Abstract
Kaolin showed as a very perspective carrier for the enzyme immobilization and it was used for the adsorption of horseradish peroxidase (HRP). The effects of the enzyme concentration and pH on the immobilization efficiency were studied in the reaction with pyrogallol and anthraquinone dye C.I. Acid Violet 109 (AV 109). In addition, Fourier transform infrared spectroscopy, scanning electron microscopy and analysis by Brunauer–Emmett–Teller were performed for kaolin, thermally activated kaolin and the immobilized enzyme. It has been shown that 0.1 IU of HRP-kaolin decolorized 87 % of dye solution, under the optimal conditions (pH 5.0, temperature 24 °C, dye concentration 40 mg/L and 0.2 mM of H2O2) within 40 min. The immobilized HRP decolorization follows the Ping Pong Bi–Bi mechanism with dead-end inhibition by the dye. The biocatalyst retained 35 ± 0.9 % of the initial activity after seven cycles of reuse in the decolorization reaction of AV 109 under optimal conditions in a batch reactor. The obtained kinetic parameters and reusability study confirmed improvement in performances of k-HRP compared to free, indicating that k-HRP has a great potential for environmental purposes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Synthetic dyes are extensively used in a wide range of industries such as paper, food, cosmetic, pharmaceutical and particularly in the textile and dyeing industries. The results of the market research show that the annual production of dyes accounts more than 7 × 105 tones, and the growth of 2 % per annum is expected in the next decade [1, 2]. Textile processing technology uses large amount of water and chemicals and since 15–20 % of the dyes do not bind to the fibers, the process wastewater is highly polluted and has to be properly purified before discharging into the natural recipient [3]. Textile dyes are highly visible in quantities of 1 ppm (several of them even of 0.005 ppm) and cause serious environmental problem because of their resistance to thermal influence, light, biodegradation remaining in the environment for an extended period of time. It is estimated that in the developing countries, 90 % of all wastewater is discharged untreated directly into rivers, lakes or oceans.
The removal of the dyes from wastewater is often very costly. However, the water quality crisis, that the world is facing, and stringent environmental legislations stimulate researchers and the industry sector to develop an effective treatment [4]. Several traditional, biological and enzymatic approaches have been used to remove organic compounds from textile wastewater and reduce the cost of the overall process [4–6]. However, there are still several shortcomings that cannot be overcome using the traditional methods of wastewater treatment such as high cost, generation of activated sludge and more toxic by-products, and the occurrence of secondary pollution problems. On the other hand, enzymatic process is eco-friendly and clean alternative for detoxification of hazardous aromatic pollutants and enzymes such as peroxidase, polyphenol oxidase, laccase, and tyrosinase have already been used [5].
The feasibility of HRP application in free form, in dyes decolorization, primarily azo dyes decolorization, has been well documented in the literature because of its ability to oxidize a large number of dyes even in the presence of contaminants commonly found in the wastewaters [7–12]. Furthermore, HRP is a natural catalyst isolated from the roots of horseradishes with high activity and broad substrate specificity finding widespread application in environmental, chemical, pharmaceutical, biotechnological industries and also has been used for detection of hydrogen peroxide in medicine, and diagnostics, since it requires the peroxide substrate as an electron acceptor.
For the industrial application of HRP in the problem of wastewater pollution resolving, an effective immobilization method is required to ensure continuous processing, improved stability, and the reuse of the biocatalyst. Although there are many immobilization techniques, physical adsorption is the most commonly used because it is a low-cost method, easy to perform and the catalytic activity of the enzyme is usually retained. The interactions that occur during the immobilization process might obviously affect performances of the immobilized enzyme, so the choice of the support is crucial for preserving adequate biocompatibility. Immobilization supports for application in wastewater treatment must meet the criteria such as biodegradability, insolubility, non-toxicity, high diffusivity and so on. Thus, the use of cheap, nontoxic, available materials, such as clay, for immobilization of enzymes by physical adsorption for a large-scale wastewater treatment is of great interest.
Natural biopolymers have a number of drawbacks such as diffusion limitations, high cost, mechanical and thermal instability, and tendency to swell. On the other hand, natural clays seem to negligible swell in water, and have physical strength and chemical resistance toward acid and alkali treatment making them an excellent support for the design of immobilized biocatalyst for wastewater treatment [13]. The most studied clay mineral is kaolin and due to eco-friendly demands in many industrial applications, kaolin has been shown to be very prospective support for the immobilization of enzymes [14]. Despite all these advantages, to the best of our knowledge, studies have not been performed to evaluate adsorption of HRP on kaolin and feasibility of the obtained biocatalyst in anthraquinone dyes decolorization.
The main topic of this paper was the development of an inexpensive, efficient, eco-friendly immobilized biocatalyst for anthraquinone dyes removal from the textile wastewater. Enzyme loading and activity of the immobilized HRP were determined as a function of pH and initial enzyme concentration. Immobilized preparation with the highest activity was used in the decolorization reaction of anthraquinone dye C.I. Acid Violet 109, often present in contaminated industrial wastewater. Decolorization parameters such as pH, contact time, dye concentration and hydrogen peroxide concentration were optimized. Optimized parameters for immobilized enzyme were compared to parameters obtained for free HRP.
