Abstract
In this study, covalent immobilization of the horseradish peroxidase (HRP) onto various polysulfone supports was investigated. For this purpose, different polysulfones were methacrylated with methacryloyl chloride, and then, nonwoven fabric samples were coated by using solutions of these methacrylated polysulfones. Finally, support materials were immersed into aquatic solution of HRP enzyme for covalent immobilization. Structural analysis of enzyme immobilization onto various polysulfones was confirmed with Fourier transform infrared spectroscopy, atomic force microscopy, and proton nuclear magnetic resonance spectroscopy. Decolorization of textile diazo (Acid Black 1) and anthraquinone (Reactive Blue 19) dyes was investigated by UV–visible spectrophotometer. Covalently immobilized enzyme has been used seven times in freshly prepared dye solutions through 63 days. Dye decolorization performance of the immobilized systems was observed that still remained high (70 %) after reusing three times. Enzyme activities of immobilized systems were determined and compared to free enzyme activity at different conditions (pH, temperature, thermal stability, storage stability). Enzyme activities of immobilized systems and free enzyme were also investigated at the different temperatures and effects of temperature and thermal resistance for different incubation time at 50 °C. In addition, storage activity of free and immobilized enzymes was determined at 4 °C at different incubation days.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Textile industry uses huge amount of water in various processes such as desizing, bleaching, mercerization, and dyeing. Colorful wastewater generally comes from dyeing processes. Dyehouse wastewater consists of high chemical oxygen demand, color, salts, unfixed pigments, and low soluble O2. These are harmful for the aquatic life and people on the ground. Legislative requirements have been increased by municipalities, national and international organizations, and governments for the discharge of wastewater which include wastewater treatment, recycling of waste effluents and reusing of treatment materials (chemicals, enzymes, etc.), and developing new methods [1]. In typical textile operations, both chemical and mechanical treatments are used on raw materials to produce a finished product. Unwanted by-products arise from many points in this cycle; the majority of which are discarded. Some solvents and colorants present a chronic health risk if prolonged exposure takes place. Many dyes and colorants are difficult to treat, but their high visibility in waste discharges means these materials attract particular attention [2]. Based on the chemical structure of the chromophoric group, dyes are classified as azo, triphenylmethane, anthraquinone, heterocyclic, and polymeric dyes, among which the versatile azo and triphenylmethane dyes account for most textile dyestuffs produced. Because these dyes are mutagenic, carcinogenic, and also cannot be completely removed by conventional wastewater treatment systems, before disposal and discharge of dye-containing effluents, they need to be treated to reduce their levels of toxicity, which will minimize their pollution impact [3]. Dyes cause public concern as even small concentrations are visible in the environment. Some dyes have been identified to be mutagenic in the Ames Salmonella mutagenicity test, and some disperse dyes have been shown to cause allergic contact eczema. Brown and Anliker summarized the effects of textile effluent on the environment and the toxicity with respect to fish and other aquatic organisms, sewage bacteria, and plants. They concluded that due to the vast number of different dyestuffs and processes in which they can be applied, an accurate environmental risk assessment can only be made on individual dyestuffs and in individual dye houses. The source of heavy metals in dyes is from premetallized dyes. The discharge for heavy metals is stringent as they can be toxic to animals and aquatic life. Dyes are stable against breakdown by many microorganisms and most dyes do not biodegrade under the aerobic biological treatments in a municipal sewage plant. Many dyes, including the azo dyes, degrade under anaerobic conditions and the aromatic amines thus formed have been found to degrade further aerobically. Therefore, the aerobic conditions of rivers and lakes should degrade the amines formed from the biodegradation of azo dyes if they accumulate in the river sediments. Moll summarized the carcinogenic potential of dye degradation products. The toxicity of these dyes was assessed in terms of azo separation, i.e., what aromatic amines are produced. This occurs through reduction of the azo bond or the influence of enzymatic systems. The main concern is for those azo colorants which can release carcinogenic amines during metabolism [4].
Several physical and chemical methods such as coagulation–flocculation, electrochemical oxidation, adsorption, and membrane filtration have been used to remove pollutants from wastewater. However, none of these conventional methods have been widely suggested because of their limitations [5–7]. Although conventional chemical and physical techniques such as precipitation, adsorption, and ozonation have been employed for the decolorization of dye effluents, they possess inherent limitations such as high cost, formation of hazardous by-products, and intensive energy requirements [8]. One promising strategy is the use of microbes including white-rot fungal and bacterial strains that possess the ability to decolorize synthetic dyes. Microbial decolorization and degradation are an environmentally friendly and cost-competitive alternative to physicochemical decomposition processes for the treatment of industrial effluents. There is a considerable number of recent reports on decolorization and degradation of individual synthetic dyes by white-rot fungi [3, 8].
