Abstract
Accumulation of P above levels that promote growth, a common plant response called “luxury consumption”, can be considered as a form of reserve to support future growth when the nutrient can subsequently be mobilized. However, the effect of P reserves on regrowth following defoliation has not been demonstrated. We tested the hypothesis that P luxury consumption increases plant tolerance to defoliation. We performed two experiments with four grass species from a continuously grazed temperate grassland in the Flooding Pampa (Argentina). The first experiment, aimed at generating P luxury consumption by fertilization, resulted in one species (Sporobolus indicus) showing luxury consumption. In this way, we were able to obtain plants of S. indicus with similar biomass but contrasting amounts of P reserves. The second experiment evaluated the subsequent regrowth following defoliation on a P-free medium of these plants differing in P reserves. Regrowth was larger for plants that had shown P luxury consumption during a previous period than for plants with lower levels of P reserves. During regrowth these plants showed a clear pattern of P remobilization from the stubble, crown, and root compartments to the regrowing tissue, in addition to a likely reutilization of P present in leaf-growth zones. This work is the first showing that high levels of P reserves can confer tolerance to defoliation by promoting compensatory growth under P deficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Accumulation of nutrients above levels that promote growth, a common plant response called “luxury consumption”, can be considered as a form of reserve to support future growth when the nutrient can subsequently be mobilized. Luxury consumption of nutrients takes place when plant growth either does not increase or increases proportionally less than root uptake of nutrients. For example, shoot growth of Agrostis setacea 6 weeks after germination was very similar between control plants and those receiving a tenfold higher rate of P supply; consequently, shoot P concentration increased up to 9 times in plants receiving the increased P (Clarkson 1967). This nutrient accumulation above levels that promote growth occurs because there is only a limited change in root absorption capacity in compensation for changing plant nutrient status, a common response of both wild and cultivated plants following fertilization (Epstein 1972; Chapin 1980; Lipson et al. 1996; Schachtman et al. 1998).
Previously stored elements, such as C and N, may be later mobilized to support growth. Combining defoliation experiments with nutrient content monitoring, several authors quantified the contribution of nutrient mobilization during regrowth following defoliation. Because remobilization and uptake are simultaneous, it was necessary to discriminate between both sources. N is remobilized to support regrowth of the lamina for several weeks, and the relative contribution of roots, rhizomes, crowns, and stubble as sources of N depends both on species and N supply (Millard et al. 1990; Thornton et al. 1993; Thornton and Millard 1993; Lipson et al. 1996). C is also remobilized and supports growth for a few days following defoliation, and the relative importance of that C source is species dependent and seems to be limited compared to N reserves (Richards and Caldwell 1985; Ourry et al. 1994; Lattanzi et al. 2004).
In contrast, the role of P reserves on plant response to defoliation is not known. Storage of P during times of luxury consumption occurs primarily as inorganic phosphate in vacuoles (Chapin 1980), and since it is a mobile chemical form (Marschner 1998), P may be remobilized to support regrowth following defoliation, as depletion of P reserves by defoliation suggests (McNaughton and Chapin 1985; Chapin and McNaughton 1989). Serengeti grass species responded more favorably to defoliation under experimental conditions of high P availability (Chapin and McNaughton 1989). However, due to the lack of stable isotopes to monitor P content (Eviner et al. 2000), experiments involving defoliation and P did not discriminate between mobilization of reserves and current uptake, which increased following defoliation (Chapin and Slack 1979). Could grass species respond less negatively to defoliation under P limitation if during a previous period they had accumulated P in their tissues? According to our knowledge, this positive effect of P reserves on regrowth following defoliation has not been demonstrated. P luxury consumption, as an exacerbated case of nutrient accumulation, is an ideal plant response to test the effect of P reserves on regrowth following defoliation.
