Abstract
The functional relationship between arbuscular mycorrhizal fungi (AMF) and their hosts is variable on small spatial scales. Here, we hypothesized that herbivore exclusion changes the AMF community and alters the ability of AMF to enhance plant tolerance to grazing. We grew the perennial bunchgrass, Themeda triandra Forssk in inoculum from soils collected in the Kenya Long-term Exclosure Experiment where treatments representing different levels of herbivory have been in place since 1995. We assessed AMF diversity in the field, using terminal restriction fragment length polymorphism and compared fungal diversity among treatments. We conducted clipping experiments in the greenhouse and field and assessed regrowth. Plants inoculated with AMF from areas accessed by wild herbivores and cattle had greater biomass than non-inoculated controls, while plants inoculated with AMF from where large herbivores were excluded did not benefit from AMF in terms of biomass production. However, only the inoculation with AMF from areas with wild herbivores and no cattle had a positive effect on regrowth, relative to clipped plants grown without AMF. Similarly, in the field, regrowth of plants after clipping in areas with only native herbivores was higher than other treatments. Functional differences in AMF were evident despite little difference in AMF species richness or community composition. Our findings suggest that differences in large herbivore communities over nearly two decades has resulted in localized, functional changes in AMF communities. Our results add to the accumulating evidence that mycorrhizae are locally adapted and that functional differences can evolve within small geographical areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The associations among mycorrhizal fungi and their hosts are ancient, dating back 400 mya when plants first colonized land (Remy et al. 1994). The vast majority of land plants form symbioses with arbuscular mycorrhizal fungi (AMF) (Brundrett 2009), which act as extended root systems, gathering inorganic nutrients from the soil that are exchanged for photosynthetic carbon (Smith and Read 2008), and there is ample evidence that these symbioses preceded the evolution of roots and allowed plants to colonize land (Remy et al. 1994; Redecker et al. 2000; Brundrett 2002). Although not nearly as old, the interactions among plants inhabiting grassland savannas and vertebrate herbivores have co-evolved through the millennia as well (McNaughton 1985; Frank et al. 2003). These below and aboveground interactions are expected to interact and alter mycorrhizal function depending on the context of herbivory (Wardle et al. 2004; Hartley and Gange 2009; A’Bear et al. 2014).
There is ample evidence that herbivores affect fungal communities, altering species richness, relative abundance of different taxa (Eom et al. 2001; Frank et al. 2003; Murray et al. 2010; van der Heyde et al. 2017), and enhancing infectivity (Petipas and Brody 2014). Yet, how AMF affect plant resistance to herbivores or tolerance of herbivore damage has been less well studied and the results have been mixed (Borowicz 2013). In some cases, inoculation with mycorrhizae enhances resistance to herbivores, reducing herbivore preference and the amount of damage incurred (Borowicz 1997; Gange 2007; Wang et al. 2015), but the effect can go the other way as well. In a long-term study of prairie plants in North America, invertebrate herbivores preferred, rather than avoided, grasses with their native compliment of mycorrhizae (Kula and Hartnett 2015).
In the few studies of AMF effects on plant tolerance (here used to denote equal growth or fitness under conditions of herbivore damage), AMF increased plants’ ability to tolerate herbivore damage in some cases (e.g., Frank et al. 2003), while others showed little or no effect (Borowicz 1997; Gange et al. 2002; Bennett and Bever 2007; Varga et al. 2009; Aguilar-Chama and Guevara 2012; González et al. 2015), and still others showed that AMF reduced tolerance (Bennett and Bever 2007; Garrido et al. 2010). These mixed results may be due to one or more of several factors. The importance of AMF to plant growth and response to damage may be highly species specific (Klironomos 2003), and dependent on the fungal species (Gange et al. 2005; Bennett and Bever 2007; Barber et al. 2013) or on the host (Kula and Hartnett 2015; Tao et al. 2016). In addition, the benefits provided by AMF may depend on soil conditions, the intensity of herbivore damage, interactions with other organisms important to plant fitness, and/or abiotic conditions (Laird and Addicott 2007; Bever et al. 2009; Bever 2015; Ji et al. 2010; Hoeksema et al. 2010; Vega-Frutis and Guevara 2013; Middleton et al. 2015; van der Putten et al. 2016).
What remains virtually unknown, however, is how long-term grazing (or lack thereof) alters mycorrhizal communities in ways that affect the plant–fungal relationship and enhance plant tolerance for damage by herbivores. Given increased grazing pressure by native herbivores whose habitat is rapidly shrinking, and under abiotic conditions that are often suboptimal for plant growth (e.g., drought), understanding these interactions has become increasingly important (Ji et al. 2013; van der Putten et al. 2016; Eldridge et al. 2017). We hypothesized that long-term access by large, vertebrate herbivores has resulted in locally adapted symbioses that enhance plant tolerance of herbivory. To examine this hypothesis, we asked the following questions: (1) does the long-term exclusion of large vertebrate herbivores affect AMF diversity or root colonization; (2) do AMF isolated from soils with different histories of herbivory affect plant tolerance of simulated damage differently, and (3) do patterns of regrowth following clipping in the field follow those in the greenhouse?
Here, we utilized the Kenya Long-term Exclosure Experiment (KLEE) to examine how the loss of grazers (through exclusion by fences) over 16 years has resulted in highly localized and functional changes in the mycorrhizal community. In East African savannas, vertebrate herbivores, such as elephants, zebra and gazelles, are important drivers of plant productivity and community composition (McNaughton 1985; Hobbs 1996; Frank et al. 1998; Veblen and Young 2010; Pringle et al. 2011; Porensky et al. 2013; Young et al. 2013; Veblen et al. 2016; Charles et al. 2017). KLEE was established in 1995 at the Mpala Research Centre (MRC) in central Kenya and consists of treatments that exclude different combinations of cattle and large vertebrate herbivores (Young et al. 1998). The KLEE treatments provide an ideal experiment to investigate how herbivory (or lack thereof) affects plant–AMF functional relationships.
Materials and methods
To test our hypotheses, we collected root and rhizosphere samples from three exclosure treatments that vary in herbivore access in the Kenya Long-term Exclosure Experiment (KLEE) located at the Mpala Research Centre in central Kenya. Resident wild mammalian herbivores include many species of large ungulates, including mega-herbivores (for a full description, see Young et al. 1998). In addition to large, wild herbivores, the area is also managed for grazing cattle (Bos taurus indicus L). The exclosures were established in 1995 and consist of six 200 m2 plots that exclude different combinations of large herbivores, replicated in three blocks (Young et al. 1998). The plots represent a gradient of herbivore access and provide an ideal experiment to investigate how herbivory (or lack thereof) affects plant–AMF associations.