Lately, the enzymatic approach has much interest in the decolorization of textile and other industrially important dyes from wastewater as an alternative strategy to conventional chemical, physical, and biological treatments. Immobilization of enzymes onto solid natural and synthetic polymer supports for the treatment of aromatic compounds have many advantages. Besides enhanced stability, enzymes can acquire additional advantageous properties via immobilization such as (a) immobilized enzymes can be reused, (b) they can be easily separated from untreated substrate and wastewater or products, (c) they involve reduced operational cost, (d) continuous removal of toxic metabolites, and (e) rapid termination of reactions, thus simplifying the work [6, 9–11]. All these advantages need for the economical enhancement of immobilized enzyme.
Reactive dyes, which have good water solubility, advantage of coloring, dyed fabric durability, and are easily hydrolyzed into insoluble forms, are extensively used in dyeing processes. Anthraquinone reactive dyes are one of the major groups among reactive dyes other than azo compounds. Reactive Blue 19 (RB 19), an anthraquinone derivative, is an industrially important dye and is being used frequently as a starting material in the production of polymeric dyes [12]. Acid Black 1 (AB 1) is an industrially important acidic diazo dye, which has a high photo and thermal stability. Due to its high degree of fastness to light, the commercial grades of AB 1 are widely used in the textile industry for dyeing wool, nylon, silk, and textile printing. Other industrial use includes coloring of soaps, anodized aluminum and casein, wood stains, and writing ink preparation. It has a structure consisting of azo, phenolic, anilino, naphthalene, and sulfonated groups [13]. Frequency of usage of these dyes in industrial dyeing process was the reason of choice in our study.
Horseradish peroxidase enzyme (HRP; EC 1.11.1.7), extracted from horseradish, is a glycoprotein with six lysine residues which can be conjugated to a labeled molecule. HRP is used extensively in biochemistry applications primarily for its ability to amplify a weak signal and increase detectability of a target molecule. Peroxidase, a heme-containing oxidoreductase, is a commercially important enzyme which catalyzes the reductive cleavage of hydrogen peroxide by an electron donor. HRP is ideal in many respects for these applications because it is smaller, more stable, and less expensive than other popular alternatives such as alkaline phosphatase. HRP is a heme-containing enzyme that utilizes hydrogen peroxide to oxidize a wide variety of organic and inorganic compounds and has great commercial importance. HRP is used as a reagent for organic synthesis, biotransformation, as well as in coupled enzyme assays, chemiluminescent assays, immunoassays, and the treatment of wastewaters [14, 15]. HRP is known to efficiently cleave aromatic azo compounds in the presence of H2O2 and to degrade and precipitate industrially important dyes [12, 13, 16–18].
Horseradish peroxidase enzyme in free form has been utilized to eliminate various dyes, for instance Remazol Blue, Red Cibacron [17], Reactive Blue 19 [12], Coomassie Brilliant Blue G 250 [16], Naphthol Blue Black [13], and Remazol Turquoise Blue G [18]. With using free form of enzyme, it has to confront some drawbacks such as low stability, obligation of one-time usage, and interestingly low specific activity values in comparison with modified or immobilized forms especially at high concentrations. In order to overcome these disadvantages, horseradish peroxidase enzyme has been also immobilized with different solid supports such as epoxy-activated acrylic polymers (Eupergit®) [19], modified chitosan beads [20], and Amberlite IRA 400 resin [21], by bioaffinity layering for wastewater treatment [22]. In light of these studies, it can be said that enzyme immobilization onto various solid support approaches gives superior result and opportunities contrary to free enzyme approach. Reusability, enhanced chemical and thermal stability, and high specific activity values can be given as example for these advantages. However, no study was found on decolorization of dyes by immobilized HRP on polysulfone supports at different circumstances before.
Polysulfone (PSU) polymers have been extensively used to prepare ultrafiltration membranes at many industrial fields because of their low cost, superior film-forming ability, good mechanical properties, strong chemical and thermal stabilities, and outstanding acidic and alkaline resistances [1]. As it is known, polysulfones are highly resistant to thermal effects, mineral acids, alkali, and electrolytes, in pH ranging from 2 to 13. They are also resistant to oxidizing agents; therefore, it can be cleaned by bleaches. It is also resistant to surfactants and hydrocarbon oils. Because of these extraordinary properties, it can be easily said that polysulfones are most promising candidate in wastewater treatment process that contain many different hazardous chemicals for support materials to immobilize enzymes onto it. Polysulfones can also be modified easily in solution phase due to dissolubility in various solvents, so they can be converted to appropriate form for possible covalent bound enzyme immobilization. Due to their superior resistance characteristics against harsh environmental conditions, polysulfones were used in this study.
In the present paper, the mechanism of immobilization of horseradish peroxidase onto modified various polysulfone polymers and dye decolorization applications has been investigated at different conditions. The decolorization of textile diazo (Acid Black 1) and anthraquinone (Reactive Blue 19) dyes by HRP immobilized onto polysulfone support materials has been studied.
