Abstract
Main conclusion
γ-Aminobutyric acid alleviates acid-aluminum toxicity to roots associated with enhanced antioxidant metabolism as well as accumulation and transportation of citric and malic acids.
Abstract
Aluminum (Al) toxicity has become the main limiting factor for crop growth and development in acidic soils and is further being aggravated worldwide due to continuous industrial pollution. The current study was designed to examine effects of GABA priming on alleviating acid-Al toxicity in terms of root growth, antioxidant defense, citrate and malate metabolisms, and extensive metabolites remodeling in roots under acidic conditions. Thirty-seven-day-old creeping bentgrass (Agrostis stolonifera) plants were used as test materials. Roots priming with or without 0.5 mM GABA for 3 days were cultivated in standard nutrient solution for 15 days as control or subjected to nutrient solution containing 5 mM AlCl3·6H2O for 15 days as acid-Al stress treatment. Roots were sampled for determinations of root characteristics, physiological and biochemical parameters, and metabolomics. GABA priming significantly alleviated acid-Al-induced root growth inhibition and oxidative damage, despite it promoted the accumulation of Al in roots. Analysis of metabolomics showed that GABA priming significantly increased accumulations of organic acids, amino acids, carbohydrates, and other metabolites in roots under acid-Al stress. In addition, GABA priming also significantly up-regulated key genes related to accumulation and transportation of malic and citric acids in roots under acid-Al stress. GABA-regulated metabolites participated in tricarboxylic acid cycle, GABA shunt, antioxidant defense system, and lipid metabolism, which played positive roles in reactive oxygen species scavenging, energy conversion, osmotic adjustment, and Al ion chelation in roots.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As the most abundant metal element in the earth's crust, aluminum (Al) is widely distributed in soils in the form of insoluble silicate or alumina, water-soluble Al, exchangeable Al, active hydroxyl Al, and organic complex Al (Ofoe et al. 2023). Generally, insoluble Al tends to transform into soluble Al when soil pH is lower than 5. It has been estimated that acidic soils account for one-third of arable land on earth (Gurmessa 2021). A substantial accumulation of soluble Al results in Al toxicity which has become the main limiting factor for crop growth and development in acidic soils (Hajiboland et al. 2023). Due to the continuous application of agricultural chemicals, fertilizers, and industrial pollution, Al toxicity is further being aggravated worldwide. Root is affected primarily by acid-Al toxicity and also acts as the first line of defense to cope with acid-Al toxicity (Liu et al. 2020). Al stress not only restrains root growth, but also decreases root vitality leading to limited uptake of water and nutrients from soils. In addition, cell membrane stability significantly declines as a result of huge amounts of accumulation of reactive oxygen species (ROS) in roots under Al stress (Fan et al. 2022). Enhanced antioxidant enzyme activities for ROS elimination such as superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and ascorbate peroxidase (APX) have been found as one of the most critical tolerance mechanisms in plants in response to Al stress (Yu et al. 2012; Liang et al. 2022).
Besides antioxidant defense system, plants have developed many other Al-detoxification strategies in the long process of evolution. For example, plants transport the extra Al to older tissues to relieve Al toxicity to young leaves. In addition, roots secrete organic acids such as malic acid, citric acid, and oxalic acid into soils to chelate Al ions, thereby reducing the bioavailable Al in the soil (Chauhan et al. 2021; Chen et al. 2022). In the tricarboxylic acid cycle (TCA), citrate synthase (CS) catalyzes biosynthesis of citrate by using acetyl CoA and oxaloacetic acid, and the malate dehydrogenase (MDH) is mainly responsible for catalyzing the mutual conversion of oxaloacetate and malic acid in animals, plants, and microorganisms. Beneficial roles of CS and MDH have been studied extensively in plants suffering from Al stress. For example, Wang et al. (2013) found that higher CS and MDH activities significantly increased concentrations of citric acid and malic acid in roots, thus alleviating acid-Al toxicity to winter rape (Brassica napus) plants. Al-activated malate transport (ALMT) and multidrug and toxic compound extrusion (MATE)-type transport have been identified as two main organic acid transporters. Multi-gene members in ALMT family are involved in various biological processes including Al tolerance, vacuolar stability, and stomatal movement (Kovermann et al. 2007; Palmer et al. 2016). MATE family members can transport citrate into vacuole or rhizosphere, which is helpful to improve the tolerance to acid-Al stress in plants (Zhou et al. 2023). It has been reported that Sensitive to proton rhizotoxicity 1 (STOP1) gene in Arabidopsis thaliana played a key role in the tolerance to acid-Al toxicity through regulating gene members of ALMT or MATE family (Iuchi et al. 2007; Liu et al. 2009).
Plant or seed priming by amino acids or other bioactive substances has been proved as an efficient and cheap technique for the improvement in stress tolerance (Jisha et al. 2013; Vijayakumari et al. 2016). As a four-carbon non-protein amino acid, γ-aminobutyric acid (GABA) exhibits a positive function in the enhancement of root adaptation to various abiotic stresses. Previous studies found that the application of exogenous GABA could reduce ROS production and improve membrane stability through increasing antioxidant enzyme activities under salt stress, thereby effectively alleviating salt-induced root growth inhibition and a decline in root activity (Shi et al. 2010; Feng et al. 2023). Exogenous GABA also could enhance the tolerance of heat-sensitive creeping bentgrass (Agrostis stolonifera) roots to heat stress (Li et al. 2023). Root growth and contents of malic acid and citric acid in leaves could be significantly promoted by exogenous GABA when Malus hupehensis plants suffered from alkaline stress (Li et al. 2020a). In addition, a recent study has demonstrated that the GABA induced accumulations of MATE1 and MATE2 proteins and also up-regulated transcript levels of MATE1 and MATE2 contribute to Al tolerance of hybrid Liriodendron (L. chinense × tulipifera) plants (Wang et al. 2021). However, the GABA-regulated adaptation mechanism of roots to Al stress is far from being fully understood in perennial plant species.
Grass species, the third most abundant flowering plants, have been widely used for phytoremediation which is an accessible technology involved in the application of plants to remove metals from contaminated soil because of its advantages such as low cost and non-destructibility to primitive environments (Prasad 2003; Huang 2021). Creeping bentgrass is a perennial grass and prefers to grow in weakly acidic soils with appropriate pH between 5.0 and 6.5. It has been used in sports turf, urban green space, and ecological management due to soft texture, stoloniferous-growing habit, strong aggressivity, and vegetation coverage. Objectives of the current study were to examine effects of root priming with GABA on alleviating acid-Al toxicity through maintaining root growth and antioxidant homeostasis and to further explore potential mechanism of GABA-regulated root adaptability to acid-Al toxicity in acidic environment associated with alterations in organic acid metabolism and global metabolites remodeling.
Materials and methods
Plant growth conditions and treatments
Seeds of creeping bentgrass cultivar ‘Penncross’ germinated and grew in white quartz sands filled with ½ Hogland’s nutrient solution (Hoagland and Arnon 1950) for 30 days. Mature plants with same sizes were removed carefully from quartz sands and then were suspended in Hogland’s nutrient solution for hydroponic cultivation by using styrofoam floating boards on the rectangular container (25 cm in length, 15 cm in width, and 20 cm in height) for 7 days. Plants were then divided into two groups. One group was cultivated in nutrient solution containing 0.5 mM GABA for 3 days as GABA priming, and another group was cultivated in nutrient solution without GABA for 3 days as non-priming. These plants with or without GABA priming were then removed into new nutrient solution without GABA for 15 days of control cultivation or into nutrient solution containing 5 mM AlCl3·6H2O for 15 days as acid-Al stress treatment. Nutrient solution was refreshed every 2 days. All plants were kept in the growth chamber at 23/19 °C (day/night) with 12 h photoperiod, 700 μmol m−2 s−1 PAR, and 65% relative humidity.