The objective of this paper is to evaluate the effect of P reserves acquired by luxury consumption on plant regrowth. We test the hypothesis that P luxury consumption increases plant tolerance to defoliation. This hypothesis predicts that regrowth following defoliation will be larger for plants that accumulated P during a previous period. We performed two experiments with four grass species which are dominant and co-dominant in the Flooding Pampa grasslands, an area where P luxury consumption is a common response, both at functional group and individual level (Ginzo et al. 1982; Rubio and Lavado 1999; Semmartin et al. 2007). The first experiment, aimed at generating P luxury consumption by fertilization, resulted in one species (Sporobolus indicus) showing luxury consumption. In this way, we tested the mentioned hypothesis only for S. indicus.
Materials and methods
In a greenhouse, we subjected individual plants of four perennial grass species to three-level P fertilizations. The aim of this fertilization was to generate P luxury consumption in aerial and belowground biomass according to the model of James et al. (2005). Luxury consumption takes place when plant biomass does not increase while plant P concentration increases following P fertilization. If these conditions are satisfied, the P content, as a product between biomass and concentration, will increase. Other permutations of biomass and concentration will also increase the P content but luxury consumption has the advantage of eliminating plant size as a cofactor of P supply. As later shown in the Results section, only one of the four species showed P luxury consumption as a response to fertilization. Fertilized and unfertilized plants of that species were subjected to a subsequent defoliation experiment (control and defoliated plants) on a P-free medium and harvested after a period of regrowth (Fig. 1). In this way, we were able to evaluate the effect of previous P supply on regrowth following defoliation.
Schematic description of the experimental protocol. The experiment started with planting one tiller per pot filled with washed sand. During the fertilization experiment, treatments were three nutrient solutions differing in P concentration [31 P mg l−1 (P × 1), 62 mg P l−1 (P × 2), and 310 mg P l−1 (P × 10)]. At the end of this experiment, some plants chosen randomly were harvested (Harvest I) to establish if luxury consumption had occurred according to the conceptual model of James et al. (2005). During the subsequent defoliation experiment, plants were transplanted to new pots filled with washed sand and watered with P-free nutrient solutions; treatment factors were defoliation (defoliated and undefoliated plants) and the nutritional level during the previous fertilization experiment. Harvest II evaluated the results
Plant material
Four perennial grass species of the Flooding Pampa grasslands were selected (Soriano 1992): Chaeototropis elongata (H.B.K.) Björk, Panicum bergii Arech., Panicum gouinii Fourn. and Sporobolus indicus (L.) R. Br. (nomenclature follows Cabrera and Zardini 1978). C. elongata is an erect tussock frequently co-dominant with highest growth rates during spring and summer. Individuals were obtained from seeds collected in a humid mesophytic prairie (36°16.8′S, 58°15.8′W), the most conspicuous community in the region (Burkart et al. 1990; Perelman et al. 2001). P. bergii and S. indicus are also erect tussocks while P. gouinii is a semi-erect and rhizomatous species. All of them show their highest growth rates during summer and autumn. P. bergii and P. gouinii are frequently co-dominants and S. indicus is a dominant species in that community. Individuals were cultivated from asexual multiplication of plants collected in the same field site detailed above. For all species, one tiller was planted per pot (1.5 l) filled with washed sand. Pots were exposed to semi-controlled conditions in a greenhouse at the Faculty of Agronomy, Buenos Aires, Argentina (34°35.4′S, 58°28.8′W), and were periodically rotated and watered when necessary. The greenhouse was a structure covered entirely by nylon with a minimum transparency of 90%, thus we did not require the use of artificial lights. Climatic conditions at the greenhouse were very similar to the grassland area where the individuals were collected (see environmental conditions below). At the time of measurements, all experimental plants were in a vegetative stage.
Generating P luxury consumption
The fertilization experiment had three levels that encompassed a gradient of P supply without substantially modifying other nutrients. These three levels of P were combined in a two-way factorial design with three levels of species (P. bergii, P. gouinii and S. indicus). The response of C. elongata to P availability was evaluated in a separate experiment. In order to create the gradient of P availability, the formulation of Hoagland solution no. 2 was modified: the salt providing N and P [(NH4)3PO4] was substituted by a salt providing K and P (KH2PO4). Therefore, the nutrient solution at the “control” level (P × 1) had (mg l−1): 274 K, 197 N, 160 Ca, 64 S, 49 Mg, 31 P, 3 Fe, 0.6 Cl, 0.4 B, 0.4 Mn, and less than 0.05 Zn, Mo, Na, Cu and Co. With respect to the Hoagland no. 2 original formulation, solution P × 1 had a 12% lower N concentration, and 17% more K, whilst P and other nutrients were similar. To generate the other two P-enriched solutions (P × 2, P × 10), this modified Hoagland no. 2 solution was in turn modified to reach 62 and 310 mg P l−1, and 493 and 822 K, respectively (all other nutrients as in P × 1).