Experimental
Materials and Characterization Methods
Methanol (Besa Chemistry) was distilled before use, and 4,4′-dichlorodiphenyl sulfone (DCDPS, Merck KGaA), 2,2-bis(4-hydroxyphenyl)propane (bisphenol A, Merck KGaA), and 2,2-bis(4-hydroxyphenyl)hexafluoropropane (bisphenol AF, Merck KGaA) were recrystallized from toluene and dried in vacuo overnight before use. Potassium carbonate (K2CO3, Fluka) was ground to fine powder and dried in a vacuum oven at 120 °C for 8 h before the polymerization. N-methyl-2-pyrrolidone (NMP, Riedel-de Haen), N,N-dimethylformamide (Sigma-Aldrich), chloroform (Merck KGaA), dichloromethane (Sigma-Aldrich), toluene (Riedel-de Haen), triethylamine (Merck KGaA) and methacryloyl chloride (Fluka), horseradish peroxidase (E.C. 1.11.1.7, molecular weight (Mw) ∼40,000 g/mol, Fluka), Acid Black 1 (Fluka), Reactive Blue 19 (Sigma-Aldrich), o-dianisidine (Sigma-Aldrich), and H2O2 (30 %) (Sigma-Aldrich) were used as received. Ultrapure water that was used in all experiments and measurements was obtained from Millipore Milli-Q Gradient system.
Gel permeation chromatography (GPC) measurements were performed with an Agilent model 1100 instrument consisting of pump and refractive index and ultraviolet (UV) detectors and three Waters Styragel columns. Differential scanning calorimetry (DSC) measurements were performed with a PerkinElmer Pyris DSC 6 Series. The DSC curves were recorded at a heating and cooling rate of 10 °C min−1 in two scans. Thermogravimetric analysis (TGA) measurements were performed on a TA SDT Q 600 instrument in nitrogen to assess the thermal and thermo-oxidative stability of samples. The samples were heated at a rate of 10 °C min−1. The thermal stability of the samples was reported at the observed temperature of 5 % weight loss. Fourier transform infrared spectroscopy (FT-IR) spectra were collected as eight scans at 4 cm−1 resolution using a PerkinElmer Spectrum One FT-IR spectrometer. NMR spectra were obtained on a Varian Unity Inova Spectrometer operating at 500 MHz. Surface morphology of samples was investigated by a scanning probe microscope SPM 9600 (Shimadzu, Japan) in dynamic mode using a silicon cantilever. Dye decolorization performance of samples was measured spectrophotometrically with a UV–visible spectrophotometer (Model UV-1700 Pharmaspec Shimadzu) based on the maximum absorbance at 620 nm in the visible range, at different pHs (3–8) and temperatures (25, 30, 35,40, 45, 50, 60, 70 °C) for 60 min. All results reported represent averages of at least three experiments. Moreover, in all cases, the experimental error was not higher than 5 %.
Preparation of Polysulfone Support Materials
In order to increase the active groups (hydroxyl) per polymer chain for covalent immobilization, stoichiometric imbalance approach was applied in the polymer synthesis. For this purpose, monomer that contains hydroxyl groups was taken with little excess in comparison to other monomer in synthesis recipes are given in the following.
Accurately weighed 12.5560 g (0.055 mol) bisphenol A and 14.3585 g (0.050 mol) DCDPS were added to 500 mL three-neck flask. K2CO3 (11.4023 g (0.0825 mol)) was used to generate in situ phenoxide ion of bisphenol A. Flask walls were rinsed with 100 mL NMP and 60 mL toluene was applied to remove the water by azeotropic distillation. The reaction mixture was stirred at 300 rpm rate with constant purge of dried argon and heated to reflux under Dean–Stark apparatus till no more water droplet is observed for 5 h. While toluene was being removed via the Dean–Stark apparatus, reaction temperature increased to 155 °C. The reaction mixture was colored and temperature was raised slowly to 190 °C by controlled removal of toluene. Polymerization has been maintained for 24 h. After this period, flask content was cooled to room temperature and diluted viscous solution with 50 mL NMP in order to remove salts via filtration. Then, the solution was precipitated into methanol/water (10:1, vol/vol) separated by filtration and reprecipitated from chloroform into methanol/water (10:1, vol/vol) finally dried in a vacuum oven at 50 °C for 8 h.
To prepare hydroxyl-terminated bisphenol AF-based polysulfone, the same synthesis and purification procedures of hydroxyl-terminated bisphenol A-based polysulfone were applied. The following amounts were taken for synthesis: 18.4932 g (0.055 mol) bisphenol AF, 14.3585 g (0.050 mol) DCDPS, 11.4023 g (0.0825 mol) K2CO3, 100 mL NMP, and 60 mL toluene.
