Abstract
A comprehensive appraisal of the mycorrhizal literature provides data for 336 plant families representing 99% of flowering plants, with regard to mycorrhizas and other nutritional adaptations. In total, arbuscular (AM), orchid, ectomycorrhizas (EM) and ericoid mycorrhizas and nonmycorrhizal (NM) roots occur in 74%, 9%, 2%, 1% and 6% of Angiosperm species respectively. Many families of NM plants have alternative nutritional strategies such as parasitism, carnivory, or cluster roots. The remaining angiosperms (8%) belong to families reported to have both AM and NM species. These are designated as NM-AM families here and tend to occur in habitats considered non-conducive to mycorrhizal fungi, such as epiphytic, aquatic, extremely cold, dry, disturbed, or saline habitats. Estimated numbers of species in each category of mycorrhizas is presented with lists of NM and EM families. Evolutionary trends are also summarised by providing data on all clades and orders of flowering and non-flowering vascular plants on a global scale. A case study of Western Australian plants revealed that plants with specialised nutritional modes such as carnivory, cluster roots, or EM were much more diverse in this ancient landscape with infertile soils than elsewhere. Detailed information on the mycorrhizal diversity of plants presented here is linked to a website (mycorrhizas.info) to allow data to remain current. Over a century of research effort has resulted in data on mycorrhizal associations of >10,000 plant species that are of great value, but also somewhat of a liability due to conflicting information about some families and genera. It is likely that these conflicts result in part from misdiagnosis of mycorrhizal associations resulting from a lack of standardisation in criteria used to define them. Families that contain both NM and AM species provide a second major source of inconsistency, but even when these are excluded there is a ∼10% apparent error rate in published lists of mycorrhizal plants. Arbuscules are linked to AM misdiagnosis since they are used less often than vesicles to recognise AM associations in roots and apparently occur sporadically in NM plants. Key issues with the diagnosis of mycorrhizal plants are discussed using the Cyperaceae as a case study. Detailed protocols designed to consistently distinguish AM from endophytic Glomeromycotan Fungus Colonisation (GFC) are provided. This review aims to stimulate debate and provide advice to researchers delving into root biology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For over a century, a substantial proportion of the research effort on mycorrhizal symbioses has focussed on identifying plant and fungal partners in samples of roots obtained from natural ecosystems. Newman and Reddell (1987), Trappe (1987) and Wang and Qiu (2006) have provided comprehensive summaries of the mycorrhizal literature [e.g. 723 papers consulted by Harley and Harley (1987) and a dataset of 3000 papers summarised by Trappe (1978)]. A second major source of information is provided by mycorrhizal surveys or data compilations on regional scales for Japan by Maeda (1954), the UK by Harley and Harley (1987) and Peat and Fitter (1993), Hawaii by Koske et al. (1992), South Africa by Allsopp and Stock (1993) and Australia by Brundrett (2008-mycorrhizas.info). Additional information is provided by mycorrhizal data compilations for hydrophytes by Khan and Belik (1995), xerophytes by Trappe (1981), ectomycorrhizal Fabaceae by Alexander (1989), the Cyperaceae by Muthukumar et al. (2004) and the Brassicaceae by DeMars and Boerner (1996).
In total, mycorrhizologists have presented data on over 10,000 plant species, which equates to about 3% of vascular plants. This comprehensive dataset is of great scientific value, but also a source of confusion, due to inconsistent reports of associations within some families and genera. Mycorrhizas are defined by microscopic features that are used to identify associations (Brundrett 2004). However, it is often not clear which structures were used to identify associations in published studies of field-collected roots. Perhaps as a consequence, it is relatively common to find examples of conflicting data on mycorrhizas for plant families, genera and even species in the scientific literature. Dickie et al. (2007) identify one example of misdiagnosis for Buddleja davidii, but there are many others. The most confusion concerns the status of plant families, such as Chenopodiaceae and Cyperaceae, that are considered to contain NM plants by most authors, but have also been reported to have AM (Hirrel et al. 1978; Muthukumar et al. 2004). Apparent misdiagnosis of EM in plants that normally have AM is also common, especially in the older mycorrhizal literature (mycorrhizas.info/ecm). Our knowledge of the mycorrhizal status of some plant families is becoming less clear over time, as errors accumulate in host plant lists. It is important that such contradictory information be resolved since data on the importance of mycorrhizas at local, regional and global scales is of great value to land mangers, for restoration ecology and conservation and also required for applied use of plants in forestry, horticulture and agriculture. Inconsistencies in the diagnosis of mycorrhizas result in a number of key questions:
-
1.
Is existing information of sufficient scope and consistency to determine which plant families contain species that typically have mycorrhizal or NM roots?
-
2.
Can plant families of NM species be allocated into categories based on nutritional or ecological strategies?
-
3.
How can inconsistencies caused by misdiagnosis be distinguished from those due to real variation in mycorrhizal associations within some plant families and genera?
-
4.
Can we resolve uncertainty about the relative importance of habitats where plants tend to be NM, relative to plant families that have variable mycorrhizas, especially when both occur together?
-
5.
Are reported inconsistencies within plant families linked to inconsistent use of criteria for identification of mycorrhizal associations such as arbuscules for AM and a Hartig net for EM?
-
6.
Are more reliable protocols for diagnosis of mycorrhizas required?
The purpose of this review is to address these questions by: (1) a critical appraisal of the literature to designate plant families with mycorrhizal or NM roots and identify families with well established mycorrhizal relationships, NM roots, or conflicting information, (2) use these data to determine the total diversity of plants with different types of mycorrhizas or alternative means of nutrition, and (3) discuss the importance of mycorrhizal survey data and suggest objectives for future surveys. The second part of this review aims to identify the most common errors that have been perpetuated in the mycorrhizal literature and recommend protocols to reduce error rates in the diagnosis of mycorrhizal associations in the future.
Methods
Two contrasting approaches were used to provide estimates of the relative diversity of mycorrhizal and other nutrition strategies in plants to provide the most accurate estimates possible and to allow comparison of results.
Mycorrhizal status of plant families, orders and clades
The first approach estimated mycorrhizal plant diversity based on current plant phylogeny data to minimise the effect of sampling biases on outcomes. A comprehensive and critical screening of the mycorrhizal literature using criteria listed below provided mycorrhizal status data for 336 families that included 99% of flowering plant species. Data on mycorrhizal and NM species was obtained from the regional survey publications listed below and many additional references only listed at mycorrhizas.info. It is conservatively estimated that over 10,000 plant species were included in the analysis. Family classification followed this approach:
-
1.
Families were not allocated to categories unless there was sufficient sampling of taxa (several species or corroborative studies).
-
2.
In families well established to be AM, occasional contradictory reports of NM roots were considered to be due to habitat conditions, sampling, or diagnosis errors.
-
3.
Families where most species consistently lack mycorrhizas are designated as NM.
-
4.
Families where both NM and AM roots were repeatedly diagnosed were assigned to the variable NM-AM category.
-
5.
Parasitic plants without roots were designated as NM.
-
6.
Studies that explain how mycorrhizal structures were used in diagnosis or illustrate such structures were given preference over other reports.
-
7.
Data for plants growing in habitats that are non-conducive for mycorrhizas (e.g. arctic, epiphytic and marine plants) were not used to determine the mycorrhizal status of families that also occur in other habitats.
-
8.
Families with substantial numbers of species with more than one root type were split across categories using estimated number of species at the genus level.
Data on the mycorrhizal status of plant families were incorporated into a table listing the 506 currently recognised flowering plant families (Soltis et al. 2000; Heywood et al. 2007), with current estimates for numbers of species in each family compiled from the data sources listed below. These data were combined with mycorrhizal records in a table to estimate of the total taxonomic diversity of all flowering plants with each type of mycorrhizas or NM roots. Data on mycorrhizas of major groups of primitive plants was compiled separately for online publication (mycorrhizas.info/evol). The estimates of mycorrhizal diversity for primitive plants and flowering plants were then combined to provide an overall estimate for all vascular plants.
A more detailed estimate of the number of species of EM plants was compiled at the genus level, using comprehensive taxonomic data from the sources listed below. Separate diversity estimates were also compiled for specialised categories on NM plants such as parasites, carnivores and species with root clusters, as was the diversity of plant families with variable NM-AM roots from different habitats. Some of these data tables were first published online at mycorrhizas.info, where they will be kept updated.
Data on estimated numbers of species in plant families were compiled primarily from Heywood et al. (2007). Additional information as provided by Florabase (florabase.calm.wa.gov.au), the Catalogue of Life (www.catalogueoflife.org), the International Plant Names Index (www.ipni.org), Angiosperm Phylogeny Website by Stevens (2001-, www.mobot.org/MOBOT/research/APweb). The diversity of lower plants was obtained from Gymnosperms Homepage (www.conifers.org) and the Tree of Life (www.tolweb.org), and Chapman (2005). Lists of parasitic plants follow Nickrent (2006, www.parasiticplants.siu.edu/ListParasites.html). Myco-heterotrophs follow Leake (1994) and Nickrent (1997-). The orchid diversity estimate is from Chase et al. (2003).
Mycorrhizal survey data summary
Data on mycorrhizas of plants in natural habitats were summarised from 128 publications, covering most major habitats and geographic regions of the world, estimated to include over 8,000 plant species. Data on habitats likely to be non-conducive to mycorrhizas, such as arctic, alpine, aquatic and epiphytic plant communities, were summarised separately for ∼2,000 plant species. This approach was used to minimise the impact of habitat conditions on overall measures of mycorrhizal occurrence. Thus, data from published lists of mycorrhizal species incorporated over 10,000 plant species. References were chosen that:
-
1.
Use modern definitions of mycorrhizal types.
-
2.
Included at least 10 species of plants from an ecosystem.
-
3.
Used roots collected in natural habitats.
-
4.
Minimised duplication of species in lists by maximising distance or habitat separation between surveys in similar habitats,
-
5.
Primarily focussed on flowering plants (gymnosperms and ferns were included in totals, but bryophytes were excluded).
Papers listing mycorrhizal plants in ecosystems were: Alarcón and Cuenca (2005), Allen et al. (1987), Allen et al. (1998), Allen et al. (2006), Allsop and Stock (1993), Andrade et al. (2000), Bagyaraj et al. (1979), Bakarr and Janos 1996), Barnola and Montilla (1997), Bauer et al. (2003), Beck-Nielsen and Madsen (2001), Bellgard (1991), Berch and Kendrick (1982), Berch et al. (1988), Béreau et al. (1997), Berliner and Torrey (1989), Bethlenfalvay et al. (1984), Blaschke (1991), Blaszkowski (1994), Bledsoe et al. (1990), Brockhoff and Allaway (1989), Brundrett and Abbott (1991), Brundrett and Kendrick (1988), Brundrett et al. (1995), Camargo-Ricalde et al. (2003), Carrillo-Garcia et al. (1999), Cázares et al. (2005), Chaudhry et al. (2005), Clayton and Bagyaraj (1984), Collier et al. (2003), Cooke and Lefor (1988), Cooper (1976), Cornwell et al. (2001), Cripps and Eddington (2005), Currah and Van Dyk (1986), da Silva et al. (2001), de Alwis and Abeynayake (1980), DeMars (1996), Dhillion et al. (1995), Dodd et al. (2002), Ducousso et al. (2008), Eriksen et al. (2002), Ernst et al. (1984), Farmer (1985), Fisher and Jayachandran (2005), Fontenla et al. (1998), Fontenla et al. (2001), Frenot et al. (2005), Frioni et al. (1999), Fuchs and Haselwandter (2004), Gai et al. (2006), Gehring and Connell (2006), Gemma and Koske (1995), Giovannetti and Nicolson (1983), Gorsi (2002), Grippa et al. (2007), Hartnett et al. (2004), Hetrick et al. (1992), Hildebrandt et al. (2001), Högberg and Piearce (1986), Högberg (1982), Hopkins (1987), Hurst and Turnbull (2002), Janos (1993), Johnson-Greene et al. (1995), Kagawa et al. (2006), Kai and Zhiwei (2006), Katenin (1964), Khan (1974), Kohn and Stasovski (1990), Koske and Gemma (1990), Koske et al. (1992), Kottke et al. (2004), Kühn et al. (1991), Kumar and Ghose (2008), Laursen et al. (1997), Lesica and Antibus (1986), Lesica and Antibus (1990), Logan et al. (1989), Louis (1990), Lovera and Cuenca (1996), Maeda (1954), Mafia et al. (1993), Malloch and Malloch (1981, 1982), Maremmani et al. (2003), McGee (1986), McGuire et al. (2008), Medve (1984), Menoyo et al. (2007), Michelsen (1993), Miller (1979, 1982), Mishra et al. (1980), Moyersoen et al. (2001), Muthukumar and Udaiyan (2000), Muthukumar et al. (2003), Muthukumar et al. (2006), Nadarajah and Nawawi (1993), Newbery et al. (1988), O'Connor et al. (2001), Olsson et al. (2004), Onguene and Kuyper (2001), Onipchenko and Zobel (2000), Pendleton and Smith (1983), Perrier et al. (2006), Peterson et al. (1985), Powlowski et al. (1996), Radhika and Rodrigues (2007), Ragupathy and Mahadevan (1993), Ragupathy et al. (1990), Rains et al. (2003), Read and Haselwandter (1981), Reddell and Milnes (1992), Reddell et al. (1996), Reeves et al. (1979), Rosales et al. (1997), Rose (1981), Ruotsalainen et al. (2002), Rowe and Pringle (2005), Saif (1975), Santos et al. (2000), Schmidt and Scow (1986), Sengupta and Chaudhuri (2002), Sharma et al. (1986), Shi et al. (2006), Siqueira et al. (1998), Šraj-Kržič et al. (2006), St John (1980), Straker et al. (2007), Tao and Zhiwei (2005), Tao et al. (2004), Thomazini (1973), Titus et al. (2002), Tori and Coley (1999), Tawaraya et al. (2003), Treu et al. (1996), Tsuyuzaki et al. (2005), Turnau et al. (1992), Väre et al. (1992), Väre et al. (1997), Weishampel and Bedford (2006), Wetzel and van der Valk (1996), Wilson and Hartnett (1998), Wubet et al. (2003), Yamato and Iwasaki (2002), Zhang et al. (2004), Zangaro et al. (2002).
Mycorrhizal studies providing survey data for 100 or more plant taxa from natural habitats were used in comparison with data summaries described above. These 14 surveys were of plants from Cameroon (Onguene and Kuyper 2001), New Zealand (Cooper 1976), China (Muthukumar et al. 2003), India (Muthukumar and Udaiyan 2000, Muthukumar et al. 2006, Ragupathy and Mahadevan 1993), Australia (Brundrett and Abbott 1991, Brundrett et al. 1995), Guyana (McGuire et al. 2008), Hawaii (Koske et al. 1992), Argentina (Fontenla et al. 2001), Canada (Currah and Van Dyk 1986), South Africa (Allsop and Stock 1993) and Japan (Maeda 1954).
Data from arctic habitats were used to investigate the relationship between latitude and mycorrhizas. These studies of arctic habitats were by Bledsoe et al. (1990), Olsson et al. (2004), Kohn and Stasovski (1990), Väre et al. (1992, 1997), Miller (1982), Treu et al. (1996), Allen et al. 2006, Ruotsalainen et al. (2002) and Katenin (1964).
A case study contrasting global averages to one of the world’s oldest landscapes in Western Australia (WA) is also presented. The ratio of expected to actual diversity in families from WA with different types of mycorrhizas, specialised roots or mycorrhiza-suppressive habitats was determined. Data on plant diversity were obtained from the Western Australian Herbarium (florabase.calm.wa.gov.au, calculated in June 2007).
The data compilation from 125 published papers described above was also used to estimate rates of errors for diagnosis of AM, EM and NM roots as well as the frequency of use of different definitions of AM (arbuscules, or hyphae, vesicles and arbuscules, or not stated). The types of data on root colonisation by mycorrhizal fungi presented is also reported. Misdiagnosis was considered likely when reports are contrary to expectations based on the mycorrhizal literature, as explained in the Section on Resolving conflicting information in published data.
Part I. the relative importance of mycorrhizas and other means of plant nutrition
Determining the total diversity of mycorrhizal and nonmycorrhizal plants
An estimation of the relative diversity of plants with different types of mycorrhizas provided by assigning mycorrhizal associations to Angiosperm families is shown in Fig. 1. In this analysis, the majority of flowering plants (>99%) belonged to families that could be reliably assigned to mycorrhizal categories with existing data. This approach is much more reliable than summaries produced from averaging published data alone, as it corrects for sampling biases (e.g. more data from the Northern Hemisphere). There are < 200 families yet to be sampled and the majority of these are very small (the average size of un-sampled families is 15 species and 1/4 are monotypic). Most of these unallocated families are likely to contain AM plants as they are sister to, or nested within clades known to predominantly contain AM plants.
The relative diversity of angiosperms with different types of mycorrhizal or nonmycorrhizal roots. The mycorrhizal status of the majority of species in plant families was determined from literature citations for >10,000 plants and combined with estimated numbers of species in each family (see “Methods” for data sources). Less than 1% of angiosperms could not be allocated (unknown). AM arbuscular mycorrhizas, NM nonmycorrhizal, NM-AM variable NM or AM, EM ectomycorrhizal
Of the 336 Angiosperm families which could be assigned to categories, 217 contained AM plants, 40 had variable NM-AM, 53 only NM, 23 included EM hosts and 3 other types of mycorrhizas were confined to one family (Orchidaceae, Ericaceae, Thysanotus in the Laxmaniaceae). A key finding is that, on a global scale, the importance of fully NM plants is less than suggested in the past (i.e. ∼6% of flowering plants). Even if families reported to contain both NM and AM species (NM-AM) is added to the NM total, 86% of flowering plants are mycorrhizal.
