Abstract
Aims
Global warming due to increasing greenhouse gas emissions intensifies frequency and duration of extreme high temperature which has become a primary threat to cool-season crops worldwide. Aim of this study was to examine root adaptability to high temperature regulated by γ-aminobutyric acid (GABA) associated with changes in antioxidant metabolism, root vitality, osmotic balance, and global metabolites remodeling in roots.
Methods
A cool-season creeping bentgrass (Agrostis stolonifera) plants were pretreated with or without the 0.5 mM GABA before being subjected to heat stress (35/30 °C) or optimal temperature condition (23/19 °C) for 15 days. Roots were sampled to analyze changes in physiological parameters and metabolomics.
Results
Heat stress significantly induced reactive oxygen species production in roots resulting in oxidative damage to proteins and cell membranes. However, the GABA could effectively alleviate heat-induced decline in total antioxidant capacity and also improve multiple antioxidant enzyme activities, root vitality, and osmotic adjustment ability in roots. Metabolomic analysis found that a total of 71 metabolites were jointly or differentially regulated by heat stress or heat stress together with the GABA application in roots. In response to heat stress, the GABA improved the accumulation of multiple amino acids, sugars, organic acids, and other metabolites (urea, putrescine, myoinositol, arbutin, campesterol, and stigmasterol) in roots.
Conclusions
GABA could effectively increase antioxidant capacity, root vitality, and osmotic adjustment associated with improved root adaptability to heat stress. In addition, the GABA-regulated metabolites remodeling could be attributed to better energy metabolism, osmotic balance, antioxidant capacity, and cellular structures in roots under heat stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global warming due to increasing greenhouse gas emissions intensifies frequency and duration of extreme high temperature which has become a primary threat to cool-season crops worldwide (Fahad et al. 2017). Maintenance of roots growth and viability is essential for heat tolerance, since roots are responsible for water and nutrients uptake as well as biosynthesis of many hormones for supply to aboveground tissue. However, roots growth and metabolic activity are more sensitive than leaves in response to elevated environmental temperature (Huang et al. 2012). High temperature stress inhibits root growth and also accelerates root senescence and programmed cell death through inducing a huge amount of reactive oxygen species (ROS) in roots (Xu et al. 2016). Enhanced antioxidant scavenging system to maintain ROS homeostasis in roots is an important adaptive mechanism for plants surviving under heat stress. It has been reported that heat-tolerant wucai (Brassica campestris) genotype could minimize heat-induced lipid peroxidation damage due to highly effectiveness of antioxidant metabolism in roots (Yuan et al. 2016). Significant up-regulation of antioxidant-relevant proteins including glutathione S-transferase (GST) and superoxide dismutase (SOD) contributed to the superior root tolerance to heat stress in thermal rough bentgrass (Agrostis scabra) (Xu and Huang 2008). 3′,5′-cyclic adenosine monophosphate (cAMP) pretreatment mitigated deleterious effects of ROS by enhancing SOD and ascorbate peroxidase (APX) activity in roots of maize (Zea mays) under high temperature stress (Zhao et al. 2021).
In addition to antioxidant metabolism, root metabolites remodeling and allocation also play important roles in growth-survival strategy under normal and stressful conditions (Atkinson et al. 2012; Shen et al. 2018). Metabolomics analysis found that the rhizobium-nodulized alfalfa (Medicago sativa) exhibited better tolerance to alkaline salt stress than the non-nodulized plants associated with the accumulation of more metabolites such as sugars, glycols, proline, succinic acid, and fumaric acid in roots (Song et al. 2017). A study of Shen et al. (2018) based on metabolomic profiling revealed that metabolic disorder occurred in roots of Tibetan wild barley (Hordeum spontaneum) under salt stress, but the salt-tolerant XZ26 genotype accumulated more proline and inositol than the salt-sensitive XZ169. Significant accumulations of betaine, melatonin, and 2-aminobutyric acid (AABA) in roots could be important adaptive responses to a long-term salt stress in sugar beet (Beta vulgaris) (Liu et al. 2020). In addition, integrated transcriptomic and metabolomic analysis also showed that melatonin induced the accumulation of linoleic acid and lecithin in roots of melon (Cucumis melo) related to enhanced tolerance to copper toxicity (Hu et al. 2020). These previous studies have elucidated possible contribution of differentially accumulated metabolites to stress tolerance in roots of different plant species under ionic stress. However, little researches have been done to understand global root metabolites reprogramming associated with the adaptation to heat stress in plants.