Materials and methods
Materials
Horseradish peroxidase (EC 1.11.1.7; donor: hydrogen peroxide oxidoreductase), with a specific activity 200–250 U/g solid, and pyrogallol were obtained commercially from Sigma-Aldrich (St. Louis, MO, USA). Kaolin was obtained from Carlo Erba. Hydrogen peroxide was purchased from E. Merck (Darmstadt), and its concentration was determined several times using its molar absorption coefficient (ε = 43.6 M−1 cm−1) at λ = 240 nm by dilution of the supplied H2O2 (30 % v/v) solution. Anthraquinone dye used in this paper, C. I. Acid Violet 109 (AV 109), was obtained from Lanaset (Lanaset Violet B). All other chemicals used in this paper were of analytical grade.
Support preparation
Due to low adsorption capacity, prior to use as the immobilization support, kaolin was activated by heating. Namely, dry powdered kaolin was mixed with distilled water (50 % of moisture) until paste was obtained. The obtained paste then was heated 2 h at 550 °C in the oven. After heating, the support was turned to powder and used for the immobilization of HRP.
HRP immobilization onto kaolin
100 mg of the activated support was immersed with 500 μL of enzyme solution with different concentrations (0.5–3 mg/mL) prepared in appropriate 0.1 M buffer (pH 2, 5, 7 and 9). The immobilization via adsorption was allowed to continue for 2 h at room temperature as previously described [15]. After the immobilization was finished, the immobilized preparation (k-HRP) was separated from the supernatant using centrifuge (MiniSpin plus, Eppendorf, Germany) at 13,000 rpm for 2 min. To remove the unbound proteins, the immobilized biocatalyst was rinsed three times with immobilization buffer (0.5 mL), until no activity was detected in the filtrate. Immobilized preparations were suspended in the immobilization buffer and stored at 4 °C until use.
Characterization of kaolin, metakaolin and k-HRP
BET and BJH
The specific surface area and pore parameters (volume and size) analysis was performed by Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) methods, respectively, using an accelerated surface area and porosimetry system—ASAP 2020 (Micromeritics, USA). The samples were previously degassed under vacuum at 150 °C for 6 h.
SEM
Scanning electron microscopy (FEG–SEM) was performed with field emission gun TESCAN MIRA3 electron microscope at an accelerating voltage of 20 kV. Prior to the observation the samples were degassed and sputter coated with gold using a Polaron SC502 Sputter Coater (Fison Instruments, United Kingdom). The average diameter and the standard deviation of the particle size for all the samples were determined using of MIRA TESCAN in situ measurement software. In each case, the sizes of 250 particles were measured.
FTIR analysis
Fourier transform infrared (FTIR) spectra were recorded using a BOMEM (Hartmann and Braun) spectrophotometer. 1 mass% of sample was grounded thoroughly with potassium bromide and the resulting powder was pressed into a transparent pellet by a hydraulic press. FTIR spectra were collected in transmission mode between 400 and 4000 cm−1 at a resolution of 4 cm−1.
Activity assay of free and k-HRP
The activity of k-HRP and its free counterpart was determined using pyrogallol as a substrate as previously described in the literature [16]. 1 mL of pyrogallol (0.013 M) in potassium-phosphate buffer (pH 7.0, 0.1 M), and 10 μL of enzyme solution were placed in the cuvette. By adding 10 μL of hydrogen peroxide (3 % v/v) reaction was started. The change of color from yellow (pyrogallol) to dark brown (purpurogallin) was monitored spectrophotometrically at 420 nm using UV–Vis spectrophotometer (Ultrospec 3300 pro, Amerischam Bioscience). The change in absorbance in the sample, against the reagent blank, was recorded each 20 s for 3 min.
The activity of the immobilized HRP was determined using 3 mL of pyrogallol (0.013 M) in the potassium-phosphate buffer (pH 7.0; 0.1 M), 5 mg of immobilized biocatalyst and 30 μL of hydrogen peroxide (3 % v/v). After suspension preparing, magnetic stirrer was set to the maximum, and samples were withdrawn every 1 min next 3 min, centrifuged at 13,000 rpm, 30 s and analytically controlled. The activity of free and immobilized peroxidase was calculated using a molar extinction coefficient of purpurogallin (12 L mmol−1 cm−1) [17]. One unit of activity was defined as the amount of peroxidase that will form 1.0 mg of purpurogallin from pyrogallol in 20 s at pH 7.0 and 20 °C.
Protein determination
Protein content in the initial solutions, supernatants and filtrates was determined according to the modified method of Lowry [18]. The amount of immobilized HRP onto kaolin was expressed as the difference in the mass of protein in the initial solution and the mass of protein in the supernatant and the filtrates.
Adsorption isotherm
The maximum amount of the enzyme that can be adsorbed on the kaolin surface was monitored using the adsorption isotherm. Adsorption isotherm was determined by varying the HRP concentration from 0.5 to 3.0 mg/mL, while temperature, contact time and pH of incubation medium were constant.