The mycorrhizal associations of the majority of large families of flowering plants are now well resolved and it is unlikely that the overall trends presented in Fig. 1 would change much with more data. However, there are several potential error sources in estimates of numbers of mycorrhizal species:
-
i.
Estimates of plant diversity in Figs. 1, 2, 3, 4 will change as taxonomy is resolved and new species are described. However, this is not expected to substantially alter the relative sizes of categories.
-
ii.
The relative diversity of the orchids varies considerably in estimates (from 18,000 to 25,000 species (Heywood et al. 2007), but the larger estimated by Chase et al. (2003) was considered to be most realistic so is used here.
The relative diversity of mycorrhizal or nonmycorrhizal plants summarised by calculating totals from published lists of mycorrhizal or nonmycorrhizal vascular plants. Data are from 128 publications from most regions of the world, estimated to include 8,000 plant species. See “Methods” for a list of these papers and Fig. 1 for abbreviations
The proportions of AM, EM and NM plants in different primitive plant clades is summarised in Fig. 2 using data provided at mycorrhizas.info. This website should be consulted for references and further information on mycorrhizas of these plant groups. Bryophytes such as mosses and liverworts included in Fig. 2, have been reported to contain AM-like associations, hyphae of other fungi, or be NM, but the nature of associations are unclear in some cases (Ligrone et al. 2007). Combined data from Figs. 1 and 2 provides an overview of the relative importance of mycorrhizas for all vascular plants in Fig. 3.
In total, over 200,000 flowering plants have AM, out of about 280,000 species in total. These 217 families are too numerous to list here. Most of the remaining families have EM or predominantly NM roots so are listed in Tables 1 and 2. Host plants with orchid (∼25,000 spp.), ericoid (∼3,900 spp.), and Thysanotus (∼50 spp. in the Laxmanniacae) mycorrhizas occur in a single family or genus, so will not be discussed further. Plants with Thysanotus mycorrhizas were excluded from Figs. 1, 2, 3, 4 as they would not be clearly visible.
Summary of mycorrhizal survey data
Estimates of the relative diversity of mycorrhizal and NM plants compiled using data from 128 published host plant lists which included about 8,000 plant taxa are shown in Fig. 4. When these results are compared with plant classification-based estimates for mycorrhizal plant diversity (Figs. 1, 2, 3), it can be seen that both approaches provide similar estimates of the relative diversity of AM plants, but surveys have tended to over-sample NM and EM plants and under-sample orchids. It is not surprising that orchids are under-sampled as their highest diversity occurs in specialised tropical epiphytic habitats (Chase et al. 2003) and mycorrhizas of epiphytes as a group are poorly studied (Janos 1993). In contrast, habitats where NM plants predominate tend to be over-represented in published lists, perhaps because they are relatively accessible or easier to sample (e.g. annual plants in disturbed habits). The relative diversity of EM hosts is also higher in Fig. 4, but this probably reflects the dominance of EM trees in many of the habitats that have been sampled most often.
Despite the fact that Fig. 4 is based on a much larger dataset than was used in earlier compilations of mycorrhizal species data [i.e. 2075 spp. in Newman and Reddell (1987), 6507 spp. in Trappe (1987), 843 spp. in Peat and Fitter (1993), 3617 spp. in Wang and Qui (2006)], some results are in close agreement as shown in Fig. 5. Only the overall importance of mycorrhizal and NM roots can be compared in Fig. 5, since the other reviews used fewer categories of mycorrhizas to summarise data. Families and taxa with variable mycorrhizas are more common in the literature summaries of Trappe 1987 and Harley and Harley 1987, which rely more heavily on older literature than the data presented here. Newman and Reddell (1987) were unable to allocate any families as totally NM, because of contradictory information in the literature, even though some families were reported to comprise ∼90% NM species. This example illustrates why it is necessary to designate such families as predominantly NM, recognise a category of families with NM-AM plants and/or exclude data that are likely to be incorrect when the majority of reports are in agreement.
Figure 6 includes data for all types of mycorrhizas from 14 large surveys for comparison with the data from Figs. 1 and 4 included for comparison. The proportion of plants with mycorrhizas varies considerable between surveys, from 100% of ferns sampled in New Zealand (Cooper 1976) to 50% of plants sampled in India (Muthukumar and Udaiyan 2000). The proportion of mycorrhizal species is substantially lower than expected in some surveys, which may be related to the habitats sampled (aquatic, epiphytic and disturbed plants were included in some surveys), but also may reflect issues with diagnosis as explained in Part II.
Ectomycorrhizal plants
There are about 6000 ectomycorrhizal (EM) plant species in 145 genera and 26 families (approximately 5600 angiosperms and 285 Gymnosperms), most of which are trees or shrubs (Table 1). Most of the families listed in Table 1 are well known EM hosts, but designation of EM hosts becomes more complex when variation occurs within large families such as the Fabaceae and Myrtaceae where numbers of EM species are most uncertain. The Sarcolaenaceae (the sister group to the Dipterocarpaceae and Cistaceae) and the Asteropeiaceae are new EM families that have been recently discovered (Ducousso et al. 2004, 2008). Table 1 is based on the data summary discussed above and additional information published online at mycorrhizas.info/ecm, which should be consulted for references and further information. Families and genera described as EM in the past, but now well established not to have EM are excluded from Table 1. Atypical EM-like associations are also excluded, as discussed in Part II.
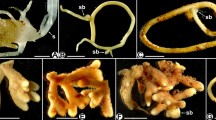
Ectomycorrhizal roots of Gnetum are substantially different from those of conifers (Pinaceae) as illustrated in Fig. 7a. The fungal interface in Gnetum EM occurs on numerous densely arranged finger-like projections, most likely derived from root hairs, embedded in matrix of hyphae. Epidermal cells are exceptionally narrow and densely packed. It is very unlikely this complex type of epidermal Hartig net evolved from the cortical Hartig net of other gymnosperms in the Pinaceae, or vice versa.
Predominantly nonmycorrhizal plant families with specialised nutrition
NM plants include about 17,000 species, or approximately 6% of flowering plants and NM-AM plants include a further 22,000 or more species, or 8% of the flowering plants. As listed in Table 2, NM or NM-AM plants occur in 90 Angiosperm families. Families were included in this list if the majority of reports are consistent and it will be updated online at mycorrhizas.info/nmplants. Despite some inconsistencies in published data, it is clear that some plant families are predominantly NM, and many of these families have been recognised for some time (e.g. Maeda 1954; Gerdemann 1968; Selivanov and Eleusenova 1974; Trappe 1981; Harley and Harley 1987; Tester et al. 1987; Brundrett 1991; Molina et al. 1992; Allsopp and Stock 1993; Schreiner and Koide 1993; Cripps and Eddington 2005). However, many of the 90 families listed in Table 2 are recognised here for the fist time. Most of the newly recognised families are parasites or carnivores that are unable or unlikely to have mycorrhizas, as explained below.
Nonmycorrhizal (NM) plants have roots that are highly resistant to mycorrhizal fungus hyphae, so usually remain free of fungi in habitats where other plants are mycorrhizal (Tester et al. 1987; Brundrett 1991; Giovannetti and Sbrana 1998). However, in many NM families, there are occasional reports of AM (usually lacking arbuscules). Families with a substantial number of reports of both NM and AM families are designated as having variable NM-AM roots. In the mycorrhizal literature, endophytic hyphae and vesicles of Glomeromycotan fungi (GFC) are interpreted as AM by some authors, but not by others, as discussed in Part II. NM plants tend to have very fine lateral roots with long root hairs, as illustrated for the NM carnivore Drosera erythrorhiza in Fig. 7b.
Figure 8 shows the relative importance of flowering plants with consistently NM roots belonging to different ecological and habitat categories. Most lineages of NM plants have evolved in directions that result in reduced benefits from mycorrhizas (loss of mycorrhizal dependency). Overall, these evolutionary trends can be summarised as “root function reduction or transformation”, where nutrient uptake by mycorrhizal roots becomes less common than other means of nutrition (Table 2). Categories of NM or NM-AM plants where mycorrhizal roots are likely to become redundant for nutrient uptake include:
-
1.
Parasites with haustoria attached to host plants,
-
2.
Carnivores that trap and digest invertebrates,
-
3.
Highly specialised hydrophytes, and
-
4.
Plants with root clusters.
These highly specialised NM plants differ from more generalist families of NM species, which acquire nutrients from soils via more conventional means. Many species in NM or NM-AM families tend to occur in specialised habitats, as discussed below. Several predominantly NM families also contain a few EM hosts. These include Kobresia spp. in the Cyperaceae, Pisonia grandis, Neea and Guapira spp. in the Nyctaginaceae and Polygonum sp. in the Polygonaceae (Table 1).
Parasites (18 families, ∼4500 spp.)
There are over 4500 parasitic plants in total and this is equivalent to about 1% of the global diversity of flowering plants (Nickrent 1997-). It is safe to assume that all holoparasites are NM due to loss of roots, or their conversion into haustoria. The most highly evolved parasitic plants grow directly attached to or within other plants, but many hemiparasites maintain a connection to the soil (Kuijt 1969). The majority of hemiparasites where roots have been assessed are NM in families such as the Orobanchaceae (Scrophulariaceae), where Lesica and Antibus (1986) found 27 species all had < 5% colonisation without arbuscules. However, there are reports of AM in hemiparasites in the Santalaceae and Krameriaceae (Lesica and Antibus 1986). It is not known if these AM associations contribute to nutrition, or are relictual but tolerated as a minor drain on resources.
Carnivores (8 families, ∼615 spp.)
Carnivores with highly specialised nutrient-capture strategies usually have NM roots, as is the case of carnivorous plants in the genera Drosera, Utricularia and Aldrovanda (the latter has no roots). Drosera species have roots that are very fine with very long root hairs (see Fig. 7b). Experiments have demonstrated that carnivorous plants acquire a substantial proportion of their nutrients by digestion of prey (Juniper et al. 1989; Schulze et al. 1997). Consequently, mycorrhizas are likely to have become partially or fully redundant. Carnivorous plants with roots that have not been examined for mycorrhizas include Brocchinia, Catopsis (Bromeliaceae) and Triphyophyllum (Dioncophyllaceae). Roridula gorgonias (Roridulaceae), a semi-carnivorous plant endemic to South Africa, has AM but also acquires nutrition from insects (Midgley and Stock 1998).
Cluster roots and related root types (8 families, ∼7000 spp.)
Some NM plants, including ∼1800 members of the Proteaceae and Myricaceae and some genera of the Fabaceae (i.e. Lupinus and Daviesia) have cluster roots—dense aggregations of lateral roots with long root hairs (Skene 1998; Lambers et al. 2006). Mycorrhizas become redundant in many plants with cluster roots, but others retain AM or EM, such as Viminaria and Aspalanthus of the Fabaceae and members of the Betulaceae, Casuarinaceae and Eleagnaceae (Allsopp and Stock 1993; Skene 1998; Lambers et al. 2006). Cluster roots can form a dense mat near the soil surface and promote nutrient uptake by their large surface area and production of exudates that increase nutrient availability (Lambers et al. 2006; Shane et al. 2006).
Some members of the Cyperaceae have root clusters that consist of swollen "dauciform" roots that are functionally similar to cluster roots (Davies et al. 1973; Shane et al. 2006). It is not clear if the Cyperaceae is a NM or NM-AM family as is discussed as the case study in Part II. Root clusters also occur in some rushes in the Restionaceae and Juncaceae (Lamont 1982; Shane et al. 2006). Other monocotyledons with NM roots that are not as well studied include the Commelinaceae, as well as the Dasypogonaceae and Haemodoraceae which have “sand-binding roots” with a thick soil sheath covering root hairs.
Predominantly nonmycorrhizal plants in mycorrhiza suppressing habitats
Plants in some families are mycorrhizal in some locations and NM in others, especially when soil conditions are not conducive to mycorrhiza formation. In other cases, families are known to include both mycorrhizal and NM species, or the family status is in doubt due to conflicting evidence. These are referred to here as NM-AM plants and plant families and Glomeromycotan fungal hyphae in roots as GFC if AM diagnosis is not certain. Possible explanations for the variable mycorrhizal status of these families are discussed in Part II. NM-AM families are included with NM plants in Fig. 8, as they often occur in the same habitats. The category of variable NM-AM mycorrhizas includes 40 families, or 8% of flowering plants (Table 2, Fig. 8). Many ferns also have variable NM-AM roots (Fig. 2). There are many NM-AM monocotyledons, especially hydrophytes (Table 2).
Situations where NM or NM-AM species are most likely to occur can be characterised as stressful and include aquatic, epiphytic, arctic, saline, disturbed, very cold (arctic and alpine) and very arid habitats (Trappe 1987; Brundrett 1991). As shown in Fig. 9, the relative importance of mycorrhizal roots is greatly reduced in arctic and alpine habitats, as well as aquatic and epiphytic habitats. These are habitats where mycorrhizal fungi may not be present, or if present, inoculum levels are likely to be low and fungal distribution very patchy. This results in a feedback loop because most mycorrhizal fungi need host plants to survive, but reduced fungal inoculum will favour non-host plants.
The relative diversity and frequency of sampling of mycorrhizal or nonmycorrhizal plants in comparatively stressful habitats. The first 5 groups of bars show the relative diversity of plants reported to have AM, NM-AM, NM, EM, ericoid (Erc) or orchid (Orc) roots in 4 habitat types. The next 5 groups of bars show the relative frequency of plant families with roots in each of these categories were sampled in the same studies. Overall results for non-stressful habitat (Fig. 4) are included for comparison as the last bar in each group. See Methods for a list of papers
Hydrophytes in aquatic, wetland or marine habitats (28 families, ∼1600 spp.)
Mycorrhizas are more likely to be absent, or sparsely/intermittently/inconsistently present in roots of hydrophytes than in other plants (Table 2, Fig. 9). However, some submerged aquatic plants rooted in sediment are typically mycorrhizal and experiments have demonstrated benefits from mycorrhizas for some of them (Clayton and Bagyaraj 1984; Beck-Nielsen and Madsen 2001; Cornwell et al. 2001; Jayachandran and Shetty 2003). The most highly specialised hydrophytes, floating plants with few or no roots, such as Ceratophyllum sp., are unlikely to ever be mycorrhizal and floating aquatic plants such as Azolla, Eichhornia, Lemna and Marsilea spp. are usually considered to be NM (Maeda 1954; Ragupathy and Mahadevan 1993; Beck-Nielsen and Madsen 2001; Kai and Zhiwei 2006; Radhika and Rodrigues 2007). In some hydrophytes, the majority of root samples with GFC lack arbuscules (Radhika and Rodrigues 2007).
Comparisons of habitats show submerged individuals are less likely to be mycorrhizal than emergent hydrophytes, or other wetland plants (Clayton and Bagyaraj 1984; Peat and Fitter 1993; Beck-Nielson and Madsen 2001; Šraj-Kržič et al. 2006). Khan and Belik (1995) list aquatic plants with NM or AM roots in different habitats, including aquatic members of the Alismataceae, Araceae, Butomacaea, Cyperaceae, Haloragaceae, Nymphaeaceae, Podostemonaceae, Pontederiaceae, Potamogetonaceae and Typhaceae. Plants in these families tend to have well developed aerenchyma and fine roots with long root hairs (Khan and Belik 1995; Beck-Nielsen and Madsen 2001). The monocotyledon families Juncaceae, Centrolepidaceae and Xyridaceae also tend to occur in wet habitats and have NM roots.
Plants with NM-AM or NM roots are even more prevalent in saline aquatic habitats. Mangroves (Avicenniaceae, Rhizophoraceae) are reported to have AM in one study, but not in 3 others (Maeda 1954; Rose 1981; Mohankumar and Mahadevan 1986; Sengupta and Chaudhuri 2002). Seagrasses (Cymodoceaceae, Hydrocharitaceae, Posidoniaceae, Zosteraceae) are NM (Nielsen et al. 1999; Brundrett and Cambridge unpublished).
Epiphytes (3 families, ∼7200 spp. of Angiosperms + many ferns)
The epiphytes that have been sampled in mycorrhizal studies predominantly belong to NM-AM families (Fig. 9). For ferns and angiosperms in families such as the Bromeliaceae, Piperaceae and Araceae, habitat is a principal determinant of mycorrhizal status, as epiphytes are often NM, while most terrestrial plants in the same families usually have AM (Lesica and Antibus 1990; Janos 1993; Maffia et al. 1993; Michelsen 1993; Gemma and Koske 1995; Grippa et al. 2007). Epiphytic ferns in a plantation had NM roots (Nadarajah and Nawawi 1993), but those growing in natural habitats are more likely to have AM (Gemma and Koske 1995; Rains et al. 2003).