Consequently, four treatments were set: (1) C (control, plants without GABA priming grew in nutrient solution, pH 6.2); (2) C + GABA (plants were primed with GABA and then grew in nutrient solution, pH 6.2); (3) Al (plants without GABA priming grew in nutrient solution containing 5 mM AlCl3·6H2O, pH 4.35); (4) Al + GABA (plants were primed with GABA and then grew in nutrient solution containing 5 mM AlCl3·6H2O, pH 4.35). All nutrient solutions were refreshed every 2 days. Each treatment included four independent biological replications (four containers) which were placed in four independent growth chambers to eliminate spatial effects. Each replication included 20 independent plants in one container. Roots were sampled on 0 day (after 3 days of priming), 7th day, and 15th day for determination of endogenous GABA and on 15th day for determination of root characteristics, physiological and bio-chemical parameters, and metabolomics.
Determinations of endogenous GABA, Al content, and growth parameters
Root activity was reflected by dehydrogenase activity which can reduce triphenyl tetrazolium chloride (TTC) to triphenyltetrazolium formate (TTF), thereby changing the color of the roots from white into pink (Fontana et al. 2020). Fresh root samples (0.2 g) were soaked in the mixed solution of 0.4% TTC and pH 7.0 phosphoric acid buffer at 37 °C for 1 h, and then 2 ml of 2 M sulfuric acid solution was added to stop the reaction. The roots were taken out from solution and the moisture on roots surface were removed carefully by using absorbent papers, and then roots were put in a mortar filled with 5 ml ethyl acetate. Finally, the absorbance of extracts was determined at 485 nm. Roots were used for determination of Al content, and dry roots (0.3 g) were ground to fine powders and put into the polytetrafluoroethylene digestion tank. Then, 5 ml of concentrated nitric acid and 2 ml of hydrogen peroxide were added into this tank. After being digested completely, the mixture was cooled down to room temperature and diluted with distilled water to 50 ml. The supernatant was used for determining the Al content by using an inductively coupled plasma emission mass spectrometer (ICP-MS). In addition, hematoxylin staining was used to dye the Al in roots. Fresh roots were rinsed with distilled water and then were immersed in hematoxylin solution including 0.1% hematoxylin, 0.01% potassium permanganate, and 0.2 mM sodium hydroxide for 30 s. The dyed roots were photographed by using a microscope after being rinsed in distilled water to remove extra colourant from roots surface. For determination of root length, root number, fresh, and dry weight of roots, 10 independent plants were randomly selected for one biological replication and each treatment included four biological replications. Assay Kit (GABA–2–W) purchased from Comin Biotechnology Co., Ltd. (Suzhou, China) was used to detect endogenous GABA content according to the label instructions.
Determinations of antioxidant enzyme activities, reactive oxygen species, and membrane stability
A total of 0.1 g roots were ground on ice with 1.5 ml of 150 mM cold phosphate buffer saline (PBS) (pH 7.0) and the homogenate was centrifuged at 12,000g for 20 min. The supernatant was collected for the determination of malondialdehyde (MDA). The 1 ml of reaction solution [20% trichloroacetic acid and 0.5% thiobarbituric acid (TBA)] was added to the supernatant. The absorbance of mixture was detected at 532 and 600 nm after the mixture was heated at 100 °C for 5 min (Dhindsa et al. 1981). The supernatant, as mentioned above, was also used to determine the SOD activity by nitrogen blue tetrazole (NBT) photoreduction method (Giannopolitis and Ries 1977) at 560 nm, guaiacol method for determination of POD activity (Chance and Maehly 1955) at 470 nm, respectively. For the CAT activity, 50 mM phos-phoric acid buffer (pH 7.0) and 45 mM hydrogen peroxide were added to the supernatant, and the absorbance value was measured at 240 nm (Chance and Maehly 1955). APX activity was determined at 290 nm based on the principle that APX reduced the amount of ascorbic acid in the presence of H2O2 (Nakano and Asada 1981). Electrolyte leakage (EL), superoxide anion (O2.−), or hydrogen peroxide (H2O2) was measured by using the method of Blum and Ebercon (1981), Elstner and Heupel (1976), or Uchida (2002), respectively. O2.− or H2O2 staining in roots was dyed by using DAB (3,3′-diaminobenzidine) or NBT (nitrotetrazolium blue chloride) (Zhang et al. 2015). Assay methods in details have been published in our previous study (Li et al. 2016).
Extraction, separation, and quantification of root metabolites
Root metabolites were extracted using the method from Roessner et al. (2000) and Rizhsky et al. (2004). Root samples were lyophilized in a lyophilizer (LGJ–10C, Chengdu, China) until the sample weight maintained a consistent weight. Freeze-dried samples were crushed into fine powder and then extracted in 80% aqueous methanol. Test procedure for metabolites extraction has been described in our previous study (Li et al. 2016). Analysis procedure of gas chromatograph-mass spectrometer (GC–MS) referred to the method of Qiu et al. (2007). Helium with a constant flow rate of 1.0 ml/min was used as the carrier gas, and metabolites were separated on DB-5MS capillary column (30 m × 250 μm I.D., 0.25 μm film thickness; Agilent J&W Scientific, Folsom, CA, USA). Implantation temperature, the temperature of transfer interface, or ion source temperature was set at 280 °C, 270 °C, or 220 °C, respectively. The initial GC temperature was maintained at 80 °C for 5 min, and then increased to 180 °C at 10 °C/min, 240 °C at 5 °C/min, 280 °C at 20 °C/min, and finally at 280 °C for 11 min. In the full scan mode (m/z 30–550), the measurement was carried out by electron impact ionization (70 eV). TURBOMASS 4.1.1 software (PerkinElmer Inc., Waltham, MA, USA) coupled with commercially available compound libraries (NIST 2005 (PerkinElmer) and Wiley 7.0 (John Wiley & Sons Ltd., Hoboken, NJ, USA) was used to identify global metabolites.
Gene expression analysis
Total RNA was extracted from 0.1 g of fresh roots by using a HiPure Universal RNA kit (Magen). Then, these RNAs were reverse-transcribed into cDNA using the MonScriptTM RTIII All-in-one Mix with dsDNase kit (Monad). cDNAs were amplified by real-time quantitative fluorescent PCR (qRT–PCR). Primers of genes involved in citric and malic acids metabolism including ALMT9-like, STOP1-like, MATE12-like, 14-like, 27-like, 29-like, and 48-like, CS-like, cMDH-like (MDH located in cytoplasm), and mMDH-like (MDH located in mitochondrion) and relevant information for qRT-PCR were shown in Table S1. PCR procedure for all genes: 5 min at 94 °C, denaturation at 95 °C for 30 s (those two steps require 40 repeats), and then annealing at 57–60 °C (Table S1) for 30 s, and extension at 72 °C for 30 s. Expression levels of genes were calculated according to the method of Livak and Schmittgen (2001).
Statistical analysis
Experimental data were analyzed by SPSS 26.0 (IBM, Armonk, NY, USA) software based on one-way analysis of variance (ANOVA). All data were analyzed with mean ± standard deviation (mean ± SD) and the t-test method was used to compare the two groups of data. The results were presented significant differences at P ≤ 0.05. All bar charts were drawn by using Origin (OriginLab, Northampton, MA, USA). The pictures of phenotype and staining were shot by camera (EOS 6D Mark II; Canon, Tokyo, Japan). TBtools (Guangzhou, Guangdong, China) was used to make the heatmap. Metabolic pathways were created using Excel (Microsoft, Redmond, WA, USA), and potential mechanism diagram was drawn using Ai (Adobe, San Jose, CA, USA).
Results
Effects of GABA priming on endogenous GABA content and root growth and activity under control condition and acid-Al stress
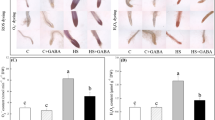
Figure 1A shows that roots pretreated with GABA grew better than roots without GABA priming under control condition or acid-Al stress. On day 0, GABA priming could significantly increase endogenous GABA content (Fig. 1B). Acid-Al stress had a positive effect on accumulation of GABA in both of GABA-primed and non-primed roots, and GABA priming could further promote the accumulation of GABA in roots on the 7th day. GABA-primed roots maintained significantly lower endogenous GABA content than the roots without GABA priming on the 15th days of acid-Al stress (Fig. 1B). Acid-Al stress significantly decreased root activity, root length, the number of root, and fresh or dry weight of root, but the decreasing trend was lower for the GABA-primed roots (Fig. 1C–G). Root activity indicated the healthy status of roots, which was reflected by dehydrogenase activity. GABA priming did not affect root activity and the number of root under control condition, but significantly increased root length, dry weight, and fresh weight under control condition. In addition, GABA priming induced a 62.23%, 23.28%, 37.14%, 25%, or 21.49% increase in root activity, root length, the number of root, dry weight, or fresh weight as compared to non-primed roots under acid-Al stress, respectively (Fig. 1C–G).