For P. bergii, P. gouinii and S. indicus, 48 individuals were grown during 95 days, from 3 March to 3 June 2004. Plants received 350 cm3 of the appropriate solution (P × 1, P × 2 or P × 10) in seven equal aliquots. Environmental conditions of the greenhouse, temperature, relative humidity, photoperiod regime and photosynthetic active radiation, varied between a minimum of 13°C, 71%, 11 h and 3 mol m−2 day−1 and a maximum of 26°C, 100%, 12 h and 41 mol m−2 day−1, respectively.
For C. elongata, 48 individuals were grown for 30 days, from 7 November to 6 December 2003. Plants received 250 cm3 of solution (P × 1, P × 2 or P × 10) in five equal aliquots. Environmental conditions of the greenhouse, temperature, relative humidity, photoperiod regime and photosynthetic active radiation, varied between a minimum of 15°C, 52%, 13 h and 6 mol m−2 day−1 and a maximum of 27°C, 100%, 14 h and 46 mol m−2 day−1, respectively.
For each species, 12 randomly chosen plants were harvested at the end of the fertilization experiment (see harvest I in Fig. 1; four replicates per P level). These plants were defoliated at 4-cm height (it simulates grazing height; Sala et al. 1986), and roots and rhizomes were washed with water to remove sand. Then, biomass was separated into roots (roots plus rhizomes in the case of P. gounii), crown (except for P. gouinii), and stubble (aerial organs within 0- to 4-cm height). Stubble was stored in humid conditions in plastic boxes and leaf area was measured no later than 2 h after harvest (leaf area meter, model Li-3000; Licor, Neb.). Dry weight was determined on oven-dried plant material (75°C for 72 h).
Total P content was measured in crowns plus stubble (0–4 cm in height), and roots (and rhizomes in the case of P. gouinii). Biomass samples were milled and 100-mg sub-samples digested in a mixed acid solution (100 ml HNO3 + 10 ml H2SO4 + 34 ml HClO4) at >100°C for 2 h. Digests were diluted to 10 ml with distilled water and colored following Fiske and Subarrow’s protocol (1925) [1 ml digest + 1 ml (NH4)6Mo7 + 1 ml hydroquinone + 1 ml Na2SO3]. Then, colored solutions were diluted to 10 ml with distilled water and analyzed colorimetrically with a flow injection autoanalyzer (Shimadzu, Kyoto). Although the treatment levels were different supply of P, a superimposed supply of K was also generated (see above composition of nutrient solutions). Thus, total K content was determined. One-hundred milligram sub-samples of milled biomass were digested in a mixed acid solution (HNO3 + HClO4; 2:1) at 235°C for 2 h (Johnson and Ulrich 1959). Digests were diluted to 50 ml with distilled water and analyzed with a flow injection autoanalyzer (ICP-AES; Shimadzu, Kyoto).
Statistical analyses were performed by two-way ANOVA (P supply × Species) for the three species grown simultaneously, and by one-way ANOVA (P supply) for C. elongata. Comparisons of means between treatments were analyzed by LSD test.