Methacrylate-terminated bisphenol A- and bisphenol AF-based polysulfones were prepared both by esterification. It was carried out by using methacryloyl chloride in the presence of base (triethylamine) according to literature [23]. Reaction procedure given was as follows: a 100-mL three-necked flask fitted with a condenser, dry argon inlet, and dropping funnel was charged with 2 g. Hydroxyl-terminated bisphenol A- or bisphenol AF-based polysulfone, 20 mL dichloromethane under constant stirring rate and dry argon atmosphere, after the complete dissolution of polymers, and excess (2 mL) triethylamine were added to the flask. Then, a solution of 0.2926 g. (2.8 mmol) methacryloyl chloride in 5 mL dichloromethane was carefully added via a funnel dropwise at 0–5 °C. From this point, stirring at room temperature has been going on for 24 h and then reaction was terminated by pouring flask content to methanol. Precipitated polymers were filtrated and washed successively with methanol and water until neutral pH was reached, and finally, obtained products were dried in vacuum overnight at 80 °C.
Immobilization of Horseradish Peroxidase Enzyme onto Polysulfone Support Materials
To prepare the support materials for covalently bound enzyme, firstly, methacrylated polysulfones were dissolved in N,N-dimethylformamide, then nonwoven fabric samples have been coated with these polymer solutions (60 mg solution per square centimeter of a nonwoven fabric). The nonwoven fabric samples have been used to enhance surface area of methacrylated polysulfones for HRP enzyme immobilization onto it and for easy application purpose to reuse these support materials in dye decolorization studies. Polymer-coated nonwoven fabric samples have been dried for 48 h at 60 °C. Horseradish peroxidase enzyme (0.1 M phosphate buffer at pH 7.0) has been applied onto support material surfaces for 48 h at 4 °C. After 48 h, the support materials have been washed twice to remove all unbound enzymes from the support material. Finally, support materials have been immersed into 0.1 M phosphate buffer at pH 7.0 for 1 h then washed with water. Immersion and washing processes were applied three times in order to be sure of the removal of unbound enzyme from immobilized enzyme materials.
Horseradish peroxidase enzyme was covalently bound to the acrylate groups of modified polysulfone support materials similarly to a previous study [24]. Connection between acrylate and amino groups occurred via Michael type reaction owing to asparagine, histidine, and arginine amino acids which took part in the molecular structure of HRP and acrylate groups of modified polysulfone support materials. All steps of syntheses are given in Fig. 1.
Enzymatic Activity Assays
The activities of free HRP and immobilized HRP (2 cm2 nonwoven polymer HRP system) were determined spectrophotometrically (UV-1700 PharmaSpec Shimadzu) by monitoring the oxidation of o-dianisidine at 30 °C and 460 nm [13, 25]. One unit of enzyme activity was defined as 1 μmol of oxidative compound produced in 1 min.
The activities of free HRP enzyme and immobilized HRP enzyme were analyzed as a function of pH and temperature and different storage time at 4 °C. The general assay procedure was as follows: buffer solution (4,850 μL) and o-dianisidine (100 μL) were transferred to centrifuge tubes (50 mL) and equilibrated to a desired temperature. Free enzyme or immobilized enzyme was added to tubes. After the addition of 50 μL H2O2 as initiator to tubes exactly 10 min later, A 460 data were recorded using cell temperature-controlled spectrophotometer. Total activities in units were calculated according to these absorbance values at A 460 (Eq. 1).
- M ε :
-
Molar absorption coefficient of o-dianisidine (11.300 M−1 cm−1)
- t :
-
Incubation time (minute)
- c HRP :
-
HRP enzyme concentration (milligrams per liter)
- A 460 :
-
Absorbance values of free enzyme or immobilized enzyme at 460 nm
All results reported represent averages of at least three experiments. Moreover, in all cases, the experimental error was not higher than 5 %.
Stability Studies
Thermal Stability
Thermal stabilities of the free or immobilized enzyme were evaluated with the activities determined according to the procedure described above. But in this procedure, activity determination was performed at different working temperatures (30, 40, 50, 60 °C) at pH 5.0. Meanwhile, thermal stabilities of the free and immolated enzyme were monitored at 50 °C. The enzyme and conjugate solutions (2 mg/mL in 0.05 M acetate buffer pH 5.0) were incubated at 50 °C for different periods of the times. All results reported represent averages of at least three experiments. Moreover, in all cases, the experimental error was not higher than 5 %.
pH Stability
The stabilities of the free or immobilized enzyme against pH factor were determined according to the same procedure for activity determination described above. The activity of the enzyme solutions at different pH values was determined at 25 °C. All enzyme experiments were performed in acetate buffer (0.05 M) at pH ranging from 3.0 to 5.0 and 0.01 M phosphate buffer of pH ranging from 6.0 to 8.0. All results reported represent averages of at least three experiments. Moreover, in all cases, the experimental error was not higher than 5 %.