Effects of GABA priming on phenotypic changes (A), endogenous GABA content (B), root activity (C), root length (D), the number of root (E), root dry weight (F), and root fresh weight (G) of creeping bentgrass under control condition and acid-aluminum stress. Different small letters above columns indicate significant differences among four treatments (P ≤ 0.05). The “*” indicates significant difference between two treatments (P ≤ 0.05). C, control; C + GABA, control + GABA; Al, acid-Al stress; Al + GABA, acid-Al stress + GABA
Effects of GABA priming on Al accumulation and antioxidant defense under control condition and Al stress
Under control condition, contents of Al, O2.−, and H2O2 remained at a low level, and there is no significant difference in these parameters between GABA-primed and non-primed roots (Fig. 2A, C, E). Acid-Al stress significantly induced the accumulation of Al, O2.−, or H2O2 in roots. GABA priming significantly inhibited acid-Al-induced increases in O2.− and H2O2 in roots, but enhanced Al accumulation in roots under acid-Al stress (Fig. 2A, C, E). Results of Al, O2.−, and H2O2 staining were consistent with contents of Al, O2.−, and H2O2 in roots (Fig. 2B, D, F). Under control condition, GABA priming did not significantly affect MDA and EL, but GABA priming significantly alleviated the accumulation of MDA and an increase in EL under acid-Al stress (Fig. 3A, B). GABA-primed roots maintained significantly higher SOD activity than roots without GABA priming under control condition or acid-Al stress (Fig. 3C). POD, CAT, and APX activities were not significantly different between treatments with and without GABA priming under control condition (Fig. 3D–F). Al stress increased POD activity, but reduced the activity of CAT or APX. GABA priming significantly increased activities of POD, CAT, and APX in roots under acid-Al stress (Fig. 3D–F).
Effects of GABA priming on Al content (A), Al staining (B), superoxide anion (O2.−) content (C), O2.− staining (D), hydrogen peroxide (H2O2) content (E), and H2O2 staining (F) in roots of creeping bentgrass under control condition and acid-aluminum stress. Different small letters above columns indicate significant differences among four treatments (P ≤ 0.05). C, control; C + GABA, control + GABA; Al, acid-Al stress; Al + GABA, acid-Al stress + GABA
Effects of GABA priming on malondialdehyde (MDA) content (A), electrolyte leakage (EL) (B), superoxide dismutase (SOD) activity (C), peroxidase (POD) activity (D), catalase (CAT) activity (E), and ascorbate peroxidase (APX) activity (F) in roots of creeping bentgrass under control condition and ac-id-aluminum stress. Different small letters above columns indicate significant differences among four treatments (P ≤ 0.05). C, control; C + GABA, control + GABA; Al, acid-Al stress; Al + GABA, acid-Al stress + GABA
Effects of GABA priming on global metabolites remodeling under control condition and acid-Al stress
A total of 70 metabolites were identified and quantified in roots based on GC–MS. Discrimination information of each metabolite is shown in Table S2. These metabolites consisted of 18 amino acids, 16 sugars, 21 organic acids, and 15 other metabolites (Fig. 4A). In order to observe changes in metabolites between two treatments, four different comparable groups were set including C + GABA vs. C, Al + GABA vs. Al, Al vs. C, and Al + GABA vs. C (Fig. 4B). Firstly, the C + GABA vs. C indicated the effect of GABA under optimal condition. In the second, the Al + GABA vs. Al indicated the effect of GABA on alleviating acid-Al toxicity to roots under acidic condition. Thirdly, the Al + GABA vs. C or Al vs. C reflected acid-Al toxicity to roots with or without GABA application, respectively. 37.23% of metabolites remained unchanged, 18.09% of metabolites increased, and 44.68% of metabolites decreased in C + GABA vs. C. 63.83%, 7.45%, or 28.72% of metabolites increased, decreased, or did not significantly change in Al + GABA vs. Al, respectively (Fig. 4B). As compared with the control condition, acid-Al stress induced a decline in 56.38% or 50.00% of metabolites in roots without or with GABA priming, respectively (Fig. 4B). GABA priming did not affect the accumulation of amino acids, sugars, and other metabolites under control condition, but significantly decreased the accumulation of organic acids under control condition (Fig. 4C). Total content of organic acids decreased significantly in roots with and without GABA priming under acid-Al stress, however acid-Al stress induced significant increases in total amino acids, sugars, and other metabolites in roots. Roots primed with GABA exhibited significantly higher organic acids, amino acids, sugars, and other metabolites than roots without GABA priming under acid-Al stress (Fig. 4C).
Heat map of 70 metabolites in different comparable groups (red represents up-regulation and green represents down-regulation) (A), the percentage of metabolites in each comparable group (B), and total contents of organic acids, amino acids, sugars, or other metabolites (C) in roots of creeping bentgrass under control condition and acid-aluminum stress. Different small letters above columns indicate significant differences among four treatments (P ≤ 0.05). C, control; C + GABA, control + GABA; Al, acid-Al stress; Al + GABA, acid-Al stress + GABA
Figure S1 shows relative contents of 18 amino acids in the roots under control condition and acid-Al stress. GABA priming significantly induced the accumulation of oxoproline, glutamic acid, cycloleucine, serine, lysine, or tyrosine, but decreased the accumulation of proline, leucine, or glutamine under stress condition. For organic acids involved in TCA cycle, the accumulation of citric acid, α-ketoglutarate, fumaric acid, or malic acid could be significantly induced by GABA priming under control and stressful conditions. Roots primed with GABA also exhibited significantly higher succinic acid content than these roots without GABA priming under acid-Al stress (Fig. S2A and B). In addition, GABA priming significantly induced accumulation of lactic acid, glyceric acid, quinic acid, oxalic acid, maleic acid, citramalic acid, threonic acid, saccharic acid, shikimic acid, or gluconic acid in roots under acid-Al stress (Fig. S2A and B). In response to acid-Al stress, roots primed with GABA had a significantly higher accumulation of 12 sugars (erythrose, glucose, ribose, talose, sucrose, glucose-6-phoshate, trehalose-6-phosphate, glucose-1-phosphhate, levoglucosan, maltose, ketose, and gentiobiose) and 10 other metabolites (dithioerythritol, dehydroascorbic acid, ascorbate, xylitol, ribitol, gluconic lactone, putrescine, galactinol, mannitol, and myo-inositol) than those roots without GABA priming under acid-Al stress (Fig. S3 and S4).
Effects of GABA priming on metabolic pathways under control condition and acid-Al stress
Changes in metabolic pathways involved in TCA cycle, GABA shunt, and metabolism of organic and amino acids, etc., in roots of creeping bentgrass in response to GABA priming under acid-Al stress are shown in Fig. 5. A total of 40 metabolites consisting of 11 amino acids, 10 organic acids, 7 sugars, and 12 other metabolites were listed in these metabolic pathways. GABA priming had greater effects on metabolites in roots under acid-Al stress than control condition. Under Al stress, GABA priming enhanced amino acids and sugars metabolism, and also increased the accumulation of citric acid, α-ketoglutaric acid, succinic acid, fumaric acid, and malic acid involved in TCA cycle (Fig. 5). Under control condition, expression levels of all genes involved in organic acid metabolism and transport kept at a low level without significant difference between treatments “C” and “C + GABA” (Fig. 6). Al stress significantly induced increases in expression levels of ALMT9-like, MATE12-like, MATE14-like, MATE27-like, MATE29-like, MATE48-like, cMDH-like, and CS-like in roots with or without GABA priming. In addition, GABA priming further improved Al-induced the expression of these genes mentioned above (Fig. 6). Expression level of STOP1-like did not increase significantly in roots without GABA priming under stressful condition, but acid-Al stress significantly induced STOP1-like expression in roots with GABA priming under acidic condition (Fig. 6). The pivotal mechanism of acid-Al tolerance induced by GABA priming in roots of creeping bentgrass associated with enhancements in antioxidant defense and accumulation and transportation of citric and malic acids is shown in Fig. 7.