Evaluating the effects of luxury consumption on regrowth following defoliation
The remaining plants were subjected to a defoliation experiment with an experimental design with two factors: previous P supply (three previous P fertilization levels; Fig. 1), and defoliation (two levels, undefoliated and defoliated plants at 4 cm in height). The number of replicates was six. Because, as will be shown in the results section, S. indicus was the only species that exhibited P luxury consumption in the fertilization experiment, the experimental details and results of the defoliation experiment will be given for this species only. The plants were washed with water to remove sand. Then, roots were immersed for 2 min in a fungicide solution of 2 g l−1 Benlate 50 (Benomyl CASRN 17804-35-2) to reduce mycorrhizal infection and colonization (Merryweather and Fitter 1996; Kahiluoto and Vestberg 2000). Washed individuals were transplanted to new pots (1.5 l) filled with washed sand. Pots were exposed to semi-controlled conditions in a greenhouse during 67 days, from 3 June to 9 August 2004. All plants received 300 cm3 of a P-free nutrient solution in six equal aliquots. The nutrient solution had (mg l−1): 235 K, 197 N, 160 Ca, 64 S, 49 Mg, 3 Fe, 0.6 Cl, 0.4 B, 0.4 Mn, and less than 0.05 Zn, Mo, Na, Cu and Co. During that period, aerial organs were sprayed 3 times with fungicide (Benlate 50, 2 g l−1) to reduce mycorrhization. Temperature, relative humidity, photoperiod regime and photosynthetic active radiation varied between a minimum of 10°C, 61%, 10 h and 3 mol m−2 day−1 and a maximum of 20°C, 97%, 11 h and 19 mol m−2 day−1, respectively. Plants were harvested (see harvest II in Fig. 1), and leaf area, total dry biomass and total P content were measured as for harvest I. Statistical analyses were performed by two-way ANOVA (previous P supply × Defoliation) and means between treatments were analyzed by LSD test.
Results
P luxury consumption
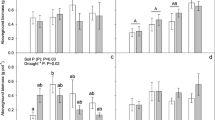
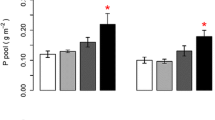
Only S. indicus showed P luxury consumption following P fertilization. C. elongata responded significantly to the medium-level fertilization as crowns plus stubble biomass, root biomass, stubble leaf area, and P concentration in crowns plus stubble increased (Table 1; Fig. 2). In contrast, P. bergii and P. gounii did not respond significantly to fertilization as biomass or P concentration were unaffected (Table 1; Fig. 2). As indicated, S. indicus showed P luxury consumption: biomass did not increase while plant P concentration increased following fertilization (Table 1; Fig. 2). Total P content for plants below 4-cm height was not significantly affected by the P × 2 treatment but was significantly increased by the P × 10 treatment where it tripled (Fig. 3a). P content discriminated by organs showed the same pattern (Fig. 3a), and the largest effects were found in crowns plus stubble (0.9, 1.6 and 2.3 mg in P × 1, P × 2 and P × 10 treatments, respectively), followed by roots (0.3, 0.5 and 0.7 mg). K content in S. indicus plants did not differ among fertilization levels (P = 0.3; data not shown).
Relative changes in biomass (y-axis) and relative changes in plant P concentration (x-axis) (n = 4) for the four selected species at harvest I (see Fig. 1). Left-hand panels show crowns plus stubble (0–4 cm in height), and right-hand panels show roots, except for Panicum gouinii (left-hand panel stubble, right-hand panel roots plus rhizomes). Relative changes were calculated as (treated − control)/control × 100. Vectors show direction and magnitude of changes generated by each P fertilization rate (P × 2 and P × 10) with respect to control (standard nutrient solution; P × 1; filled point at origin). See Table 1 for absolute values and statistical differences. For abbreviations, see Fig. 1
a Total P content in crowns plus stubble (0–4 cm in height), and roots of Sporobolus indicus plants (n = 4) grown under three nutrient solutions differing in P concentration (P × 1, P × 2, P × 10) at harvest I (Fig. 1). b Total biomass of undefoliated and defoliated S. indicus plants at harvest II (n = 6) that had grown at three different P fertilization treatments before the defoliation treatment. c Total P content in crowns plus stubble, and roots of defoliated S. indicus plants at harvest II (n = 6). Bars are SEs and different letters indicate significant differences (differences in a and c are indicated on total P content). For abbreviations, see Fig. 1
Effects of luxury consumption on post-defoliation regrowth
Post-defoliation regrowth of S. indicus plants was increased by previous P fertilization at a P × 10 rate. Total production of undefoliated plants was very similar across the three previous fertilization treatments (Fig. 3b). In contrast, total production of defoliated plants, which includes final live and clipped off biomass, was significantly affected by previous fertilization: it was higher in plants that had been previously fertilized at a P × 10 rate (Fig. 3b), and, as a consequence, had 3 times more P at the beginning of regrowth (Fig. 3a). Total leaf area showed the same pattern as total production (data not shown).