We collected samples from three exclosures: (1) plots where wild large herbivores and cattle were allowed (MWC); (2) plots where only wild large herbivores were allowed (MW); and (3) plots that excluded all large herbivores (0). We assessed AMF diversity in field-collected roots from the different herbivory treatments, and assessed the influence of AMF from these exclosures on plant growth and tolerance to herbivory, by conducting a simulated herbivory experiment in the greenhouse using Themeda triandra Forssk (hereafter Themeda), a grass abundant throughout the area (Young et al. 1998). We also complimented the greenhouse experiment by clipping Themeda in the same exclosures in the field.
AMF diversity
To examine AMF diversity, we sampled Themeda roots in June 2012 in each of the three treatment plots at 10-m intervals along 50-m transects. Sampling was replicated in each block such that 135 root samples were collected (N = 45 per treatment). We used terminal restriction fragment length polymorphism (T-RFLP) of PCR-amplified large ribosomal subunit rRNA-gene DNA gene fragments (Verbruggen et al. 2010) to characterize the AMF communities in the roots. To isolate fungal DNA, we performed a PCR using primer pair LR1-FLR2, followed by a nested PCR to isolate AMF DNA, using primer pair FLR3–FLR4 labeled with fluorescent dyes 6-FAM (FLR3) and VIC (FLR4), for visualization in the fragment analysis step.
Data were analyzed using Peak Scanner (v1.0, Applied Biosystems; Foster City, CA), and the T-REX web application (Culman et al. 2009). To assess T-RF community composition, we performed a nonparametric MANOVA (PERMANOVA), using the ADONIS function in the vegan package of R (version 3.3.1) (PERMANOVA; Anderson 2001; Oksanen et al. 2011). Data were visualized using non-metric multidimensional scaling (metaMDS function in R, version 2.13.1). We used a nested ANOVA with herbivory treatment as the main fixed effect, treatment nested within replication block and transect nested within treatment and species richness as the response variable. For a more detailed description of field sampling, sample preparation, and analyses see Methods Supplement (Online Resource).
Effects of herbivory history on plant growth, tolerance of clipping, and leaf P
To obtain experimental inoculum from the field, Themeda rhizospheres were collected in May 2011 from herbivory treatments in the North block of KLEE by placing a 10 × 10 cm square frame around Themeda plants and extracting the soil and roots within that frame to a depth of 15 cm. The rhizospheres of two neighboring plants were sampled from three different locations selected haphazardly within each herbivory treatment (six samples per herbivory treatment). Samples were air-dried and shipped in sealed, plastic bags to the University of Vermont. To stimulate production of viable AMF propagules for use as experimental inocula, we prepared trap cultures using sorghum-sudan (Sorghum bicolor var sudanense) as a host (Morton et al. 1995). For a detailed description of trap pot preparation, see Methods Supplement (Online Resource).
We also collected Themeda seeds from a large area (~ 4 ha) adjacent to the KLEE experimental plots, where plants experience natural levels of herbivory by both wild herbivores and cattle. Seeds were germinated in a growth chamber using a 12L:12D photoperiod cycle with temperatures of 30 °C day and 20 °C night. Seedlings were randomly assigned to one of three inoculum source treatments (MWC, MW, or O); and one of two AMF treatments consisting of (1) a 300 mL sterilized sand:clay substrate amended with 10 mL live inoculum (AMF +) and (2) 300 mL sterilized sand:clay substrate amended with 10 mL sterilized inoculum (AMF −; autoclaved at 121 °C for 60 min), and one of two clipping treatments (clipped or non-clipped), resulting in 12 treatment combinations. Twenty milliliter of microbial filtrate was added back to pots to control for the effects of non-AMF soil microbes (Koide and Li 1989). Nineteen plants were used for each treatment combinations (228 plants).
To simulate herbivory, we clipped plants to 2.5 cm in height after 7 weeks, and again 10 weeks later. Clipped material was weighed after drying at 65 °C for 72–84 h. Because it was impossible to process all plants at once, plants were harvested over a 2-week period after randomly assigning harvest date. The total length of the experiment was thus 22–24 weeks per plant. For each plant, 0.15 g of roots were collected haphazardly to assess AMF colonization, and stored in 70% ethanol at 4 °C until processing. The remaining roots, as well as aboveground material were dried (65 °C for 72–84 h) and weighed. The total root dry weight was calculated by applying the dry:wet ratio of the oven-dried roots to the roots separated for assessment. Total accumulated biomass (biomass at harvest combined with material collected at clipping) and root:shoot ratio at harvest were then calculated.
We also analyzed leaf phosphorous (P) concentration as a proxy for nutritional benefit of the AMF symbiosis. For several clipped plants, we had insufficient leaf material to conduct the analysis and thus randomly paired plants within treatments and combined their leaf material, resulting in 7–9 samples per treatment combination. Nutrient analysis was conducted by the University of Vermont Agriculture and Environmental Testing Laboratory by nitric acid digestion and inductively coupled plasma-optical emission spectrometry. For a detailed description of experimental preparation, see Methods Supplement (Online Resource).
Our statistical approach involved a combination of a priori LS Means Contrasts conducted within ANCOVA frameworks, designed to compare the effect of AMF inoculation within herbivory treatments (AMF + vs AMF −), and a posteriori (post hoc) Tukey’s HSD tests to evaluate differences in treatment means for effects found to be significant, but not the object of our study, and so for which we had no specific hypotheses. We evaluated the influence of AMF from areas of differing herbivory histories on plant total accumulated biomass, leaf P, and root:shoot ratio using three-way ANCOVAs with inoculum source (MWC, MW, O), AMF treatment (AMF + and AMF −), clipping (non-clipped and clipped), and their interactions as effects. Harvest date was included as a covariate in biomass analyses but not in leaf P as plants from different harvest dates had been combined. To evaluate the effect of AMF on plant growth and response to clipping, differences in AMF + and AMF − treatment means within each inoculum source were evaluated by a priori LS Means Contrasts for both non-clipped and clipped plants separately. For leaf P, the clipping effect was not significant and did not interact with inoculum source, and so we conducted the LS Means Contrasts inclusive of non-clipped and clipped plants together, and compared leaf P among inoculum sources (MWC, MW, O) by conducting a post hoc Tukey’s HSD (main effect of inoculum source) within the three-way ANCOVA framework. Total biomass and leaf P data fit assumptions of normality of residuals and homogeneity of variance reasonably well and no transformation improved assumptions, so raw values were used in the analysis. Root:shoot values were square root transformed to improve assumptions.