The Araceae (aroids) have complex mycorrhizal relationships as they include terrestrial plants with AM, as well as NM hydrophytes such as Lemna and Pistia spp. and NM-AM epiphytes such as Philodendron spp. (Maeda 1954; Santos et al. 2000). Species in the Araceae were split between AM and AM-NM categories in Table 2. Epiphytic orchids are also often mycorrhizal, but require further study (Hadley and Williamson 1972; Otero et al. 2002). Ericoid mycorrhizas also occur in epiphytic Ericaceae in South America (Rains et al. 2003). The overall importance of epiphytic mycorrhizas is not as well resolved, as that of other plants, due to limited sampling (Janos 1993). In some cases the significance of GFC in epiphytes is unclear since only hyphae and vesicles were observed (Nadjarajah and Nawawi 1993; Maffia et al. 1993).
Arctic and alpine plants (1 family, ∼650 spp.)
Many alpine and arctic plants belong to variable NM-AM or NM families such as the Cyperaceae, Brassicaceae and Caryophyllaceae, but only the Saxifragaceae occurs most often in these habitats. Nonmycorrhizal plants tend to become more dominant at high latitudes (Väre et al. 1997), as is also the case in sub-antarctic islands (Laursen et al. 1997). Figure 10a uses data from studies of arctic plants to illustrate this point. Extremely cold habitats also seem to induce some plants to switch to EM, as is the case with arctic species of Kobresia, Dryas and Polygonum. Plants belonging to families which are typically AM elsewhere are also likely to have NM roots in the coldest arctic sites (Fig. 10b). The impact of altitude on mycorrhizas in alpine habitats seems to be less pronounced than the impact of latitude in arctic sites (Fig. 9), but this could result from the choice of sampling locations.
Correlations between arctic latitude (degrees N) and the relative frequency of inclusion of plants with nonmycorrhizal roots in publications. a Plants lacking mycorrhizas. b Nonmycorrhizal plants in families that are typically mycorrhizal in other habitats. See “Methods” for the list of papers
Arid and arid saline habitats (12 families, ∼7800 spp.)
Non-succulent predominantly NM plant families with species that often occur in salt-affected areas, such as desert saltpans and salt lake margins, include the Amaranthaceae, Chenopodiaceae, Cleomaceae, Frankeniaceae, Plumbaginaceae, Tamaricaceae and Zygophyllaceae (Table 2). Selivanov and Eleusenova (1974) summarised data for 234 desert plants, of which a comparatively high proportion (35%) were NM. They observed that families such as the Brassicaceae, Caryophyllaceae, Frankeniaceae, Juncaceae and Polygonaceae were fully NM while the Chenopodiaceae, Cyperaceae, Plumbaginaceae and Papaveraceae had a majority of NM plants.
The families Aizoaceae, Crassulaceae, Mesembranthaceae, Portulacaceae and Molluginaceae have succulent leaves and NM-AM or NM roots. They also frequent arid habitats where mycorrhizas may be less beneficial than elsewhere because plant productivity is very low and periods of root activity are brief. However, many other succulents, such as members of the Agavaceae, Cactaceae and Euphorbiaceae have AM roots (Bethlenfalvay et al. 1984; Carrillo-Garcia et al. 1999; Camargo-Ricalde et al. 2003).
Disturbed habitats and weedy plants (5 families, ∼7000 spp.)
It is well known that many NM plants are herbs that occur in disturbed habitats (Trappe 1987; Harley and Harley 1987; Peat and Fitter 1993). Families that include many annual weeds and often have NM roots include the Amaranthaceae, Brassicaceae, Capparaceae, Caryophyllaceae, Chenopodiaceae, Cyperaceae, Molluginaceae, Papaveraceae, Polygonaceae, Portulacaceae, Urticaceae and Zygophyllaceae (Hirrel et al. 1978; Pendleton and Smith 1983; DeMars and Boerner 1996). These families are fully NM, or include some AM hosts such as Atriplex which is a shrub in the NM-AM family Chenopodiaceae (Miller 1979; Schmidt and Reeves 1984; Asghari et al. 2005). Nonmycorrhizal families also tend to be early colonisers of habitats created by disturbances such as volcanism, glaciation or erosion, but mycorrhizal plants soon become established in these new habitats (Allen 1988; Gemma and Koske 1990; Cázares et al. 2005). It has been well established that severe soil disturbance reduces the inoculum potential of mycorrhizal fungi, but they tend to be present in all but the most recently/severely impacted sites (Brundrett et al. 1996a; Jasper 2007). Consequently, lack of mycorrhizal fungus inoculum may contribute to the NM/AM status of plants in some habitats, where more intensive sampling is required to resolve the mycorrhizal status of plants (see Section on Resolving issues with diagnosis of mycorrhizas).
In a comprehensive study of 649 taxa in the Brassicaceae, DeMars and Boerner (1996) observed that 20 taxa contained hyphae and vesicles and the rest were fully resistant to an aggressive root colonising AM fungus. No samples had arbuscules. In contrast, Orlowska et al. (2002) considered Biscutella laevigata in the Brassicaceae to be AM, but arbuscules were only present in mature specimens of these annual plants. A detailed study of Thlaspi spp. in the Brassicaceae by Regvar et al. (2003) found GFC in some samples, but arbuscules were very rare. They concluded these associations were probably of no functional significance.
The Papaveraceae include many weeds and species well established to be NM such as Chelidonium majus (e.g. 43 samples all NM—Brundrett and Kendrick 1988), as well as those with AM such as Sanguinaria canadensis (18 samples all AM—Brundrett and Kendrick 1988). However, mycorrhizal relations of the later species are complex, because AM occurs in fine laterals, but not in coarser roots where orange-coloured metabolites that include fungistatic alkaloids are most visible (Brundrett and Kendrick 1988; Brundrett 1991). This example where AM and NM roots apparently occur simultaneously in a single host is worthy of further study. The Hydrophyllaceae are another family reported to include both NM and AM plants in separate genera.
Other NM or NM-AM families (8 families, ∼1,700 spp.)
Other families reported to have NM species where the majority of species are not associated with harsh habitats are the Adoxaceae, Erythroxylaceae, Quiinaceae, Resedaceae, Capparaceae (sister to Brassicaceae), Hydrophyllaceae, Nyctaginaceae and Loasaceae (Table 2). Of these families, the Adoxaceae and Loasaceae, are poorly sampled. Members of the Erythroxylaceae and Quiinaceae accumulate very toxic alkaloids (Heywood et al. 2007). Fungistatic chemical accumulation is characteristic of many NM plant families (see Brundrett 1991).
Case Study 1: The relative importance of mycorrhizal and nonmycorrhizal roots in an ancient landscape with nutrient-poor soils
This case study is presented to demonstrate how knowledge of the nutrition of plant families can be scaled to a regional scale.
The Southwest Floristic Region of Western Australia is an internationally recognised biodiversity hotspot (Myers et al. 2000). High plant diversity in this region is linked to highly infertile soils and a long geological history without major tectonic or glacial disturbance (Hopper and Gioia 2004). Figure 11 clearly shows that certain functional categories of roots or plant nutrition have become much more important in the ancient landscapes of Western Australia (WA) than elsewhere. These include NM plants in the cluster root, carnivore and marine plant categories, as well as EM hosts. Parasitic plants and orchids are less diverse than other highly specialised plants in WA (their centres of diversity are in the humid tropics). Western Australia has about 150 species of carnivores, most of which are endemic, which represents almost 1/4 of all carnivorous plants. These include over 100 Drosera spp. and 39 Utricularia spp (Florabase 2007). Western Australia is also a hotspot of diversity for marine angiosperms (seagrasses) with about 30% of known species (M. Cambridge pers. comm.).
The relative importance of specialised nutritional strategies for flowering plants in Western Australia (WA) in comparison with the whole World. Data are the ratio of actual over expected numbers of species, as explained in the text. Categories of plants with bars extending above the expected line (1) are more diverse in WA than they are on a global scale
It is well known that highly leached soils in the ancient landscapes of WA include many habitats where plants with cluster roots tend to be more abundant than elsewhere in the world (Lamont 1982; Lambers et al. 2006). However, plants with AM roots are also common in these habitats and there is a much higher diversity of plants with EM roots than would be expected (e.g. the Myrtaceae and Fabaceae are often dominant). In conclusion, it seems that the ancient landscapes and infertile soils of WA are linked to an exceptionally high relative diversity of plants with specialised means of mineral nutrition. It is likely that the former provided time for a high degree of speciation of plants in these categories, while the latter could explain their increased relative diversity relative to other ecosystems.
Mycorrhizal evolution revisited
The evolution of mycorrhizal associations has been discussed in considerable detail elsewhere (Pirozynski and Malloch 1975; Trappe 1978; Read et al. 2000; Brundrett 2002; Bidartondo 2005; Wang and Qui 2006), so only updated information is provided here. Detailed summaries of the relative importance to clades and orders of flowering and non-flowering plants are discussed in the previous sections and summarised in Figs. 12 and 13. Figure 13b includes the same data as Fig. 13a with AM hosts omitted to allow other categories to be seen more clearly. Only a very small basal group of flowering plants is poorly sampled and there is a high degree of consistency within many clades of angiosperms (Figs. 12, 13). As has already been well established, AM is the basal condition in all major groups of vascular plants and is still dominant in most orders and clades. At the clade level, most contain 2 or more nutrient strategies, but, the Euasterids I and II are predominantly AM plants (Fig. 13). Of the 54 orders of flowering plants, 22 are predominantly AM hosts.
The occurrence of mycorrhizas in clades of flowering plants (see Fig. 1 for Abbreviations)
The occurrence of mycorrhizas in orders of flowering plants. a All types of mycorrhizas. b Data as in a, but omitting AM to allow other categories to be seen more clearly. c Orders of plants with highly specialised nonmycorrhizal or myco-heterotrophic (MH) plants (see Fig. 1 for other abbreviations)
Ectomycorhizas occur in at least 10 separate lineages of the angiosperms and 2 in the Gymnosperms, but there almost certainly are multiple origins of EM within orders such as the Caryophyllales, and within families such as the Ericaceae, Fabaceae and Myrtaceae.
There are 90 families of NM or NM-AM plants (Table 2, Figs. 12, 13). These are dispersed throughout the angiosperms in at least 30 clades (the NM strategy likely originated more than once in some clades). The largest aggregations of NM or NM-AM families within a clade are in:
-
The Alismatales with 13 families including many hydrophytes.
-
The Poales with 8 families including the Restionaceae, Cyperaceae and Juncaceae, with the Poaceae being an AM in-group. This clade also includes many variable NM-AM aquatic or epiphytic plants in the Typhaceae, Bromeliaceae, Sparganiaceae.
-
The Caryophyllales with 14 families including halophytes and carnivores.
-
The Santalales with 7 families of parasites (Der and Nickrent 2008).
-
The Brassicales with 4 families.
-
The Lamiales with 6 families of carnivores, parasites, or aquatic plants.
There are also many NM families that are isolated within clades of predominantly mycorrhizal plants, such as the Proteaceae and a number of other isolated groups of parasites, epiphytes and aquatic plants (some are unplaced within clades due to unresolved phylogeny). In other cases NM plants exist as a group or groups within families that also include AM hosts such as the Papaveraceae and Hydrophyllaceae. This situation also occurs in families such as the Araceae, Piperaceae, Bromeliaceae that include substantial numbers of both terrestrial and epiphytic species, as well as in families with both aquatic and terrestrial members.
Orders with highly specialised NM plants, or myco-heterotrophs are displayed in Fig. 13c. Each of these strategies has arisen more than once in distantly related species including; 6 or more lineages of parasites, 4 of epiphytes, 5 of myco-heterotrophs outside the Orchidaceae, 3 of NM cluster roots, 6 or more of aquatics and 4 or more of carnivores. Assessment of the phylogeny of carnivorous plants has shown that Drosera, Dionaea, Aldrovanda, Drosophyllum, Nepenthes and Triphyophyllum are 2 closely related clades in the Caryophyllales (Cameron et al. 2002). Thus, the majority of carnivorous plants belong to a single lineage of predominantly NM plants in an order including many other NM plants (Aizoaceae, Caryophyllaceae, Chenopodiaceae, Polygonaceae, etc.). There are also many parasitic plants in a single lineage within the Santalales (Der and Nickrent 2008). In some orders, plants that have lost the capacity to host AM seem to be more likely to evolve new nutrient acquisition mechanisms such as carnivory. In other cases, specialisations such as cluster roots or parasitism probably preceded the loss of mycorrhizas, which became redundant or impossible in the case of those parasites or aquatic plants that lack soil contacting roots at maturity.
Earlier reviews by Trappe (1987) and Wang and Qui (2006) also summarised data for substantial numbers of flowering plants. Reorganisation of the “tree of life” for plants makes it difficult to compare clades in Trappe (1987) with those presented here. However, results are in agreement for aquatic monocots with NM or NM-AM roots (Alismatales, etc.) and for some orders of NM or EM plants (Fagales, Santalales, etc.). Wang and Qui (2006) list families as NM that are known to have mycorrhizal roots (Isoetaceae, Adoxaceae, Bromeliaceae, Butomaceae, Cannaceae, Erythroxylaceae, Loasaceae, Menyanthaceae and Nymphaeaceae), or were only represented by a single report (Cyclanthaceae, Limnocharitaceae, Bataceae, Butomaceae). As stated by Harley and Harley (1987), single reports are not sufficiently reliable to make a diagnosis about the presence or absence of mycorrhizas for a family. Wang and Qui (2006) also list several families as AM that are considered by most mycorrhizologists to consists predominantly of NM species (Brassicaceae, Juncaceae, Proteaceae, Restionaceae) and classify some NM-AM families as AM (Cyperaceae, Papaveraceae). The approach in the current review differs from that of Wang and Qui (2006) by developing a consensus view of the literature for each family, by discounting occasional contradictions that are likely to be errors and by allocating inconsistently mycorrhizal families in the NM-AM category. In summary, the majority of NM families designated by Wang and Qui (2006) are not in accordance with those recognised here (i.e. they only recognised 21 NM families, of which 9 are probably incorrect, while over 90 are recognised here).
The evolution of nutrient-uptake mechanisms, such as new types of mycorrhizas or NM cluster roots, seems to have coincided with the origin of many plant families which apparently became more competitive in certain habitats (Brundrett 2002). We would assume that these mechanisms provided a selective advantage due to increased nutritional efficiency relative to associated costs. However, analysis of the costs and benefits of root nutrient-uptake mechanisms is complex, because mycorrhizal plants remain dominant in most habitats, while most NM plants are marginalised in wet, saline, dry, disturbed, or cold habitats or extremely infertile soils, where plant productivity is low and inoculum of mycorrhizal fungi could be scarce (Brundrett 1991).
Conclusions
Different approaches have been used to summarise data on the relative diversity of plants with mycorrhizas and other plant nutrition adaptations at different scales. These scales include locations, habitats, regions, ecosystems, or the whole world. Data on the relative dominance of mycorrhizal plants at the ecosystem level provides the most accurate indication of the ecological importance of these associations (St John and Coleman 1983), but is available for few locations and cannot be determined on a global scale. In this review, a summary of mycorrhizal association data for families, orders and clades of flowering plants allowed the total diversity of all plants with mycorrhizal roots to be accurately calculated on a global scale for the first time. The same approach was also applied at a regional scale in Western Australia to reveal major trends in plant adaptation in ancient landscapes.
There now is sufficient data to establish the category or categories of mycorrhizal association or other nutritional strategies of most families and orders of flowering plants. Consequently there is little need of further studies that only produce list of mycorrhizal plants unless they target gaps in existing knowledge by including:
-
1.
Poorly sampled habitats.
-
2.
Un-sampled plant families.
-
3.
Families with complex root strategies, such as the Fabaceae in Australia where the relative diversity of plants with AM, EM or NM-cluster roots is unresolved.
-
4.
Plants in variable NM-AM families, especially if detailed information about seasonal variation or habitat effects on colonisation is provided.
-
5.
Mycorrhizal colonisation data linked to data on plant diversity, ecology or physiology at the ecosystem scale.
-
6.
Corrections to the status of families or genera published in earlier studies.
Part II. Mycorrhizal Diagnosis and Misdiagnosis
Quantifying methods and estimating error rates in published data
Compilation of data from 128 published lists of mycorrhizal plants, as reported in Part I, also allowed the relative importance of different criteria for diagnosis of AM and data used to make these diagnoses to be categorised (Figs. 14, 15). This analysis revealed that despite considerable improvement in our knowledge of how mycorrhizal associations work over more than a century of progress, no consensus has emerged about how they should be identified. Problems with mycorrhizal definitions can contribute to confusion about which families have mycorrhizal or NM roots, as is discussed below.
Apparent error rates for diagnosis of arbuscular mycorrhizas (amAM), nonmycorrhizal roots (amNM), or ectomycorrhizas (amEM) in mycorrhizal studies are shown in the first three double columns. The remaining 5 double bars show the frequency of publication for different categories of data used in mycorrhizal diagnosis. In all cases, the first bar represents the proportion of taxa across all studies (128 publications) and the second column represents the proportion of studies in each category. Data are from the same sources used in Fig. 4
Since mycorrhizas formed by Glomeromycotan fungi are now routinely described as arbuscular mycorrhizas (AM) rather than as vesicular-arbuscular mycorrhizas (VAM), we would expect that most reports of the occurrence of these associations to be based on observations of arbuscules. However, as Fig. 14 shows this is not the case. In fact, arbuscules were only used to identify about 1/4 of AM species listed in publications (it is unlikely studies which do not state which criteria were used relied on arbuscules). The reasons why arbuscules are important, but should not be the only criteria used for diagnosis are explained in Part J below.