The Langmuir isotherm model can be represented using the following equation:
where Q e is the amount of the adsorbed protein on the kaolin at equilibrium (mg/g), C e is the protein amount in the solution at equilibrium (mg/L), Q max is the maximum adsorbent capacity (mg/g), K L is the Langmuir isotherm model constant (L m/g). Using the dimensionless parameter R L, it can be evaluated the feasibility of adsorption on adsorbent; if the adsorption is unfavorable R L > 1, R L = 1 linear, 0 < R L < 1 favorable, R L = 0 irreversible. Experimental results were analyzed using Origin Pro software version 7.0 (Origin Lab Corporation, Northampton, MA, USA) and showed the correspondence with the Langmuir isotherm model by virtue of the high regression coefficient.
Desorption studies
The solutions of CaCl2 (1 M) and non-ionic surfactant TRITON X-100 (1 %, w/w) were used in desorption studies. The obtained immobilized preparations were incubated for half an hour in the solution of CaCl2, rinsed with the immobilization buffer (acetate, pH 5, 0.1 M) and submitted to activity determination, after which the samples were incubated in the solution of TRITON for half an hour too. After the treatment with CaCl2 and TRITON X-100 solutions, activities were compared to assume the type of interactions that prevail between HRP and support.
Dye decolorization
To determine the optimum pH for the decolorization reaction, stock solutions (30 mg/L) of AV 109 were prepared in different buffers (pH between 3 and 12). Generally, into 5 mL of each dye solution, 0.4 mM of hydrogen peroxide and 0.1 IU of free or immobilized enzyme were added. Samples were taken every 5 min and analyzed using UV–Vis spectrophotometer at maximum wavelength for tested dye (λ max 590 nm AV 109). After the optimal pH and contact time were determined, the concentration of dye and hydrogen peroxide was varied in the range of 10–100 mg/L and 0.05–1.0 mM, respectively.
Percent of decolorization was calculated using the following Eq. (2) [19]:
where A 0 is the initial absorbance of untreated dye solutions (control) and A t is the absorbance of dye solutions after enzymatic treatment.
Reusability of the immobilized enzyme
The reusability (or operational stability) of the immobilized k-HRP was studied in an isothermal batch type reactor at 5 mL scale under constant conditions (pH 5.0, temperature 24 °C, dye concentration 40 mg/L and 0.2 mM of H2O2 %) using 0.1 IU of the immobilized enzyme. At the end of each reaction, the immobilized HRP was separated from the reaction medium by centrifugation (2 min, 13,000 rpm), washed with the immobilization buffer twice to remove any remaining substrate or a product and used in the next catalytic cycle in fresh medium. The reusability study was repeated until decolorization of testing dye was detected.
Results and discussion
Support characterization
In this study, to complete the structure changes over phase transformation and get metakaolin, kaolin was subjected to thermal treatment (2 h, 550 °C) [20]. The phase transformation of kaolin was carried out due to increase in specific surface area and reactivity as a consequence of the presence of silica (SiO2) and amorphous alumina (Al2O3) in reactive forms [20–22]. Using BET method, it was estimated that the obtained metakaolin had a specific surface area of 9.26 m2/g. The porosity of the support after thermal treatment was substantially improved suggesting that the amount of possible attachment sites for HRP molecule increased. Namely, the total pore volume of kaolin was 0.041 mL/g, before phase transformation and it has reached a value of 0.054 mL/g after the transformation.
The FEG–SEM images of kaolin deliver us the information regarding the morphology of the samples before, after thermal treatment and after HRP immobilization, and are shown in Fig. 1 at different magnifications. The average particle diameter determined using MIRA TESCAN in situ measurement software, before thermal treatment was determined to be 12.6 µm, with standard deviation (STD) of 6.8 µm, meanwhile after thermal treatment average particle diameter decreased on 10.1 µm, with STD of 5.7 µm, suggesting an increase of the specific surface area available for the HRP adsorption. The FEG–SEM micrographs (Fig. 1) indicate that before and after thermal treatment kaolin is composed of irregular shape particles which surface morphologies change after thermal treatment. Namely, from the Fig. 1b it appeared that the heating increased the porosity and friability of the kaolin layered structure. After the adsorption of HRP, a significant change was observed in the structure of clay support. It is clearly seen from the Fig. 1c that the support has irregular and rough surface [21] and, also, the filling of the space between leaf layers of the support and compact agglomerations has become evident that can be assumed to be due to the enzyme adsorption.
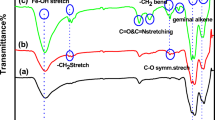
Adsorption of HRP was also confirmed with FTIR analysis. Figure 2 contains spectra of kaolin before and after heating as well as HRP immobilized on pretreated kaolin. The spectrum of kaolin and pretreated kaolin by heating has all the characteristic bands previously described in the literature [21, 23]. By heating at 550 °C kaolin lost water and aluminum and seemed to become tetrahedrally coordinated. Fading the bands at 3701, 3616 and 1620 cm−1 that originated from stretching of hydroxyl group and stretching and bending of adsorbed water molecules, and disappearance of bands at 905 and 530 along with appearance of new band at 800 cm−1 that originate from change in aluminum coordination unambiguously showed that dehydroxylation occurred [23]. Compared to spectrum of heated kaolin, appearance of two bands at 1638 and 1575 cm−1 in spectrum of immobilized enzyme confirmed the adsorption of HRP because these bands are the most prominent bands of the protein and originate from the –C=O stretching and –NH bending vibration of the peptide bonds [24].