Effects of herbivory history on mycorrhizal colonization of roots
To assess AMF colonization, roots were stained using 5% ink and vinegar solution (v/v) (Sheaffer Skrip Bottled Ink, black, product SHF94231, Sheaffer Pen, Shelton, CT, USA; methods in Vierheilig et al. 1998). The percentage of root length colonized was quantified using the gridline intersect method (Giovannetti and Mosse 1980), examining over 100 intersections per plant. Percent root length colonization (PRLC) was calculated as the percentage of intersections containing any apparent mycorrhizal structure (referred to as non-negative intersections). Arbuscules were rarely observed in Themeda roots; therefore, arbuscules, arbusculate coils, and coiled hyphae were tabulated together and referred to as percent “nutrient exchange site colonization” (NEXC), calculated as the percentage of non-negative intersections containing these structures. Spherical and oblong vesicle-like structures observed within roots were likewise tabulated together as percent vesicle colonization (VC). We evaluated mycorrhizal colonization (PRLC, NEXC, VC) using separate two-way ANCOVAs, with inoculum source (MWC, MW, O), clipping (non-clipped and clipped), and their interaction as effects, and harvest date as a covariate. The inoculum source by clipping interaction was not significant for any response; we thus compared inoculum sources (MWC, MW, O) using post hoc Tukey’s HSD (main effect of inoculum source). PRLC was logit transformed to improve normality. All analyses were performed in JMP® Pro version 11.2.0 (SAS Institute Inc, 2013).
Field clipping experiment
To examine the response of Themeda to simulated herbivory in the field, we clipped plants on June 4th, 2011 in the aforementioned herbivory treatments in the North and South blocks. Three different locations were selected haphazardly within each plot and at each location we randomly selected eight plants. We clipped half of the plants to 3 cm (N = 24, per treatment), leaving the remainder as controls. Plant height was measured prior to clipping and 10 days post-clipping. Ten days provided sufficient time for plants to regrow, but avoided confounding results of clipping with natural herbivory. Relative growth rate (RGR) was calculated as: \(\ln (H_{2} ) - \ln (H_{1} )/(t_{2} - t_{1} )\), where H1 and H2 are the plant height at time t1 and t2, with t1 being the start of the experiment and t2 the end of the experiment (10 days later). Many of the non-clipped controls did not grow appreciably over a 10-day period (i.e., zero growth rate), and thus our data did not fit the assumptions to perform an ANOVA. We therefore analyzed the results using a hurdle model compartmentalized into a logistic regression and a generalized linear model. To perform the logistic regression, we re-coded our data as 0 if the plant grew less than 0.001 cm per day and as a 1 if the plant grew more than 0.001. We used a model selection process to evaluate the effects of clipping and herbivory on whether the plant grew (1) or not (0). The interaction term (clipping × herbivory) was not tested for lack of sufficient zero values in all categories. In the second stage of the hurdle model, we analyzed only plants that grew during our experimental period using a generalized linear model. In the GLM model, we evaluated the effects of clipping and herbivory and the interaction between clipping and herbivory. In both models we treated clipping, herbivory, and KLEE block as fixed effects. To avoid pseudoreplication, sampling location was treated as a random effect nested within herbivory treatment and location. Analyses were performed in R version 3.3.1 (R Development Core Team 2016) using packages lme4 (Bates et al. 2014) for the logistic regression and nlme (Pinheiro et al. 2017) for the GLM.
Results
Effect of herbivory history on AMF diversity
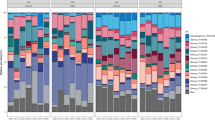
The history of herbivory had no significant effect on AMF T-RF community composition (PERMANOVA: F(2,24) = 0.68 P = 0.86, Supp Fig. S1a, Online Resource). Likewise, in all three treatments, we found approximately equal T-RF richness (approximately 13 T-RFs per treatment; ANOVA: F(2,113) = 0.17, P = 0.85, Supp Fig. S1b, Online Resource) (Fig. 1).
Mycorrhizal colonization. AMF from areas of different grazing histories exhibited different patterns of plant root colonization in both the extent (PRLC) and composition (NEXC and VC) of colonization. Two-way ANCOVA, main effect of inoculum source: PRLC: F(2,101) = 22.14, P < 0.0001; NEXC: F(2,101) = 19.42, P < 0.0001; VC: F(2,101) = 34.59, P < 0.0001. Treatments not sharing same letter are significantly different (Tukey’s HSD within two-way ANOVA framework). Bars represent LS means ± 1 SE
AMF effects on plant growth
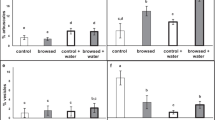
The main effects of herbivory history (inoculum source) and AMF inoculation (AMF treatment) were significant for all responses. For ease of interpretation, we report here significant higher order interactive effects, as well as results of LS Means Contrasts, and main effects where interactions were not significant. The table of full statistical results can be found in Supp Table S1, Online Resource). The effect of AMF on total accumulated biomass depended upon herbivory history (three-way ANCOVA, inoculum source × AMF interaction, F(2,197) = 6.31, P = 0.002). Among plants in the non-clipped treatment, plants grown with AMF + inoculum from MWC produced more total biomass than plants grown in sterilized, AMF −, inoculum (Fig. 2a, LS Means Contrasts, F(1,197) = 12.54, P = 0.0005). However, the differences in non-clipped plant biomass between the AMF + or AMF − treatments for MW or O plots were not significant (Fig. 2a, LS Means Contrasts, MW: F(1,197) = 0.28, P = 0.60), O: F(1,197) = 0.44, P = 0.51).
Total accumulated biomass and root:shoot ratio at harvest of non-clipped plants (left panels) and clipped plants (right panels). Asterisks indicate difference in AMF + vs AMF − means within KLEE treatment (LS Means Contrasts conducted within three-way ANOVA framework, P < 0.05). Bars represent LS means ± 1 SE
The effect of AMF on root:shoot ratio at harvest also depended on herbivory history (three-way ANCOVA, inoculum source × AMF interaction F(2,197) = 7.23, P = 0.0009). Among plants in the non-clipped treatment, plants grown with AMF + inoculum from MW had higher root:shoot ratios than plants grown in sterilized, AMF −, MW inoculum (Fig. 2b, LS Means Contrasts, F(1,197) = 27.10, P < 0.0001), whereas root:shoot ratios of plants grown in MWC and O inoculum were not affected by AMF (Fig. 2b, LS Means Contrasts, MWC: F(1,197) = 1.63, P = 0.20), O: F(1,197) = 1.26, P = 0.26).