Error rates in mycorrhizal diagnosis can only be estimated by assuming that most plant families consistently have mycorrhizal or NM roots, when there is sufficient sampling for this to be determined. Even when problematic families and habitats are excluded, the overall error rates in diagnosis of AM, NM and EM roots are higher than might be expected (Fig. 15). In Fig. 15, possible misdiagnoses of mycorrhizas are referred to as;
-
1.
amAM—which is apparently misdiagnosed AM in a family of predominantly NM plants,
-
2.
amNM—which is apparently misdiagnosed NM in a family of mycorrhizal plants, or
-
3.
amEM—which is apparently misdiagnosed EM.
The overall apparent rate of misdiagnoses for mycorrhizas of AM, NM and EM is about 10% (Fig. 15). The largest category for potential errors (amNM) results when plants in typically mycorrhizal families are not found to have AM. This could result from inadequate methods (processing of roots), or poor samples, and indicates mycorrhizologists are most likely to err on the side of under-detection. Overly stringent diagnostic criteria result in an increased probability of failure to diagnose AM (Section Resolving issues with diagnosis of mycorrhizas). For example, a survey of over 300 species had a high amNM rate of about 30%, but this can be reduced to 10% if samples with vesicles but no arbuscules are considered AM, as is most often the case in other studies. The errors reported in Fig. 15 result from both a low overall rate across all studies and a much higher rate of apparent errors in several large surveys that included many taxa with limited sampling of each. However, any apparent correlation between the size of surveys and apparent errors is contradicted by other large studies with low apparent error rates, such as Maeda (1954) who sampled >1000 spp. and had very few contradictory results at the family level.
Types of data presented in publications on mycorrhizal plants are also summarised in Fig. 15. As was the case with diagnosis, data on the occurrence of arbuscules in root samples is only presented in 1/4 of studies, so it is often not possible to check how diagnosis was performed when results are unexpected. Mycorrhizal data presentation in the literature is inconsistent and usually does not allow diagnostic criteria to be applied retrospectively (Fig. 15). In particular:
-
Colonisation data may be presented as % RLC, or as a scale, or only presence or absence is noted.
-
Sampling replication is often low, or not stated.
-
Data on variability or consistency within species is rarely presented. Standard errors are sometimes presented, but may to be pseudo-replication in some cases.
-
Arbuscular and vesicular data are often not presented separately from RLC data.
-
Morphological criteria for diagnosis of mycorrhizas are often not applied or are not stated in methods.
-
In the majority of cases, vesicles and hyphae are of equal or greater importance to arbuscules in the diagnosis of AM.
-
Diagnosis of EM is also not always reliable, as some reported associations lack a Hartig net (see Section on Diagnosis of ectomycorrhizas (EM) below).
Case study 2: Resolving conflicting data for the Cyperaceae, Juncaceae and allied families
The purpose of this case study is to summarise data on the Cyperaceae in an attempt to understand why this plant family has been repeatedly diagnosed as NM by many authors, while others consider sedges to be AM hosts. Detailed information on sedge roots from published reports that included many species or samples of sedges is summarised below.
-
Powell (1975) found sedges and rushes (Cyperaceae, Juncaceae) were predominantly NM, 36 of 88 spp. had sparse GFC and arbuscules were not reported. They also did not form AM in pots after inoculation.
-
Brundrett and Kendrick (1988) found 2 upland Carex species were consistently NM throughout the year (28 samples).
-
Meney et al. (1993) sampled 12 species of sedges and rushes (Restionaceae) in Western Australia and found 4 species had GFC, but only later in the growing season.
-
Cooke and Lefor (1998) found the Cyperaceae (18 spp.), Juncaceae (6 spp.) had highly variable GFC across taxa and sites (AM not defined by arbuscules).
-
Miller et al. (1999) reported GFC in 16 of 23 Carex spp., but only 9 contained arbuscules.
-
Muthukumar and Udaiyan (2002) studied the phenology of 2 tropical sedges and found GFC varied seasonally, but no arbuscules were formed.
-
Fuchs and Haselwandter (2004) reported Carex sp. in bogs had GFC colonisation that varied seasonally and between sites (4% RLC without arbuscules).
-
Ruotsalainen and Aikio (2004) found that the presence of AM fungi reduced the growth of a Carex sp. when it was growing in competition with a host plant.
-
Gai et al. (2006) found 9 sedges in Tibet had GFC, but only 10 of 22 samples had arbuscules (Kobresia spp. apparently lacked EM in this study).
-
Perrier et al. (2006) found 3 tropical sedges in New Caledonia had variable GFC, but low mycorrhizal intensity. Arbuscules were not quantified separately.
-
Weishanpel and Bedford (2006) studied 10 sedge species that were primarily NM, but 5 of 17 samples had low GFC (2–18% RLC), including 3 of 17 with traces of arbuscular colonisation (<3% RLC).
-
A detailed literature review by Muthukumar et al. (2004) summarised data from 221 sedges of which 40% were considered to have AM. However, they noted that the majority of root samples lacked arbuscules and concluded that sedges have a low capacity for mycotrophy.
Several alternative hypotheses that could explain the variable reports of AM and NM in sedges and rushes and may apply to other families with NM-AM roots are:
-
1.
These are NM plants with occasional endophytic GFC (sometimes with arbuscules) due to specificity errors or resistance breakdown in older roots that is misdiagnosed as AM.
-
2.
There is a continuum extending from fully NM to AM species in the Cyperaceae, and some other families resulting from continuing adaptation to variable habitat conditions.
-
3.
Sedges are potentially AM plants, but often occur in mycorrhiza-suppressive habitats.
-
4.
Error rates in published data are too great to allow reliable conclusions.
Some of the evidence cited above supports the first hypothesis, in that GFC is more likely to occur in older sedge roots and most reports of putative AM in sedges state these are inconsistent or sparse and they usually lack arbuscules. This would imply that sedges are functionally NM plants that cannot fully exclude relictual GFC (perhaps the carbon drain from sparse colonisation is negligible). Comprehensive studies of mycorrhizal root phenology using a rigorously applied definition of AM and a high degree of sampling replication, both within species and throughout the growing season, are required to investigate this hypothesis.
The second hypothesis is supported by the fact that the Cyperaceae is a very large family that occupies diverse habitats. Variable mycorrhizal relationships could be linked to habitat factors that result in adaptation to stressful conditions such as waterlogged and cold soils. However, this could also be indicative of flaws in the processes used to designate AM and NM plants, as explained below. Examples that support a high capacity for evolution of new root types in sedges include arctic sedges in the genus Kobresia which are the only monocots to have acquired EM associations, and are presumably descended from a NM ancestor.
There is good evidence to refute the third hypothesis, as some sedges have consistently NM roots in warm dry habitats. The fourth hypothesis seems likely to be a major contributor to conflicting reports of AM in sedges, but is unlikely to be its sole cause.
When assessing the literature on putative AM of sedges it seems most likely that conflicting data results, at least in part, from a failure to use consistent criteria to identify mycorrhizal associations. In this review a precautionary approach has been taken that classifies the Cyperaceae as a variable NM-AM family. However, the balance of information seems to suggest they are a predominantly NM family, with only sporadic GFC in roots. It is possible that all arbuscules in sedges occur in older roots and that young sedge roots are highly resistant to mycorrhizal fungi. If the Cyperaceae are designated as a true NM plants (as opposed to variable NM-AM) the error rate for mycorrhizal diagnoses in published data will increase substantially. This also requires us to acknowledge that arbuscules occur sporadically in NM plants, further weakening the role of arbuscules in defining AM. The confusion about the mycorrhizal status of families such as the Cyperaceae needs to be resolved by more rigorous approaches to diagnosis, as is explained below.
Glomeromycotan fungus colonisation (GFC) in predominantly NM plant families
Since the majority of reports of AM in AM-NM families such as the Cyperaceae did not use arbuscules to diagnose associations, inconsistent colonisation data of these families, may be indicative of misdiagnosis of endophytic growth of hyphae and vesicles without arbuscules in roots as AM. Endophytic activity by Glomeromycotan fungi (which is often referred to as saprophytic growth) is relatively common in various plant organs (e.g. Stasz and Sakai 1984; Warner 1984; Smith et al. 1998; Brundrett 2006; Zhang and Guo 2007). Humans like to have clearly defined boundaries between alternatives we consider important (i.e. we have a tendency to see the world in black and white). However, fungi are not constrained by our world-view and are opportunists that constantly seek to exploit new situations as endophytes or mycorrhizal partners.
Endophytic GFC is likely to be of only minor ecological significance to plants or fungi and needs to be distinguished from more important plant-fungal associations. It is recommended that Glomeromycotan fungi in roots be labelled GFC in cases where a mycorrhizal association cannot be confirmed (Appendix 2). Fungi known to be mycorrhizal in roots should not be called endophytes, as this contradicts the diagnosis of mycorrhizas. Consistent use of terminology, especially in titles and keywords, is required to avoid confusion and allow knowledge to be retrieved by computerised literature searches.
While it is safe to diagnose roots that never contain arbuscules, but may contain some hyphae and vesicles as NM roots with GFC, the diagnosis of roots with occasional arbuscules is more difficult to resolve, as revealed by the Cyperaceae case study. For example, Hildebrandt et al. (2001) found occasional GFC in roots of members of the Juncaceae, Juncaginaceae and Caryophyllaceae (mostly under 10% RLC) with a few arbuscules (3% RLC in 1 sample). Other examples of occasional arbuscules in plants normally considered NM include the Brassicaceae (Orlowska et al. 2002; Regvar et al. 2003) and Proteaceae (Bellgard 1991; Boulet and Lambers 2005) and epiphytic bromeliads (Rowe and Pringle 2005). In some cases we need to be cautious about calling the observed structures arbuscules, as published images are inconclusive, or no images are provided. Brundrett and Kendrick (1988) distinguished AM and endophytic growth by Glomeromycotan fungi by sampling roots throughout the year to develop an understanding of root and mycorrhizal phenology. They observed that hyphae and vesicles were only present in senescent roots in NM plants, so were not the remnants of mycorrhizal associations.
A key question that arises from the frequent apparent misdiagnosis of AM in sedges reported in case study 2 is: How many arbuscules are required for a functional AM association? If arbuscules are rare, or only occur in old roots they are unlikely to be of major functional significance, but further anatomical and physiological research is required to determine if this is the case. There may also be varying degrees of endophytic activity by Glomeromycotan fungi, perhaps due to differing exclusion mechanisms by non-hosts. GFC could also be strongly influenced by environmental factors. The declining resistance of NM plants (e.g. annuals growing in seasonal environment) to AM fungi with age often seems to coincide with changes in environmental conditions, such as drying out of aquatic habitats, making them more favourable to GFC. Thus is may be essential to sample roots at different phases of the growing season to resolve association types. The diagnosis of NM and AM roots is inextricably linked, as any roots not diagnosed with mycorrhizas are considered to be NM.
There are several possible explanations for reports of AM in predominantly NM plant families:
-
i.
There are no fully NM plants since they all have the capacity to occasionally form AM,
-
ii.
There are no fully NM families since they all contain a few species with AM,
-
iii.
The occasional reports of AM in NM families are errors in sampling, assessment, or diagnoses that fall within the expected error rate (∼10%), and/or
-
iv.
True NM plants have occasional AM that can include a few arbuscules, but these are not functional mycorrhizas.
The first of these alternatives has been shown to be incorrect by detailed studies demonstrating that roots of NM plants were resistant to high inoculum levels of Glomeromycotan fungi (e.g. Brundrett and Abbott 1991; Hirrell et al. 1978; DeMars and Boerner 1996). The second alternative has been confirmed for a few NM-AM families such as the Papaveraceae, but seems unlikely for most others. There is strong evidence to support both the third and fourth alternatives for the majority of NM families, as summarised above. Consequently, we should be prepared to expect occasional GFC in the roots of NM plants and may also have to acknowledge that these roots may contain a few arbuscules without having functional AM associations.
Diagnosis of ectomycorrhizas (EM)
Misdiagnoses of EM associations are much less common than misdiagnoses of AM, presumably due to the less frequent occurrence of EM and the major alterations in root structure that normally occur. However, atypical EM-like associations that are difficult to categorise do occur, as shown by examples in Table 3. Misdiagnosis of EM could have major consequences to our understanding of ecosystem processes if dominant trees in ecosystems are involved. However, most EM hosts belong in families well documented to have these associations and nothing else (or have both EM and AM in roots), but some are restricted to particular genera within a family of AM or NM hosts and others have both EM and AM (listed in Table 1).
Most examples listed in Table 3 seem to result from application of an imprecise definition of EM (where the Hartig net is not required), or associations that appear to be intermediate between EM and ericoid or saprobic growth of hyphae on roots (Brundrett 2006). Many of the unusual associations in Table 3 occur in alpine habitats. One example is Pedicularis spp. which are hemiparasites in the Orobanchaceae (Scrophulariaceae) reported to be EM in some alpine studies (Kohn and Stasovski 1990; Li and Guan 2007), but not others (Cázares et al. 2005; Gardes and Dahlberg 1996).
It is reasonable to expect that the EM interface (Hartig net) must be connected to the apoplastic space of roots and also must be sealed or enclosed to prevent loss of metabolites into the soil to allow effective nutrient exchange. For example, a strong relationship between the degree of Hartig net formation and growth responses was observed when screening isolates in a glasshouse trial (Burgess et al. 1994). In EM roots the zone of exchange is usually delimited by a suberised exodermis or the endodermis within the root and a well developed mantle on the outside and there are substantial morphological responses by host cells to produce an effective interface (cell enlargement, transfer cells, etc.) (see Brundrett et al. 1990; Vesk et al. 2000; Peterson et al. 2004). In Table 3 it is assumed roots are not EM if they lack a substantial plant-fungus interface.
There are many cases of probable EM misdiagnoses in literature published before morphological definitions of associations to became standardised. These are not errors as such since they represent the state of knowledge at that time they were originally published, but cause confusion when perpetuated in more recent publications. For example, Acer, Fraxinus, Ulmus, the Cupressaceae, etc. were included in EM hosts lists by Trappe (1962) and subsequent authors (e.g. Smith and Read 1997). The Cupressaceae were once assumed to be hosts for EM fungi that fruited in habitats where they co-occur with conifers in the Pinaceae (roots were not sampled). Plants with beaded roots are also more susceptible to confusion than other plants, as they appear heterorhizic if not examined carefully (e.g. Acer, Ulmus, and members of the Podocarpaceae), but the AM status of these trees is now well resolved. There are also cases where it seems likely that field-collected root samples were contaminated by roots of other species, such as reports of EM in ferns. Warcup and McGee (1983) observed unusual associations in families such as the Asteraceae and Stylidiaceae, which were not found to be EM by other investigators (Table 4).
As discussed above, the Hartig net-like structures on the root surface will not function as exchange site if substances produced by the fungus escape from the root, but the fungus may benefit by capturing exudates. In the case of Graffenreda sp. (Haug et al. 2004) the root primarily hosts AM, which would have much greater access to internally released metabolites. Epiphytic Ericaceae with “cavendishoid mycorrhizas” (Setaro et al. 2006) are unlikely to function as EM since contact between the putative Hartig net, which is inconstantly present and weakly developed, primarily occurs on the outer surfaces of epidermal cells containing ericoid mycorrhizas. Rains et al. (2003) considered these associations to be ericoid mycorrhizas. A patchy mantle has also been observed on other ericoid roots (Massicotte et al. 2005). Other EM-like associations that appear to be non-functional include those of Morchella sp. on Pinaceae (Dahlstrom et al. 2000), Cortinarius sp. on Carex (Harrington and Mitchell 2002) and Tricholoma sp. on Pinus (Gill et al. 1999). Some fungi considered to be EM associates may actually be parasitic on EM roots (Yun and Hall 2004).
The opportunistic colonisation of root surfaces by fungal hyphae is common in nature and perhaps should be considered a form of endophytism where fungi feed on root exudates without penetrating cells (see Brundrett 2006). In conclusion, there needs to be a reasonable prospect that mycorrhizal associations can function by providing balanced two-way exchange before we should call them EM. It is not possible to resolve if some of the associations listed in Table 3 are diagnosis errors or unusual new associations without additional anatomical and physiological studies. A list of EM host plants that is as accurate as possible is provided in Table 1 and will be maintained online (mycorrhizas.info/ecm).
Practical definitions of mycorrhizal associations
Anatomical features of mycorrhizas must be observed to distinguish them from other fungi in roots (Brundrett 2004). Any attempt to define mycorrhizas by physiological parameters such growth responses would be impractical, since such information is usually not available. For example, mycorrhizal growth responses have been measured for about 200 host plants from natural ecosystems grown at realistic soil fertility levels (Brundrett and Abbott 2002). In contrast, the anatomy of the root-fungus interface has been used to identify mycorrhizas in over 10,000 plants (see Section on Summary of mycorrhizal survey data). Thus, mycorrhizas are defined by anatomy alone > 99.9% of the time. See Brundrett (2004) for a more comprehensive discussion of this topic.
A revised definition of mycorrhizas was provided by Brundrett (2004) to exclude non-mycorrhizal symbioses in roots and to encompass all types of these symbioses. This definition is based on developmental and functional features that distinguish and unify mycorrhizas, so these features can also be used for diagnosis of associations. These criteria are summarised in Table 4 and explained in the list below:
-
1.