Conditions for immobilization of HRP
Optimum pH
pH of the reaction mixture for the adsorption appeared to be one of the most important parameters because of its intrinsic influence on the surface charge densities of enzyme and support, but also on enzyme conformation, and structure. Thus, the pH determines the type and intensity of enzyme-support interactions. Adsorption behavior of HRP under different pHs is depicted on Fig. 3a.
As can be seen from the Fig. 3a, the specific activity of the immobilized HRP varied significantly with pH. Thus, the activity increased with increasing pH in the range from 2.0 to 5.0 reaching maximum value of 38.89 ± 0.05 IU/mg and then gradually decreased. At pH 2.0, the immobilized preparation showed the lowest activity of only 0.45 ± 0.02 IU/mg, suggesting that at pH ≤ 3.0 the acid-induced conformational changes in enzyme molecules occurred. This process has been shown to be followed by hydrogen bonding disruption around heme causing the release of the heme from the protein cavity [25] and loose of the catalytic activity. The mentioned structural changes were confirmed by recording the UV–Vis spectrum of HRP in the solutions at different pHs. When the secondary structure of the HRP is disrupted, displacement or disappearance of Soret peak at 403 nm can be observed as it was recorded at pH 2.0 [26]. The maximum activity at pH 5.0 can be explained by the fact that under these conditions the enzyme binding was in a way that allowed the conservation of the catalytic function. At pH above 7.0, the activity of obtained immobilized preparation was reduced significantly. Experimentally, it was found that the isoelectric point (pI) of metakaolin used for the immobilization is 1.36 (experimental procedure not shown) reveling that the support surface was negatively charged at pH 2.0 and 5.0. Considering that the isoelectric point of HRP used in this study is 7.2 [27], the electrostatic interaction apparently played an important role in the adsorption mechanism because protein molecule surfaces are positively charged at these pHs. This is in agreement with the literature [28, 29]. The results are presented in Table 1.
It seemed that at pH 5.0, electrostatic interactions were predominant. Namely, almost 88 % of bound enzyme was desorbed from the support after incubation with 1 M solution of CaCl2 for 90 min. This provided evidence that most of the enzyme was adsorbed by electrostatic interactions on the support rather than by hydrophobic interactions or covalently. At pH 7.0, protein molecules were slightly neutral to positively charged, while support was negatively charged, so the contribution of electrostatic interactions was less pronounced (around 62 % of bound enzyme was desorbed after incubation with 1 M solution of CaCl2), resulting in immobilized preparation with significantly reduced activity. Contrary, at pH 9.0, net charges of both protein surface and support surface were negative and it could be expected that the electrostatic repulsion led to the lower immobilization yield. The substantially weaker adsorption of proteins on the clay surface when the pH is above the isoelectric point of the protein has been already reported in the literature [30]. Even though at pH above pI, the protein surface carries a negative charge as well as support surface, there is a small portion of surface with positive charge available for some binding interactions [31]. In addition, the enzymes are flexible and can occupy conformation complementary to the vicinity on the support surface, so the same charge repulsion is minimized and adsorption of the protein is possible [32, 33]. The pH 5.0 was selected as suitable for immobilization of HRP onto kaolin since the obtained immobilized enzyme showed around fourfold higher activity than the preparations obtained at pH 7.0 and 9.0.
Adsorption isotherm
To determine the maximum capacity of the support and the type of interactions, the adsorption isotherm of HRP was measured at pH 5. The concentration of the offered protein was varied in the range 0.5–3.0 mg/mL and the results have been presented in Fig. 3b. Experimental data were fitted with both Langmuir and Freundlich adsorption isotherms (data not shown) and based on the value of the linear regression coefficient it was calculated that this set of results can be adequately described with Langmuir model (R 2 = 0.97). Langmuir isotherm model assumed that the support surface consists of definite binding sites, with equal adsorption energy, where adsorption and desorption are in equilibrium. At once occupied binding place, no further adsorption can take place. Thus, the well agreement of equilibrium data with Langmuir isotherm model confirmed the monolayer coverage of enzyme molecules onto kaolin surface, and the maximum adsorption capacity was found to be 4.63 mg/g. The formation of protein adsorption monolayer on the clay surfaces has also been confirmed by other researchers too [34].