Dye Decolorization Experiments
RB 19 (4 mg mL−1) and AB 1 (4 mg mL−1) were prepared in distilled water as stock. Synthetic dye solutions were prepared 100 μL for AB 1 (10 mg L−1) and 25 μL for RB 19 (40 mg L−1) at pH 5.0. The optimum pH value of horseradish peroxidase enzyme for dye decolorization experiments had been determined as pH 5.0 in our previous studies [12–14]. Decolorization of dyes has been examined with UV–visible spectrophotometer at maximum wavelength values of textile dyes (for AB 1,594 nm, for RB 19 618 nm) at 25 °C for 1 h. The decolorization was carried out directly in a 50-mL beaker. The reaction was followed by adding a buffer solution at pH 5.0 (50 mM acetate buffer), 25 (AB 1)/100 (RB 19) μL dye HRP solution (0.41 mg/60 mg nonwoven fabric, 8,440 U mg−1), and finally 100 μL H2O2 (3 %) as the initiator to beaker, respectively, in a total volume of 10 mL. Finally, immobilized enzyme support system is washed with water and kept in this medium at 4 ◦C for reusing dye decolorization experiments until further use. Percent dye decolorization was calculated following mathematical Eq. 2:
- Abs(i):
-
Initial dye absorbance at λ max
- Abs(t):
-
Dye absorbance at λ max after reaction started with H2O2 for each incubation times
Experiments were performed three times and results are expressed as the mean values.
Results and Discussion
Preparation of Polysulfone Support Materials
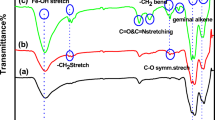
Polymerizations of hydroxyl-terminated bisphenol A-based and bisphenol AF-based polysulfones were achieved via nucleophilic substitution reactions by using stoichiometric imbalance approach. The characterizations of the obtained hydroxyl-terminated bisphenol A-based and bisphenol AF-based polysulfones were carried out by using attenuated total reflectance (ATR)-FT-IR, proton nuclear magnetic resonance spectroscopy (1H-NMR), GPC, DSC, and TGA. ATR-FT-IR and 1H-NMR spectra show the characteristic bands for the polyether sulfone backbone (Fig. 2). It is determined that obtained polysulfones have sufficient molecular weights capable of forming a film (bisphenol A-based polysulfone Mw = 13,100, HI = 1.56; bisphenol AF-based polysulfone, Mw = 17,100; heterogeneity index (HI) = 1.62), and they have also high glass transition temperatures (T g) and outstanding decomposition temperatures (T d) (bisphenol A-based polysulfone T g = 165 °C, T d = 460 °C; bisphenol AF-based polysulfone T g = 181 °C, T d = 510 °C).
The derivatizations of hydroxyl-terminated bisphenol A-based and bisphenol AF-based polysulfone samples were carried out with methacryloyl chloride in the presence of triethylamine as a base.
The modified polysulfone samples were characterized by ATR-FT-IR. Substitution of methacrylate functional groups over the hydroxyl end groups of different polysulfones via esterification process was observed. Evidence of the formation of the new ester carbonyl peak at around 1,735 cm−1 can be seen in the ATR-FT-IR spectra of bisphenol A- and bisphenol AF-based polysulfones (Fig. 2).
Molecular structures of the modified polysulfone samples were also examined by 1H-NMR. It was confirmed that methacrylation was achieved for both bisphenol A- and bisphenol AF-based polysulfones. In the examination of bisphenol A-based polysulfone spectra, firstly, a peak belonging to methyl functionalities of isopropylidene group at 1.65 ppm can be seen and then three peaks between 6.70 and 7.86 ppm correspond to the aromatic protons of the poly(ethersulfone) backbone. With the appearance of the new peaks at around 5.80, 6.40, and 2.10 ppm in methacrylated bisphenol A-based polysulfone spectra, it was confirmed that methacrylation was achieved (Fig. 2).
Similarly, new peaks belonging methacrylate group at 5.85, 6.40, and 2.10 ppm in methacrylated bisphenol AF-based polysulfone spectra can be seen, while these peaks did not exist in unmodified bisphenol AF-based polysulfone spectra (Fig. 2).
Immobilization of Horseradish Peroxidase Enzyme onto Polysulfone Support Materials
It is well known that atomic force microscopy (AFM) is a well-established technique for imaging surfaces even single biomacromolecules under physiological conditions. The exceptionally high spatial resolution and signal-to-noise ratio of the AFM make it possible to observe the substructure of individual molecules. In particular, specimens prepared for AFM remain in a plastic state, which enables the direct observation of the dynamic molecular response, perturbed film structure, and conformation changes of single proteins, as well as their assembly in real time, in an aqueous environment [26]. In order to determine the roughness parameter values of polysulfones, methacrylated polysulfones, and covalently enzyme bound polysulfone samples, AFM technique was used.