The structure and development of mycorrhizal fungus hyphae is substantially altered in the presence of roots of host plants. These root-inhabiting hyphae are structurally and functionally distinct from hyphae formed in soil by the same fungus.
-
2.
Mycorrhizas require intimate contact between hyphae and plant cells in an enclosed interface where nutrient exchange occurs.
-
3.
The primary role of mycorrhizas is the symbiotic transfer of mineral nutrients from fungus to plant. In most cases there also is substantial reciprocal transfer of metabolites from plant to fungus (i.e. mutualism).
-
4.
Mycorrhizas require synchronised plant-fungus development for ongoing nutrient exchange, since hyphae normally only colonise young roots in mutualistic associations.
-
5.
Plants control mycorrhizal associations by growth of new roots, digestion of old interface hyphae in plant cells (AM, orchid), or altered root system form (EM).
Existing published reports often provide several lines of evidence for mycorrhizal diagnosis (e.g. percentage root length colonised, and arbuscular colonisation), but rarely link the diagnosis of mycorrhizas to such evidence in a reproducible way. As Figs. 14 and 15 show there are more published reports that do not state which criteria were used and lack detailed root colonisation data than those that do. Consequently, we should not be surprised that there are many examples of contradictory data in the literature that probably arise from differences in interpretation of such data. The misdiagnosis of AM and NM roots is particularly common, as discussed in Part L above. The following subsections discuss criteria for diagnosis of mycorrhizal roots and more detailed protocols are provided in Appendix 2. Methods for processing root samples are available elsewhere (e.g. Brundrett et al. 1996b, mycorrhizas.info/method).
Arbuscular mycorrhizas(AM)
It is ironical that as we increasingly tend to drop the V (vesicles) from the name of arbuscular mycorrhizas (from VAM to AM), evidence is accumulating that some of these associations lack arbuscules, that vesicles are used more often than arbuscules in diagnosis of associations, and that arbuscules may occur in non-host plants where Glomeromycotan fungi grow as endophytes. Examples of AM associations without arbuscules include non-photosynthetic, myco-heterotrophs with exploitative AM and primitive ferns such as Psilotum and Botrychium (Peterson et al. 1981; Imhof 1999b; Winther and Friedman 2007). A new approach to the diagnosis of AM is required to reconcile discrepancies in lists of host and non-host plants. Thus, while it is fairly safe to use arbuscules as the main diagnostic criterion in most cases, other evidence is also required to show that the morphology of associations are consistent with AM, as discussed in Appendix 2.
Protocols in Appendix 2 are designed to help prevent errors in the diagnosis of AM. These errors result primarily because arbuscules are not used to define AM in most field-collected roots, since they are difficult to observe in older roots. A more inclusive approach using arbuscules in combination with other diagnostic criteria listed in Table 4 should help distinguish endophytic GFC activity in NM roots from old AM associations. However, in some cases it may not be possible to distinguish AM or endophytic activity by Glomeromycotan fungi and these should be referred to as GFC. However, diagnosis of fungi in roots as GFC does not resolve its mycorrhizal status as it can imply that either a plant is NM with endophytic fungi or that knowledge of its mycorrhizal status is unresolved due to inadequate data.
Ectomycorrhizas (EM)
The presence of a Hartig net defines EM associations (Brundrett 2004). It is easy to recognise typical associations with a prominent mantle and thickened roots with an altered branching pattern. However, there are cases where root branching is not greatly altered, the mantle is thin or absent, or a well-developed Hartig net is not present (Table 3). As explained above, EM-like associations without a normal Hartig net may lack functional significance so should not be recognised as EM, especially when they are not the main type of mycorrhizal association present.
Nonmycorrhizal plants
Nonmycorrhizal plants have roots that remain free of mycorrhizal fungi in habitats where other plants are mycorrhizal (Selivanov and Eleusenova 1974; Tester et al. 1987; Brundrett 1991; Koide and Schreiner 1992). However, in some cases these roots of NM plants contain traces of endophytic hyphae and vesicles of Glomeromycotan fungi and sometimes may also have a few arbuscules. Absence of mycorrhizas in these species is not regulated by habitat conditions, even though they often occur in harsh habitats (Table 2).
Facultative or variable mycorrhizas
The recognition of NM-AM plants in this review as a category of variable mycorrhizas as defined by habitats as well as plant phylogeny differs from earlier approaches where facultatively mycorrhizal plants where defined by inconsistent or sparse mycorrhizal colonisation, or by soil fertility, as is summarised below.
-
1.
Facultative mycorrhizal species were originally defined as plants with roots that remain poorly colonised in soils where other species are highly mycorrhizal (usually < 25% of suitable roots) (Janos 1980; Brundrett 1991). These plants typically have fine roots with long root hairs as observed by Baylis (1975), St John (1980) and many others.
-
2.
More recently, physiological definitions of facultative mycorrhizas have been defined using nutrient response curves regulated by P availability (Abbott and Robson 1984; Schweiger et al. 1995; Janos 2007). In these experiments species with fine roots and long root hairs were less dependant on mycorrhizas than plants with coarse roots and few root hairs. This is a valuable approach for cultivated plants, but is less applicable to plants growing in natural habitats where soil P levels cannot be manipulated.
-
3.
A third concept presented in this review concerns variable mycorrhizas of a species, genus or family where colonisation is regulated by habitat conditions, as explained in Part I. These are designated as variable NM-AM plants. This variability may be linked to sampling time, or habitat conditions and is especially common in epiphytes, hydrophytes and arctic plants. A similar definition of facultative AM was used by Trappe (1987) to deal with contradictions in the mycological literature.
-
4.
A few families are known to include plants in different genera that are consistently either AM or NM plants. Examples include Atriplex species in the predominantly NM family Chenopodiaceae and the NM-AM family Papaveraceae (see Section on Predominantly nonmycorrhizal plant families with specialised nutrition).
The first 2 definitions of facultative mycorrhizas listed above are usually only applied at the species level, while the third concept (NM-AM) can apply to species or families. The fourth concept can only be applied to variability within families. In the current review, only NM-AM families are recognised, since facultative AM as defined by 1 or 2 above can not be designated in most publications due to insufficient data on root colonisation consistency. Facultative mycorrhizas should be suspected if plants have relatively fine roots with long root hairs and are weakly colonised by mycorrhizal fungi.
Resolving issues with diagnosis of mycorrhizas
How do we minimise both Type 1 and Type 2 errors in mycorrhizal diagnosis
In statistics we need to minimise both Type 1 (rejecting a true hypothesis) and Type II (accepting a false hypothesis) errors in analysis of data. In the case of mycorrhizal associations we will commit a Type I error if we overlook, or misinterpret diagnostic criteria, or a Type II error if we reach conclusions not supported by these criteria. Type I errors could include failure to diagnosis AM in old roots due to the absence of arbuscules, but Type 2 errors seem to be more common (i.e. identification of AM from endophytic hyphae and vesicles in non-host plants). In EM associations, examples of Type II errors would also result if EM hosts were designated without a Hartig net. In statistics it is also recognised that these errors cannot be avoided entirely, but analysis protocols are designed to ensure they will be minimised at an acceptably low rate. Mycorrhizal research protocols can be designed to minimise Type I and Type II errors (Appendix 2) and we need to acknowledge that these errors occur in published data.
Use of multiple sources of evidence
It is very difficult to distinguish between endophytic and mutualistic colonization of roots by Glomeromycotan fungi (AM vs GFC), as is also the case for some EM-like associations. The presence of arbuscules is normally used to identify AM and the presence of a Hartig net to define EM associations. However, these definitions are not always applied and careful judgement may be required when examining roots collected from the field, particularly if they are old, or have atypical associations. In Appendix 2, the use of arbuscules is recommended as the main criterion for AM wherever possible, but other evidence such as consistency of root occupation and indirect evidence of metabolite transfer to the fungus (vesicles or sporulation) should also be used to support diagnosis.
Adequate sampling and processing of roots
In most cases the diagnosis of AM or NM roots is straightforward, but it is more difficult if roots are inconsistently or sparsely colonised by fungi. In these cases, roots often contain mixtures of fungi, especially dark septate endophytes, as is most common in arctic and alpine habitats (Ruotsalainen et al. 2002; Cázares et al. 2005). The alternative hypotheses that fungi are (1) endophytic GFC in non-hosts, or (2) AM without arbuscules in older host roots need to be tested when we examine such roots. These problems can be minimised by understanding the phenology of roots to sample active roots, or by sampling at different times to observe colonisation trends. As explained in Appendix 2, It is essential that mycorrhizal diagnosis is based on adequate samples that include young roots and histological procedures used to examine roots reveal diagnostic features (many published images are not sufficiently clear). In some cases better results were obtained by growing plants from seed in soil from natural habitat or applying inoculum of known fungi, than were obtained by excavation of roots of unknown age from the field (Maeda 1954; Brundrett and Abbott 1991). This also avoids the possibility of cross-contamination of root samples with other species.
An understanding of the functional significance of GFC in natural ecosystems may require more comprehensive mycorrhizal colonisation intensity data than is normally obtained by mycorrhizologists. Currently a singe arbuscule in a km or roots, which is unlikely to provide much benefit, can be scored equally to 1000 arbuscules! McGonigle et al. (1990) developed a procedure for detailed assessment of arbuscule, vesicles and hyphae in root segments. However, it is recommended that this approach be modified to distinguish single occurrences from multiple occurrences of fungal structures in each root segment (see Table 6 in Appendix 2). Sufficient sampling replication, examination of seasonal colonisation tends and colonisation intensity data (especially for arbuscules) are all required to resolve the mycorrhizal status of families such as the Cyperaceae and to distinguish facultative mycorrhizal associations. Physiological data confirming that mycorrhizas are beneficial to plants would also provide valuable supporting evidence of AM associations, but cannot be used alone for diagnosis.
Results
As reported above, few published reports included sufficient data to allow results to be verified. In the future it is recommended that publications about mycorrhizal associations in ecosystems rigorously apply and state definitions used and include the data used to make these diagnoses (listed in Appendix 2). This requires additional columns to be included in results tables, and clear statements in the methods section of papers. This information could also be included in a supplemental table linked to publications available on the web. It is also recommended that result tables organize plants within families and genera to allow comparisons with other published data.
Resolving conflicting information in published data
Misdiagnosis usually results in low error rate, which is acceptable in the context of individual surveys, as it usually does not affect our understanding of the overall importance of mycorrhizas in particular locations. However, the significance of errors in diagnoses are magnified when data from many sources are compiled causing errors to accumulate in lists. These errors have the potential to limit our understanding of the importance of mycorrhizas at the plant family, ecosystem and global scales. Some of this can be resolved by giving detailed studies greater weight than observational studies without sampling replication across habitats or times when interpreting published data. As Harley and Harley (1987) stated, the mycorrhizal status of a family should not be decided by a single record, especially if the habitat is not conducive to mycorrhizal formation. One example is Batis sp. (Bataceae) designated as a NM plant by Wang and Qui (2006) after Gemma and Koske (1990) who collected it in a disturbed habitat (sand dunes), but this family may not be NM elsewhere.
Several approaches can be used to resolve conflicting published data on mycorrhizas:
-
1.
A majority rules (consensus) approach, where a family is considered to be AM or NM when most reports are in agreement even if there is some conflicting evidence (within a 10%) error rate in the published data. This works for most plant families if relatively recent data sources are consulted.
-
2.
An “expert system” approach where data are carefully reinterpreted or discarded by using evidence to support diagnosis, if it is provided in publications.
Both approaches require a greater burden of proof when published results contradict expected outcomes based on the phylogeny and habitats of plants. It is probably common for mycorrhizologists to expend additional effort checking unexpected results, but there are no defined protocols for dealing with “outliers” in mycorrhizal data.
This review used a consensus approach to develop lists of mycorrhizal plants that are consistent with plant phylogeny in most cases (see Part I). It is also important to avoid circular reasoning where preconceived ideas help determine conclusions leading to entrenchment of ideas that may not be entirely correct (but it can also be argued that this is how scientific progress normally occurs).
Diagnosis of mycorrhizal fungi
This review primarily concerns the diagnosis of mycorrhizal hosts and not fungi. However, since the propensity for mycorrhizal fungi to grow as endophytes in (or on) non-hosts can result in misdiagnosis of associations, it is necessary to briefly consider the potential impacts of this on the designation of mycorrhizal fungi. It is now common for fungi in roots to be identified by molecular means using extracted DNA, but much harder to establish what the roles of these fungi are by these means. The fact that some of the fungi that are detected may be endophytes needs to be considered, especially if results are contrary to expectations. A relevant study by Allen et al. 2003 found two groups of fungi in ericoid roots, (1) ascomycetes which could be isolated, but were rarely detected by DNA, and (2) Sebacina isolates which dominated DNA samples but could not be isolated. In this study only the ascomycetes were confirmed to be mycorrhizal. Sebacina isolates have been detected in the roots of plants with most types of mycorrhizas, but their roles in these roots are rarely tested. While some of these associations are likely to be mycorrhizal, the alternative hypothesis that they may be endophytes also needs to be considered. Despite these issues, most clades of mycorrhizal fungi are now well known (see links in Appendix 1 for lists of taxa).
Conclusions
While there is little doubt about the mycorrhizal status of the majority of large plant families (as established decades ago) there are some whose mycorrhizal status have become more uncertain over time. In part, this results from sampling over a wider range of plant diversity, environmental conditions and habitat types, but it also results because definitions of association types are not rigorously applied and errors accumulate in lists of host plants. It should be possible to eliminate the second source of contradictory data by more consistent protocols for mycorrhizal diagnosis in the future, as recommended here. We also need to address problems with data consistency (adequate replication, providing data used for diagnosis, etc.) and sampling effort, especially in environments where edaphic conditions restrict fungal activity (e.g. epiphytic, aquatic, alpine, arctic, saline and arid habitats).
We should also consider the statistical concept of Type 1 and Type 2 errors that result in either under- or over-allocation of significant results in lists of mycorrhizal plants. For example, we will tend to under-allocate taxa if we only use reports that cite arbuscules, but are likely to over-allocate mycorrhizal plants if reports that do not state if arbuscules were seen are all considered to be correct. In most cases, closely related plants (families and genera) share mycorrhizal associations or other nutrition strategies. However, there are exceptions to any generalisation, so assumptions about mycorrhizal relationships based on plant phylogeny need to be checked.
This review identifies the most common errors that have been perpetuated in the mycorrhizal literature and recommends protocols to reduce error rates in the future. The most frequent cause of misdiagnosis of plants in NM families as AM seems to be caused by misidentification of endophytic growth of Glomeromycotan fungi in non-hosts as AM. There also is increasing evidence that arbuscules occasionally occur in roots of predominantly NM plants, but their functional significance in these roots is unclear. It is anticipated that in future, more consistent approaches should reduce the misdiagnosis rate for mycorrhizas and resolve the inconsistencies in published list of host plants. After all, it is better to identify mycorrhizal association types accurately for a few species than inaccurately for many.