The assignment of 40 metabolites into integrative metabolic pathways involved in TCA cycle, GABA shunt, amino acids and sugars metabolism in roots of creeping bentgrass. Metabolic pathways were made based on four different comparable groups. Red represents up-regulation; green represents down-regulation; gray represents no significant change
Effects of GABA priming on transcript levels of genes related to organic acid metabolism in roots of creeping bentgrass under control condition and aluminum stress. Different small letters above columns indicate significant differences among four treatments (P ≤ 0.05). C, control; C + GABA, control + GABA; Al, acid-Al stress; Al + GABA, acid-Al stress + GABA
Potential mechanism regulated by GABA priming in roots of creeping bentgrass under aluminum stress. Results were demonstrated based on the comparable group Al + GABA vs. Al. Solid lines represent that pathways have been confirmed, while dashed lines represent that pathways still need to be further studied
Discussion
Although different plant species have different tolerance to Al stress, inhibitions of root elongation, root activity, and root biomass are first symptoms when plants suffer from acid-Al toxicity (Hui et al. 2011; Choudhury and Sharma 2014). As an economic and environmentally-friendly plant growth regulator, GABA could significantly promote roots growth under multi-stress conditions including alkaline stress and salt stress (Wang et al. 2017; Li et al. 2020a). Similar results were found between GABA-pretreated and unpretreated creeping bentgrass roots in response to Al stress. GABA priming significantly improved root elongation, root activity, and root biomass of creeping bentgrass under acid-Al stress. It is well known that acid-Al stress induces a substantial production of ROS which causes lipid peroxidation, accelerated programmed cell death and damage to nucleic acids and protein, leading to root growth inhibition (Yamamoto et al. 2003; Hao et al. 2022). Therefore, it is of primary importance to remove extra ROS to control growth and development of roots under Al stress. One of the most important mechanisms of ROS scavenging is the antioxidant defense system involved in multiple enzymes (Dumanović et al. 2021). It has been reported that exogenous GABA reduced accumulations of ROS and MDA by increasing activities of SOD, CAT, and APX in rice (Oryza sativa) under salinity, osmotic stress, and their combination (Sheteiwy et al. 2019). Exogenous application of GABA alleviated an increase in EL and the accumulation of H2O2 associated with significant increases in activities of SOD, POD, and CAT in Malus hupehensis under alkaline stress (Li et al. 2020a). Acid-Al stress led to the accumulation of MDA and ROS (H2O2 and O2.−) in creeping bentgrass roots, however, roots primed with GABA maintained significantly higher activities of SOD, POD, CAT, and APX as well as lower oxidative damage to roots than those roots without GABA priming under acid-Al stress. These findings supported that enhanced antioxidant defense induced by GABA priming is of primary importance for ROS homeostasis to ensure a well oxidation–reduction environment for root growth and development under acid-Al stress.
Many studies have demonstrated that exogenous application of GABA could significantly induce the accumulation of endogenous GABA in roots or leaves of various plant species under abiotic stress (Li et al. 2017a, 2020b; Çekiç 2018). Similar findings were demonstrated in roots of creeping bentgrass during 7 days of acid-Al stress. However, roots primed with GABA could not maintain higher endogenous GABA content than those roots without GABA priming on 15th day of acid-Al stress, which indicated that GABA priming might accelerate the transformation of endogenous GABA into other substances during the later stage of acid-Al stress. It has been found that plants often undergo metabolic imbalance and energy shortages due to enhanced respiratory pathway under stressful conditions (Heinemann and Hildebrandt 2021). GABA could be metabolized into different amino acids in plants, which is known as GABA shunt (Bouche and Fromm 2004; Ansari et al. 2021). Enhanced metabolic circulation induced by GABA priming was propitious to maintenance of osmotic adjustment (OA) and metabolites homeostasis under environmental stress (Li et al. 2016, 2017b). Findings from metabolome also showed that GABA priming significantly increased accumulations of total metabolites, organic acids, amino acids, sugars, and other metabolites in roots of creeping bentgrass in response to acid-Al stress. The recent study of Tan et al. (2022) demonstrated that exogenous application of spermine significantly improved transformation and utilization of endogenous GABA to support TCA cycle for energy metabolism in creeping bentgrass under drought stress. Our study found that GABA priming induced significant increases in glutamate (an important intermediate metabolite of GABA shunt) and other amino acids including oxyproline, cycloleucine, serine, lysine, and tyrosine in roots of creeping bentgrass under acid-Al stress. Significant accumulation of these amino acids played positive roles in OA, osmoprotectant, and metabolic homeostasis (Reddy and Shad Ali 2011; Bhutto et al. 2023).
Enhanced GABA shunt has been proved to be beneficial to better maintenance of intermediate metabolites including citric acid, malic acid, fumaric acid, and succinic acids in TCA cycle (Hijaz and Killiny 2019; Nehela and Killiny 2019). Most of organic acids involved in the TCA cycle exhibit important functions of redox regulation, energy production, OA, and cation homeostasis in higher plants (Igamberdiev and Eprintsev 2016). As early as 1998, Zheng et al. (1998) found that high levels of organic acid secretion were associated with high Al resistance (pH 4.8). More and more studies also further proved positive roles of citric acid and malic acid in acid-Al tolerance due to their ability to chelate Al3+ in cells and rhizosphere, thereby decreasing acid-Al toxicity to roots (Wang et al. 2022, 2023). Therefore, the improvement in Al tolerance induced by GABA priming could be related to significant increases in contents of citric and malic acids in roots of creeping bentgrass. MDH isozymes exist in mitochondria (mMDH), cytoplasm (cMDH), and other organellae (Tomaz et al. 2010; Zheng et al. 2021). The mMDH catalyzes the oxidation of malic acid to oxaloacetic acid, while cMDH is mainly involved in the hydrogenation of oxaloacetic acid to malic acid as the last step of the TCA cycle (Chinopoulos 2020). Oxaloacetic acid is then catalyzed by CS to form citric acid in plants. Overexpression of cMDH or CS could improve the resistance of transgenic crops to cold, salt, and acid-Al stress (Tesfaye et al. 2001; Anoop et al. 2003; Yao et al. 2011; Wang et al. 2016). In addition, increased activities of cMDH or CS promoted the production of malic acid or citric acid, thus improving the acid-Al tolerance in different plant species (Hidayah et al. 2020; Yao et al. 2020). GABA priming significantly promoted transcript levels of cMDH and CS, which was consistent with changes in accumulations of malate and citric acid in roots of creeping bentgrass under acid-Al stress. These findings indicated that enhanced metabolism of citric and malic acids was an important regulatory mechanism of acid-Al tolerance induced by GABA priming in roots. This might explain the reason why GABA-primed roots accumulated more Al, but exhibited better root growth and activity than non-primed roots in response to acid-Al stress. In addition, plants store Al in the root apex instead of transferring Al to the aboveground parts in favor of photosynthesis for growth maintenance. For example, a recent research of Silva et al. (2020) found that soybean (Glycine max) cultivar Conquista has good adaptability to acid-Al stress due to a great capacity of Al immobilization in roots. However, it still deserves to be further investigated whether GABA regulates Al immobilization in roots to decrease accumulation of Al in aboveground tissues or not in our further study.