AMF effects on plant response to clipping
Clipped plants accumulated less total biomass than non-clipped plants (three-way ANCOVA, main effect of clipping, F(1,197) = 51.14, P < 0.0001), regardless of herbivory history (KLEE × clipping interaction, F(1,197) = 1.42, P = 0.24) or AMF inoculation (AMF treatment × clipping interaction, F(1,197) = 0.57, P = 0.45). However, among clipped plants, plants grown with AMF + inoculum from MW produced more total biomass than plants grown in sterilized, AMF −, MW inoculum (Fig. 2c, LS Means Contrasts, F(1,197) = 4.18, P = 0.042), whereas total biomass accumulation did not differ between the AMF + or AMF − treatments for MWC or O plots (Fig. 2a, LS Means Contrasts, MWC: F(1,197) = 1.30, P = 0.25), O: F(1,197) = 2.50, P = 0.12).
Root:shoot ratios were also affected by clipping (F(1,197) = 91.51, P < 0.0001), and were dependent on both herbivory history and AMF inoculation (significant three-way interaction of inoculum source × AMF treatment × clipping: F(2,197) = 5.18, P < 0.006). Among clipped plants, plants grown with AMF + inoculum from MWC and MW had higher root:shoot ratios than plants grown in sterilized, AMF −, MWC and MW inoculum (Fig. 2d, LS Means Contrasts, F(1,197) = 26.66, P < 0.0001 and F(1,197) = 9.12, P < 0.003, respectively), whereas root:shoot ratio was not affected by AMF in the O plot (Fig. 2d, LS Means Contrasts, F(1,197) = 0.09, P = 0.76).
Effects of herbivory history on leaf phosphorous accumulation
Plants grown in inoculum from areas where all large herbivores were excluded (O), accumulated less leaf P than plants grown in inoculum from areas where large herbivores were allowed (main effect of inoculum source, F(2,84) = 22.41, P < 0.0001; Tukey’s HSD). Regardless of inoculum source, plants grown with AMF had higher concentrations of leaf P at harvest than plants grown without AMF (Fig. 3, F(1,84) = 103.57; P = 0.0001, and LS Means Contrasts: MWC: F(1,84) = 42.87, P < 0.0001), MW: F(1,84) = 43.23, P < 0.0001), O: F(1,84) = 19.98, P < 0.0001). The effect of clipping on leaf P was dependent on AMF inoculation (clipping × AMF treatment interaction: F(1,84) = 13.36; P = 0.0004); when grown without AMF, clipped plants had less leaf P relative to non-clipped plants, whereas when grown with AMF, clipped plants did not differ from non-clipped plants in leaf P concentration.
Leaf phosphorous concentration. Asterisks indicate difference in AMF + vs AMF − means within KLEE treatment (LS Means Contrasts conducted within three-way ANOVA framework, P < 0.05). Inoculum source groupings not sharing same letter are significantly different (Tukey’s HSD). Bars represent LS means ± 1 SE
Effects of herbivory history on mycorrhizal colonization of roots
AMF from areas of different herbivory histories exhibited different patterns of plant root colonization in both the extent (PRLC) and composition (NEXC and VC) of colonization in the clipping experiment (Fig. 1, Two-way ANCOVA, with main effects of inoculum source: PRLC: F(2,101) = 22.14, P < 0.0001; NEXC: F(2,101) = 19.42, P < 0.0001; VC: F(2,101) = 34.59, P < 0.0001). Root colonization intensity differed among the three inoculum sources (Tukey’s HSD, Fig. 1a); with AMF from MWC having the greatest extent of percent of root length colonized, and MW the lowest. The composition of colonization (NEXC and VC) was similar for AMF from MWC and O, but MW had a significantly higher percentage of nutrient exchange sites (Fig. 1b), and fewer vesicles (Fig. 1c) compared to MWC and O (Tukey’s HSDs). Mycorrhizal colonization of roots was also effected by clipping, in that clipped plants had more nutrient exchange sites than non-clipped plants (Fig. 1b, inset, two-way ANCOVA, main effect of clipping, F(1,101) = 10.77, P = 0.0014).
Field clipping experiment
Results from our field clipping experiments were in line with those found in the greenhouse. Clipping significantly enhanced growth rate of plants across all treatments (logistic regression, main effect of clipping, Χ2 (1) = 68.73, P < 0.0001). Furthermore, clipped plants growing in areas with only wild herbivores (MW) had significantly higher growth rates than clipped plants growing in areas with wild herbivores plus cattle (MWC) and in areas with no herbivores (O) (Fig. 4, GLM, clipping × herbivory interaction, F(2,81) = 14.30, P < 0.0001).
Discussion
The outcome of the association between arbuscular mycorrhizal fungi and their hosts is dynamic, with the quality, quantity, and timing of resource exchange linked to environmental context (Klironomos 2002, 2003; Wardle et al. 2004; Ji et al. 2010, 2013; Johnson et al. 2010; Bever 2015). Recent evidence suggests that adaptation on a highly localized scale can alter the AMF–plant relationship in ways that benefit both partners (Johnson et al. 2010; Ji et al. 2013; Petipas et al. 2017).
Our objective was to determine whether herbivory by large, vertebrate herbivores has resulted in locally adapted symbioses that enhance plant tolerance of herbivore damage and whether removal or a reduction of herbivory results in a response by the AMF community. We predicted that AMF diversity and/or functionality would be altered by long-term differences in vertebrate herbivory in ways that would affect plant tolerance to herbivore damage. Although we did not detect differences in AMF species diversity among herbivory treatments in field collections, our findings suggest that, indeed, different levels and/or types of grazing has resulted in functional changes in AMF communities that affect plant growth, investment in roots versus shoots, P accumulation, and response to herbivory, although in ways not fully aligned with our expectations. These results are consistent with earlier work that demonstrated that species richness between fenced and unfenced areas was similar, but AMF infectivity was higher in areas with herbivore access (Petipas and Brody 2014).
In the current study, we found that AMF root colonization, biomass production, root:shoot ratios, leaf P uptake, and plant response to clipping were all affected by AMF from soils with different levels of herbivory. These functional differences may be attributable to rapid diversification or plasticity among fungal genotypes in response to the different environmental conditions within the exclosures. Such rapid genetic change has been demonstrated and may underlie the ubiquity and tremendous success of AMF in responding to environmental change (Angelard et al. 2014). Thus, our assessment of genetic diversity may have been too coarse to elucidate functional diversity which can occur within species or even among nucleotypes of the same AMF clone (Koch et al. 2004, 2006; Johnson et al. 2012).