Roughness parameter values are determined for bisphenol A-based polysulfone as 6.05, 11.68, and 72.52 nm and for bisphenol AF-based polysulfone as 2.04, 43.14, and 62.79 nm for virgin, methacrylated, and covalently enzyme bound polysulfones, respectively. Roughness parameter values of virgin, methacrylated, and covalently enzyme bound polysulfone samples show an increasing trend in comparison to unmodified polysulfone samples. This observed trend supports that confirmation of methacrylation and enzyme binding onto polysulfone samples, similarly in literature [24].
Effect of Different pHs for Dye Decolorization by Using Immobilized Horseradish Peroxidase
Most enzymes have a characteristic pH value at which their activity is maximized. The interrelation of enzymatic activity with pH, for any enzyme, depends on the acidic–basic behavior of the substrate, as well as other factors which are, in general, difficult to analyze quantitatively [14, 18]. At the extreme pH value (i.e., pH 3.0), low decolorization values were observed because the activity of HRP was lost at these pHs (Fig. 3). No color change was observed in all control flasks which contain only aqueous solutions of dyes and not any enzyme or immobilized enzyme at different pH values. It has been reported that HRP showed the best activity at pH 5.0 [13, 18, 27]. The results of this study are in agreement with the ones obtained by Dong et al. [27], suggesting that decolorization might be due to the HRP activity. Bhunia et al. [17] studied the enzymatic decolorization of the dyes Remazol Blue and Red Cibacron, at different pH values, and it was concluded that at pH values above 6.0, the HRP activity was inhibited. Zang et al. [28] studied immobilization of horseradish peroxidase on polyacrylamide gel for the removal of pentachlorophenol and the optimum pH observed was 5.0. From these results, it can be concluded that the optimum pH may vary for the free and immobilized horseradish peroxidase enzyme, highlighting the need to study the pH to be used. In addition, the ideal pH of immobilized enzyme for the decolorization of Reactive Blue 19 and Acid Black 1 dyes was also found to be 5.0 in this study.
Activities of free HRP enzyme were determined higher than immobilized enzymes. Decreasing of activities of immobilized enzymes in comparison to free enzyme is due to covalent binding. It comes across often in literature that covalent binding of enzymes onto solid supports restricts activities because with covalent binding, chemical structure of enzyme and working active center changes; thus, it is observed decreasing at enzyme activities.
As you can see in Fig. 3, the highest activity values of free enzyme and immobilized enzymes were observed at pH 5.0 similarly literature [3, 12, 16, 18, 27]. It can be said easily that the reason of decreasing activity values of free and immobilized enzymes at higher pH, above pH 5.0, or lower pH, below pH 5.0, is the denaturation of HRP enzyme. It can also be observed in Fig. 3 that free enzyme is more resistant to denaturation with increasing or decreasing pH values than immobilized enzymes. If it is compared with stability of immobilized enzymes against higher or lower pH values according to polysulfone support material type, bisphenol AF-based polysulfone support materials are more successful than bisphenol A-based polysulfone support materials. This characteristic of bisphenol AF-based polysulfone support materials can be explained with its chemical structure; bulky flourine groups provide more protection to enzyme structures against higher or lower pH values.
Temperature Effect and Storage activity of Immobilized Enzymes and Free Enzyme
Enzyme immobilization can improve performance of enzymes such as activity, stability, or selectivity [29]. Most enzymes are not stable at high temperatures. This property limits their usage and storage under such conditions [30]. To develop stable enzymes using protein engineering, immobilization techniques, stabilizing additives, and chemical modification offer opportunities for practical applications [31].
Storage activities of free HRP and immobilized HRP onto polysulfone support materials at different days at 4 °C (activities were measured at pH 5.0 and 25 °C). Storage lifetimes of free horseradish peroxidase enzyme and immobilized horseradish peroxidases were studied for 4 °C (Fig. 4) at pH 5.0. Free enzyme lost its activity after 16 days at 4 °C, whereas immobilized enzymes were stable, showing higher activity values for 46 days at 4 °C. As a result, immobilized enzymes had very long storage lifetime compared to free horseradish peroxidase enzyme at 4 °C which is considered a favorable feature for usage in applications.
It was studied with free and immobilized enzymes at different temperatures (30, 40, 50, and 60 °C). Bisphenol AF-based polysulfone-immobilized enzyme system was more stable at high temperatures (50 and 60 °C). High resistance against the temperature may be due to thermal protective effects of bulky flourine groups and formation of thick polymer cover around enzyme molecules. The polymer molecules are preferentially excluded from the surface layer of the protein molecule, and the water shell around the protein molecule is preserved, so that the conformation of the protein becomes more rigid.
In addition, thermal resistance was also investigated for different incubation time at 50 °C. Immobilized enzymes were more thermal resistant than the free enzyme at a different incubation for 60 min. In addition, bisphenol AF-based polysulfone-immobilized enzyme was observed to have extreme thermal resistance for 60 min incubation at 50 °C (Figs. 5 and 6). Multipoint covalent bonding of polymer to horseradish peroxidase caused the formation of an enzyme whose thermal stability was increased especially at pH 5.