ALMTs, known as plant-specific anion channel proteins that can be activated by Al, protect plant roots from Al toxicity through regulating malate transport to chelate with the Al3+ (Magalhaes et al. 2018). MATEs are a novel family of secondary transporter genes encoding citrate transporters associated with Al tolerance in plants. Ye et al. (2017) indicated that a significant increase in vacuolar membrane-localized SL-ALMT9 expression enhanced malate transport and acid-Al resistance of tomato (Solanum lycopersicum). In addition, Liu et al. (2016) found that the expression of multiple MATEs in Al-tolerant soybean varieties was twice as high as that in sensitive varieties in response to acid-Al toxicity. It has also been confirmed that AtALMT1 or AtMATE were regulated by the transcription factor STOP1 in A. thaliana (Liu et al. 2009; Ito et al. 2019). A. thaliana stop1 mutants significantly repressed expression of MATE and ALMT1 leading to significant declines in exudations of malate and citrate in roots under acid-Al stress (Jiang et al. 2017). A. thaliana stop1 mutants exhibited hypersensitivity to Al rhizotoxicity due to a lack of ability to induce AtALMT1 expression in roots (Ohyama et al. 2013). Ectopic expression of STOP1 could significantly alleviate acid-Al damage to A. thaliana stop1 mutation (Silva-Navas et al. 2021). In addition, overexpression of GmSTOP1a increased the expression of GmALMT1 related to enhanced acid-Al tolerance of soybean hairy roots (Zhou et al. 2018). Our findings showed that GABA priming further activated acid-Al-induced expression of ALMT9-like and five MATE genes (MATE12-like, MATE14-like, MATE27-like, MATE29-like and MATE48-like) and also significantly up-regulated STOP1-like expression in roots of creeping bentgrass under acid-Al stress. A previous study has shown that the GABA could directly regulate the activity of TaALMT1 to affect plant growth (Ramesh et al. 2015). However, the GABA as a signaling molecule directly regulates STOP1-like expression or indirectly affects its expression through regulating metabolic homeostasis under acid-Al stress, which cannot be fully explained in our current study. The regulatory role of GABA in transport and secretion of organic acids in roots associated with enhanced acid-Al tolerance deserves to be further investigated in our future studies.
In addition to amino acids and organic acids, GABA priming also improved accumulation of many sugars including erythrose, glucose, ribose, talose, sucrose, glucose-6-phosphate, trehalose-6-phosphate, glucose-1-phosphate, levoglucosan, maltose, kestose, and gentiobiose in roots of creeping bentgrass. Sugars are important osmo-regulators, membrane stabilizer, and metabolic resources for energy cycle under stressful conditions (Jouve et al. 2004; Khan et al. 2020). Soluble sugars also act as one of the major messengers to regulate gene expression and enzymatic activities involved in plant development, cell metabolism, and stress tolerance (Khan et al. 2020; Afzal et al. 2021). Al-tolerant maize (Zea mays) genotype minimized acid-Al-induced membrane lipid peroxidation and inhibition of root growth, which was correlated with increased accumulation of carbohydrates in roots, but carbohydrates content in roots of Al-sensitive genotype remained unchanged in response to acid-Al stress (Giannakoula et al. 2008). Similar results were demonstrated in the study of Khan et al. (2000) who found that Al-tolerant maize accessions accumulated more carbohydrates in roots than sensitive genotypes under acid-Al stress. Moreover, phosphorus addition could promote oil tea (Camellia aleifera) root growth under acid-Al stress by increasing accumulation of various sugars including arabinose, glucose, glucose-1-phosphate, glucose-6-phosphate, mannose, and sucrose (Qu et al. 2020). Other metabolites like putrescine (Put), myo-inositol, ascorbate, and galactinol were significantly improved by GABA priming in roots of creeping bentgrass subjected to acid-Al stress. Previous studies have demonstrated positive function of these metabolites in regulating acid-Al tolerance in plants. In rice roots, Put enhanced acid-Al tolerance associated with the decrease in Al retention in root cell walls (Zhu et al. 2019). Myo-inositol was implicated in various physiological and biochemical processes such as antioxidant defense, hormonal regulation, and lipid signaling transduction, and its accumulation helped plants to overcome environmental stresses (Valluru and Van den Ende 2011; Hu et al. 2020; Li et al. 2020c). Ascorbate was an important antioxidant which alleviated acid-Al-induced oxidative damage to wheat (Triticum aestivum) roots (Sun et al. 2015; Liu et al. 2018). As the precursor of raffinose family oligosaccharides (RFOs), galactinol exhibited positive roles in OA, DNA repair, and protein protection in plants under abiotic stress (Taji et al. 2002), and enhanced synthesis of galactol conferred salt tolerance of poplar (Populus trichocarpa) (Liu et al. 2021). GABA priming induced accumulations of Put, myo-inositol, ascorbate, and galactinol in roots of creeping bentgrass under acid-Al stress, which could be important strategies to improve the acid-Al tolerance of roots because of their roles in antioxidant, OA, and metabolic regulation.
Conclusion
GABA priming significantly mitigated Al-induced inhibition of root growth and oxidative damage through maintaining high SOD, POD, CAT, and APX activities under acid-Al stress. In response to acid-Al stress, enhanced accumulations of multiple amino acids, organic acids, carbohydrates, and other metabolites such as myo-inositol, Put, ascorbate, and galactinol in roots were induced by exogenous GABA priming. These metabolites participated in TCA cycle, GABA shunt, and lipids metabolism, which played positive roles in energy conversion, OA, and ion chelation in roots. In addition, the GABA priming particularly up-regulated accumulation and transportation of citric and malic acids in roots under acid-Al stress. The current findings promote understanding of GABA-regulated roots growth and tolerance to acid-Al stress in perennial plant species. GABA-enhanced Al immobilization in roots in favor of photosynthesis for maintenance of aboveground growth needs further research in future.
Data availability
Not applicable.
Abbreviations
- ALMT9-like:
-
Al-activated malate transport 9-like
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- cMDH-like:
-
Malate dehydrogenase, cytoplasmic-like
- CS-like:
-
Citrate synthase-like
- MATEs-like:
-
Multidrug and toxic compound extrusions-like
- MDA:
-
Malondialdehyde
- POD:
-
Peroxidase
- SOD:
-
Superoxide dismutase
- STOP1-like:
-
Sensitive to proton rhizotoxicity 1-like
References
Afzal S, Chaudhary N, Singh NK (2021) Role of soluble sugars in metabolism and sensing under abiotic stress. In: Aftab T, Hakeem KR (eds) Plant growth regulators. Springer International Publishing, Cham, 305–334. https://doi.org/10.1007/978-3-030-61153-8_14
Anoop VM, Basu U, McCammon MT, McAlister-Henn L, Taylor GJ (2003) Modulation of citrate metabolism alters aluminum tolerance in yeast and transgenic canola overexpressing a mitochondrial citrate synthase. Plant Physiol 132(4):2205–2217. https://doi.org/10.1104/pp.103.023903
Ansari MI, Jalil SU, Ansari SA, Hasanuzzaman M (2021) GABA shunt: a key-player in mitigation of ROS during stress. Plant Growth Regul 94(2):131–149. https://doi.org/10.1007/s10725-021-00710-y