It is evident from Fig. 3b that an increase of offered enzyme concentration to 1.5 mg/mL led to an increase in mass of the adsorbed enzyme up to 2.61 mg/g of the support (42.6 %). At higher concentrations, the saturation of the kaolin surface with the HRP molecules can be observed and the dynamic equilibrium between the adsorbed and desorbed molecules has been established. During the adsorption, the protein molecules can occupy one or more binding places depending on the protein molecule orientation. If the protein molecule is orientated in such a way when occupies more places, the adsorbing capacity of the support decreases [35]. Therefore, an inadequate HRP molecules orientation on the kaolin surface can be the reason why the kaolin adsorbed only 2.61 mg/g protein which was lower than theoretically predicted value of 4.63 mg/g. The parameters of Langmuir model could also give us information about the type of the adsorption. Using the dimensionless factor R L it was calculated that R L = 0.391, i.e. 0 < R L < 1 which means that the adsorption of HRP onto kaolin was favorable process. Unlike the enzyme loading, the activity of the immobilized preparation decreased with further increase in the initial enzyme concentration (Fig. 3b). Namely, an increase in the mass of bound protein resulted in specific activity increase until the initial concentration of 1.5 mg/L was reached. This could be explained by the fact that an increase in mass of bound protein intensified the protein–protein interactions causing protein structural rearrangement and activity decline [36, 37]. Thus, the immobilized HRP obtained at 1.5 mg/mL of the initial enzyme concentration, with specific activity 39.1 IU/mg, was applied in the subsequent experiments.
Optimization of process parameters for AV 109 decolorization
Primarily, it is important to emphasize that there are no data in the literature about the adsorption of HRP onto kaolin and its further application in the decolorization of the anthraquinone dye. To exclude the possibility of the dye adsorption on support, kaolin was applied in the decolorization reaction under the same reaction conditions as the immobilized enzyme. The result of this experiment was negative, confirming that the dye degradation is solely result of the enzymatic reaction. Potential and the effectiveness of k-HRP preparations were examined in the decolorization reaction of AV 109 anthraquinone dye. 0.1 IU of immobilized enzyme was applied in the reaction and the results were compared with the decolorization reaction catalyzed by the same amount of free HRP. Optimal pH for the decolorization was evaluated and the results are depicted in Fig. 4.
It was showed that the immobilization of HRP onto kaolin resulted in a slight shifting and broadening of the pH optimum (Fig. 4). This can be associated with the ionic changes around the enzyme active center caused by the immobilization process. The fact that the immobilized enzyme exposed almost constant decolorization potential in the range of 1 pH unit in the acidic environment could be of high importance from the application point of view. Primary application of k-HRP was the remediation of industrial wastewater polluted with anthraquinonic dyes, which is generally slightly acidic [38, 39]. Although the highest decolorization was attained with the free HRP at pH 4 (90.9 %), even small variation in solution pH led to decrease in the efficiency (Fig. 4c). In contrast, with immobilized HRP the decolorization efficiency remained unchanged from pH 4 to 5, revealing that the immobilization of HRP onto kaolin had stabilizing and protecting effects on the enzyme. After 40 min of incubation at pH 5.0, the immobilized HRP removed 69.3 % of AV 109 dye. Free HRP exhibited the highest activity at pH 4.0, where after 15 min of contact between enzyme and dye, 90.9 % of AV 109 dye was decolorized, under similar conditions. One of the seldom examples of anthraquinone dyes decolorization with immobilized HRP was decolorization via HRP immobilized onto various polysulfone supports. Herein, optimum pH for anthraquinone dye RB 19 decolorization was found to be 5.0, like in this study [40]. Similar behavior was also noticed when HRP was immobilized on electrospun microfibrous membranes where optimum pH for immobilized HRP was 5.0. The activity of free as well as immobilized enzyme was in correlation with pH, which affected the acidic–basic behavior of the substrate, indicating the importance of finding an optimum pH for performing the desired reaction [41].
After pH and contact time optimization, the initial hydrogen peroxide and dye concentration influence on immobilized and free enzyme was explored (Fig. 5a, b). Besides radical species formed in the HRP catalytic cycle, phenoxy radicals, or polymerized phenols that obscures active center, HRP molecule can be inactivated with hydrogen peroxide [42]. So, there is a need for finding the adequate hydrogen peroxide dose, to avoid its negative influence on the HRP molecule, increasing the efficiency of the immobilized HRP in the treatment of wastewater polluted with dyes.
a The effect of the initial H2O2 concentration on the decolorization of AV 109 with free and k-HRP, b the effect of the initial dye concentration on the decolorization of AV 109 with free and k-HRP, c the initial reaction rate versus dye concentration for free HRP (fixed H2O2 concentration 0.1 mM, dotted lines) and for k-HRP (fixed H2O2 concentration 0.2 mM, solid line)
The influence of the initial hydrogen peroxide concentration was examined by varying the concentration in the range 0.05–1.0 mM. It was apparent from Fig. 5a that k-HRP required higher concentration of hydrogen peroxide than free HRP. It was found that optimal concentrations of hydrogen peroxide for decolorization reaction were 0.1 and 0.2 mM with free and k-HRP, achieving the AV 109 decolorization of 90.7 and 80.3 %, respectively. By increasing the initial hydrogen peroxide concentration, small decrease in decolorization percentage was observed, so that k-HRP retained 96 % of maximal activity at 1 mM of hydrogen peroxide applied, which is another proof that immobilization attenuated inhibitory effect of HRP co-substrate. HRP immobilized in β-cyclodextrin–chitosan matrix, exhibited highest activity when 0.6 mM hydrogen peroxide was applied, and removed around 61 % of tested azo dye after 2 h of the reaction [12].
