion and phosphorus recycling options. Glob Environ Change 23:1454–1466
Escobin-Mopera L, Ohtani M, Sekiguchi S, Sone T, Abe A, Tanaka M et al (2012) Purification and characterization of phytase from Klebsiella pneumoniae 9-3B. J Biosci Bioeng 113:562–567
Joshi S, Satyanarayana T (2014) Optimization of heterologous expression of the phytase (PPHY) of Pichia anomala in P. pastoris and its applicability in fractionating allergenic glycinin from soy protein. J Ind Microbiol Biotechnol 41:977–987
Kumar A, Chanderman A, Singh S (2015) Microbial production of phytases for combating environmental phosphate pollution and other diverse applications. Crit Rev Environ Sci Technol. doi:10.1080/10643389.2015.1131562
Howson SJ, Davis RP (1983) Production of phytate-hydrolysing enzyme by some fungi. Enzyme Microb Technol 5:377–382
Heinonen JK, Lahti RJ (1981) A new and convenient colorimetric determination of inorganic orthophosphate and its application to the assay of inorganic pyrophosphatase. Anal Biochem 113:313–317
Singh B, Satyanarayana T (2006) Phytase production by thermophilic mold Sporotrichum thermophile in solid-state fermentation and its application in dephytinization of sesame oil cake. Appl Biochem Biotechnol 133:239–250
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Bae HD, Yanke LJ, Cheng KJ, Selinger LB (1999) A novel staining method for detecting phytase activity. J Microbiol Methods 39:17–22
Bhavsar K, Ravi Kumar V, Khire JM (2011) High level phytase production by Aspergillus niger NCIM 563 in solid state culture: response surface optimization, up-scaling, and its partial characterization. J Indus Microbiol Biotechnol 38:1407–1417
Sapna, Singh B (2013) Improved production of protease-resistant phytase by Aspergillus oryzae and its applicability in the hydrolysis of insoluble phytates. J Ind Microbiol Biotechnol 40:891–899
Gacula MC, Singh J (1984) Statistical methods in food and consumer research. Academic Press, New York
Greiner R (2007) Phytate-degrading enzymes: regulation of synthesis in microorganisms and plants. Inositol phosphates. Linking agriculture and the environment. CAB International, Wallingford
Williams SG (1970) The role of phytic acid in the wheat grain. Plant Physiol 45:376–381
Guo J, Bian YY, Zhu KX, Guo XN, Peng W, Zhou HM (2015) Activation of endogenous phytase and degradation of phytate in wheat bran. J Agric Food Chem 63:1082–1087
Karthic P, Joseph S, Arun N, Kumaravel S (2013) Optimization of biohydrogen production by Enterobacter species using artificial neural network and response surface methodology. J Renew Sustain Energy 5:033104
Torres CA, Marques R, Ferreira AR, Antunes S, Grandfils C, Freitas F, Reis MA (2014) Impact of glycerol and nitrogen concentration on Enterobacter A47 growth and exopolysaccharide production. Int J Biol Macromol 71:81–86
Son MK, Hong SJ, Lee YH (2007) Acetate-mediated pH-stat fed-batch cultivation of transconjugant Enterobacter sp. BL-2S over-expressing glmS gene for excretive production of microbial polyglucosamine PGB-1. J Ind Microbiol Biotechnol 34:799–805
Li L, Li K, Wang Y, Chen C, Xu Y, Zhang L, Han B, Gao C, Tao F, Ma C (2015) Metabolic engineering of Enterobacter cloacae for high-yield production of enantiopure (2R, 3R)-2, 3-butanediol from lignocellulose-derived sugars. Meta Eng 28:19–27
Kleist S, Miksch G, Hitzmann B, Arndt M, Friehs K, Flaschel E (2003) Optimization of the extracellular production of a bacterial phytase with Escherichia coli by using different fed-batch fermentation strategies. Appl Microbiol Biotechnol 61:456–462
Verma D, Satyanarayana T (2012) Phytase production by the unconventional yeast Pichia anomala in fed batch and cyclic fed batch fermentations. Afr J Biotechnol 11:13705–13709
Coban HB, Demirci A (2014) Enhanced submerged Aspergillus ficuum phytase production by implementation of fed-batch fermentation. Bioproc Biosyst Engin 37:2579–2586