S. indicus plants that had been fertilized at a P × 10 rate showed a clear pattern of P remobilization from the stubble, crown, and root compartments to the regrowing tissue. During regrowth, the P content of stubble, crown, and root tissue of plants previously fertilized at a P × 10 rate dropped from 3.06 mg (Fig. 3a) to 0.7 mg (Fig. 3c). Therefore, during the regrowth after defoliation these plants remobilized on average 2.36 mg of P from the initial biomass, i.e., 79% of the accumulated P. This was a consequence of a sharp decrease in the P concentration in these organs during the regrowth period, from 0.15 to 0.20% (harvest I; Table 1) to 0.03–0.04% (harvest II).
Regrowth of plants previously fertilized at a P × 2 rate did not significantly differ from control, P × 1 plants (Fig. 3b), although the difference between initial and final total P content, a measure of the P remobilization, was higher in plants previously fertilized at a P × 2 than P × 1 rate (1.7 and 0.6 mg, respectively; Fig. 3a–c).
Discussion
Luxury consumption of P during a previous period increased the post-defoliation regrowth of S. indicus plants growing in a P-free medium (Figs. 2, 3). During the regrowth these plants showed a clear pattern of P remobilization from the stubble, crown, and root compartments to the regrowing tissue, in addition to a likely reutilization of P present in leaf-growth zones. This remobilization may have originated from sheaths present in the stubble, crowns, and roots (i.e., true remobilization), or from leaf blades and leaf-growth zones in emerging new leaves that were also present in the stubble at the time of defoliation (i.e., reutilization). However, leaf blades were a minor component of the stubble at harvest I (Table 1; 23 cm2 of leaf area represents about 0.08–0.15 g, which in turn accounts for 5–9% of total biomass). This strongly suggests that most P for regrowth was remobilized from sheaths present in the stubble, crowns, or roots. Total P reserves remaining in crowns and roots of Serengeti grasses were strongly depleted by defoliation, while absorption capacity was stimulated, unaffected or reduced by defoliation depending on species (McNaughton and Chapin 1985; Chapin and McNaughton 1989). Although suggestive, previous studies did not isolate the effect of reserves from the effect of uptake on regrowth after defoliation (Chapin and Slack 1979; McNaughton and Chapin 1985; Chapin and McNaughton 1989). Our work then shows that high levels of P reserves confer greater postdefoliation regrowth. Low levels of P reserves as shown by plants previously fertilized at a medium rate (P × 2), although they were remobilized (Fig. 3a–c), did not increase post-defoliation regrowth. This pattern suggests that the effect of P reserves on regrowth depends on the level of reserves, as shown for N reserves (Hamilton et al. 1998).
Previous works had shown that plant tolerance to defoliation depends on species, C and N reserves, recovery conditions, soil nutrient levels, tissue N level, evolutionary mechanisms, and light environment (Chapin and McNaughton 1989; Georgiadis et al. 1989; McNaughton 1992; Ourry et al. 1994; Oesterheld and McNaughton 1988; Volenec et al. 1996; Hamilton et al. 1998; Ferraro and Oesterheld 2002). Under P deficiency, total biomass yield of grazing-adapted grasses from the Serengeti Plains was reduced by defoliation (Chapin and McNaughton 1989). In contrast, the growth of these same species was maintained or enhanced by defoliation under adequate nutrient supply (McNaughton et al. 1983). Our work suggests that plant tolerance to defoliation of S. indicus also depends on P reserves and that reutilization and remobilization confer tolerance to defoliation by promoting compensatory growth under P deficiency.