Anthraquinone dyes consist of fused aromatic rings, and resonance effects in their cyclic structures are well known, which makes them harder to decolorize than azo dyes. They are recalcitrant substrates and require carbon–carbon bonding breakage that is more difficult than the breakage of nitrogen bonds in azo dyes structure due to their less electronegativity [43]. Due to the substrate specificity, the maximum concentration of AV 109 that can be decolorized was examined with free and immobilized HRP, under defined conditions. The initial dye concentration was varied in 10–100 mg/L range and results have been presented in Fig. 5b.
It was clear from Fig. 5b that tested anthraquinone dye exerted inhibitory effect on HRP. The dye decolorization for free HRP increased by increasing dye concentration up to 30 mg/L and then gradually decreased. On the other hand, k-HRP showed higher affinity toward tested dye, so the maximum dye concentration that can be decolorized in high percentage, under specific conditions was 40 mg/L. This indicated that immobilization of HRP onto kaolin provided a biocompatible and inert environment for enzyme molecules whose active centers were less exposed to the inhibitory effect of tested dye. Thus, k-HRP was possible to decolorize 87 % (40 mg/L) of the dye, as opposed 92 % (30 mg/L) with free HRP. When applied in 100 mg/L of AV 109 solution, k-HRP retained 86 % of activity when free HRP only 23 %.
The catalytic efficiency of free and immobilized enzyme was determined in terms of three kinetic parameters, K m, V m and K i. The experimentally obtained results are summarized in Table 2, and graphically presented in Fig. 5c. All results from this set of experiments showed good agreement with Ping Pong Bi–Bi model which considers the inhibition with dye. Mathematical model can be expressed using the following Eq. (3) [19]
where \(\vartheta_{0}\) is the initial rate of the reaction, V max is the maximum rate, [H2O2]0, [D]0 are initial concentrations of hydrogen peroxide concentration and dye, respectively, K mb, K ma are Michaelis constants for dye and hydrogen peroxide, respectively, K ib inhibition constant for dye.
The data presented in Table 2, obtained by modeling the experimental results with Ping Pong Bi–Bi model inhibition with dye, clearly indicated the improved performances of the immobilized HRP. First, by comparing the maximal velocities of the reaction catalyzed with free enzyme (1.27 mM/min), and with immobilized enzyme (0.87 mM/min), it was evident that immobilization resulted in difficulties with the access of the substrate to the enzyme active center. This was confirmed by comparing the values of apparent kinetic constants K mb for free and k-HRP. Namely, higher value of K mb for k-HRP approved its reduced affinity toward dye. This can be due to steric hindrance of enzyme active center by the support or conformational changes of the immobilized enzyme [43]. Steric hindrance can be associated with the fact that some of the HRP molecules were entrapped inside of the kaolin pores. Furthermore, this behavior of k-HRP resulted also in inhibition diminishment. The inhibition constant for free HRP was less than for k-HRP, which means that kaolin seemed protective on the HRP active center. HRP was immobilized onto electrospun microfibrous membranes, and kinetics of degradation of bisphenol A was monitored following the Michaelis–Menten equation without inhibition [41]. Generally, in the literature there are a little data about the kinetics of dye decolorization with free either immobilized HRP, and all of them neglected the substrate inhibition presence.
Reusability study
Economic validity for the application of enzymes in the decolorization of textile dyes from wastewater at industrial scale significantly increases with the possibility of utilizing the same enzyme repeatedly in the desired reaction. Reusability of k-HRP was examined through consecutive cycles in the decolorization reaction of AV 109. The obtained results are presented in Fig. 6.
The stability profile revealed that the activity of k-HRP dropped initially after four cycles, remaining almost constant during the next three batches. Namely, the immobilized enzyme retained 35 ± 0.9 % of the initial activity after seven successive cycles. By comparing the productivity of free and immobilized HRP, it appeared that that the immobilized HRP was 4.2-fold more productive than the free enzyme. Particularly, in the first treatment cycle, both free and immobilized HRP showed high capacity for the tested dye decolorization (percent of decolorization was 92 and 87 % for free and k-HRP, respectively). However, k-HRP was recycled and in the second and third cycle decolorized 88 and 53 % of the offered dye amount after 210 min, respectively. Next four cycles were prolonged even more, but resulted in almost constant AV 109 decolorization percentage of 35 ± 0.9 %. Ultimately, free enzyme decolorized 2.7 mg, meanwhile k-HRP 11.3 mg of AV 109 dye. The operation stability was higher than previously reported for the HRP immobilized on various supports which also shown significant loss of activity after fourth cycle [8, 10, 38].
However, besides being highly efficient and stable, a support used for industrial applications must also be produced at affordable costs. A rough cost analysis based on support material prices (Catalog prices, Sigma-Aldrich) revealed that the kaolin used in our research is many times cheaper than the commercially polymers such as Diaion®, 2-hydroxyethyl methacrylate (HEMA) or Eupergit C (8.6, 21.1 and 1464.5 times, respectively). Furthermore, in the current analysis, additional costs including labor cost, labor requirements, equipment cost for enzyme immobilization have not been considered which is important for a large-scale wastewater treatment because of the reduction of the additional costs permitted by eliminating the support or enzyme activation step in this case. Comparing to several covalent binding protocols reported in the literature, the k-HRP is preferred to use as a biocatalyst because of its low cost, easy production, and efficient removal from the reaction medium for reuse.
