Abstract
The extracellular production of Escherichia coli phytase was studied in fed-batch fermentations. Two different feeding strategies were compared: control by keeping the glucose concentration constant, and control by keeping a low constant oxygen level in the medium. For the feeding control based on glucose concentration, a recently developed rapid glucose controlling system was tested for the first time in bacterial cultivations and used to establish the fermentative production of extracellular phytase with E. coli. High activity levels (120 U ml−1) at short cultivation times (14 h) were obtained. Even higher activity levels—albeit at longer cultivation times—were reached by applying a feeding control, the main characteristic of which was a constant low oxygen concentration. The optimum oxygen level for the production of phytase was in the range of 5–10% saturation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phytase (myo-inositol hexakisphosphate phosphohydrolase, EC 3.1.3.8 for 3-phytase and 3.1.8.26 for 6-phytase) has become an important feed additive in the nutrition of monogastric animals since it is able to enhance plant phosphorus utilization and to eliminate the negative effects of phytic acid (Mroz et al. 1994). For several years, pig and poultry feed has already been supplemented with phytases from Aspergillus sp. In order to obtain better phytases, several bacterial species (Greaves et al. 1967; Irving and Cosgrove 1971; Shimizu 1992; Greiner et al. 1993; Kim et al. 1998; Yanke et al. 1998), fungi (Mitchell et al. 1997; Pasamontes et al. 1997; Ullah and Sethumadhavan 1998) as well as plants (Hayakawa et al. 1989; Houde et al. 1990; Nagai and Funahashi 1998) have been screened for the enzyme. From all organisms tested so far the phytase from Escherichia coli had the highest specific activity—eight times that of the commercially used Aspergillus niger phytase (Wyss et al. 1999; Miksch et al. 2002). Furthermore, in contrast to the fungal enzyme, E. coli phytase was found to be both highly resistant to proteolytic degradation in the stomach of monogastric animals, and more resistant to high temperatures during the pelleting process. Therefore, it would be highly desirable to produce the phytase of E. coli for practical applications. Unfortunately, only very low phytase activity was detectable in normally growing cells of E. coli (Greiner et al. 1993). Although the activity increased after a shift from aerobic to anaerobic conditions, the yield was too low for any practical application.
Recently, some of the present authors presented a system for the overexpression and extracellular production of phytase in E. coli and demonstrated that, in principle, it was feasible to develop a fermentative production system (Miksch et al. 2002).
The main purpose of the investigations reported here was to compare different fermentation strategies for the optimization of the extracellular production of phytase, particularly by applying a newly developed rapid glucose controlling (RGC) system for glucose analysis during fermentation.
Materials and methods
Bacterial strain
E. coli strain BL21(DE3)pPhyt109 (Miksch et al. 2002) was used for all fermentation experiments. Plasmid pPhyt109, an expression vector derived from the multi copy plasmid pUC19, contains the gene for E. coli phytase under the constitutive promoter of the bglA gene of Bacillus amyloliquefaciens. In addition, the expression vector contained a secretion cassette of 2.5 kb providing the competence for the secretion of phytase into the culture medium based on the action of the Kil protein expressed under the control of the stationary-phase promoter of the fic gene (Miksch et al. 1997a, 1997b, 2002).
Analytical methods
Phytase activity was determined as previously described (Miksch et al. 2002). Optical density was measured at 600 nm with a Biochrom 4060 photometer (Pharmacia, Uppsala, Sweden). To determine the bacterial dry mass, 1 ml bacterial culture was centrifuged in Eppendorf vials at 14,000 rpm for 10 min. The pellet was washed in an aqueous NaCl solution, pelleted again, and dried overnight at 60°C under vacuum.
Acetate concentrations were analyzed using a commercial kit (No. 0148261, R-Biopharma, Darmstadt, Germany) according to the supplier's instructions. For off-line determinations of glucose concentrations, a test kit from Sigma (Infinity Glucose Reagent, No. 18-20; Sigma Diagnostics, Deisenhofen, Germany) was used.