We further predicted that AMF from areas in which large herbivores have access, would enhance tolerance to herbivory, whereas AMF from areas protected from large herbivores would not. Unexpectedly, the addition of cattle appears to have a different effect on the functioning of AMF than that of the wild herbivores alone, differing in every response measured in our study from those of plots grazed by only wild herbivores. Plants inoculated with AMF from areas where cattle graze along with wild herbivores (MWC), had a lower proportion of nutrient exchange sites with their fungal partners and a greater proportion of fungal vesicles—characteristics that are generally interpreted as less beneficial to plant hosts (Johnson 1993; Johnson et al. 1997; Nijjer et al. 2010; Verbruggen and Kiers 2010; Kiers et al. 2011; Knegt et al. 2016). Despite this, plants grown with AMF from MWC clearly benefited from the symbiosis in the production of more total biomass and increased leaf P uptake. However, these benefits did not translate into a commensurate response to clipping.
In contrast, plants inoculated with AMF from areas where only wild herbivores have access (MW), had higher proportions of nutrient exchange sites, and the lowest proportion of vesicles, suggesting a more beneficial association for the plant. While no benefit of the association was evident in terms of biomass production in non-clipped plants, plants inoculated with AMF from MW had greater overall biomass when clipped, relative to plants grown without AMF. Interestingly, these plants had consistently higher root:shoot ratios regardless of clipping status; an effect also observed in clipped plants of the MWC treatment, but not in plants grown with AMF from areas protected from large herbivores. Plasticity in plant resource partitioning may enable plants to mediate nutrient limitation (Weiner 2004), and thus infection by AM fungi could lead to decreased allocation of resources to root growth (e.g., reduced root/shoot ratio). However, the influence of AMF on plant resource partitioning depends on many factors, including the species of plant and AM fungi, and the surrounding biotic and abiotic environment (Veresoglou et al. 2012), and is thus context dependent. Here we found that AM fungi from areas where ungulates graze cause increases in root/shoot ratios, whereas AM fungi without historical exposure to herbivory do not affect root/shoot ratio. This result is important because roots can be a source of nutrients for remobilization during regrowth (Ourry et al. 1988; Oyarzabal and Oesterheld 2009), and thus enhance plant regrowth following herbivory. Our results suggest that this strategy may be mediated by grazing-adapted mycorrhizal fungi. Despite not having an increase in overall biomass when mycorrhizal, AMF associations in MW may be facilitating compensation by posturing the plant to withstand herbivory prior to clipping by enhancing root/shoot ratios and increasing P accumulation.
The addition of cattle to areas in which large wild herbivores are present appears to have a different effect on the functioning of AMF than that of the wild herbivores alone. While these differences cannot be explained by dung deposition (Kimuyu et al. 2017), or soil pH or nutrient status (unpublished data), they may be driven by differences in grazing pressure among treatments (Veblen et al. 2016) and/or by changes in the plant host community. Cattle grazing can enhance plant species diversity and aggregation (Porensky et al. 2013), and reduce grass and forb cover (Young et al. 2005; Kimuyu et al. 2017); suggesting that Themeda may be involved in different plant–plant competitive interactions under different herbivore regimes. Themeda triandra is a weak competitor which thrives in areas of reduced competition. In those areas it may rely less on its fungal symbionts for access to resources. Alternatively, enhanced grazing pressure by cattle could increase soil compaction and/or carbon dynamics via the amount of plant material removed at each grazing bout, which could affect AM functionality (Entry et al. 2002; Wearn and Gange 2007).
Our prediction that T. triandra grown in inoculum with AMF from areas where vertebrate herbivores were excluded would show a lower tolerance of herbivory than those from the other treatments was largely supported. Not only did these plants show a lack of tolerance to clipping, they also showed less beneficial composition of AMF colonization (lower nutrient exchange sites, and higher proportion of vesicles), and were unresponsive to AMF colonization in terms of biomass production or plant resource partitioning. Although they displayed the general increase in leaf P accumulation associated with AMF that was observed in all treatments, the plants in O had lower overall leaf P than plants in either MWC or MW.
Surprisingly, plants inoculated with non-AMF microbes (as a microbial wash) from areas where herbivores have been excluded consistently grew the largest regardless of AMF inoculation status. As plants grown with AMF from this area had lower numbers of nutrient exchange sites, we speculate that they may have been released from the resource costs of AMF association, and instead may interact with beneficial non-AMF microbes, resulting in enhanced plant growth in the competitive environment. In our study, we sought to uncover how grazing history has influenced plant–AMF associations; however, it is likely that the gradient of grazing intensity established in the KLEE plots has affected other non-AMF microbial associations with plants as well. For example, Eldridge et al. (2017) recently showed that grazing affects both the bacterial and fungal communities, favoring some taxa over others and altering community composition. Eldridge et al. (2017) found that the diversity of soil bacterial communities increased while the diversity of fungal communities decreased with increased grazing intensity in semi-arid woodlands of eastern Australia. Further, dark septate root endophytes may increase (Saravesi et al. 2014) or decrease (Piippo et al. 2011; Ruotsalainen and Eskelinen 2011) with herbivory. However, to date there is limited information on how dark septate endophytes limit or enhance plant response to herbivory. While information regarding the non-AMF microbial contingent would have undoubtedly been informative, assessment of non-AMF microbial diversity was outside the scope of this study, and is currently being investigated by other researchers in this system (Kelly Gravuer and Grace Charles, pers. comm.). The specificity and functional outcome of changes in these communities under different grazing pressure will be exciting areas for future work.
In summary, our findings suggest that differences in large herbivore grazing for nearly two decades has resulted in functional changes in AMF communities that feedback to plant growth, P accumulation, and response to herbivory. There is growing evidence for ecological history giving rise to locally adapted plant–AMF associations (Johnson et al. 2010; Ji et al. 2013). There is independent evidence that both plants and fungi can mediate nutrient and carbon allocations (Bever et al. 2009; Kiers et al. 2011; Fellbaum et al. 2012), and that the trade between AMF and hosts is influenced by the environmental context (Verbruggen et al. 2012; Zheng et al. 2015; Klironomos 2002, 2003; Ji et al. 2010, 2013; Johnson et al. 2010; Bever 2015). Thus, the ability of plants to select for mycorrhizal partners that enhance growth or tolerance to stress such as chronic and repeated herbivory, may allow AMF associations to become optimized to local conditions (Read 2002; Klironomos 2003; Friesen et al. 2011; Revillini et al. 2016).