Conclusion
In this study kaolin, as a cheap support, adsorption as a mild immobilization method, HRP as a versatile enzyme, proved to be a clean alternative for the decolorization of wastewater polluted with dyes. Detailed examination showed that the performances of immobilized HRP were substantially improved. The performance improvement is reflected in the increased resistance to the suicidal effects of co-substrate as well as the AV 109 dye. Kinetic parameters values were the confirmation of immobilized HRP advantages already mentioned above. The next research step could be stability increase of immobilized HRP, i.e. covalent immobilization, which opens the possibility of application of this biocatalyst in the appropriate configuration of bioreactors, for the purpose of continuous use.
References
Vojinović V, Carvalho RH, Lemos F, Cabral JMS, Fonseca LP, Ferreira BS (2007) Kinetics of soluble and immobilized horseradish peroxidase-mediated oxidation of phenolic compounds. Biochem Eng J 35:126–135
Abdel-Aty AM, Hamed MB, Fahmy AF, Mohamed SA (2013) Comparison of the potential of Ficus sycomorus latex and horseradish peroxidase in the decolorization of synthetic and natural dyes. J Genet Eng Biotehnol 11:95–102
O’Neil C, Hawkes FR, Lourenco ND, Pinheiro HM, Delee W (1999) Colour in textile effluents-sources, measurement, discharge consents and simulation: a review. J Chem Technol Biotechnol 74:1009–1018
Robinson T, McMullan G, Marcant R, Nigam P (2001) Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour Technol 77:247–255
Husain Q (2006) Potential applications of the oxidoreductive enzymes in the decolorization and detoxification of textile and other synthetic dyes from polluted water: a review. Crit Rev Biotechnol 26:201–221
Mishra A, Malik A (2014) Novel fungal consortium for bioremediation of metals and dyes from mixed waste stream. Bioresour Technol 171:217–226
Yu SM, Cheng J, Zuo P (2006) Horseradish peroxidase immobilized on aluminum pillared interlayered clay for the catalytic oxidation of phenolic wastewater. Water Res 40:283–290
Guo SV, Zheng F, Zhang B, Zhang JL, Huang XL, Liu H, Zhang JY (2010) Horseradish peroxidase immobilized on graphene oxide: physical properties and applications in phenolic compound removal. J Phys Chem C 114:8469–8473
Almezadeh I, Nejati S (2009) Phenols removal by immobilized horseradish peroxidase. J Hazard Mater 166:1082–1086
Shakeri M, Shoda M (2008) Decolorization of an anthraquinone dye by the recombinant dye-decolorizing peroxidase (rDyP) immobilized on mesoporous materials. J Mol Catal B Enzym 54:42–49
Jiang Y, Tang W, Gao J, Zhou L, He Y (2014) Immobilization of horseradish peroxidase in phospholipid-teplatedtitania and its application in phenolic compounds and dye removal. Enzyme Microb Technol 55:1–6
Celebi M, Altikatoglu M, Akdeste ZM, Yildrim H (2012) Determination of decolorization properties of Reactive Blue 19 using horseradish peroxidase enzyme. Turk J Biochem 37:200–206
Martins SCS, Martin CM, Fiuza LMCG, Santaella SD (2013) Immobilization of microbial cells: a promising tool for treatment of toxic pollutants in industrial wastewater. Afr J Biotechnol 12:4412–4418
Karagulyan HK, Gasparayan GP, Deckre SR (2008) Immobilization of fungal β-galactosidase on silica gel and kaolin carriers. Appl Biochem Biotechnol 146:39–47
Kim HJ, Suma Y, Lee SH, Kim JA, Kim HS (2012) Immobilization of horseradish peroxidase onto clay minerals using soil organic matter for phenol removal. J Mol Catal 83:8–15
Prodanović O, Prokopijević M, Spasojević D, Stojanović Ž, Radotić K, Knežević-Jugović ZD, Prodanović R (2012) Improved covalent immobilization of horseradish peroxidase on macroporous glycidyl methacrylate-based copolymers. Appl Biochem Biotechnol 168:1288–1301
Cvetić T, Sabovljević A, Pristov JB, Sabovljević M (2009) Effects of day length on photosynthetic pigments and antioxidative metabolism of in vitro cultured moss Atrichumundulatum (Hedw.) P. Beauv. (Bryophyta). Bot Serb 33:83–88
Hartree EF (1972) Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem 48:422–427
Šekuljica NŽ, Prlainović NŽ, StefanovićAB, Žuža MG, Čičkarić DZ, Mijin DŽ, Knežević-Jugović ZD (2015) Decolorization of anthraquinonic dyes from textile effluent using horseradish peroxidase: optimization and kinetic study. Sci World J Article ID 371625, 12 pages
Plešingerova B, Sučik G, Fabian M (2011) Surface area change of kaolin causing annealing. Acta Metall Slov 17(16):9–176