P luxury consumption is a common plant response with potential multiple effects at different levels. At the individual level, species that show luxury consumption during nutrient flushes may use these reserves to support growth after soil reserves are exhausted (Chapin 1980) and/or to buffer the impact of adverse conditions such as defoliation events (our work). Previous work suggested that compensatory growth was not a viable strategy in P-deficient environments because plant production was decreased by defoliation (Chapin and McNaughton 1989). Our work suggests that under P deficiency, plant growth could be stimulated by defoliation if plants accumulate P during a previous period. Interestingly, total production of undefoliated plants was very similar across the three previous fertilization treatments (Fig. 3b). It suggests that previous luxury consumption of P only conferred an advantage on withdrawal of the P supply when plants were defoliated (Fig. 3b), similarly to N mobilization (Volenec et al. 1996).
At the community level, luxury consumption could modify plant interactions. For slow-growing species, luxury consumption in aboveground organs could be advantageous to limit nutrient availability to other competitive fast-growing species, as seen in arctic and alpine vegetation responses to N fertilization (Lipson et al. 1996; Wijk et al. 2003). At the ecosystem level, P luxury consumption could modify the risk of herbivory, similarly to the significantly higher browse rates on saplings with luxury N (Tripler et al. 2002).
Grazing by livestock in a context of spatial and temporal variation of soil P availability is common in Flooding Pampa grasslands. During the last two centuries, grazing by introduced herbivores has been a common disturbance in the region, and has modified both the structure and functioning of grasslands by promoting changes in canopy structure, species richness and above-ground primary production (Sala et al. 1986; Rusch and Oesterheld 1997). Other common disturbances such as fertilizations and natural flooding events increase soil P availability (Ginzo et al. 1982; Rubio et al. 1997; García et al. 2002; Semmartin et al. 2007). Thus, in this system, accumulation of P reserves during nutrient fluxes likely represents an ecological advantage when considering frequent defoliation by herbivores. S. indicus is considered a grazing-tolerant species since it is the only grass species increasing its importance under grazing (Hidalgo and Cauhépé 1991). Its response to increases in soil P availability may be one factor responsible for this tolerance to grazing. Thus, our results should be carefully interpreted because the greater tolerance conferred by P reserves could be offset if plants with higher P content were consumed to a greater extent.
Although we did not demonstrate P remobilization by tracing stable isotopes, as is possible for C and N (Avice et al. 1996; De Visser et al. 1997; Meuriot et al. 2004), we used a reliable experimental protocol that indicates that high levels of P reserves acquired by luxury consumption confer greater post-defoliation regrowth under P deficiency. Analogously, the etiolated technique is often used to examine soluble carbohydrate mobilization by quantifying regrowth in the absence of current photosynthesis (Richards and Caldwell 1985). We used the same logic to separate current P uptake effects from remobilization effects because in our defoliation experiment plants grew in a medium without P. Our protocol could be a solution to the problem posed by the lack of stable isotopes for monitoring P content (Eviner et al. 2000).