Our results and those of others suggest that changes in the herbivore community and its cascading effects on plant communities and carbon and nutrient status, all influence the strength and direction of feedbacks between plants and their mycorrhizal partners (Bardgett and Wardle 2003; Kula and Hartnett 2015; Eldridge et al. 2017). Our work also underscores that of others in demonstrating the importance of grazing history in affecting AMF functional associations and suggests ecological specificity among them (Read 2002; Johnson et al. 2010; Antunes et al. 2011; Ruotsalainen and Eskelinen 2011). Furthering our understanding of how long-term, rapid evolutionary changes affect interactions in below and aboveground communities, is an important frontier that will allow us to predict community response to change (van der Putten et al. 2013; Eldridge et al. 2017; Smith-Ramesh and Reynolds 2017). Future work that investigates the influence of microbial communities, and AMF in particular, on plant responses to herbivores will be well-served by considering the long-term, ecological context of the interactions.
References
A’Bear AD, Johnson SN, Jones TH (2014) Putting the “upstairs–downstairs” into ecosystem service: what can aboveground–belowground ecology tell us? Biol Control 75:97–107
Aguilar-Chama A, Guevara R (2012) Mycorrhizal colonization does not affect tolerance to defoliation of an annual herb in different light availability and soil fertility treatments but increases flower size in light-rich environments. Oecologia 168(1):131–139
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Angelard C, Tanner CJ, Fontanillas P, Niculita-Hirzel H, Masclaux F, Sanders IR (2014) Rapid genotypic change and plasticity in arbuscular mycorrhizal fungi is caused by a host shift and enhanced by segregation. ISME J 8(2):284–294
Antunes PM, Koch AM, Morton JB, Rillig MC, Klironomos JN (2011) Evidence for functional divergence in arbuscular mycorrhizal fungi from contrasting climatic origins. New Phytol 189:507–514
Barber NA, Kiers ET, Theis N, Hazzard RV, Adler LS (2013) Linking agricultural practices, mycorrhizal fungi, and traits mediating plant–insect interactions. Ecol Appl 23(7):1519–1530
Bardgett RD, Wardle DA (2003) Herbivore-mediated linkages between aboveground and belowground communities. Ecology 84:2258–2268
Bates D, Maechler M, Bolker B, Walker S (2014) lme4: Linear mixed-effects models using Eigen and S4. R Pack Vers 1(7):1–23
Bennett AE, Bever JD (2007) Mycorrhizal species differentially alter plant growth and response to herbivory. Ecology 88:210–218
Bever JD (2015) Preferential allocation, physio-evolutionary feedbacks, and the stability and environmental patterns of mutualism between plants and their root symbionts. New Phytol 205:1503–1514
Bever JD, Richardson SC, Lawrence BM, Holmes J, Watson M (2009) Preferential allocation to beneficial symbiont with spatial structure maintains mycorrhizal mutualism. Ecol Lett 12:13–21
Borowicz VA (1997) A fungal root symbiont modifies plant resistance to an insect herbivore. Oecologia 112:534–542
Borowicz VA (2013) The impact of arbuscular mycorrhizal fungi on plant growth following herbivory: a search for pattern. Acta Oecol 52:1–9
Brundrett MC (2002) Coevolution of roots and mycorrhizas of land plants. New Phytol 154:275–304
Brundrett MC (2009) Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320:37–77
Charles GK, Porensky LM, Riginos C, Veblen KE, Young TP (2017) Herbivore effects on herbaceous productivity vary by guild: cattle increase mean productivity while wildlife reduce variability. Ecol Appl 27:143–155
Culman SW, Bukowski R, Gauch HG, Cadillo-Quiroz H, Buckley DH (2009) T-Rex: software for the processing and analysis of T-RFLP data. BMC Bioinform 10:171
Eldridge DJ, Delgado-Baquerizo M, Travers SK, Val J, Oliver I, Hamonts K, Singh BK (2017) Competition drives the response of soil microbial diversity to increased grazing by vertebrate herbivores. Ecology 98:1922–1931
Entry JA, Rygiewicz PT, Watrud LS, Donnelly PK (2002) Influence of adverse soil conditions on the formation and function of arbuscular mycorrhizas. Adv Environ Res 7(1):123–138
Eom A-H, Wilson GWT, Hartnett DC (2001) Effects of ungulate grazers on arbuscular mycorrhizal symbiosis and fungal community structure in tallgrass prairie. Mycologia 93:233–242
Fellbaum CR, Gachomo EW, Beesetty Y, Choudhari S, Strahan GD, Pfeffer PE, Kiers ET, Bücking H (2012) Carbon availability triggers fungal nitrogen uptake and transport in arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci 109:2666–2671
Frank DA, McNaughton SJ, Tracy BF (1998) The ecology of the Earth’s grazing ecosystems. Bioscience 48:513–521
Frank DA, Gehring CA, Machut L, Phillips M (2003) Soil community composition and the regulation of grazed temperate grassland. Oecologia 137:603–609
Friesen ML, Porter SS, Stark SC, von Wettberg EJ, Sachs JL, Martinez-Romero E (2011) Microbially mediated plant functional traits. Annu Rev Ecol Evol S 42:23–46
Gange AC (2007) Insect-mycorrhizal interactions: patterns, processes, and consequences. In: Ohgushi T, Craig TP, Price PW (eds) Ecological communities: plant mediation in indirect interaction webs, vol 6. Cambridge University Press, New York, pp 124–144
Gange AC, Bower E, Brown VK (2002) Differential effects of insect herbivory on arbuscular mycorrhizal colonization. Oecologia 131:103–112
Gange AC, Brown VK, Aplin DM (2005) Ecological specificity of arbuscular mycorrhizae: evidence from foliar- and seed-feeding insects. Ecology 86:603–611
Garrido E, Bennett AE, Fornoni J, Strauss SY (2010) Variation in arbuscular mycorrhizal fungi colonization modifies the expression of tolerance to above-ground defoliation. J Ecol 98(1):43–49
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
González JB, Clarke GL, Brody AB (2015) Lack of sex-specific differences in mycorrhizal associations and response to herbivory in the gynodioecious herb, Polemonium foliosissimum. Plant Ecol 216:951–962
Hartley SE, Gange AC (2009) Impacts of plant symbiotic fungi on insect herbivores: mutualism in a multitrophic context. Annu Rev Entomol 54:323–342