Abbreviations
- AM:
-
arbuscular mycorrhizas (vesicular-arbuscular mycorrhizas VAM)
- NM:
-
nonmycorrhizal plants
- EM:
-
ectomycorrhizas (ECM)
- NM-AM:
-
plants with variable AM or NM roots
- GFC:
-
endophytic or unspecified colonisation by Glomeromycotan Fungi
- RLC:
-
root length colonised
References
Abbott LK, Robson AD (1984) The effect of VA mycorrhizae on plant growth. In: Conway LP, Bagyaraj DJ (eds) VA Mycorrhiza. CRC, Boca Raton, pp 113–130
Alarcón C, Cuenca G (2005) Arbuscular mycorrhizas in coastal sand dunes of the Paraguaná Peninsula, Venezuela. Mycorrhiza 16:1–9
Alexander IJ (1989) Systematics and ecology of ectomycorrhizal legumes. In: Stirton CH, Zarucchi JL (eds) Advances in Legume Biology. Missouri Botanical Garden, Missouri, pp 607–624
Allen EB, Chambers JC, Connor KF, Allen MF, Brown RW (1987) Natural re-establishment of mycorrhizae in disturbed alpine ecosystems. Arctic Alpine Res 19:11–20
Allen EB, Rincon E, Allen MF, Perezjimenez A, Huante P (1998) Disturbance and seasonal dynamics of mycorrhizae in a tropical deciduous forest in Mexico. Biotropica 30:261–274
Allen MF (1988) Re-establishment of VA mycorrhizas following severe disturbance: comparative patch dynamics of a shrub desert and a subalpine volcano. Proc Roy Soc Edinburgh 94B:63–71
Allen MF, Egerton-Warburton LM, Allen EB, Karen O (1999) Mycorrhizae in Adenostoma fasciculatum Hook. & Arn.: a combination of unusual ecto- endo-forms. Mycorrhiza 8:225–228, doi:10.1007/s005720050238
Allen N, Nordlander M, McGonigle T, Basinger J, Kaminsjy S (2006) Arbuscular mycorrhizae on Axel Heiberg Island (80°N) and at Saskatoon (52°N) Canada. Can J Bot 84:1094–1100
Allen TR, Millar T, Berch SM, Berbee ML (2003) Culturing and direct DNA extraction find different fungi from the same ericoid mycorrhizal roots. New Phytol 160:255–272
Allsop N, Stock WD (1993) Mycorrhizal status of plants growing in the Cape Floristic Region, South Africa. Bothalia 23:91–104
Andrade ACS, Queiroz MH, Hermes RA, Oliveira VL (2000) Mycorrhizal status of some plants of the Araucaria forest and the Atlantic rainforest in Santa Catarina, Brazil. Mycorrhiza 10:131–136
Asghari HR, Marschner P, Smith SE, Smith FA (2005) Growth response of Atriplex mummularia to inoculation with arbuscular mycorrhizal fungi at different salinity levels. Plant Soil 273:245–256, doi:10.1007/s11104-004-7942-6
Bagyaraj DJ, Manjunath A, Patil RB (1979) Occurrence of vesicular-arbuscular mycorrhizas in some tropical aquatic plants. Trans Br Mycoll Soc 72:164–167
Bakarr MI, Janos DP (1996) Mycorrhizal associations of tropical legume trees in Sierra Leone, West Africa. For Ecol Manage 89:89–92
Barnola LG, Montilla MG (1997) Vertical distribution of mycorrhizal colonization, root hairs, and belowground biomass in three contrasting sites from the tropical high mountains, Mérida, Venezuela. Arctic Alpine Res 29:206–212
Bauer CR, Kellogg CH, Bridgham SD, Lamberi GA (2003) Mycorrhizal colonization across hydrologic gradients in restored and reference freshwater wetlands. Wetlands 23:961–968, doi:10.1672/0277-5212(2003)023[0961:MCAHGI]2.0.CO;2
Baylis GTS (1975) The magnolioid mycorrhiza and mycotrophy in root systems derived from it. In: Sanders FE, Mosse B, Tinker PB (eds) Endomycorrhizas. Academic, New York, pp 373–389
Beck-Nielsen D, Madsen TV (2001) Occurrence of vesicular-arbuscular mycorrhiza in aquatic macrophytes from lakes and rivers. Aquat Bot 71:141–148, doi:10.1016/S0304-3770(01)00180-2
Bellgard SE (1991) Mycorrhizal associations of plant species in Hawksbury sandstone vegetation. Aust J Bot 39:357–364, doi:10.1071/BT9910357
Berliner R, Torrey JG (1989) Studies on mycorrhizal associations in Harvard Forest, Massachusetts. Can J Bot 67:2245–2251
Berch SM, Kendrick B (1982) Vesicular-arbuscular mycorrhizae of southern Ontario ferns and fern-allies. Mycologia 74:769–776
Berch SM, Gamiet S, Deom E (1988) Mycorrhizal status of some plants in south-western British Columbia. Can J Bot 66:1924–1928
Béreau M, Gazel M, Garbaye J (1997) Les symbioses mycorhiziennes des arbres de la forêt tropicale humide de Guyane francaise. Can J Bot 75:711–716
Berliner R, Torrey JG (1989) Studies on mycorrhizal associations in Harvard Forest, Massachusetts. Can J Bot 67:2245–2251
Bethlenfalvay GJ, Dakessian S, Pacovsky RS (1984) Mycorrhizae in a southern California desert: ecological implications. Can J Bot 62:519–524, doi:10.1139/b84-077
Bidartondo MI (2005) The evolutionary ecology of myco-heterotrophy. New Phytol 167:335–352, doi:10.1111/j.1469-8137.2005.01429.x
Blaschke H (1991) Multiple mycorrhizal associations of individual calcicole host plants in the alpine grass-heath zone. Mycorrhiza 1:31–34
Blaszkowski J (1994) Arbuscular fungi and mycorrhizae (Glomales) of the Hel Peninsula, Poland. Mycorrhiza 5:71–88, doi:10.1007/BF00204022
Bledsoe C, Klein P, Bliss LC (1990) A survey of mycorrhizal plants on Truelove Lowland, Devon Island, N.W.T., Canada. Can J Bot 68:1848–1856
Brockhoff JO, Allaway WG (1989) Vesicular-arbuscular mycorrhizal fungi in natural vegetation and sand-mined dunes at Bridge Hill, New South Wales. Wetlands 8:47–54
Boulet FM, Lambers H (2005) Characterisation of arbuscular mycorrhizal fungi colonisation in cluster roots of Hakea verrucosa F. Muell (Proteaceae), and its effect on growth and nutrient acquisition in ultramafic soil. Plant Soil 269:357–367, doi:10.1007/s11104-004-0908-x
Brundrett MC (1991) Mycorrhizas in natural ecosystems. Adv Ecol Res 21:171–313, doi:10.1016/S0065-2504(08)60099-9
Brundrett MC (2002) Coevolution of roots and mycorrhizas of land plants. New Phytol 154:275–304, doi:10.1046/j.1469-8137.2002.00397.x
Brundrett MC (2004) Diversity and classification of mycorrhizal associations. Biol Rev Camb Philos Soc 79:473–495, doi:10.1017/S1464793103006316
Brundrett MC (2006) Understanding the roles of multifunctional mycorrhizal and endophytic fungi. In: Schulz B, Boyle C, Sieber TN (eds) Microbial root endophytes. Springer, Berlin, pp 281–298
Brundrett MC, Abbott LK (1991) Roots of jarrah forest plants. I. Mycorrhizal associations of shrubs and herbaceous plants. Aust J Bot 39:445–457, doi:10.1071/BT9910445
Brundrett MC, Kendrick WB (1988) The mycorrhizal status, root anatomy, and phenology of plants in a sugar maple forest. Can J Bot 66:1153–1173, doi:10.1139/b88-166
Brundrett MC, Murase G, Kendrick B (1990) Comparative anatomy of roots and mycorrhizae of common Ontario trees. Can J Bot 68:551–578, doi:0.1139/b90-076
Brundrett M, Abbott L, Jasper D, Malajczuk N, Bougher N, Brennan K, Ashwath N (1995) Mycorrhizal Associations in the Alligator Rivers Region. Part II Results of Experiments. Final Report. Office of the Supervising Scientist, Jabiru, NT, Australia
Brundrett MC, Ashwath N, Jasper DA (1996a) Mycorrhizas in the Kakadu region of tropical Australia. II. Propagules of mycorrhizal fungi in disturbed habitats. Plant Soil 184:173–184, doi:10.1007/BF00029286
Brundrett M, Bougher N, Dell B, Grove T, Malajczuk N (1996b) Working with Mycorrhizas in Forestry and Agriculture. ACIAR Monograph 32. Australian Centre for International Agricultural Research, Canberra
Burgess T, Dell B, Malajczuk N (1994) Variations in mycorrhizal development and growth stimulation by 20 Pisolithus isolates inoculated on to Eucalyptus grandis W. Hill ex Maiden. New Phytol 127:731–739, doi:10.1111/j.1469-8137.1994.tb02977.x
Camargo-Ricalde SL, Dhillion SS, Jimenéz-Gonzáles C (2003) Mycorrhizal perennials of the “matorral xerófilo” and the “selva bja caducifolia” communities in the semiarid Tehuacán-Cuicatlán Valley, Mexico. Mycorrhiza 13:77–83
Cameron KM, Wurdack KJ, Jobson RW (2002) Molecular evidence for the common origin of snap-traps among carnivorous plants. Am J Bot 89:1503–1509, doi:10.3732/ajb.89.9.1503
Carrillo-Garcia Á, León de la Luz J-L, Bashan Y, Bethlenfalvay GJ (1999) Nurse plants, mycorrhizae and plant establishment in a disturbed area of the Sonoran Desert. Restor Ecol 7:321–335, doi:10.1046/j.1526-100X.1999.72027.x
Cázares E, Trappe JM, Jumponnen A (2005) Mycorrhiza-plant colonization patterns on a subalpine glacier forefront as a model system of primary succession. Mycorrhiza 15:405–416, doi:10.1007/s00572-004-0342-1
Chapman AD (2005) Numbers of Living Species in Australia and the World. Australian Biodiversity Information Services, Toowoomba, Australia. A Report for the Department of the Environment and Heritage, September 2005. (www.environment.gov.au/biodiversity/abrs/publications/other/species-numbers/03-03-groups-plants.html)
Chase MW, Cameron KM, Barrett RL, Freudenstein JV (2003) DNA data and Orchidaceae systematics: a new phylogenetic classification. In: Dixon KW, Kell SP, Barrett RL, Cribb PJ (eds) Orchid conservation. Natural History Publications, Kota Kinabalu, pp 69–89
Chaudhry MS, Batool Z, Khan AG (2005) Preliminary assessment of plant community structure and arbuscular mycorrhizas in rangeland habitats of Cholistan desert, Pakistan. Mycorrhiza 15:606–611
Clayton JS, Bagyaraj DJ (1984) Vesicular-arbuscular mycorrhizas in submerged aquatic plants of New Zealand. Aquat Bot 19:251–262, doi:10.1016/0304-3770(84)90043-3
Collier SC, Yarnes CT, Herman RP (2003) Mycorrhizal dependency of Chihuahuan Desert plants is influenced by life history strategy and root morphology. J Arid Environ 55:223–229
Cooke JC, Lefor MW (1988) The mycorrhizal status of selected plant species from Connecticut wetlands and transition zones. Restor Ecol 6:214–222, doi:10.1111/j.1526-100X.1998.00628.x
Cooper KM (1976) A field survey of mycorrhizas in New Zealand ferns. NZ J Bot 14:169–181
Cornwell WK, Bedford BL, Chapin CT (2001) Occurrence of arbuscular mycorrhizal fungi in a phosphorus-poor wetland and mycorrhizal responses to phosphorus fertilization. Am J Bot 88:1824–1829, doi:10.2307/3558359
Cripps CL, Eddington LH (2005) Distribution of mycorrhizal types among alpine vascular plant families on the Beartooth Plateau, Rocky Mountains, U.S.A., in reference to large-scale patterns in arctic-alpine habitats. Arct Antarct Alp Res 37:177–188, doi:10.1657/1523-0430(2005)037[0177:DOMTAA]2.0.CO;2
Currah RS, Van Dyk M (1986) A survey of some perennial vascular plant species native to Alberta for occurrence of mycorrhizal fungi. Can Field Nat 100:330–342
Dahlstrom JL, Smith JE, Weber NS (2000) Mycorrhiza-like interaction by Morchella with species of the Pinaceae in pure culture synthesis. Mycorrhiza 9:279–285, doi:10.1007/PL00009992
Davies J, Briarty LG, Rieley JO (1973) Observations on the swollen lateral roots of the Cyperaceae. New Phytol 72:167–174, doi:10.1111/j.1469-8137.1973.tb02022.x
da Silva, dos Santos BA, Alves MV, Maia LC (2001) Arbuscular mycorrhiza in species of Commelinidae (Liliopsida) in the state of Pernambuco (Brazil). Acta Bot Brasilia 15:155–165
DeMars BG (1996) Vesicular-arbuscular mycorrhizal status of spring ephemerals in Two Ohio forests. Ohio J Sci 96:97–99
DeMars BG, Boerner REJ (1996) Vesicular arbuscular mycorrhizal development in the Brassicaceae in relation to plant life span. Flora 191:179–189
Der JP, Nickrent D (2008) A Molecular Phylogeny of Santalaceae (Santalales). Syst Bot 33:107–116, doi:10.1600/036364408783887438
de Alwis DP, Abeynayake K (1980) A survey of mycorrhizae in some forest trees of Sri Lanka. In: Mikola P (ed) Tropical Mycorrhiza Research. Clarendon Press, Oxford, pp 146–153
Dhillion SS, Vidiella PE, Vidiella PE, Aquilera LE, Friese CF, De Leon E, Armesto JJ, Zak JC (1995) Mycorrhizal plants and fungi in the fog-free Pacific coastal desert of Chile. Mycorrhiza 5:381–386
Dickie IA, Thomas MM, Bellingham PJ (2007) On the perils of mycorrhizal status lists: the case of Buddleja davidii. Mycorrhiza 17:687–688, doi:10.1007/s00572-007-0146-1
Dodd JC, Dougall TA, Clapp JP, Jeffries P (2002) The role of arbuscular mycorrhizal fungi in plant community establishment at Samphire Hoe, Kent, UK – the reclamation platform created during the building of the Channel tunnel between France and the UK. Biodivers Cons 11:39–58
Ducousso M, Bourgeois C, Buyck B, Eyssartier G, Vincelette M, Rabevohitra R, Béna G, Randrihasipara L, Dreyfus B, Prin Y (2004) The last common ancestor of Sarcolaenaceae and Asian dipterocarp trees was ectomycorrhizal before the India-Madagascar separation, about 88 million years ago. Mol Ecol 13:231–236, doi:10.1046/j.1365-294X.2003.02032.x
Ducousso M, Ramanankierana H, Duponnois R, Rabévohitra R, Randrihasipara L, Vincelette M, Dreyfus B, Prin B (2008) Mycorrhizal status of native trees and shrubs from eastern Madagascar littoral forests with special emphasis on one new ectomycorrhizal endemic family, the Asteropeiaceae. New Phytol 178:233–238, doi:10.1111/j.1469-8137.2008.02389.x
Eriksen M, Bjureke KE, Dhillion SS (2002) Mycorrhizal plants of traditionally managed boreal grasslands in Norway. Mycorrhiza 12:117–123
Ernst WHO, Van Duin WE, Oolbekking GT (1984) Vesicular-arbuscular mycorrhiza in dune vegetation. Acta Bot Neerl 33:151–160
Farmer AM (1985) The occurrence of vesicular-arbuscular mycorrhiza in isoetoid-type submerged aquatic macrophytes under naturally varying conditions. Aquat Bot 21:245–249
Fisher JB, Jayachandran K (2005) Presence of arbuscular mycorrhizal fungi in South Florida native plants. Mycorrhiza 15:580–588
Florabase (2007) Census of Plants of Western Australia. Western Australian Herbarium (url: florabase.dec.wa.gov.au/statistics)
Fontenla S, Godoy R, Rosso P, Havrylenko M (1998) Root associations in Austrocedrus forests and seasonal dynamics of arbuscular mycorrhizas. Mycorrhiza 8:29–33
Fontenla S, Puntieri J, Ocampo JA (2001) Mycorrhizal associations in the Patagonian steppe, Argentina. Plant Soil 223:13–29
Frenot Y, Bergstrom DM, Gloaguen JC, Tavenard R, Strullu DG (2005) The first record of mycorrhizae on sub-Antarctic Heard Island: a preliminary examination. Antarctic Sci 17:205–210
Frioni L, Minasian H, Volfovicz (1999) Arbuscular mycorrhizae and ectomycorrhizae in native tree legumes in Uruguay. For Ecol Manag 115:41–47
Fuchs B, Haselwandter K (2004) Red list plants: colonisation by arbuscular mycorrhizal fungi and dark septate endophtes. Mycorrhiza 14:277–281, doi:10.1007/s00572-004-0314-5
Gai JP, Cai XB, Feng G, Christie P, Li XL (2006) Arbuscular mycorrhizal fungi associated with sedges on the Tibetan plateau. Mycorrhiza 16:151–157, doi:10.1007/s00572-005-0031-8
Gardes M, Dahlberg A (1996) Mycorrhizal diversity in arctic and alpine tundra: an open question. New Phytol 133:147–157, doi:10.1111/j.1469-8137.1996.tb04350.x
Gehring CA, Connell JH (2006) Arbuscular mycorrhizal fungi in the tree seedlings of two Australian rain forests: occurrence, colonization, and relationships with plant performance. Mycorrhiza 16:89–98, doi:10.1007/s00572-005-0018-5
Gemma JN, Koske RE (1990) Mycorrhiae on recent Volcanic substrates in Hawaii. Am J Bot 79:1193–1200, doi:10.2307/2444630
Gemma JN, Koske RE (1995) Mycorrhizae in Hawaiian epiphytes. Pac Sci 49:175–180
Gill WM, Lapeyrie F, Gomi T, Suzuki K (1999) Tricholoma matsutake—an assessment of in situ and in vitro infection by observing cleared and stained roots. Mycorrhiza 9:227–231, doi:10.1007/s005720050271
Giovannetti M, Nicolson TH (1983) Vesicular-arbuscular mycorrhizas in Italian and dunes. Trans Br Mycol Soc 80:552–557
Giovannetti M, Sbrana C (1998) Meeting a non-host: the behaviour of AM fungi. Mycorrhiza 8:123–130, doi:10.1007/s005720050224
Gorsi MS (2002) Studies on mycorrhizal association in some medicinal plants of Azad Jammu and Kashmir. Asian J Plant Sci 1:383–387
Grippa CR, Hoeltgebaum MP, Stürmer SL (2007) Occurrence of arbuscular mycorrhizal fungi in bromeliad species from the tropical Atlantic forest biome in Brazil. Mycorrhiza 17:235–240, doi:10.1007/s00572-006-0090-5