References
Avice JC, Ourry A, Lemaire G, Boucaud J (1996) Nitrogen and carbon flows estimated by 15N and 13C pulse-chase labelling during regrowth of alfalfa. Plant Physiol 112:281–290
Burkart SE, León RJC, Movia CP (1990) Inventario fitosociológico del pastizal de la Depresión del Salado (Prov. Bs. As.) en un área representativa de sus principales ambientes. Darwiniana 30:27–69
Cabrera AL, Zardini EM (1978) Manual de la flora de los alrededores de Buenos Aires, 2nd edn. Acme, Buenos Aires
Chapin FS III (1980) The mineral nutrition of wild plants. Annu Rev Ecol Syst 11:233–260
Chapin FS III, McNaughton SJ (1989) Lack of compensatory growth under phosphorus deficiency in grazing-adapted grasses from the Serengeti Plains. Oecologia 79:558–562
Chapin FS III, Slack M (1979) Effect of defoliation upon root growth, phosphate absorption and respiration in nutrient-limited tundra graminoids. Oecologia 42:67–79
Clarkson DT (1967) Phosphorus supply and growth rate in species of Agrostis L. J Ecol 55:111–118
De Visser R, Vianden H, Schnyder H (1997) Kinetics and relative significance of remobilized and current C and N incorporation in leaf and root growth zones of Lolium perenne after defoliation: assessment by C-13 and N-15 steady-state labelling. Plant Cell Environ 20:37–46
Epstein HE (1972) Mineral nutrition of plants: principles and perspectives, 3rd edn. Wiley, New York
Eviner VT, Chapin FS III, Vaughn CE (2000) Nutrient manipulations in terrestrial ecosystems. In: Sala OE, Jackson RB, Mooney HA, Howarth RW (eds) Methods in ecosystem science. Springer, New York, pp 291–307
Ferraro DO, Oesterheld M (2002) Effect of defoliation on grass growth. A quantitative review. Oikos 98:125–133
Fiske C, Subarrow Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66:375–381
García F, Micucci F, Rubio G, Ruffo M, Daverede I (2002) Fertilización de Forrajes en la Región Pampeana, 1st edn. INPOFOS Cono Sur, Buenos Aires
Georgiadis NJ, Ruess RW, McNaughton SJ, Western D (1989) Ecological conditions that determine when grazing stimulates grass production. Oecologia 81:316–322
Ginzo HD, Collantes M, Caso OH (1982) Fertilization of a native grassland in the “Depresión del Río Salado”, Province of Buenos Aires: herbage dry matter accumulation and botanical composition. J Range Manage 35:35–39
Hamilton EW III, Giovannini MS, Moses SA, Coleman JS, McNaughton SJ (1998) Biomass and mineral element responses of a Serengeti short-grass species to nitrogen supply and defoliation: compensation requires a critical [N]. Oecologia 116:407–418
Hidalgo LG, Cauhépé MA (1991) Effects of seasonal rest in aboveground biomass for a native grassland of the Flooding Pampa, Argentina. J Range Manage 44:471–475
James JJ, Tiller RL, Richards JH (2005) Multiple resources limit plant growth and function in a saline–alkaline desert community. J Ecol 93:113–126
Johnson CM, Ulrich A (1959) Analytical methods for use in plant analysis. Calif Agric Exp Stn Bull 766:25–78
Kahiluoto H, Vestberg M (2000) Creation of a non-mycorrhizal control for a bioassay of AM effectiveness. II. Benomyl application and soil sampling time. Mycorrhiza 9:259–270
Lattanzi FA, Schnyder H, Thornton B (2004) Defoliation effects on carbon and nitrogen substrate import and tissue-bound efflux in leaf growth zones of grasses. Plant Cell Environ 27:347–356
Lipson DA, Bowman WD, Monson RK (1996) Luxury uptake and storage of nitrogen in the rhizomatous alpine herb, Bistorta bistortoides. Ecology 77:1277–1285
Marschner H (1998) Mineral nutrition of higher plants, 2nd edn. Academic Press, London
McNaughton SJ (1992) Laboratory-simulated grazing: interactive effects of defoliation and canopy closure on Serengeti grasses. Ecology 73:170–182
McNaughton SJ, Chapin FS III (1985) Effects of phosphorus nutrition and defoliation on C4 graminoids from the Serengeti Plains. Ecology 66:1617–1629
McNaughton SJ, Wallace LL, Coughenour MB (1983) Plant adaptation in an ecosystem context: effects of defoliation, nitrogen, and water on growth of an African C4 sedge. Ecology 64:307–318
Merryweather J, Fitter A (1996) Phosphorus nutrition of an obligately mycorrhizal plant treated with the fungicide benomyl in the field. New Phytol 132:307–311