Culture media and cultivation of bacteria
Fermentation experiments were carried out in a bioreactor with a total volume of 7 l and a working volume of 5 l equipped with direct digital control (DDC) from MBR (Multiple Bioreactors and Sterile Plants, Zurich, Switzerland). PCS software (MBR), executed by a VME (Versa Module Europe)-bus computer operating under OS/9, was used to implement all supervisory control tasks. Bacterial cultures were prepared by inoculating 200 ml TB medium in shake flasks (1 l) with bacteria from glycerol stock (−80°C) and shaking the flasks on a rotary shaker overnight at 150 rpm at 37°C. These cultures were used to inoculate 5 l medium for batch and 3 l for fed-batch cultivations.
A glucose mineral salt medium (Horn et al. 1996) was used as growth medium with the exception that the culture medium for the initial batch operating phase contained only 6 g l−1 glucose instead of 25 g l−1. Bacteria were grown overnight to an OD600 of 25 and the initial culture medium was inoculated 1:10 (v/v). The pH was maintained at 6.9 by controlled addition of 4 N NaOH. Antifoam (PE8100, BASF, Germany) was added automatically when required. Air flow was kept constant at 10 l min−1. During the batch phase of fermentation, the concentration of dissolved oxygen (pO2) was kept constant at 30% saturation by automatically adjusting the stirrer speed. The feeding of feed solutions FS1 and FS2 (Horn et al. 1996) was performed in different modes: either keeping the glucose concentration constant by feeding according to the analysis of the glucose concentration by means of the RGC system and flow injection analysis (FIA), or keeping pO2 constant by feeding. The equipment necessary for controlling the glucose concentration is described separately below. With respect to the feeding strategy based on constant oxygen concentration, the feeding pump was operated under a controller with proportional integral (PI) behavior. The stirrer speed was kept constant at 500 rpm.
For off-line sample collection during the fermentation process, an automatic sampler (FC205; Gilson, Middleton, Wis.) was used. The collected samples (20–24 ml each) remained in test tubes at 4°C until they were analyzed.
RGC system
In contrast to commonly used FIA systems, the RGC system did not use a special sample module, i.e., the sample stream contained bacterial cells (Hitzmann and Arndt 2001). The frequency of analysis could thus be increased significantly. The samples (24 µl) were injected into the carrier stream and mixed with glucose oxidase (36 µl, 400 U ml−1) by a second injector located in front of the sample injector (Fig. 1). The amount of metabolized oxygen was determined by an oxygen electrode (Anasyscon, Hannover, Germany) and was a measure for the glucose concentration (see "glucose" in Fig. 1). The oxygen assimilated by cells had to be determined in a separate measurement and this value had to be subtracted from the total oxygen consumption (see "cells" in Fig. 1). This variable was measured only every 4 min since it changed very slowly. Both the calculation of glucose concentrations and the control of the RGC system were accomplished by means of the program CAFCA (Computer Assisted Flow Control and Analysis, Hitzmann et al. 1993).
To minimize data noise Kalman filter was applied (Hitzmann et al. 2000). The balance equation of a batch cultivation in a well-mixed stirred tank and growth on the limiting substrate glucose with Monod kinetics was applied as model for the bioprocess. The parameters of the Kalman filter were optimized by simulations. The Kalman filter not only provided estimated data of glucose concentrations but also estimated data for bacterial biomass and the maximal specific growth rate. The flow rate of the feeding pump was controlled based on these parameters and a PI controller.