Bhutto L, Osborne C, Quick W (2023) Osmotic adjustment and metabolic changes under drought stress conditions in wheat (Triticum aestivum L.) genotypes. Pak J Bot 55(3):915–923. https://doi.org/10.30848/PJB2023-3(22)
Blum A, Ebercon A (1981) Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci 21(1):43–47. https://doi.org/10.2135/cropsci1981.0011183x002100010013x
Bouche N, Fromm H (2004) GABA in plants: just a metabolite? Trends Plant Sci 9(3):110–115. https://doi.org/10.1016/j.tplants.2004.01.006
Çekiç F (2018) Exogenous GABA stimulates endogenous GABA and phenolic acid contents in tomato plants under salt stress. Celal Bayar Univ J Sci 14(1):61–64. https://doi.org/10.18466/cbayarfbe.348935
Chance B, Maehly A (1955) Assay of catalases and peroxidases. Methods Enzymol 2:764–775
Chauhan DK, Yadav V, Vaculik M, Gassmann W, Pike S, Arif N, Singh VP, Deshmukh R, Sahi S, Tripathi DK (2021) Aluminum toxicity and aluminum stress-induced physiological tolerance responses in higher plants. Crit Rev Biotechnol 41(5):715–730. https://doi.org/10.1080/07388551.2021.1874282
Chen W, Tang L, Wang J, Zhu H, Jin J, Yang J, Fan W (2022) Research advances in the mutual mechanisms regulating response of plant roots to phosphate deficiency and aluminum toxicity. Int J Mol Sci 23(3):1137. https://doi.org/10.3390/ijms23031137
Chinopoulos C (2020) Acute sources of mitochondrial NAD+ during respiratory chain dysfunction. Exp Neurol 327:113218. https://doi.org/10.1016/j.expneurol.2020.113218
Choudhury S, Sharma P (2014) Aluminum stress inhibits root growth and alters physiological and metabolic responses in chickpea (Cicer arietinum L.). Plant Physiol Biochem 85:63–70. https://doi.org/10.1016/j.plaphy.2014.10.012
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32(1):93–101
Dumanović J, Nepovimova E, Natić M, Kuča K, Jaćević V (2021) The significance of reactive oxygen species and antioxidant defense system in plants: a concise overview. Front Plant Sci 11:552969. https://doi.org/10.3389/fpls.2020.552969
Elstner EF, Heupel A (1976) Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal Biochem 70(2):616–620
Fan J, Chen K, Xu J, Khaldun A, Chen Y, Chen L, Yan X (2022) Physiological effects induced by aluminium and fluoride stress in tall fescue (Festuca arundinacea Schreb). Ecotoxicol Environ Saf 231:113192. https://doi.org/10.1016/j.ecoenv.2022.113192
Feng D, Gao Q, Sun X, Ning S, Qi N, Hua Z, Tang J (2023) Effects of foliage-applied exogenous γ-aminobutyric acid on seedling growth of two rice varieties under salt stress. PLoS ONE 18(2):e0281846. https://doi.org/10.1371/journal.pone.0281846
Fontana JE, Wang G, Sun R, Xue H, Li Q, Liu J, Davis KE, Thornburg TE, Zhang B, Zhang Z, Pan X (2020) Impact of potassium deficiency on cotton growth, development and potential microRNA-mediated mechanism. Plant Physiol Biochem 153:72–80. https://doi.org/10.1016/j.plaphy.2020.05.006
Giannakoula A, Moustakas M, Mylona P, Papadakis I, Yupsanis T (2008) Aluminum tolerance in maize is correlated with increased levels of mineral nutrients, carbohydrates and proline, and decreased levels of lipid peroxidation and Al accumulation. J Plant Physiol 165(4):385–396. https://doi.org/10.1016/j.jplph.2007.01.014
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol 59(2):309–314. https://doi.org/10.1104/pp.59.2.309
Gurmessa B (2021) Soil acidity challenges and the significance of liming and organic amendments in tropical agricultural lands with reference to Ethiopia. Environ Dev Sustain 23(1):77–99. https://doi.org/10.1007/s10668-020-00615-2
Hajiboland R, Panda CK, Lastochkina O, Gavassi MA, Habermann G, Pereira JF (2023) Aluminum toxicity in plants: present and future. J Plant Growth Regul 42(7):3967–3999. https://doi.org/10.1007/s00344-022-10866-0
Hao J, Peng A, Li Y, Zuo H, Li P, Wang J, Yu K, Liu C, Zhao S, Wan X, Pittman JK, Zhao J (2022) Tea plant roots respond to aluminum-induced mineral nutrient imbalances by transcriptional regulation of multiple cation and anion transporters. BMC Plant Biol 22(1):203. https://doi.org/10.1186/s12870-022-03570-4
Heinemann B, Hildebrandt TM (2021) The role of amino acid metabolism in signaling and metabolic adaptation to stress-induced energy deficiency in plants. J Exp Bot 72(13):4634–4645. https://doi.org/10.1093/jxb/erab182
Hidayah AN, Yahya S, Sopandie D (2020) The tolerance of oil palm (Elaeis guineensis) seedlings to Al stress is enhanced by citric acid and natural peat water. Biodiversitas 21(10):4850–4858. https://doi.org/10.13057/BIODIV/D211051
Hijaz F, Killiny N (2019) Exogenous GABA is quickly metabolized to succinic acid and fed into the plant TCA cycle. Plant Signal Behav 14(3):e1573096. https://doi.org/10.1080/15592324.2019.1573096
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Calif Agric Exp Stat Circ. https://doi.org/10.1097/00010694-193910000-00022. (2nd edn)
Hu L, Zhou K, Ren G, Yang S, Liu Y, Zhang Z, Li Y, Gong X, Ma F (2020) Myo-inositol mediates reactive oxygen species-induced programmed cell death via salicylic acid-dependent and ethylene-dependent pathways in apple. Hortic Res 7:138–150. https://doi.org/10.1038/s41438-020-00357-2
Huang B (2021) Grass research for a productive, healthy and sustainable society. Grass Res 1(1):1–2. https://doi.org/10.48130/GR-2021-0001
Hui NY, Liu P, Wang ZY, Chen WR, Xu GD (2011) The effect of aluminum treatments on the root growth and cell ultrastructure of two soybean genotypes. Crop Prot 30(3):323–328. https://doi.org/10.1016/j.cropro.2010.11.024
Igamberdiev AU, Eprintsev AT (2016) Organic acids: the pools of fixed carbon involved in redox regulation and energy balance in higher plants. Front Plant Sci 7:205704. https://doi.org/10.3389/fpls.2016.01042
Ito H, Kobayashi Y, Yamamoto YY, Koyama H (2019) Characterization of NtSTOP1-regulating genes in tobacco under aluminum stress. Soil Sci Plant Nutr 65(3):251–258. https://doi.org/10.1080/00380768.2019.1603064
Iuchi S, Koyama H, Iuchi A, Kobayashi Y, Kitabayashi S, Kobayashi Y, Ikka T, Hirayama T, Shinozaki K, Kobayashi M (2007) Zinc finger protein STOP1 is critical for proton tolerance in Arabidopsis and coregulates a key gene in aluminum tolerance. Proc Natl Acad Sci USA 104(23):9900–9905. https://doi.org/10.1073/pnas.0700117104
Jiang F, Wang T, Wang Y, Kochian LV, Chen F, Liu J (2017) Identification and characterization of suppressor mutants of stop1. BMC Plant Biol 17(1):128. https://doi.org/10.1186/s12870-017-1079-2
Jisha K, Vijayakumari K, Puthur JT (2013) Seed priming for abiotic stress tolerance: an overview. Acta Physiol Plant 35(5):1381–1396. https://doi.org/10.1007/s11738-012-1186-5
Jouve L, Hoffmann L, Hausman J-F (2004) Polyamine, carbohydrate, and proline content changes during salt stress exposure of aspen (Populus tremula L.): involvement of oxidation and osmoregulation metabolism. Plant Biol 6(1):74–80. https://doi.org/10.1055/s-2003-44687
Khan A, McNeilly T, Collins J (2000) Accumulation of amino acids, proline, and carbohydrates in response to aluminum and manganese stress in maize. J Plant Nutr Soil 23(9):1303–1314. https://doi.org/10.1080/01904160009382101
Khan N, Ali S, Zandi P, Mehmood A, Ullah S, Ikram M, Ismail I, Shahid M, Babar M (2020) Role of sugars, amino acids and organic acids in improving plant abiotic stress tolerance. Pak J Bot 52(2):355–363. https://doi.org/10.30848/PJB2020-2(24)