Hadley G, Williamson B (1972) Features of mycorrhizal infection in some Malayan orchids. New Phytol 71:1111–1118, doi:10.1111/j.1469-8137.1972.tb01989.x
Harley JL, Harley EL (1987) A check-list of mycorrhiza in the British flora. New Phytol 105(Supplement 2):1–102, doi:10.1111/j.1469-8137.1987.tb00674.x
Harrington TJ, Mitchell DT (2002) Colonization of root systems of Carex flacca and C. pilulifera by Cortinarius (Dermocybe) cinnamomeus. Mycol Res 106:452–459, doi:10.1017/S0953756202005713
Hartnett DC, Potgieter AF, Wilson GWT (2004) Fire effects on mycorrhizal symbiosis and root system architecture in southern African savanna grasses. Afric J Ecol 42:328–337
Haug I, Lempe J, Homeier J, Weiss M, Setaro S, Oberwinkler F, Kottke I (2004) Graffenrieda emarginata (Melastomataceae) forms mycorrhizas with Glomeromycota and with a member of the Hymenoscyphus ericae aggregate in the organic soil of a neotropical mountain rain forest. Can J Bot 82:340–356, doi:10.1139/b03-153
Hetrick BAD, Wilson GWT, Todd TC (1992) Relationships of mycorrhizal symbiosis, rooting strategy, and phenology among tallgrass prairie forbs. Can J Bot 70:1521–1528
Heywood VH, Brummitt RK, Culham A, Selberg O (2007) Flowering plants families of the world. Royal Botanic Gardens, Kew
Hildebrandt U, Janetta K, Ouziad F, Renne B, Nawrath Bothe KH (2001) Arbuscular mycorrhizal colonization of halophytes in Central European salt marshes. Mycorrhiza 10:175–183, doi:10.1007/s005720000074
Hirrel MC, Mehravaran H, Gerdemann JW (1978) Vesicular-arbuscular mycorrhizae in the Chenopodiaceae and Cruciferae: do they occur? Can J Bot 56:2813–2817, doi:10.1139/b78-336
Högberg P (1982) Mycorrhizal associations in some woodland and forest trees and shrubs in Tanzania. New Phytol 92:407–415
Högberg P, Piearce GD (1986) Mycorrhizas in Zambian trees in relation to host taxonomy, vegetation type and successional patterns. J Ecol 74:775–785
Hopkins NA (1987) Mycorrhizae in a California serpentine grassland community. Can J Bot 65:484–487
Hopper SD, Gioia P (2004) The Southwest Australian Floristic Region: evolution and conservation of a global hot spot of biodiversity. Annu Rev Ecol Syst 35:623–650, doi:10.1146/annurev.ecolsys.35.112202.130201
Hurst SE, Turnbull MH (2002) The effect of plant light environment on mycorrhizal colonisation in field-grown seedlings of podocarp angiosperm tree species. N Z J Bot 40:65–72
Imhof S (1999a) Root morphology, anatomy and mycotrophy of the achlorophyllous Voyria aphylla (Jacq.) Pers. (Gentianaceae). Mycorrhiza 9:33–39, doi:10.1007/s005720050260
Imhof S (1999b) Subterranean structures and mycorrhiza of the achlorophyllous Burmannia tenella Bentham (Burmanniaceae). Can J Bot 77:637–643, doi:10.1139/cjb-77-5-637
Janos DP (1980) Vesicular-arbuscular mycorrhizae affect lowland tropical rain forest plant growth. Ecology 61:151–162, doi:10.2307/1937165
Janos DP (1993) Vesicular-arbuscular mycorrhizae of epiphytes. Mycorrhiza 4:1–4, doi:10.1007/BF00203242
Janos DP (2007) Plant responsiveness to mycorrhizas differs from dependence upon mycorrhizas. Mycorrhiza 17:75–91, doi:10.1007/s00572-006-0094-1
Jasper DA (2007) Beneficial soil microorganisms in the jarrah forest and their recovery in bauxite mine restoration in southwestern Australia. Restor Ecol 15:S74–S84
Jayachandran K, Shetty KG (2003) Growth response and phosphorus uptake by arbuscular mycorrhizae of wet prairie sawgrass. Aquat Bot 76:281–290, doi:10.1016/S0304-3770(03)00075-5
Johnson-Green PC, Kenkel NC, Booth T (1995) The distribution and phenology of arbuscular mycorrhizae along an inland salinity gradient. Can J Bot 73:1318–1327
Juniper BE, Robins RJ, Joel DM (1989) The Carnivorous Plants. Academic, London
Kagawa A, Fujiyoshi M, Tomita M, Masuzawa T (2006) Mycorrhizal status of alpine plant communities on Mt. Maedake Cirque in the Japan South Alps. Polar Biosci 20:92–102
Kai W, Zhiwei Z (2006) Occurence of arbuscular mycorrhizas and dark septate endophytes in hydrophytes from lakes and streams in southwest China. Int Rev Hydrobiol 91:29–37, doi:10.1002/iroh.200510827
Katenin AE (1964) Mycorrhiza of arctic plants. Problemy Severa 8:148–154 [In Russian]
Khan AG (1974) The occurrence of mycorrhizas in halophytes, hydrophytes and xerophytes, and of Endogone spores in adjacent soils. J Gen Microbiol 81:7–14
Khan AG, Belik M (1995) Occurrence and ecological significance of mycorrhizal symbioses in aquatic plants. In: Verma A, Hock B (eds) Mycorrhiza: Structure, function, molecular biology and biotechnology. Springer, Heidelberg, pp 627–666
Kohn LM, Stasovski E (1990) The mycorrhizal status of plants at Alexander fiord, Ellesemere Island, Canada, a high arctic site. Mycologia 82:23–35, doi:10.2307/3759959
Koide RT, Schreiner RP (1992) Regulation of vesicular-arbuscular mycorrhizal symbiosis. Annu Rev Plant Physiol Plant Mol Biol 43:557–581, doi:10.1146/annurev.pp.43.060192.003013
Kope HH, Warcup JH (1986) Synthesised ectomycorrhizal associations of some Australian herbs and shrubs. New Phytol 104:591–599, doi:10.1111/j.1469-8137.1986.tb00659.x
Koske RE, Gemma JN (1990) VA mycorrhizae in strand vegetation of Hawaii: evidence for long-distance codispersal of plants and fungi. Am J Bot 77:466–474
Koske RE, Gemma JN, Flynn T (1992) Mycorrhizae in Hawaiian angiosperms: a survey with implications for the origin of the native flora. Am J Bot 79:853–862, doi:10.2307/2444994
Kottke I, Beck A, Oberwinkler F, Homeier J, Neill D (2004) Arbuscular endomycorrhizae are dominant in the organic soil of a neotropical montane cloud forest. J Trop Ecol 20:125–129
Kuijt J (1969) The biology of parasitic flowering plants. University of California Press, Berkeley
Kumar T, Ghose M (2008) Status of arbuscular mycorrhizal fungi (AMF) in the Sundarbans of India in relation to tidal inundation and chemical properties of soil. Wetland Ecol Manage 16:471–483
Kühn KD, Weber HC, Dehne HW, Gworgwor NA (1991) Distribution of vesicular-arbuscular mycorrhizal fungi on a fallow agriculture site I. Dry habitat. Agnew Botanik 65:169–185
Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas J (2006) Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann Bot (Lond) 98:693–713, doi:10.1093/aob/mcl114
Lamont B (1982) Mechanisms for enhancing nutrient uptake in plants, with particular reference to mediterranean South Africa and Western Australia. Bot Rev 48:597–689, doi:10.1007/BF02860714
Laursen GA, Treu R, Seppelt RD, Stephenson SL (1997) Mycorrhizal assessment of vascular plants from subantarctic Macquarie Island. Arct Alp Res 29:483–491, doi:10.2307/1551996
Leake JR (1994) The biology of myco-heterotrophic (‘saprophytic’) plants. New Phytol 127:171–216, doi:10.1111/j.1469-8137.1994.tb04272.x
Lesica P, Antibus RK (1986) Mycorrhizal status of hemiparasitic vascular plants in Montana U. S. A. Trans Br Mycol Soc 86:341–343
Lesica P, Antibus RK (1990) The occurrence of mycorrhizae in vascular epiphytes of two Costa Rican rain forests. Biotropica 22:250–258, doi:10.2307/2388535
Li A-R, Guan K-Y (2007) Mycorrhizal and dark septate endophytic fungi of Pedicularis species from northwest of Yunnan Province, China. Mycorrhiza 17:103–109, doi:10.1007/s00572-006-0081-6
Ligrone R, Carafa A, Lumni E, Bianciotti V, Bonfante P, Duckett J (2007) Glomeromycotan associations in liverworts: a molecular, cellular and taxonomic analysis. Am J Bot 94:1756–1777, doi:10.3732/ajb.94.11.1756
Logan VS, Clarke PJ, Allaway WG (1989) Mycorrhizas and root attributes of plants of coastal sand-dunes of New South Wales. Aust J Plant Physiol 16:141–146
Louis I (1990) A mycorrhizal survey of plant species colonizing coastal reclaimed land in Singapore. Mycologia 82:772–778
Lovera M, Cuenca G (1996) Arbuscular mycorrhizal infection in Cyperaceae and Gramineae from natural disturbed and restored savannas in La Gran Sabana, Venezuela. Mycorrhiza 6:111–118
Maeda M (1954) The meaning of mycorrhiza in regard to systematic botany. Kumamoto J Sci B 3:57–84
Maffia B, Nadkarni NM, Janos DP (1993) Vesicular-arbuscular mycorrhizae of epiphytic and terrestrial Piperaceae under field and greenhouse conditions. Mycorrhiza 4:5–9
Malloch D, Malloch B (1981) The mycorrhizal status of boreal plants: species from northeastern Ontario. Can J Bot 59:2167–2172
Malloch D, Malloch B (1982) The mycorrhizal status of boreal plants: additional species from northeastern Ontario. Can J Bot 60:1035–1040
Maremmani A, Bedini S, Matoševic I, Tomai PE, Giovannetti M (2003) Type of mycorrhizal associations in two coastal nature reserves of the Mediterranean basin. Mycorrhiza 13:33–40
Massicotte HB, Melville LH, Peterson RL (2005) Structural characteristics of root-fungal interactions for five ericaceous species in eastern Canada. Can J Bot 83:1057–1064, doi:10.1139/b05-046
McGee P (1986) Mycorrhizal associations of plant species in a semiarid community. Aust J Bot 34:585–593, doi:10.1071/BT9860585
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501, doi:10.1111/j.1469-8137.1990.tb00476.x
McGuire KL, Henkel TW, Granzow de la Cerda I, Villa G, Edmund F, Andrew C (2008) Dual mycorrhizal colonization of forest-dominating tropical trees and the mycorrhizal status of non-dominant tree and liana species. Mycorrhiza 18:217–222
Medve RJ (1984) The mycorrhizae of pioneer species in disturbed ecosystems in Western Pennsylvania. Am J Bot 71:787–794
Meney KA, Dixon KW, Scheltema M, Pate JS (1993) Occurrence of vesicular mycorrhizal fungi in dryland species of Restionaceae and Cyperaceae from south-west Western Australia. Aust J Bot 41:733–737, doi:10.1071/BT9930733
Menoyo E, Becarra AG, Renison D (2007) Mycorrhizal associations in Polylepis woodlands of Central Argentina. Can J Bot 85:526–631
Michelsen A (1993) The mycorrhizal status of vascular epiphytes in Bale Mountains National Park, Ethiopia. Mycorrhiza 4:11–15, doi:10.1007/BF00203244
Midgley JJ, Stock WD (1998) Natural abundance of ∂15N confirms insectivorous habit of Roridula gorgonias, despite it having no proteolytic enzymes. Ann Bot (Lond) 8:387–388
Miller OK Jr (1982) Mycorrhizae, mycorrhizal fungi, and fungal biomass in subalpine tundra at Eagle Summit, Alaska. Holarctic Ecol 5:125–134
Miller RM (1979) Some occurrences of vesicular-arbuscular mycorrhiza in natural and disturbed ecosystems of the Red Desert. Can J Bot 57:619–623, doi:10.1139/b79-079
Miller RM, Smith CR, Jastrow JD, Bever JD (1999) Mycorrhizal status of the genus Carex (Cyperaceae). Am J Bot 86:547–553, doi:10.2307/2656816
Mishra RR, Sharma GD, Gatphoh AR (1980) Mycorrhizas in the ferns of north eastern India. Proc Ind Nat Sci Acad B 46:546–551
Mohankumar V, Mahadevan A (1986) Survey of vesicular-arbuscular mycorrhizae in mangrove vegetation. Curr Sci 55:936
Molina R, Massicotte H, Trappe JM (1992) Specificity phenomena in mycorrhizal symbioses: community-ecological consequences and practical implications. In: Allen MJ (ed) mycorrhizal functioning an integrative plant-fungal process. Chapman & Hall, New York, pp 357–423
Moyersoen B, Becker P, Alexander IJ (2001) Are ectomycorrhizas more abundant than arbuscular mycorrhizas in tropical heath forests? New Phytol 150:591–599
Muthukumar T, Sha LQ, Yang XD, Cao M, Tang JW, Zheng Z (2003) Distribution of roots and arbuscular mycorrhizal associations in tropical forest types of Xishuangbanna, southwest China. Appl Soil Ecol 22:241–253
Muthukumar T, Senthilkumar M, Rajangam M, Udian K (2006) Arbuscular mycorrhizal morphology and dark septate fungal associations in medicinal and aromatic plants of Western Ghats, Southern India. Mycorrhiza 17:11–24
Muthukumar T, Udaiyan K (2000) Arbuscular mycorrhizas of plants growing in the Western Ghats region, Southern India. Mycorrhiza 9:297–313, doi:10.1007/s005720050274
Muthukumar T, Udaiyan K (2002) Seasonality of vesicular-arbuscular mycorrhizae in sedges in a semi-arid tropical grassland. Acta Oecol 23:337–247, doi:10.1016/S1146-609X(02)01165-7
Muthukumar T, Udaiyan K, Shanmughavel P (2004) Mycorrhiza in sedges—an overview. Mycorrhiza 14:65–77, doi:10.1007/s00572-004-0296-3
Myers N, Mittermeier RA, Mittermeier CG, de Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858, doi:10.1038/35002501
Nadarajah P, Nawawi A (1993) Mycorrhizal status of epiphytes in Malaysian oil palm plantations. Mycorrhiza 4:21–25, doi:10.1007/BF00203246
Newbery DM, Alexander IJ, Thomas DW, Gartlan JS (1988) Ectomycorrhizal rainforest legumes and soil phosphorus in Korup National Park, Cameroon. New Phytol 109:433–450
Newman EI, Reddell P (1987) The distribution of mycorrhizas among families of vascular plants. New Phytol 106:745–751, doi:10.1111/j.1469-8137.1987.tb00175.x
Nickrent DL (1997)- onward. The parasitic plant connection. http:// www.parasiticplants.siu.edu/
Nielsen SL, Thingstrup I, Wigand C (1999) Apparent lack of vesicular–arbuscular mycorrhiza (VAM) in the seagrasses Zostera marina L. and Thalassia testudinum Banks ex König. Aquat Bot 63:261–266, doi:10.1016/S0304-3770(98)00123-5
O’Connor PJ, Smith SE, Smith FA (2001) Arbuscular mycorrhizal associations in the southern Simpson Desert. Aust J Bot 49:493–499
Olsson PA, Eriksen B, Dahlberg A (2004) Colonisation by arbuscular mycorrhizal and fine endophytic fungi in herbaceous vegetation in Arctic Canada. Can J Bot 82:1547–1556
Onguene NA, Kuyper TW (2001) Mycorrhizal associations in the rain forest of South Cameroon. For Ecol Manage 140:277–287
Onipchenko VG, Zobel M (2000) Mycorrhia, vegetative mobility and responses to disturbance of alpine plants in the northwestern Caucasus. Folia Geobotanica 35:1–11
Orlowska E, Zubek Sz, Jurkiewicz A, Szarek-Lukaszewska G, Turnau K (2002) Influence of restoration of arbuscular mycorrhiza of Biscutella laevigata L. (Brassicaceae) and Plantago lanceolata L. (Plantaginaceae) from calamine spoil mounds. Mycorrhiza 12:153–160, doi:10.1007/s00572-001-0155-4
Otero JT, Ackerman JD, Bayman P (2002) Diversity and host specificity of endophytic Rhizoctonia-like fungi from tropical orchids. Am J Bot 89:1852–1858, doi:10.3732/ajb.89.11.1852
Peat HJ, Fitter AH (1993) The distribution of arbuscular mycorrhizas in the British flora. New Phytol 125:845–854, doi:10.1111/j.1469-8137.1993.tb03933.x
Pendleton RL, Smith BN (1983) Vesicular-arbuscular mycorrhizae of weedy and colonizer plant species at disturbed sites in Utah. Oecologia 59:296–301, doi:10.1007/BF00378852
Perrier N, Amier, Colin F (2006) Occurrence of mycorrhizal symbioses in the metal-rich lateritic soils of the Koniambo Massif, New Caledonia. Mycorrhiza 16:449–458, doi:10.1007/s00572-006-0057-6
Peterson RL, Ashford AE, Allaway WG (1985) Vesicular-arbuscular mycorrhizal associations of vascular plants on Heron Island, a Great Barrier Reef coral cay. Aust J Bot 33:69–76
Peterson RL, Howarth MJ, Whittier DP (1981) Interactions between a fungal endophyte and gametophyte cells in Psilotum nudum. Can J Bot 59:711–720, doi:10.1139/b81-101
Peterson RL, Massicotte HB, Melville LH F Phillips F (2004) Mycorrhizas: Anatomy and Cell Biology. NRC Research, Canada
Pirozynski KA, Malloch DW (1975) The origin of land plants: a matter of mycotrophism. Biosystems 6:153–164, doi:10.1016/0303-2647(75)90023-4
Powell CL (1975) Rushes and sedges are non-mycotrophic. Plant Soil 42:481–484, doi:10.1007/BF00010023
Powlowska TE, Blaszkowski J, Rühling Å (1996) The mycorrhizal status of plants colonizing a calamine spoil mound in southern Poland. Mycorrhiza 6:499–505
Radhika KP, Rodrigues BF (2007) Arbuscular mycorrhizae in association with aquatic and marshy plant species in Goa, India. Aquat Bot 86:291–294, doi:10.1016/j.aquabot.2006.10.009