Results
Feeding control based on glucose concentration
Fermentation experiments were performed with three different constant low glucose concentrations (0.3, 0.2 and 0.1 g l−1) according to the RGC system. Since the results with a glucose concentration of 0.3 g l−1 were much less impressive compared with those at 0.2 and 0.1 g l−1, only the latter two strategies are described here. Feeding was started after a cultivation time of 4.5 h, when the glucose concentration decreased to 0.2 g l−1 and 0.1 g l−1, respectively (Figs. 2A, 3A). The glucose concentration shows that it was possible to maintain a rather constant glucose level during the whole feeding period. Data from off-line measurements of the glucose concentration (Fig. 2B) were lower than data from on-line measurements during the exponential growth phase (until 10 h) of the bacteria. The difference was due to the very high metabolic activity of the bacteria during that period. Although the samples were kept on ice and measured immediately, glucose was metabolized rapidly. In contrast, after entry into stationary phase, the off-line data corresponded very well to the on-line data obtained by the RGC system. Figures 2B and 3B demonstrate that bacterial dry matter concentrations estimated by the RGC system corresponded exactly to that determined manually during the exponential growth phase. After some time of fed-batch operation, acetate ions began to accumulate, which obviously affected the growth rate. During the first phase of cultivation, pO2 was maintained constant by increasing the stirrer speed (Figs. 2C, 3C). During the later phase of cultivation, pO2 varied over a broader range. At both glucose concentrations (0.2 and 0.1 g l−1) similar bacterial growth characteristics were observed. An OD600 of approximately 60 and a bacterial dry matter concentration of 35 g l−1 were reached in both cases (Figs. 2A, B, 3A, B). The bacterial growth rate was relatively high (µ=0.5 h−1) during the first phases of cultivation; however, the growth rate decreased rapidly in the later phase of cultivation, as mentioned above, presumably due to the accumulation of acetic acid (Figs. 2C, 3C).
Fed batch fermentation of Escherichia coli strain BL21(DE3)pPhyt109 at a glucose concentration of 0.2 g l−1 showing different variables of the cultivation. A Kinetics of glucose concentration, optical density (OD600) and phytase activity (total and extracellular). B Kinetics of glucose concentration, acetate concentration and dry matter. C Stirrer speed and concentrations of dissolved oxygen (pO2) and CO2 in the off-gas. D Feeding flow rate and specific growth rate
Fed batch fermentation of E. coli strain BL21(DE3)pPhyt109 at a glucose concentration of 0.1 g l–1. A, B, C and D as in Fig. 2
With respect to phytase production, the experiments at these two glucose concentrations (0.2 and 0.1 g l−1) differed significantly. During the cultivation with a glucose concentration of 0.2 g l−1, the phytase concentration increased rapidly over the entire feeding period (Fig. 2A). Approximately 50% of the total phytase activity was produced during the second phase of cultivation, which resembled a stationary phase with respect to biomass concentration. The phytase was almost completely secreted into the culture medium. Using this feeding strategy approximately 120 U ml−1 extracellular phytase was produced in a relatively short cultivation time of 14 h. The phytase concentrations found in the periplasm or in the cytoplasm (data not shown) were negligible compared with those found in the culture medium. Continuation of the fermentation did not lead to higher phytase concentrations (data not shown). In contrast, at a glucose concentration of 0.1 g l−1 the phytase concentration increased very fast already during the first growth phase. After entry into some kind of stationary phase no further increase of phytase activity occurred. The maximal concentrations of total and extracellular phytase were only 60% of those obtained for the cultivation at 0.2 g l−1 glucose. The lower phytase production correlated with a much lower CO2 output (Figs. 2C, 3C) indicating a lower metabolic activity at 0.1 g l−1 glucose than at 0.2 g l−1. Lower phytase production also correlated with an earlier accumulation of acetate ions. Acetate appeared during the second growth phase. At the end of the apparent stationary phases, acetate concentrations of about 1.5 g l−1 were attained after an operating time of 12 h (Figs. 2B, 3B). Finally, the specific growth rates calculated by the RGC system and the corresponding feeding profiles, in terms of flow rate of the feed solution, are shown in Figs. 2D and 3D, respectively.