Kovermann P, Meyer S, Hörtensteiner S, Picco C, Scholz-Starke J, Ravera S, Lee Y, Martinoia E (2007) The Arabidopsis vacuolar malate channel is a member of the ALMT family. Plant J 52(6):1169–1180. https://doi.org/10.1111/j.1365-313x.2007.03367.x
Li Z, Yu J, Peng Y, Huang B (2016) Metabolic pathways regulated by γ-aminobutyric acid (GABA) contributing to heat tolerance in creeping bentgrass (Agrostis stolonifera). Sci Rep 6(1):30338. https://doi.org/10.1038/srep30338
Li Y, Fan Y, Ma Y, Zhang Z, Yue H, Wang L, Li J, Jiao Y (2017a) Effects of exogenous γ-aminobutyric acid (GABA) on photosynthesis and antioxidant system in pepper (Capsicum annuum L.) seedlings under low light stress. J Plant Growth Regul 36(2):436–449. https://doi.org/10.1007/s00344-016-9652-8
Li Z, Yu J, Peng Y, Huang B (2017b) Metabolic pathways regulated by abscisic acid, salicylic acid and γ-aminobutyric acid in association with improved drought tolerance in creeping bentgrass (Agrostis stolonifera). Physiol Plant 159(1):42–58. https://doi.org/10.1111/ppl.12483
Li Y, Liu B, Peng Y, Liu C, Zhang X, Zhang Z, Liang W, Ma F, Li C (2020a) Exogenous GABA alleviates alkaline stress in Malus hupehensis by regulating the accumulation of organic acids. Sci Hortic 261:108982. https://doi.org/10.1016/j.scienta.2019.108982
Li Z, Cheng B, Peng Y, Zhang Y (2020b) Adaptability to abiotic stress regulated by γ-aminobutyric acid in relation to alterations of endogenous polyamines and organic metabolites in creeping bentgrass. Plant Physiol Biochem 157:185–194. https://doi.org/10.1016/j.plaphy.2020.10.025
Li Z, Fu J, Shi D, Peng Y (2020c) Myo-inositol enhances drought tolerance in creeping bentgrass through alteration of osmotic adjustment, photosynthesis, and antioxidant defense. Crop Sci 60(4):2149–2158. https://doi.org/10.1002/csc2.20186
Li Z, Zhou M, Zeng W, Zhang Y, Liu L, Liu W, Peng Y (2023) Root metabolites remodeling regulated by γ-aminobutyric acid (GABA) improves adaptability to high temperature in creeping bentgrass. Plant Soil. https://doi.org/10.1007/s11104-023-05905-y
Liang Y, Bai T, Liu B, Yu W, Teng W (2022) Different antioxidant regulation mechanisms in response to aluminum-induced oxidative stress in Eucalyptus species. Ecotoxicol Environ Saf 241:113748. https://doi.org/10.1016/j.ecoenv.2022.113748
Liu J, Magalhaes JV, Shaff J, Kochian LV (2009) Aluminum-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. Plant J 57(3):389–399. https://doi.org/10.1111/j.1365-313X.2008.03696.x
Liu J, Li Y, Wang W, Gai J, Li Y (2016) Genome-wide analysis of MATE transporters and expression patterns of a subgroup of MATE genes in response to aluminum toxicity in soybean. BMC Genom 17(1):223. https://doi.org/10.1186/s12864-016-2559-8
Liu W, Xu F, Lv T, Zhou W, Chen Y, Jin C, Lu L, Lin X (2018) Spatial responses of antioxidative system to aluminum stress in roots of wheat (Triticum aestivum L.) plants. Sci Total Environ 627:462–469. https://doi.org/10.1016/j.scitotenv.2018.01.021
Liu C, Liu Y, Wang S, Ke Q, Yin L, Deng X, Feng B (2020) Arabidopsis mgd mutants with reduced monogalactosyldiacylglycerol contents are hypersensitive to aluminium stress. Ecotoxicol Environ Saf 203:110999. https://doi.org/10.1016/j.ecoenv.2020.110999
Liu L, Wu X, Sun W, Yu X, Demura T, Li D, Zhuge Q (2021) Galactinol synthase confers salt-stress tolerance by regulating the synthesis of galactinol and raffinose family oligosaccharides in poplar. Ind Crop Prod 165:113432. https://doi.org/10.1016/j.indcrop.2021.113432
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Magalhaes JV, Piñeros MA, Maciel LS, Kochian LV (2018) Emerging pleiotropic mechanisms underlying aluminum resistance and phosphorus acquisition on acidic soils. Front Plant Sci 9:1420. https://doi.org/10.3389/fpls.2018.01420
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22(5):867–880
Nehela Y, Killiny N (2019) ‘Candidatus Liberibacter asiaticus’ and its vector, Diaphorina citri, augment the tricarboxylic acid cycle of their host via the γ-aminobutyric acid shunt and polyamines pathway. Mol Plant Microbe Interact 32(4):413–427. https://doi.org/10.1094/mpmi-09-18-0238-r
Ofoe R, Thomas RH, Asiedu SK, Wang-Pruski G, Fofana B, Abbey L (2023) Aluminum in plant: benefits, toxicity and tolerance mechanisms. Front Plant Sci 13:1085998. https://doi.org/10.3389/fpls.2022.1085998
Ohyama Y, Ito H, Kobayashi Y, Ikka T, Morita A, Kobayashi M, Imaizumi R, Aoki T, Komatsu K, Sakata Y, Iuchi S, Koyama H (2013) Characterization of AtSTOP1 orthologous genes in tobacco and other plant species. Plant Physiol 162(4):1937–1946. https://doi.org/10.1104/pp.113.218958
Palmer AJ, Baker A, Muench SP (2016) The varied functions of aluminium-activated malate transporters-much more than aluminium resistance. Biochem Soc Trans 44(3):856–862. https://doi.org/10.1042/bst20160027
Prasad M (2003) Phytoremediation of metal-polluted ecosystems: hype for commercialization. Russ J Plant Physiol 50(5):686–701. https://doi.org/10.1023/A:1025604627496
Qiu Y, Su M, Liu Y, Chen M, Gu J, Zhang J, Jia W (2007) Application of ethyl chloroformate derivatization for gas chromatography-mass spectrometry based metabonomic profiling. Anal Chim Acta 583(2):277–283. https://doi.org/10.1016/j.aca.2006.10.025
Qu X, Zhou J, Masabni J, Yuan J (2020) Phosphorus relieves aluminum toxicity in oil tea seedlings by regulating the metabolic profiling in the roots. Plant Physiol Biochem 152:12–22. https://doi.org/10.1016/j.plaphy.2020.04.030
Ramesh SA, Tyerman SD, Xu B, Bose J, Kaur S, Conn V, Domingos P, Ullah S, Wege S, Shabala S, Feijó JA, Ryan PR, Gilliham M (2015) GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat Commun 6(1):7879. https://doi.org/10.1038/ncomms8879
Reddy AS, Shad Ali G (2011) Plant serine/arginine-rich proteins: roles in precursor messenger RNA splicing, plant development, and stress responses. Wires RNA 2(6):875–889. https://doi.org/10.1002/wrna.98
Rizhsky L, Liang H, Shuman J, Shulaev V, Davletova S, Mittler R (2004) When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol 134(4):1683–1696. https://doi.org/10.1104/pp.103.033431
Roessner U, Wagner C, Kopka J, Trethewey RN, Willmitzer L (2000) Simultaneous analysis of metabolites in potato tuber by gas chromatography–mass spectrometry. Plant J 23(1):131–142. https://doi.org/10.1046/j.1365-313x.2000.00774.x
Sheteiwy MS, Shao H, Qi W, Hamoud YA, Shaghaleh H, Khan NU, Yang R, Tang B (2019) GABA-alleviated oxidative injury induced by salinity, osmotic stress and their combination by regulating cellular and molecular signals in rice. Int J Mol Sci 20(22):5709. https://doi.org/10.3390/ijms20225709
Shi SQ, Shi Z, Jiang ZP, Qi LW, Sun XM, Li CX, Liu JF, Xiao WF, Gong S (2010) Effects of exogenous GABA on gene expression of Caragana intermedia roots under NaCl stress: regulatory roles for H2O2 and ethylene production. Plant Cell Environ 33(2):149–162. https://doi.org/10.1111/j.1365-3040.2009.02065.x
Silva CO, Brito DS, da Silva AA, do Rosário Rosa V, Santos MFS, de Souza GA, Azevedo AA, Dal-Bianco M, Oliveira JA, Ribeiro C (2020) Differential accumulation of aluminum in root tips of soybean seedlings. Braz J Bot 43:99–107. https://doi.org/10.1007/s40415-020-00593-9
Silva-Navas J, Salvador N, Pozo J, Benito C, Gallego FJ (2021) The rye transcription factor ScSTOP1 regulates the tolerance to aluminum by activating the ALMT1 transporter. Plant Sci 310(3):110951. https://doi.org/10.1016/j.plantsci.2021.110951