Hobbs NT (1996) Modification of ecosystems by ungulates. J Wildl Manag 60:695–713
Hoeksema JD, Chaudhary VB, Gehring CA, Johnson NC, Karst J, Koide RT, Wilson GW (2010) A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol Lett 13(3):394–407
Ji B, Bentivenga SP, Casper BB (2010) Evidence for ecological matching of whole AM fungal communities to the local plant–soil environment. Ecology 91:3037–3046
Ji B, Gehring CA, Wilson GWT, Miller RM, Flores-Rentería L, Johnson NC (2013) Patterns of diversity and adaptation in Glomeromycota from three prairie grasslands. Mol Ecol 22:2573–2587
Johnson NC (1993) Can fertilization of soil select less mutualistic mycorrhizae? Ecol Appl 3:749–757
Johnson NC, Graham JH, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism–parasitism continuum. New Phytol 135:575–585
Johnson NC, Wilson GWT, Bowker MA, Wilson JA, Miller RM (2010) Resource limitation is a driver of local adaptation in mycorrhizal symbioses. Proc Natl Acad Sci 107:2093–2098
Johnson D, Martin F, Cairney JW, Anderson IC (2012) The importance of individuals: intraspecific diversity of mycorrhizal plants and fungi in ecosystems. New Phytol 194(3):614–628
Kiers ET, Duhamel M, Beesetty Y, Mensah JA, Franken O, Verbruggen E, Fellbaum CR, Kowalchuk GA, Hart MM, Bago A, Palmer TM, West SA, Vandenkoornhuyse P, Jansa J, Bucking H (2011) Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 333:880–882
Kimuyu DM, Veblen KE, Riginos C, Chira RM, Githaiga JM, Young TP (2017) Influence of cattle on browsing and grazing wildlife varies with rainfall and presence of megaherbivores. Ecol Appl 27:786–798
Klironomos JN (2002) Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 418:67–70
Klironomos JN (2003) Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 84:2292–2301
Knegt B, Jansa J, Franken O, Engelmoer DJ, Werner GD, Bücking H, Kiers ET (2016) Host plant quality mediates competition between arbuscular mycorrhizal fungi. Fungal Ecol 20:233–240
Koch AM, Kuhn G, Fontanillas P, Fumagalli L, Goudet J, Sanders IR (2004) High genetic variability and low local diversity in a population of arbuscular mycorrhizal fungi. Proc Natl Acad Sci 101(8):2369–2374
Koch AM, Croll D, Sanders IR (2006) Genetic variability in a population of arbuscular mycorrhizal fungi causes variation in plant growth. Ecol Lett 9(2):103–110
Koide RT, Li M (1989) Appropriate controls for vesicular–arbuscular mycorrhiza research. New Phytol 111:35–44
Kula AAR, Hartnett DC (2015) Effects of mycorrhizal symbiosis on aboveground arthropod herbivory in tallgrass prairie: an in situ experiment. Plant Ecol 216(4):589–597
Laird RA, Addicott JF (2007) Arbuscular mycorrhizal fungi reduce the construction of extrafloral nectaries in Vicia faba. Oecologia 152(3):541–551
McNaughton SJ (1985) Ecology of a grazing ecosystem: the Serengeti. Ecol Monogr 55:259–294
Middleton EL, Richardson S, Koziol L, Palmer CE, Yermakov Z, Henning JA, Bever JD (2015) Locally adapted arbuscular mycorrhizal fungi improve vigor and resistance to herbivory of native prairie plant species. Ecosphere 6(12):1–16
Morton JB, Bentivenga SP, Bever JD (1995) Discovery, measurement, and interpretation of diversity in arbuscular endomycorrhizal fungi (Glomales, Zygomycetes). Can J Bot 73(Suppl 1):S25–S32
Murray TR, Frank DA, Gehring CA (2010) Ungulate and topographic control of arbuscular mycorrhizal fungal spore community composition in a temperate grassland. Ecology 91:815–827
Nijjer S, Rogers WE, Siemann E (2010) The impacts of fertilization on mycorrhizal production and investment in western gulf coast grasslands. Am Midl Nat 163:124–133
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson PR, Solymos P, Stevens HH, Szoecs E, Wagner H (2011) Vegan: community ecology package. R package version 20-2
Ourry A, Boucaud J, Salette J (1988) Nitrogen mobilization from stubble and roots during re-growth of defoliated perennial ryegrass. J Exp Bot 39(6):803–809
Oyarzabal M, Oesterheld M (2009) Phosphorus reserves increase grass regrowth after defoliation. Oecologia 159:717–724
Petipas RH, Brody AK (2014) Termites and ungulates affect arbuscular mycorrhizal richness and infectivity in a semiarid savanna. Botany 92(3):233–240
Petipas RH, González JB, Palmer TM, Brody AK (2017) Habitat-specific AMF symbioses enhance drought tolerance of a native Kenyan grass. Acta Oecol 78:71–78
Piippo S, Markkola A, Härmä E, Tuomi J (2011) Do compensatory shoot growth and mycorrhizal symbionts act as competing above- and below-ground sinks after simulated grazing? Plant Ecol 212:33–42
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2017) nlme: Linear and nonlinear mixed effects models. R package version 31-131
Porensky LM, Wittman SE, Riginos C, Young TP (2013) Herbivory and drought interact to enhance spatial patterning and diversity in a savanna understory. Oecologia 173:591–602
Pringle RM, Palmer TM, Goheen JR, McCauley DJ, Keesing F (2011) Ecological importance of large herbivores in the Ewaso ecosystem. Smithson Control Zool 632:43–54
R Development Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Read DJ (2002) Towards ecological relevance—progress and pitfalls in the path towards an understanding of mycorrhizal functions in nature. In: van der Heijden MGA, Sanders IR (eds) Mycorrhizal ecology. Springer, Berlin, pp 3–29
Redecker D, Morton JB, Bruns TD (2000) Ancestral lineages of arbuscular mycorrhizal fungi (Glomales). Mol Phylogenet Evol 14(2):276–284
Remy W, Taylor TN, Hass H, Kerp H (1994) Four hundred-million-year-old vesicular arbuscular mycorrhizae. Proc Natl Acad Sci 91:11841–11843
Revillini D, Gehring CA, Johnson NC (2016) The role of locally adapted mycorrhizas and rhizobacteria in plant–soil feedback systems. Funct Ecol 30(7):1086–1098
Ruotsalainen AL, Eskelinen A (2011) Root fungal symbionts interact with mammalian herbivory, soil nutrient availability and specific habitat conditions. Oecologia 166(3):807–817
Saravesi K, Ruotsalainen AL, Cahill JF (2014) Contrasting impacts of defoliation on root colonization by arbuscular mycorrhizal and dark septate endophytic fungi of Medicago sativa. Mycorrhiza 24:239–245