Feeding control at low oxygen levels
During these fed batch cultivation experiments, the addition of feeding solution was controlled in order to keep the oxygen saturation in the medium at a constant level. The set points chosen were 2, 5, 10, and 20% oxygen saturation at an operating temperature of 37°C. The evolution of the main operating variables is shown in Fig. 4. For all fermentations with constant pO2 the bacterial growth rates were low, mainly due to limiting oxygen transfer rates, because of a low constant stirrer speed of 500 min−1. The bacterial dry matter increased continuously during the entire fermentation time of 60 h. The feeding profiles are given in Fig. 4. During all experiments the glucose concentration was very low during the entire cultivation process and attained approximately 0.1 g l−1. Acetate accumulation was not observed during these experiments (data not shown). Phytase production obviously depended on the oxygen level. In general, as with the RGC system, the phytase was almost completely released into the medium.
In general, most of the phytase was produced during the first 15 h of cultivation. Highest phytase concentrations were observed for experiments with oxygen saturation set points of 5% and 10%. Extracellular phytase concentrations of 140–180 U ml−1 were reached.
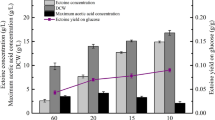
Figure 5 shows the performance of the fermentation processes for different oxygen levels in terms of the yield coefficient with respect to the product phytase relative to the limiting substrate glucose (Y P/S). As expected, the yield coefficients were much better at oxygen saturations of 5 and 10% compared to the other two experiments.
Yield coefficient of product (relative to glucose consumption) during fed batch fermentations of E. coli strain BL21(DE3)pPhyt109. Feeding was controlled at different constant low pO2 as indicated in Fig. 4
The effectivity of fermentation can also be characterized by calculation of the selectivity, i.e., the ratio of phytase activity produced to the amount of biomass produced. As already expected from the yield coefficients, the cultivations at 5 and 10% oxygen saturation level yielded higher selectivities than those at 2 and 10% (data not shown). Figure 6 presents a comparison of the selectivities of the two best fed batch processes with different feeding strategies, i.e., control by a glucose concentration of 0.2 g l−1 and control by an oxygen saturation level of 5%. It shows that the strategy of oxygen-dependent control favored the formation of the target product phytase over formation of biomass.
Discussion
Comparing different feeding strategies—such as feeding control by glucose concentrations or by constant levels of dissolved oxygen—for fed-batch cultivation processes, it has been demonstrated that the effectivity of fermentation and the yield of extracellular phytase can be improved significantly by maintaining the glucose concentration at a low level. Although there are several reports on controlling the substrate concentration by means of the pO2 (Cutayar and Poillon 1989; Rani and Rao 1999) the direct determination of substrate concentrations has been applied much less due to the complex requirements with respect to the analytical methods. The glucose concentration can in principle be measured by well known FIA systems. However, the time constant for sampling is rather high for conventional FIA systems (Dochain and Perrier 1997). For controlling cultivations of fast-growing organisms such as E. coli a faster method of on-line glucose analysis would therefore be most welcome. Consequently, a combination of FIA combined with the RGC system was developed and tested for the cultivation of yeast (Hitzmann et al. 2000). Since this system does not need a sampling device, the time interval from sampling to the presentation of the result could be reduced from 5–10 min down to 1 min. Here, we report for the first time the application of this system for the cultivation of bacteria, specifically E. coli.
Using the RGC system it was shown that a high level of extracellular phytase (120 U ml−1) was reached in the relatively short cultivation time of 14 h. Almost all of the phytase produced was secreted into the culture medium. This meant that the RGC system was much more effective than the commonly used means of controlling the substrate concentration using pO2 (Rani and Rao 1999).
A major problem in fermentations with E. coli is the formation of acetate. Acetate is produced when E. coli is grown under anaerobic or oxygen-limited conditions. However, E. coli cultures growing in the presence of excess glucose can also produce acetate even under aerobic conditions (El-Mansi and Holms 1989; Luli and Strohl 1990; Kleman and Strohl 1994). A high concentration of acetate (i.e., above 5 g l−1 at pH 7) reduces the growth rate and biomass yield (Pan et al. 1987; Jensen and Carlsen 1990; Han et al. 1992). Our data show clearly that acetate did not appear during fed-batch cultivation controlled by means of low oxygen levels. This effect may be due to low growth rates, because the cultivations were limited by the oxygen transfer capacity. However, acetate clearly arose during processes controlled by direct measurement of glucose. In the latter case, the initial growth rate was high and the presence of acetate, although at low concentrations, seemed to affect the growth of the E. coli culture. Thus, limiting the growth rate in addition may lead to an improved strategy.