Dancer G, Mah JH, Rhee MS, Hwang IG, Kang DH (2009) Resistance of Enterobacter sakazakii (Cronobacter spp.) to environmental stresses. J Appl Microbiol 107:1606–1614
Rowbury R, Goodson M, Wallace A (1992) The PhoE porin and transmission of the chemical stimulus for induction of acid resistance (acid habituation) in Escherichia coli. J Appl Bacteriol 72:233–243
Gauthier MJ, Flatau GN, Clément RL, Munro PM (1993) The loss of culturability by Escherichia coli cells in seawater depends on availability of phosphate ions and phosphate transport systems. Microb Ecol 26:29–35
Zhao W, Xiong A, Fu X, Gao F, Tian Y, Peng R (2010) High level expression of an acid-stable phytase from Citrobacter freundii in Pichia pastoris. Appl Biochem Biotechnol 162:2157–2165
Ghatak A, Guha AK, Ray L (2010) β-d-Galactosidase from Enterobacter cloacae: production and some physicochemical properties. Appl Biochem Biotechnol 162:1678–1688
Cha J, Jung JH, Park SE, Cho MH, Seo DH, Ha SJ et al (2009) Molecular cloning and functional characterization of a sucrose isomerase (isomaltulose synthase) gene from Enterobacter sp. FMB-1. J Appl Microbiol 107:1119–1130
Farrokh P, Yakhchali B, Asghar Karkhane A (2014) Cloning and characterization of newly isolated lipase from Enterobacter sp. Bn12. Braz J Microbiol 45:677–687
Graminho ER, Takaya N, Nakamura A, Hoshino T (2015) Purification, biochemical characterization, and genetic cloning of the phytase produced by Burkholderia sp. strain a13. J Gen Appl Microbiol 61:15–23
Yu P, Chen Y (2013) Purification and characterization of a novel neutral and heat-tolerant phytase from a newly isolated strain Bacillus nealsonii ZJ0702. BMC Biotechnol 13:78
Park I, Lee J, Cho J (2012) Degradation of phytate pentamagnesium salt by Bacillus sp. T4 phytase as a potential eco-friendly feed additive. Asian Australas J Anim Sci 10:1466–1472
Yao MZ, Wang X, Wang W, Fu YJ, Liang AH (2013) Improving the thermostability of Escherichia coli phytase, appA, by enhancement of glycosylation. Biotechnol Lett 10:1669–1676
Reddy CS, Achary VM, Manna M, Singh J, Kaul T, Reddy MK (2015) Isolation and molecular characterization of thermostable phytase from Bacillus subtilis (BSPhyARRMK33). Appl Biochem Biotechnol 175:3058–3067
Tang J, Leung A, Leung C, Lim BL (2006) Hydrolysis of precipitated phytate by three distinct families of phytases. Soil Biol Biochem 38:1316–1324
Santos T, Connolly C, Murphy R (2015) Trace element inhibition of phytase activity. Biol Trace Elem Res 163:255–265
Sarıbuga E, Nadaroglu H, Dikbas N, Senol M, Cetin B (2015) Purification, characterization of phytase enzyme from Lactobacillus plantarum bacteria and determination of its kinetic properties. Afr J Biotechnol 13:2373–2378
Bar A, Shinder D, Yosefi S, Vax E, Plavnik I (2003) Metabolism and requirements for calcium and phosphorus in the fast-growing chicken as affected by age. Braz J Nutr 89:51–61
Sugiura SH, Dong FM, Hardy RW (2000) A new approach to estimating the minimum dietary requirement of phosphorus for large rainbow trout based on nonfecal excretions of phosphorus and nitrogen. J Nutr 130:865–872
Tran TT, Hashim SO, Gaber Y, Mamo G, Mattiasson B, Hatti-Kaul R (2011) Thermostable alkaline phytase from Bacillus sp. MD2: effect of divalent metals on activity and stability. J Inorg Biochem 105:1000–1007
Haros M, Rosell CM, Benedito C (2001) Fungal phytase as a potential bread making additive. Eur Food Res Technol 213:317–322
Acknowledgments
The financial support from the National Research Foundation, Republic of South Africa and Durban University of Technology is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chanderman, A., Puri, A.K., Permaul, K. et al. Production, characteristics and applications of phytase from a rhizosphere isolated Enterobacter sp. ACSS. Bioprocess Biosyst Eng 39, 1577–1587 (2016). https://doi.org/10.1007/s00449-016-1632-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-016-1632-7