Smith SE, Read D (2008) Mycorrhizal symbiosis, 3rd edn. Academic Press, Amsterdam, p 787
Smith-Ramesh LM, Reynolds HL (2017) The next frontier of plant–soil feedback research: unraveling context dependence across biotic and abiotic gradients. J Veg Sci 28(3):484–494
Tao L, Ahmad A, Roode JC, Hunter MD (2016) Arbuscular mycorrhizal fungi affect plant tolerance and chemical defences to herbivory through different mechanisms. J Ecol 104(2):561–571
van der Heyde M, Bennett JA, Pither J, Hart M (2017) Longterm effects of grazing on arbuscular mycorrhizal fungi. Agric Ecosyst Environ 243:27–33
van der Putten WH, Bardgett RD, Bever JD, Bezemer TM, Casper BB, Fukami T, Kardol P, Klironomos JN, Kulmatiski A, Schweitzer JA, Suding KN, van de Voorde TFJ, Wardle DA (2013) Plant–soil feedbacks: the past, present and future challenges. J Ecol 101:265–276
van der Putten WH, Bradford MA, Pernilla Brinkman E, Voorde TF, Veen GF (2016) Where, when and how plant–soil feedback matters in a changing world. Funct Ecol 30(7):1109–1121
Varga S, Kytöviita MM, Siikamäki P (2009) Sexual differences in response to simulated herbivory in the Gynodioecious herb Geranium sylvaticum. Plant Ecol 202(2):325–336
Veblen KE, Young TP (2010) Contrasting effects of cattle and wildlife on the vegetation development of a savanna landscape mosaic. J Ecol 98:993–1001
Veblen KE, Porensky LM, Riginos C, Young TP (2016) Are cattle surrogate wildlife? Savanna plant community composition explained by total herbivory more than herbivore type. Ecol Appl 26:1610–1623
Vega-Frutis R, Guevara R (2013) Greater mycorrhizal colonization of unisexual morphs than of hermaphroditic morphs of Jacaratia mexicana during flowering and fruiting in central Mexico. Symbiosis 59:173–181
Verbruggen E, Kiers ET (2010) Evolutionary ecology of mycorrhizal functional diversity in agricultural systems. Evol Appl 3:547–560
Verbruggen E, Röling WFM, Gamper HA, Kowalchuk GA, Verhoef HA, van der Heijden MGA (2010) Positive effects of organic farming on below-ground mutualists: large-scale comparison of mycorrhizal fungal communities in agricultural soils. New Phytol 186:968–979
Verbruggen E, Mouden CE, Jansa J, Akkermans G, Bücking H, West SA, Kiers ET (2012) Spatial structure and interspecific cooperation: theory and an empirical test using the mycorrhizal mutualism. Am Nat 179:E133–E146
Veresoglou SD, Menexes G, Rillig MC (2012) Do arbuscular mycorrhizal fungi affect the allometric partition of host plant biomass to shoots and roots? A meta-analysis of studies from 1990 to 2010. Mycorrhiza 22(3):227–235
Vierheilig H, Coughlan AP, Wyss U, Piche Y (1998) Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl Environ Microbiol 64:5004–5007
Wang M, Bezemer TM, van der Putten WH, Biere A (2015) Effects of the timing of herbivory on plant defense and insect performance in ribwort plantain (Plantago lanceolate L) depend on plant mycorrhizal status. J Chem Ecol 41:1006–1017
Wardle DA, Bardgett RD, Klironomos JN, Setälä H, van der Putten WH, Wall DH (2004) Ecological linkages between aboveground and belowground biota. Science 304:1629–1633
Wearn JA, Gange AC (2007) Above-ground herbivory causes rapid and sustained changes in mycorrhizal colonization of grasses. Oecologia 153(4):959–971
Weiner J (2004) Allocation, plasticity and allometry in plants. Perspect Plant Ecol 6(4):207–215
Young TP, Okello B, Kinyua D, Palmer TP (1998) KLEE: a long-term, large scale herbivore exclusion experiment in Laikipia, Kenya. Afr J Range For Sci 14:94–102
Young TP, Palmer TM, Gadd ME (2005) Competition and compensation among cattle, zebras, and elephants in a semi-arid savanna in Laikipia, Kenya. Biol Conserv 122:351–359
Young HS, McCauley DJ, Helgen KM, Goheen JR, Otarola-Castillo E, Palmer TM, Pringle RM, Young TP, Dirzo R (2013) Effects of mammalian herbivore declines on plant communities: observations and experiments in an African savanna. J Ecol 101:1030–1041
Zheng C, Ji B, Zhang J, Zhang F, Bever J (2015) Shading decreases plant carbon preferential allocation towards the most beneficial mycorrhizal mutualist. New Phytol 205:261–368
Acknowledgements
We thank, G. Clarke, C. Armstrong, D. Heleba for help in the greenhouse and comments on JBG’s thesis. Special thanks to JBG’s MSc committee, J.M. Harris and J.O. Vigoreaux, for constructive criticism of the analysis and interpretation of the results, and to A. Howard (UVM) and F. Vermeylen (Cornell) for help with the statistical analysis. We thank J. Morton (INVAM, WVU) for his mentorship in the field of AMF and for constructive remarks on this and other AMF projects. Our work was supported by NSF DEB-0519223 to A.K. Brody. Mpala Research Centre and staff provided logistical support. KLEE was carried out under Government of Kenya research clearance permit No. NCST/RCD/12B/012/42. KLEE was built and maintained by grants from the James Smithson Fund of the Smithsonian Institution (to A.P. Smith), The National Geographic Society (Grants 4691-91 and 9106-12), NSF (LTREB DEB 97-07477, 03-16402, 08-16453, 12-56004, and 12-56034) and the African Elephant Program of the US Fish and Wildlife Service (98210-0-G563) (to T.P. Young, C. Riginos, and K.E. Veblen).
Author information
Authors and Affiliations
Contributions
JBG, RHP and AKB conceived of the ideas, designed and conducted the experiments, and wrote the initial manuscript. OF and ETK provided expertise, laboratory materials and equipment and assistance in the molecular identification of the fungal communities, and helped write the final manuscript. KEV provided expert knowledge of KLEE and helped write the final manuscript.
Corresponding author
Additional information
Communicated by Corné Pieterse.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
González, J.B., Petipas, R.H., Franken, O. et al. Herbivore removal reduces influence of arbuscular mycorrhizal fungi on plant growth and tolerance in an East African savanna. Oecologia 187, 123–133 (2018). https://doi.org/10.1007/s00442-018-4124-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-018-4124-4