Experiments following the strategy of feeding controlled by constant oxygen concentrations has shown that the level of oxygen saturation has a major impact on the results. Highest extracellular phytase concentrations were obtained with oxygen saturation levels in the range of 5–10%. The reason for this cannot be explained at the present status of knowledge since little is yet known about the regulatory network under the growth conditions used.
In addition, much less glucose was consumed at 5% oxygen. In all cases the phytase was almost completely released into the medium. The operating times shown in Fig. 4 would not be applied in practice. The fermentation could be finished after a cultivation time of 30 h, since the production of phytase did not increase further. It would seem reasonable to shorten the operating time to 15–20 h by increasing the oxygen supply in order to raise the growth rate.
References
Cutayar JM, Poillon D (1989) High cell density culture of Escherichia coli in a fed-batch system with dissolved oxygen as substrate feed inductor. Biotechnol Lett 11:155–160
Dochain D, Perrier M (1997) Dynamical modelling, analysis, monitoring and control design for nonlinear bioprocesses. In: Scheper T (ed) Advances in biochemical engineering biotechnology, vol 56. Springer, Berlin Heidelberg New York, pp 147–197
El-Mansi EMT, Holms WH (1989) Control of carbon flux to acetate excretion during growth of Escherichia coli in batch and continuous cultures. J Gen Microbiol 135:2875–2883
Greaves MP, Anderson G, Klebley DM (1967) The hydrolysis of inositol phosphatates by Aerobacter aerogenes. Biochim Biophys Acta 132:412–418
Greiner R, Konietzny U, Jany Kl-D (1993) Purification and characterization of two phytases from Escherichia coli. Arch Biochem Biophys 303:107–113
Han K, Lim HC, Hong J (1992) Acetic acid formation in Escherichia coli fermentation. Biotechnol Bioeng 39:663–671
Hayakawa T, Toma Y, Igaue I (1989) Purification and characterization of acid phosphatases with and without phytase activity from rice bran. Agric Biol Chem 53:1475–1483
Hitzmann B, Arndt M (2001) Eine schnelle Glucoseanalytik für die Regelung biotechnischer Prozesse. Bioforum 10:658–661
Hitzmann B, Lammers F, Weigel B, van Putten A (1993) Die Automatisierung von Fließinjektionsanalyse-Systemen. Bioforum 12:450–454
Hitzmann B, Broxtermann O, Cha Y-L, Sobieh O, Stärk E, Scheper T (2000) The control of glucose concentration during yeast fed-batch cultivation using a fast measurement complemented by an extended Kalman filter. Bioprocess Eng 23:337–341
Horn U, Strittmatter W, Krebber A, Knüpfer U, Kujau M, Wenderoth R, Müller K, Matzku S, Plückthun A, Riesenberg D (1996) High volumetric yields of functional dimeric miniantibodies in Escherichia coli, using an optimized expression vector and high-cell-density fermentation under non-limited growth conditions. Appl Microbiol Biotechnol 46:524–532
Houde RL, Alli I, Kermasha S (1990) Purification and characterization of Canola seed (Brassica sp.) phytase. J Food Biochem 14:331–351
Irving GCJ, Cosgrove DJ (1971) Inositol phosphate phosphatases of microbiological origin. Some properties of a partially purified bacterial (Pseudomonas sp.) phytase. Aust J Biol Sci 24:547–557
Jensen EB, Carlsen S (1990) Production of recombinant human growth hormone in Escherichia coli. Expression of different precursors and physiological effects of glucose. Biotechnol Bioeng. 36:1–11