Sun C, Liu L, Yu Y, Liu W, Lu L, Jin C, Lin X (2015) Nitric oxide alleviates aluminum-induced oxidative damage through regulating the ascorbate-glutathione cycle in roots of wheat. J Integr Plant Biol 57(6):550–561. https://doi.org/10.1111/jipb.12298
Taji T, Ohsumi C, Iuchi S, Seki M, Kasuga M, Kobayashi M, Yamaguchi-Shinozaki K, Shinozaki K (2002) Important roles of drought- and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant J 29(4):417–426. https://doi.org/10.1046/j.0960-7412.2001.01227.x
Tan M, Hassan MJ, Peng Y, Feng G, Huang L, Liu L, Liu W, Han L, Li Z (2022) Polyamines metabolism interacts with γ-aminobutyric acid, proline and nitrogen metabolisms to affect drought tolerance of creeping bentgrass. Int J Mol Sci 23(5):2779. https://doi.org/10.3390/ijms23052779
Tesfaye M, Temple SJ, Allan DL, Vance CP, Samac DA (2001) Overexpression of malate dehydrogenase in transgenic alfalfa enhances organic acid synthesis and confers tolerance to aluminum. Plant Physiol 127(4):1836–1844. https://doi.org/10.1104/pp.010376
Tomaz T, Bagard M, Pracharoenwattana I, Lindén P, Lee CP, Carroll AJ, Ströher E, Smith SM, Gardeström P, Millar AH (2010) Mitochondrial malate dehydrogenase lowers leaf respiration and alters photorespiration and plant growth in Arabidopsis. Plant Physiol 154(3):1143–1157. https://doi.org/10.1104/pp.110.161612
Uchida A, Jagendorf AT, Hibino T, Takabe T, Takabe T (2002) Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Sci 163(3):515–523. https://doi.org/10.1016/s0168-9452(02)00159-0
Valluru R, Van den Ende W (2011) Myo-inositol and beyond-emerging networks under stress. Plant Sci 181(4):387–400. https://doi.org/10.1016/j.plantsci.2011.07.009
Vijayakumari K, Jisha K, Puthur JT (2016) GABA/BABA priming: a means for enhancing abiotic stress tolerance potential of plants with less energy investments on defence cache. Acta Physiol Plant 38:230. https://doi.org/10.1007/s11738-016-2254-z
Wang Y, Xu H, Kou J, Shi L, Zhang C, Xu F (2013) Dual effects of transgenic Brassica napus overexpressing CS gene on tolerances to aluminum toxicity and phosphorus deficiency. Plant Soil 362(1):231–246. https://doi.org/10.1007/s11104-012-1289-1
Wang Q, Sun H, Dong Q, Sun T, Jin Z, Hao Y, Yao Y (2016) The enhancement of tolerance to salt and cold stresses by modifying the redox state and salicylic acid content via the cytosolic malate dehydrogenase gene in transgenic apple plants. Plant Biotechnol J 14(10):1986–1997. https://doi.org/10.1111/pbi.12556
Wang Y, Gu W, Meng Y, Xie T, Li L, Li J, Wei S (2017) γ-Aminobutyric acid imparts partial protection from salt stress injury to maize seedlings by improving photosynthesis and upregulating osmoprotectants and antioxidants. Sci Rep 7:43609. https://doi.org/10.1038/srep43609
Wang P, Dong Y, Zhu L, Hao Z, Hu L, Hu X, Wang G, Cheng T, Shi J, Chen J (2021) The role of γ-aminobutyric acid in aluminum stress tolerance in a woody plant, Liriodendron chinense× tulipifera. Hortic Res 8:80. https://doi.org/10.1038/s41438-021-00517-y
Wang P, Zhou S, Li A, Xie L (2022) Influence of aluminum at low pH on the rhizosphere processes of Masson pine (Pinus massoniana Lamb). Plant Growth Regul 97:499–510. https://doi.org/10.1007/s10725-022-00816-x
Wang C, Bian C, Li J, Han L, Guo D, Wang T, Sun Z, Ma C, Liu X, Tian Y, Zheng X (2023) Melatonin promotes Al3+ compartmentalization via H+ transport and ion gradients in Malus hupehensis. Plant Physiol 193(1):821–839. https://doi.org/10.1093/plphys/kiad339
Yamamoto Y, Kobayashi Y, Devi SR, Rikiishi S, Matsumoto H (2003) Oxidative stress triggered by aluminum in plant roots. Plant Soil 101:39–243. https://doi.org/10.1023/a:1026127803156
Yao YX, Dong QL, Zhai H, You CX, Hao YJ (2011) The functions of an apple cytosolic malate dehydrogenase gene in growth and tolerance to cold and salt stresses. Plant Physiol Biochem 49(3):257–264. https://doi.org/10.1016/j.plaphy.2010.12.009
Yao H, Zhang S, Zhou W, Liu Y, Liu Y, Wu Y (2020) The effects of exogenous malic acid in relieving aluminum toxicity in Pinus massoniana. Int J Phytoremed 22(6):669–678. https://doi.org/10.1080/15226514.2019.1707162
Ye J, Wang X, Hu T, Zhang F, Wang B, Li C, Yang T, Li H, Lu Y, Giovannoni JJ, Zhang Y, Ye Z (2017) An indel in the promoter of Al-activated malate transporter 9 selected during tomato domestication determines fruit malate contents and aluminum tolerance. Plant Cell 29(9):2249–2268. https://doi.org/10.1105/tpc.17.00211
Yu L, Sun J, Guo S, Yan J, Zhu W (2012) Antioxidant enzyme activities in root tips of Vigna unguiculata L. seedlings under aluminum stress. Acta Botan Boreali-Occiden Sin 32(11):2299–2304. https://doi.org/10.3969/j.issn.1000-4025.2012.11.022
Zhang Q, Wang M, Hu J, Wang W, Fu X, Liu J (2015) PtrABF of Poncirus trifoliata functions in dehydration tolerance by reducing stomatal density and maintaining reactive oxygen species homeostasis. J Exp Bot 66(19):5911–5927. https://doi.org/10.1093/jxb/erv301
Zheng SJ, Ma JF, Matsumoto H (1998) Continuous secretion of organic acids is related to aluminium resistance during relatively long-term exposure to aluminium stress. Physiol Plant 103(2):209–214. https://doi.org/10.1034/j.1399-3054.1998.1030208.x
Zheng Y, Cabassa-Hourton C, Planchais S, Lebreton S, Savouré A (2021) The proline cycle as an eukaryotic redox valve. J Exp Bot 72(20):6856–6866. https://doi.org/10.1093/jxb/erab361
Zhou Y, Yang Z, Gong L, Liu R, Sun H, You J (2018) Molecular characterization of GmSTOP1 homologs in soybean under Al and proton stress. Plant Soil 427:213–230. https://doi.org/10.1007/s11104-018-3645-2
Zhou M, Yuan Y, Lin J, Lin L, Zhou J, Li Z (2023) γ-Aminobutyric acid priming alleviates acid-aluminum toxicity to creeping bentgrass by regulating metabolic homeostasis. Int J Mol Sci 24(18):14309. https://doi.org/10.3390/ijms241814309
Zhu CQ, Cao XC, Bai ZG, Zhu LF, Hu WJ, Hu AY, Abliz B, Zhong C, Liang QD, Huang J, Zhang JH, Jin QY (2019) Putrescine alleviates aluminum toxicity in rice (Oryza sativa) by reducing cell wall Al contents in an ethylene-dependent manner. Physiol Plant 167(4):471–487. https://doi.org/10.1111/ppl.12961
Acknowledgements
This research was supported by the National Natural Science Foundation of China (32171684).
Author information
Authors and Affiliations
Contributions
Min Zhou: data curation, investigation, formal analysis, and writing—original draft. Cheng Huang: methodology and investigation. Junnan Lin, Yan Yuan, Long Lin, and Jianzhen Zhou: investigation and material cultivation. Zhou Li: conceptualization, technical guidance, writing—review and editing, methodology, formal analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Communicated by Dorothea Bartels.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
425_2024_4461_MOESM1_ESM.docx
Supplementary Information Table S1 Details of primer sequences of tested genes. Table S2 Relative retention time and mass to charge ratios of 70 identified metabolites in creeping bentgrass. Figure S1-4. Contents of different amino acids, organic acids, sugars, and other metabolites in creeping bentgrass roots under control condition and acid-aluminum stress (DOCX 500 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, M., Huang, C., Lin, J. et al. γ-Aminobutyric acid (GABA) priming alleviates acid-aluminum toxicity to roots of creeping bentgrass via enhancements in antioxidant defense and organic metabolites remodeling. Planta 260, 33 (2024). https://doi.org/10.1007/s00425-024-04461-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-024-04461-8