Dye Decolorization by Using Reusing of Immobilized Horseradish Peroxidases
The same immobilized enzymes (horseradish peroxidase enzyme immobilized onto bisphenol A-based and bisphenol AF-based PSU support) were reused seven times for different days (1, 4, 12, 21, 42, 49, and 63 days) after storage at 4 °C in the refrigerator (Figs. 7 and 8). Immobilized enzyme was applied in a 50-mL beaker of synthetic dye solution for 1 h. After 1 h, immobilized enzyme was removed from synthetic dye solution and washed with distilled water for other usage. After usage of immobilized enzyme on the first day, it was stored for other usages at 4 °C until other applications. Dye decolorization values were calculated at a decreasing of maximum absorbance of dye after 1 h. After three times of reusing immobilized enzymes, dye decolorization values decreased remarkably. However, immobilized enzymes were reused seven times at low decolorization values (4, 5, 6, and 7 reusing; Figs. 7 and 8).
Conclusions
Hydroxyl-terminated bisphenol A- and bisphenol AF-based polysulfones were synthesized successfully by condensation polymerization and subsequently methacrylated via esterification. Support materials were prepared for HRP enzyme immobilization by casting methacrylated polysulfone solutions onto nonwoven fabric samples. Finally, HRP enzyme was immobilized to support materials via Michael addition type reaction.
In the dye decolorization experiments shown using immobilized horseradish peroxidase enzyme in the dyes (AB 1 and RB 19), removal presented good results in 1 h at pH 5.0. Immobilized horseradish peroxidase enzyme was more stable than free enzyme at 50 °C and storage activity (4 °C). Horseradish peroxidase immobilized onto polysulfone supports can be reused seven times for the decolorization of AB 1 and RB 19 dyes. The covalent horseradish peroxidase enzyme immobilization process leads to an insoluble, reusable, and quite stable form of the enzyme that allows us to work in high-temperature values because of thermal resistance of immobilized enzyme than the free form.
References
Lau, W. J., & Ismail, A. F. (2009). Polymeric nanofiltration membranes for textile dye wastewater treatment: preparation, performance evaluation, transport modelling, and fouling control—a review. Desalination, 245, 321–348.
Modak, P. (1994). The textile industry and the environment, United Nations Environment Programme, Industry and Environment, Technical, Report No. 16, United Nations Publication, E93-111-D5.
Hadibarata, T., Yusoff, A. R. M., Aris, A., Salmiati, Hidayat, T., & Kristanti, R. A. (2012). Decolorization of azo, triphenylmethane and anthraquinone dyes by laccase of a newly isolated Armillaria sp. F022. Water, Air, and Soil Pollution, 223, 1045–1054.
Barclay, S., & Buckley, C. (2000). Waste minimisation guide for the textile industry: a step towards cleaner production, Technical Report, University of Natal, The South African Water Research Commission.
Pala, A., & Tokat, E. (2002). Color removal from cotton textile industry wastewater in an activated sludge system with various additives. Water Research, 36, 2920–2925.
Matto, M., & Husain, Q. (2009). Decolorization of textile effluent by bitter gourd peroxidase immobilized on concanavalin a layered calcium alginate–starch beads. Journal of Hazardous Materials, 164(2–3), 1540–1546.
Amini, M., Arami, M., Mahmoodi, N. M., & Akbari, A. (2011). Dye removal from colored textile wastewater using acrylic grafted nanomembrane. Desalination, 267, 107–113.
Hadibarata, T., Yusoff, A. R. M., & Kristanti, R. A. (2012). Decolorization and metabolism of anthraquinone-type dye by laccase of white-rot fungi Polyporus sp. S133. Water, Air, and Soil Pollution, 223, 933–941.
Husain, Q. (2006). Potential applications of the oxidoreductive enzymes in the decolorization and detoxification of textile and other synthetic dyes from polluted water: a review. Critical Reviews in Biotechnology, 26(4), 201–21.
Won, K., Kim, S., Kima, K. J., Park, H. W., & Moon, S. J. (2005). Optimization of lipase entrapment in Ca-alginate gel heads. Process Biochemistry, 40, 2149–2154.
Krajewska, B. (2004). Application of chitin- and chitosan-based materials for enzyme immobilizations: a review. Enzyme and Microbial Technology, 35, 126–139.
Çelebi, M., Altıkatoğlu, M., Akdeste., Z., & Yıldırım, H (2012). Determination of decolorization properties of Reactive Blue 19 dye using horseradish peroxidase. Turkish Journal of Biochemistry, 37(2), 200–206.
Onder, S., Celebi, M., Altikatoglu, M., Hatipoglu, A., & Kuzu, H. (2011). Decolorization of Naphthol Blue Black using the horseradish peroxidase. Applied Biochemistry and Biotechnology, 163, 433–443.
Altikatoglu, M., Arioz, C., Basaran, Y., & Kuzu, H. (2009). Stabilization of horseradish peroxidase by covalent conjugation with dextran aldehyde against temperature and pH changes. Central European Journal of Chemistry, 7(3), 423–428.