Kim YO, Lee JK, Kim HK, Yu JH, Ho TK (1998) Cloning of the thermostable phytase gene (phy) from Bacillus sp. DS11 and its overexpression in Escherichia coli. FEMS Microbiol Lett 162:185–191
Kleman GL, Strohl WR (1994) Acetate metabolism by Escherichia coli in high-cell-density fermentation. Appl Environ Microbiol 60:3952–3958
Luli GW, Strohl WR (1990) Comparison of growth, acetate production, and acetate inhibition of Escherichia coli strains in batch- and fed-batch fermentations. Appl Environ Microbiol 56:1004–1011
Miksch G, Fiedler E, Dobrowolski P, Friehs K (1997a) The kil gene of the ColE1 plasmid of Escherichia coli controlled by a growth-phase-dependent promoter mediates the secretion of a heterologous periplasmic protein during the stationary phase. Arch Microbiol 167:143–150
Miksch G, Neitzel R, Fiedler E, Flaschel E (1997b) Extracellular production of a hybrid β-glucanase from Bacillus by Escherichia coli under different cultivation conditions in shaking cultures and bioreactors. Appl Microbiol Biotechnol 47:120–126
Miksch G, Kleist S, Friehs K, Flaschel E (2002) Overexpression of the phytase from Escherichia coli and its extracellular production in bioreactors. Appl Microbiol Biotechnol 59:685–694
Mitchell DB, Vogel K, Weimann BJ, Pasamontes L, Van Loon APGM (1997) The phytase subfamily of histidine acid phosphatases: isolation of genes for two novel phytases from the fungi Aspergillus terreus and Myceliophthora thermophila. Microbiology 143:245–252
Mroz Z, Jongbloed AW, Kemme PA (1994) Apparent digestibility and retention of nutrients bound to phytate complexes as influenced by microbial phytase and feeding regimen in pigs. J Anim Sci 72:126–132
Nagai Y, Funahashi S (1998) Phytase from wheat bran. Part II. Successive dephosphorylation of myo-inositol hexaphosphate by wheat bran phytase. Agric Biol Chem 27:619–624
Pan JG, Rhee JS, Lebeault JM (1987) Physiological constraints in increasing biomass concentration of Escherichia coli B in fed-batch culture. Biotechnol Lett 9:89–94
Pasamontes L, Haiker M, Wyss M, Tessier M, Van Loon APGM (1997) Gene cloning, purification, and characterization of a heat-stable phytase from the fungus Aspergillus fumigatus. Appl Environ Microbiol 63:1696–1700
Rani KY, Rao VSR (1999) Control of fermenters—a review. Bioprocess Eng 21:77–88
Shimizu M (1992) Purification and characterization of phytase from Bacillus subtilis (Natto) N-77. Biosci Biotechnol Biochem 56:1266–1269
Ullah AHJ, Sethumadhavan K (1998) Differences in the active site environment of Aspergillus ficuum phytases. Biochem Biophys Res Commun 243:458–462
Wyss M, Brugger R, Kronenberger A, Remy R, Fimbel R, Oesterhelt, G. Lehmann M, Van Loon APGM (1999) Biochemical characterization of fungal phytases (myo-inositol hexakisphosphate phosphohydrolases): catalytic properties. Appl Environ Microbiol 65:367–373
Yanke LJ, Bae HD, Selinger LB, Cheng K-J (1998) Phytase activity of anaerobic ruminal bacteria. Microbiology 144:1565–1573
Acknowledgements
The financial support of the Deutsche Bundesstiftung Umwelt (DBU) is gratefully acknowledged. S.K. is grateful to the Max-Buchner-Stiftung for receipt of a scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kleist, S., Miksch, G., Hitzmann, B. et al. Optimization of the extracellular production of a bacterial phytase with Escherichia coli by using different fed-batch fermentation strategies. Appl Microbiol Biotechnol 61, 456–462 (2003). https://doi.org/10.1007/s00253-003-1229-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-003-1229-3