Ragupathy S, Mahadevan A (1993) Distribution of vesicular-arbuscular mycorrhizae in the plants and rhizosphere soils of the tropical plains, Tamil Nadu, India. Mycorrhiza 3:123–136, doi:10.1007/BF00208920
Ragupathy S, Mohankumar V, Mahadevan A (1990) Occurrence of vesicular arbuscular mycorrhizae in tropical hydrophytes. Aquat Bot 36:287–291
Rains KC, Nadkarni NM, Bledsoe CS (2003) Epiphytic and terrestrial mycorrhizas in a lower montane Costa Rican cloud forest. Mycorrhiza 13:257–264, doi:10.1007/s00572-003-0224-y
Read DJ, Haselwandter K (1981) Observations on the mycorrhizal status of some alpine plant communities. New Phytol 88:341–352, doi:10.1111/j.1469-8137.1981.tb01729.x
Read DJ, Duckett JG, Francis R, Ligrone R, Russell A (2000) Symbiotic fungal associations in ‘lower’ land plants. Philos Trans R Soc Lond B Biol Sci 355:815–831, doi:10.1098/rstb.2000.0617
Reddell P, Milnes AR (1992) Mycorrhizas and other specialised nutrient-acquisition strategies: their occurrence in woodland plants from Kakadu and their role in rehabilitation of waste rock dumps at a local uranium mine. Aust J Bot 40:223–242
Reddell P, Hopkins MS, Graham AW (1996) Functional association between apogeotropic aerial roots, mycorrhizas and paper-barked stems in a lowland tropical rainforest in North Queensland. J Trop Ecol 12:763–777
Reeves FB, Wagner D, Moorman T, Keil J (1979) The role of endomycorrhizae in revegetation practices in the semi-arid west I. A comparison of incidence of mycorrhizae in severely disturbed vs. natural environments. Am J Bot 66:6–13
Rosales J, Cuenca G, Ramirez N, De Andrade Z (1997) Native colonizing species and degraded land restoration in La Gran Sabrana, Venezuela. Restor Ecol 5:147–155
Rose SL (1981) Vesicular-arbuscular endomycorrhizal associations of some desert plants of Baja California. Can J Bot 59:1056–1060, doi:10.1139/b81-144
Regvar M, Vogel K, Irgel N, Wraber T, Hildebrandt U, Wilde P, Bothe H (2003) Colonization of pennycress (Thlaspi spp.) of the Brassicaceae by arbuscular mycorrhizal fungi. J Plant Physiol 160:615–626, doi:10.1078/0176-1617-00988
Rowe AR, Pringle A (2005) Morphological and molecular evidence of arbuscular mycorrhizal fungi associations in Costa Rican epiphytic bromeliads. Biotropica 37:245–250, doi:10.1111/j.1744-7429.2005.00033.x
Ruotsalainen AL, Aikio S (2004) Mycorrhizal inoculum and performance of nonmycorrhizal Carex bigelowii and mycorrhizal Trientalis europea. Can J Bot 82:443–449, doi:10.1139/b04-011
Ruotsalainen AL, Väre H, Vestberg M (2002) Seasonality of root fungal colonisation in low-alpine herbs. Mycorrhiza 12:29–36, doi:10.1007/s00572-001-0145-6
Saif SR (1975) The occurrence of mycorrhizas and Endogone spores in the rhizospheres of plants growing around university campus Islamabad. Pak J Bot 7:175–182
Santos BA, Silva GA, Maia LC, Alves MV (2000) Mycorrhizas in Monocotyledonae of northeast Brazil: subclasses Alismatidae, Arecidae and Zingiberidae. Mycorrhiza 10:151–153, doi:10.1007/s005720000068
Schweiger PF, Robson AD, Barrow NJ (1995) Root hair length determines beneficial effect of a Glomus species on shoot growth of some pasture species. New Phytol 131:247–254, doi:10.1111/j.1469-8137.1995.tb05726.x
Schmidt SK, Reeves FB (1984) Effect of the non-mycorrhizal pioneer plant Salsola kali L. (Chenopodiaceae) on vesicular-arbuscular mycorrhizal (VAM) fungi. Am J Bot 71:1035–1039, doi:10.2307/2443378
Schmidt SK, Scow KM (1986) Mycorrhizal fungi on the Galapagos Islands. Biotropica 18:236–240
Schreiner R, Koide RT (1993) Antifungal compounds from roots of mycotrophic and nonmycotrophic plant species. New Phytol 123:99–105
Schulze W, Schulze ED, Pate JS, Gillison AN (1997) The nitrogen supply from soil and insects during growth of the pitcher plants Nepenthes mirabilis, Cephalotus follicularis and Darlingtonia californica. Oecologia 112:464–471, doi:10.1007/s004420050333
Selivanov & Eleusenova (1974) [Characteristics of mycosymbiotic relations in the plant communities of north Kazakhstan deserts.] (In Russian). Botanicheskii Zhyrnal 59, 18–35
Sengupta A, Chaudhuri S (2002) Arbuscular mycorrhizal relations of mangrove plant community at the Ganges river estuary in India. Mycorrhiza 12:169–174
Setaro S, Weiss M, Oberwinkler F et al (2006) Sebacinales form ectendomycorrhizas with Cavendishia nobilis, a member of the Andean clade of Ericaceae, in the mountain rain forest of southern Ecuador. New Phytol 169:355–365, doi:10.1111/j.1469-8137.2005.01583.x
Sharma SK, Sharma GD, Mishra RR (1986) Status of mycorrhizae in sub-tropical forest ecosystem of Meghalaya. Acta Bot Indica 14:87–92
Shane MW, Cawthray GR, Cramer MD, Kuo J, Lambers H (2006) Specialised ‘dauciform’ roots of Cyperaceae are structurally distinct, but functionally analogous with ‘cluster’ roots. Plant Cell Environ 29:1989–1999, doi:10.1111/j.1365-3040.2006.01574.x
Shi ZY, Feng G, Christie P, Li XL (2006) Arbuscular mycorrhizal status of spring ephemerals in the desert ecosystem of Junggar Basin, China. Mycorrhiza 16:269–275
Siqueira JO, Carneiro MAC, Curi N, Rosado SCS, Davide AC (1998) Mycorrhizal colonization and mycotrophic growth of native woody species as related to successional groups in southeastern Brazil. For Ecol Manage 107:241–252
Skene KR (1998) Cluster roots: some ecological considerations. J Ecol 86:1060–1064, doi:10.1046/j.1365-2745.1998.00326.x
Smith SE, Read DJ (1997) Mycorrhizal symbiosis. Academic, London
Smith JE, Johnson KA, Cázares E (1998) Vesicular mycorrhizal colonisation of seedlings of Pinaceae and Betulaceae after spore inoculation with Glomus intraradices. Mycorrhiza 7:279–285, doi:10.1007/s005720050193
Soltis DE, Soltis PS, Chase MW, Mort ME, Albach DC, Zanis M, Savolainen V, Hahn WH, Hoot SB, Fay MF, Axtell M, Swensen SM, Prince LM, Kress WJ, Nixon KC, Farris JS (2000) Angiosperm phylogeny inferred from 18S rDNA, rbcL, and atpB sequences. Bot J Linn Soc 133:381–461
Šraj-Kržič N, Pongrac P, Klemenc M, Kladnik A, Regvar M, Gaberščik A (2006) Mycorrhizal colonisation in plants from intermittent aquatic habitats. Aquat Bot 85:331–336, doi:10.1016/j.aquabot.2006.07.001
St John TV (1980) Root size, root hairs and mycorrhizal infection: a re-examination of Baylis’s hypothesis with tropical trees. New Phytol 84:483–487, doi:10.1111/j.1469-8137.1980.tb04555.x
Straker CJ, Weiersbye IM, Witkowski ETF (2007) Arbuscular mycorrhiza status of gold and uranium tailings and surrounding soils of South Africa’s deep level gold mines: I. Root colonization and spore levels. S Afric J Bot 73:218–225
St John TV, Coleman DC (1983) The role of mycorrhizae in plant ecology. Can J Bot 61:1005–1014, doi:10.1139/b83-108
Stasz TE, Sakai WS (1984) Vesicular-arbuscular mycorrhizal fungi in the scale-leaves of Zingiberaceae. Mycologia 76:754–757, doi:10.2307/3793236
Tao L, Zhiwei Z (2005) Arbuscular mycorrhizas in a hot and arid ecosystem in southwest China. Applied Soil Ecology 29:135–141
Tao L, Jianping L, Zhiwei Z (2004) Arbuscular mycorrhizas in a valley-type savanna in southwest China. Mycorrhiza 14:323–327
Tawaraya K, Takaya Y, Turjaman M, Tuah SJ, Limin SH, Tamaid Y, Chae JY, Wagatsuma T, Osakid M (2003) Arbuscular mycorrhizal colonization of tree species grown in peat swamp forests of Central Kalimantan, Indonesia. For Ecol Manage 182:381–386
Tester M, Smith SE, Smith FA (1987) The phenomenon of “nonmycorrhizal” plants. Can J Bot 65:419–431, doi:10.1139/b87-051
Thomazini LI (1973) Mycorrhizas in plants of the “Cerrado”. Plant Soil 41:707–711, doi:10.1007/BF02185833
Titus JH, Titus PJ, Nowak RS, Smith SD (2002) Arbuscular mycorrhizae of Mojave Desert plants. West N Am Nat 62:327–334
Tori SD, Coley PD (1999) Tropical monodominance: a preliminary test of the ectomycorrhizal hypothesis. Biotropica 31:220–228
Trappe JM (1962) The fungus associates of ectotrophic mycorrhizae. Bot Rev 28:538–606, doi:10.1007/BF02868758
Trappe JM (1981) Mycorrhizae and productivity of arid and semiarid rangelands. pp 581–599 in Advances in food producing systems for arid and semi arid lands. Academic. New York
Trappe JM (1987) Phylogenetic and ecologic aspects of mycotrophy in the angiosperms from an evolutionary standpoint. In: Safir GR (ed) Ecophysiology of VA mycorrhizal plants. CRC, Boca Raton, pp 5–25
Treu R, Laursen GA, Stephenson SL, Landolt JC, Densmore R (1996) Mycorrhizae from Denali National Park and Preserve, Alaska. Mycorrhiza 6:21–29
Tsuyazaki S, Hase A, Niinuma H (2005) Distribution of different mycorrhizal classes on Mount Koma, northern Japan. Mycorrhiza 15:93–100, doi:10.1007/s00572-004-0304-7
Turnau K, Mitka J, Kedzierska A (1992) Mycorrhizal status of herb-layer plants in a fertilized oak-pine forest. Plant Soil 143:148–152
Väre H, Vestberg M, Eurola S (1992) Mycorrhiza and root associated fungi in Spitsbergen. Mycorrhiza 1:93–104
Väre H, Vesterg M, Ohtonen (1997) Shifts in mycorrhiza and microbial activity along an oroarctic gradient in northern Fennoscandia. Arct Alp Res 29:93–104, doi:10.2307/1551839
Vesk PA, Ashford AE, Markovina AL, Allaway WG (2000) Apoplastic barriers and their significance in the exodermis and sheath of Eucalyptus pilularis-Pisolithus tinctorius ectomycorrhizas. New Phytol 145:333–346, doi:10.1046/j.1469-8137.2000.00583.x
Wang B, Qiu YL (2006) Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16:299–363, doi:10.1007/s00572-005-0033-6
Warcup JH, McGee PA (1983) The mycorrhizal associations of some Australian Asteraceae. New Phytol 95:667–672, doi:10.1111/j.1469-8137.1983.tb03531.x
Warner A (1984) Colonization of organic matter by vesicular-arbuscular mycorrhizal fungi. Trans Br Mycol Soc 82:352–354
Weishampel PA, Bedford BL (2006) Wetland dicots and monocots differ in colonization by arbuscular mycorrhizal fungi and dark septate endophytes. Mycorrhiza 16:495–502, doi:10.1007/s00572-006-0064-7
Wetzel PR, van der Valk AG (1996) Vesicular-arbuscular mycorrhizae in prairie pothole wetlands vegetation in Iowa and North Dakota. Can J Bot 74:883–890, doi:10.1139/b96-110
Wilson GWT, Hartnett DC (1998) Interspecific variation in plant responses to mycorrhizal colonization in tallgrass prairie. Am J Bot 85:1732–1738
Winther JL, Friedman WE (2007) Arbuscular mycorrhizal symbionts in Botrychium (Ophioglossaceae). Am J Bot 94:1248–1255, doi:10.3732/ajb.94.7.1248
Wubet T, Kottke I, Teketay D, Oberwinkler F (2003) Mycorrhizal status of indigenous trees in dry Afromontane forests of Ethiopia. For Ecol Manage 179:387–399
Yamato M, Iwasaki M (2002) Morphological types of arbuscular mycorrhizal fungi in roots of understorey plants in Japanese deciduous broadleaved forests. Mycorrhiza 12:291–296
Yun W, Hall IR (2004) Edible ectomycorrhizal mushrooms: challenges and achievements. Can J Bot 82:1063–1073, doi:10.1139/b04-051
Zhang Y, Guo L (2007) Arbuscular mycorrhizal structures and fungi associated with mosses. Mycorrhiza 17:319–325, doi:10.1007/s00572-007-0107-8
Zhang Y, Guo LD, Liu RJ (2004) Arbuscular mycorrhizal fungi associated with common pteridophytes in Dujiangyan, southwest China. Mycorrhiza 14:25–30
Zangaro W, Nisizaki SMA, Domingos JCB, Nakano EM (2002) Micorriza arbuscular em espécies arbóreas nativas da bacia do Rio Tibagi, Paraná. Cerne 8:77–87
Acknowledgements
I would especially like to thank my wife Karen Clarke for her endless patience while this review was compiled. This review would not have been possible without the support of Lotterywest and the School of Plant Biology at The University of Western Australia. I am also very grateful to Hans Lambers for suggesting this data be made available and providing detailed comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Yongguan Zhu.
Appendices
Appendix 1
List of relevant tables and other data with direct links
Information | Link |
|---|---|
Ectomycorrhizal families and genera | |
Nonmycorrhizal families | |
Mycorrhizas of primitive plants | |
Methods for identifying mycorrhizas | |
Ectomycorrhizal fungi | |
Arbuscular mycorrhizal fungi (Arthur Schüßler's site) |
Appendix 2
Practical advice for the diagnosis of mycorrhizal associations
Processes required to obtain, process and evaluate samples for accurate mycorrhizal diagnosis are listed in Table 5. It is advisable to use several criteria to identify mycorrhizal associations, especially when roots are of unknown age (field collected). The first criteria (presence of a mycorrhizal interface) should always be used, as it provides the most reliable evidence, but should not be the only evidence required for diagnosis. Consistency of colonisation is another key criteria. If interface hyphae (arbuscules, Hartig net, or coils) were not observed in roots, reliable identification mycorrhizas may not be possible and it should be stated that further sampling is required for that species. It is important to clearly state which criteria were used in diagnosis in published reports. Lists of mycorrhizal species should be organised into plant families to allow comparison with other studies.
A protocol for diagnosis of AM or NM roots is presented in Fig. 16. Many mycorrhizal studies are already at least partially compliant with these requirements if they include data that allows multiple evidence of diagnosis (e.g. arbuscules, vesicles and colonisation levels). It is most difficult to distinguish functional AM from endophytic root colonisation, especially in extreme habitats where mycorrhizal activity may be suppressed. These habitats usually require more samples or sampling times to determine if plants are mycorrhizal. In some cases it will not be possible to conclusively state if samples are mycorrhizal or not—in which case sparse associations are likely to be of minor importance.
Diagnosis becomes easier with experience. It is unrealistic to expect accurate diagnosis without experience or guidance from an experience mycorrhizologists. Accuracy in mycorrhizal diagnosis is linked to the following factors:
-
Experience and training.
-
Sampling intensity.
-
Use of standard diagnosis criteria.
-
Adequate samples with sufficient replication that include young roots.
-
Higher sampling intensity in habitats where NM-AM plants are common.
-
Minimising cross contamination of roots by different plant species, but acknowledging it may still occur, especially with fine-rooted species.
-
Acknowledging when diagnosis cannot be resolved by GFC designation. It is better to err on the side of caution rather than publish an incorrect diagnosis.
Table 6 lists categories of data that should be used to diagnose AM associations. It is best to list all data and protocols used for diagnosis in publications. Protocols used to diagnose AM should be fully explained in the methods section. Detailed information can be presented as supplemental data if not included in the main document. Arbuscule density information is especially important if plants belong to families suspected to have NM-AM roots, have NM roots with some GFC, or are from habitats where NM plants tend to occur. However, in many cases a statement that plants designated as AM contained typical associations with many arbuscules in their roots will be sufficient to confirm diagnosis.
A similar process to that described above can be used to present data used to support diagnosis of EM associations (see Table 4), but usually is not required unless associations are atypical, or occur in an unexpected host plant. Table 3 also provides criteria that could be used for the diagnosis of ericoid or orchid mycorrhizas.
Rights and permissions
About this article
Cite this article
Brundrett, M.C. Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320, 37–77 (2009). https://doi.org/10.1007/s11104-008-9877-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-008-9877-9