Veitch, N. C. (2004). Horseradish peroxidase: a modern view of a classic enzyme. Phytochemistry, 65(3), 249–259.
Altikatoglu, M., & Celebi, M. (2011). Enhanced stability and decolorization of Coomassie Brilliant Blue R-250 by dextran aldehyde-modified horseradish peroxidase. Artificial Cells, Blood Substitutes, and Biotechnology: An International Journal, 39(3), 185–190.
Bhunia, A., Durani, S., & Wangikar, P. P. (2007). Horseradish peroxidase catalyzed degradation of industrially important dyes. Biotechnology and Bioengineering, 72, 562–567.
De Souza, S. M. A. G. U., Forgiarini, E., & de Souza, A. A. U. (2007). Toxicity of textile dyes and their degradation by the enzyme horseradish peroxidase (HRP). Journal of Hazardous Materials, 147, 1073–1078.
Pramparo, L., Stuber, F., Font, J., Fortuny, A., Fabregat, A., & Bengoa, C. (2010). Immobilisation of horseradish peroxidase on Eupergit® for the enzymatic, elimination of phenol. Journal of Hazardous Materials, 177, 990–1000.
Monier, M., Ayad, D. M., Wei, Y., & Sarhan, A. A. (2010). Immobilization of horseradish peroxidase on modified chitosan beads. International Journal of Biological Macromolecules, 46, 324–330.
Peralta-Zamora, P., Esposito, E., Pelegrini, R., Groto, R., Reyes, J., & Durán, N. (1998). Effluent treatment of pulp and paper, and textile industries using immobilised horseradish peroxidase. Environmental Technology, 19(1), 55–63.
Dalal, S., & Gupta, M. N. (2007). Treatment of phenolic wastewater by horseradish peroxidase immobilized by bioaffinity layering. Chemosphere, 67, 741–747.
Dizman, C., Ates, S., Torun, L., & Yagci, Y. (2010). Synthesis, characterization and photoinduced curing of polysulfones with (meth)acrylate functionalities. Beilstein Journal of Organic Chemistry, 6, 56.
Cano, A., Minguillion, C., & Palet, C. (2006). Immobilization of endo-1,4-beta-xylanase on polysulfone acrylate membranes: synthesis and characterization. Journal of Membrane Science, 280, 383–388.
Altikatoglu, M., & Basaran, Y. (2011). Additive effect of dextrans on the stability of horseradish peroxidase. The Protein Journal, 30, 84–90.
Shi, J. X., Zhang, X. E., Xie, W. H., Zhou, Y. F., Zhang, Z. P., Deng, J. Y., Cass, A. E. G., Zhang, Z. L., Pang, D. W., & Zhang, C. G. (2004). Improvement of homogeneity of analytical biodevices by gene manipulation. Analytical Chemistry, 76, 632–638.
Dong, Y. C., Chen, J. L., Li, C. H., & Zhu, H. X. (2007). Decoloration of three azo dyes in water by photocatalysis of Fe(III) oxalate complexes/H2O2 in the presence of inorganic salts. Dyes and Pigments, 73, 261–268.
Zang, J. B., Ye, P., Chen, S., & Wang, W. J. (2007). Removal of pentachlorophenol by immobilized horseradish peroxidase. International Biodeterioration and Biodegradation, 59, 307–314.
Altikatoglu, M., Basaran, Y., Arioz, C., Ogan, A., & Kuzu, H. (2010). Glucose oxidase-dextran conjugates with enhanced stabilities against temperature and pH. Applied Biochemistry and Biotechnology, 160, 2187–2197.
Liu, J. Z., Song, H. Y., Weng, L. P., & Ji, L. N. (2002). Increased thermostability and phenol removal efficiency by chemical modified horseradish peroxidase. Journal of Molecular Catalysis B: Enzyme, 18, 225–232.
Betancor, L., Gallego, F. L., Hidalgo, A., Alonso-Morales, N., Fuentes, M., Fernández-Lafuente, R., & Guisán, J. M. (2004). Prevention of interfacial inactivation of enzymes by coating the enzyme surface with dextran-aldehyde. Journal of Biotechnology, 110, 201–207.
Acknowledgments
The authors thank the 11th Committee of Istanbul Chamber of Industry for their financial support. We also want to thank Yeliz Başaran for the AFM studies, Feyza KARASU KILIÇ for GPC measurements, and Ahmet Lütfi UĞUR for TGA-DSC studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Celebi, M., Kaya, M.A., Altikatoglu, M. et al. Enzymatic Decolorization of Anthraquinone and Diazo Dyes Using Horseradish Peroxidase Enzyme Immobilized onto Various Polysulfone Supports. Appl Biochem Biotechnol 171, 716–730 (2013). https://doi.org/10.1007/s12010-013-0377-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0377-x