Tahir H, Sultan M, Qadir Z (2013) Physicochemical modification and characterization of bentonite clay and its application for the removal of reactive dyes. Int J Chem 5:19–32
Kakali G, Perraki T, Tsilvis S, Badogiannis E (2001) Thermal treatment of kaolin: the effect on mineralogy on the pozzolanic activity. Appl Clay Sci 20:73–80
Ilić BR, Mitrović AA, LjR Miličić (2010) Thermal treatment of kaolin clay to obtain metakaolin. Hem Ind 64:351–356
Prlainović NŽ, Bezbradica DI, Knežević-Jugović ZD, Stevanović SI, Avramov-Ivić MI, Uskoković PS, Mijin DŽ (2013) Adsorption of lipase from Candida rugosa on multi walled carbon nanotubes. J Ind Eng Chem 19:279–285
Chattopadhyay K, Mazumdar S (2000) Structural and conformational stability of horseradish peroxidase: effect of pH and temperature. Biochemistry 39:263–270
Park Byung-Wook, Kyoung-A K, Do-Young Y, Dong-Shik K (2012) Enzyme activity assay for horseradish peroxidase encapsulated in peptide nanotubes. Enzym Microb Technol 51:81–85
Zhang J, Zhang F, Yang H, Huang X, Zhang J (2010) Graphene oxide as a matrix for enzyme immobilization. Amer Chem Soc 26:6083–6085
Theng BKG (1982) Clay–polimer interactions: summary and perspectives. Clays Clay Miner 30:1–10
Yu HW, Li N, Tong DS, Zhou LinCX, Xu CY (2013) Adsorption of proteins and nucleic acids on clay minerals CH and their interactions: a review. Appl Clay Sci 80–81:443–452
McLaren AD, Peterson GH, Barshad I (1958) The adsorption and reactions of enzymes and proteins on clay minerals. Soil Sci Soc Am J 22:239–244
McLaren AD (1954) The adsorption and reactions of enzymes and proteins on kaolinite. J Phys Chem 58:129–137
Matuszevska B, Norde W, Luklema J (1981) Comptetitive adsorption of human plasma albumin and dextran on silver iodide. J Colloid Interface Sci 84:403–408
Luklema J (1984) Proteins at solid–liquid interfaces: a colloid-chemical review. Colloids Surf 10:33–42
Fiorito TM, Icoz I, Stotzky G (2005) Adsorption and binding of the transgenic plant proteins, human serum albumin, β-glucuronidase, and Cry3Bb1, on montmorillonite and kaolinite: microbial utilization and enzymatic activity of free and clay-bound proteins. Appl Clay Sci 39:142–150
Roach P, Farrar D, Perry CC (2005) Interpretation of protein adsorption: surface-induced conformational changes. Am Chem Soc 127:8168–8173
Sethuraman A, Belfort G (2005) Protein structural perturbation and aggregation on homogenous surfaces. Biophys J 88:1322–1333
Sethuraman A, Vendanthan G, Imoto T, Przybycien T, Belfort G (2004) Protein unfolding and interfaces: slow dynamics of alpha-helix to beta-sheet transition. Proteins Struct Funct Bioinform 56:669–678
Cheng J, Yu SM, Zuo P (2006) Horseradish peroxidase immobilized on aluminium-pillared interlayered clay for the catalytic oxidation of phenolic wastewater. Water Res 40:283–290
Jamal F, Pandey PK, Qidwai T (2010) Potential of peroxidase enzyme from Trichosantes diocia to mediate disperse dye decolorization in conjuction with redox mediators. J Mol Catal B Enzym 66:177–181
Celebi M, Kaya MA, Altikatoglu M, Yildrim H (2013) Enzymatic decolorization of anthraquinone and diazo dyes using horseradish peroxidase enzyme immobilized onto various polysulfone supports. Appl Biochem Biotechnol 171:716–730
Xu R, Chi C, Li F, Zhang B (2013) Immobilization of horseradish peroxidase on electrospunmicrofibrous membranes for biodegradation and adsorption of bisphenol A. Bioresour Technol 149:111–116
Melgarejo FR, Lopez JNR, Canovas FG, Ruiz PAG (2004) Immobilization of horseradish peroxidase on cinnamic carbohydrate esters. Process Biochem 39:1455–1464
Jamal F, Qidwal T, Pandey PK, Singh R, Singh S (2011) Azo and anthraquinone dye decolorization in relation to its molecular structure using soluble Trichosantes dioica peroxidase supplemented with redox mediator. Catal Commun 12:1218–1233
Acknowledgments
Researchers engaged in this paper development would like to acknowledge Ministry of Education, Science and Technological Development of the Republic of Serbia for the great financial support (Project Numbers III-46010 and 172013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Šekuljica, N.Ž., Prlainović, N.Ž., Jovanović, J.R. et al. Immobilization of horseradish peroxidase onto kaolin. Bioprocess Biosyst Eng 39, 461–472 (2016). https://doi.org/10.1007/s00449-015-1529-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-015-1529-x