Meuriot F, Avice J-C, Simon J-C, Laine P, Decau M-L, Ourry A (2004) Influence of initial organic N reserves and residual leaf area on growth, N uptake, N partitioning and N storage in alfalfa (Medicago sativa) during post-cutting regrowth. Ann Bot 94:311–321
Millard P, Thomas R, Buckland ST (1990) Nitrogen supply affects the remobilization of nitrogen for the regrowth of defoliated Lolium perenne L. J Exp Bot 41:941–947
Oesterheld M, McNaughton SJ (1988) Intraspecific variation in the response of Themeda triandra to defoliation. The effect of time of recovery and growth rates on compensatory growth. Oecologia 77:181–186
Ourry A, Kim TH, Boucaud J (1994) Nitrogen reserve mobilization during regrowth of Medicago sativa L. Relationships between availability and regrowth yield. Plant Physiol 105:831–837
Perelman S, León RJC, Oesterheld M (2001) Cross-scale vegetation patterns of Flooding Pampa grasslands. J Ecol 89:562–577
Richards JH, Caldwell MM (1985) Soluble carbohydrates, concurrent photosynthesis and efficiency in regrowth following defoliation: a field study with Agropyron species. J Appl Ecol 22:907–920
Rubio G, Lavado RS (1999) Acquisition and allocation of resources in two waterlogging-tolerant grasses. New Phytol 143:539–546
Rubio G, Oesterheld M, Alvarez CR, Lavado RS (1997) Mechanisms for the increase in phosphorus uptake of waterlogged plants: soil phosphorus availability, root morphology and uptake kinetics. Oecologia 112:150–155
Rusch GM, Oesterheld M (1997) Relationship between productivity, and species and functional group diversity in grazed and non-grazed Pampas grassland. Oikos 78:519–526
Sala OE, Oesterheld M, León RJC, Soriano A (1986) Grazing effects upon plant community structure in subhumid grasslands of Argentina. Vegetatio 67:27–32
Schachtman DP, Reid RJ, Ayling SM (1998) Phosphorus uptake by plants: from soil to cell. Plant Physiol 116:447–453
Semmartin M, Oyarzabal M, Loreti JM, Oesterheld M (2007) Controls of primary productivity and nutrient cycling in a temperate grassland with year-round production. Austral Ecol 32:416–435
Soriano A (1992) Rio de la Plata grasslands. In: Coupland RT (ed) Ecosystems of the world 8A. Natural grasslands. Introduction and western hemisphere, 1st edn. Elsevier, Amsterdam, pp 367–407
Thornton B, Millard P (1993) The effects of nitrogen supply and defoliation on the seasonal internal cycling of nitrogen in Molinia caerulea. J Exp Bot 44:531–536
Thornton B, Millard P, Duff EI, Buckland ST (1993) The relative contribution of remobilization and root uptake in supplying nitrogen after defoliation for regrowth of laminae in four grass species. New Phytol 124:689–694
Tripler CE, Canham CD, Inouye RS, Schnurr JL (2002) Soil nitrogen availability, plant luxury consumption, and herbivory by white-tailed deer. Oecologia 133:517–524
Volenec JJ, Ourry A, Joern BC (1996) A role for nitrogen reserves in forage regrowth and stress tolerance. Physiol Plant 97:185–193
Wijk MTV, Williams M, Gough L, Hobbie SE, Shaver GR (2003) Luxury consumption of soil nutrients: a possible competitive strategy in above-ground and below-ground biomass allocation and root morphology for slow-growing arctic vegetation? J Ecol 91:664–676
Acknowledgments
This research was funded by grants from the University of Buenos Aires (G071) and the Agencia Nacional de Promoción Científica y Tecnológica (PICT 08-12186 and 32415). M. Oyarzabal was supported by a doctoral fellowship from CONICET (Argentina). We thank Dr. Gerardo Rubio for initial discussions and Dr. Agustín Grimoldi, Prof. Dr. Russell Monson, Prof. Dr. Hermann Heilmeier, and two reviewers for valuable comments on the manuscript. Chemical determinations were made by the Laboratorio de Servicios Analíticos Especiales (LABFAUBA), Facultad de Agronomía, Universidad de Buenos Aires, Argentina.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Hermann Heilmeier.
Rights and permissions
About this article
Cite this article
Oyarzabal, M., Oesterheld, M. Phosphorus reserves increase grass regrowth after defoliation. Oecologia 159, 717–724 (2009). https://doi.org/10.1007/s00442-008-1263-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-008-1263-z