Creeping bentgrass (Agrostis stolonifera) is perennial cool-season turfgrass and used widely in sports turf due to many excellent features such as soft texture, creeping growth habit, and capacity of rapid establishment. However, high temperature stress damages to roots and also induces declines in turf quality leading to increased turf maintenance and management costs worldwide (Fry and Huang 2004; Liu and Huang 2000). γ-Aminobutyric acid (GABA) is known as a non-proteinogenic amino acid and also recognized as a key metabolite for carbon and nitrogen metabolism, pollen tube growth, and signaling transduction in recent decades (Khan et al. 2021). It has been reported widely about regulatory role of the GABA in heat tolerance associated with changes in antioxidant defense, heat shock factor pathway, and metabolic homeostasis in plants (Liu et al. 2019; Ren et al. 2021; Wang et al. 2022). Our previous study has also proved that foliar application of the GABA conferred heat tolerance involved in the enhancement of sugars and amino acids accumulation and organic acids metabolism in leaves of creeping bentgrass (Li et al. 2016b). Although positive function of the GABA on thermotolerance has been extensively documented in leaves of different plant species, the GABA-regulated potential mechanism of root tolerance to high temperature is much less investigated. Current study focuses on investigation into regulatory effects of the GABA on root vitality, antioxidant metabolism, osmotic adjustment (OA), primary and secondary metabolites profiling, and relevant metabolic pathways associated with root thermotolerance in a cool-season horticultural crop creeping bentgrass. Current findings will be contributed to better understand adaptive mechanism of the GABA-regulated heat tolerance in roots.
Materials and methods
Plant material and treatments
Seeds of creeping bentgrass (cv. Penncross) were sowed evenly in a container (25 cm length, 15 cm width, and 10 cm height) that was filled with quartz sands because roots could be taken out from quartz sand easily without mechanical damage to roots. Seeds germinated in controlled growth chambers (23/19 °C (day/night), 65% relative humidity, and 750 μmol·m−2·s−1 PAR) for 7 days and then watered with Hoagland’s solution (Hoagland and Arnon 1950). All containers were waterproofed and had good water holding capacity. Hoagland’s solution was refreshed every other day. After being cultivated in Hoagland’s solution for 20 days, all plants were divided into two groups: one group was fed with Hoagland’s solution containing 0.5 mM GABA in roots for 2 days as the GABA-pretreated plants; another group was fed with normal Hoagland’s solution without GABA for 2 days as the non-pretreated plants. Optimal dose of GABA (0.5 mM) for improvement in heat tolerance of creeping bentgrass has been screened in our previous study (Li et al. 2016b). GABA-pretreated and non-pretreated plants were then subjected to high temperature stress (35/30 °C) or cultivated under normal condition (23/19 °C) for 15 days in growth chambers. Four treatments were set: 1) C, the plants without GABA pretreatment were cultivated under normal condition for 15 days; 2) C + GABA, the GABA-pretreated plants were cultivated under normal condition for 15 days; 3) HS, the plants without GABA pretreatment were cultivated under heat stress condition for 15 days; and 4) HS + GABA, the GABA-pretreated plants were cultivated under heat stress condition for 15 days. Four biologic replicates (four containers) were used for each treatment. For the analysis of physiological parameters and metabolomics, roots collected from more than 20 independent plants were used for each replicate, which indicated that more than 80 plants were used for each treatment. Each replicate was grown in independent batch and four replicates were repeated in four growth chambers.
Determination of oxidative damage and antioxidant metabolism
For superoxide anion radical (O2.-) dyeing or hydrogen peroxide (H2O2) staining, fresh roots were immerged in 1 mM nitrotetrazolium blue chloride (NBT) for 3 h or in 0.1% (w/v) 3-diaminobenzinidine for 8 h, respectively. These roots were cleaned up by distilled water and then photographed by a microscope (Dunand et al. 2007; Thordal-Christensen et al. 1997). The O2.- and H2O2 contents were detected by using assay methods of Elstner and Heupel (1976) and Velikova et al. (2000), respectively. Total antioxidant capacity (TAC) and protein carbonyl content were detected by using the assay kits that were purchased from Suzhou Comin Biotechnology, Suzhou, China according to manufacturer’s instructions. Electrolyte leakage (EL) was detected based on the method of Blum and Ebercon (1981). Fresh roots (0.2 g) were cleaned and immerged in 10 ml of distilled water for 12 h to detect the initial conductivity (Cinitial) of solution by using a conductivity meter. These roots were autoclaved at 120 °C for 20 min to detect maximum conductance (Cmax) of solution. The EL was calculated as the percentage of Cinitial /Cmax.
For analysis of malondialdehyde (MDA) and antioxidant enzyme activities including SOD, peroxidase (POD), catalase (CAT), APX, dehydroascorbate reductase (DHAR), and monodehydroascorbate reductase (MDHAR), 0.15 g of clean and fresh roots were ground with 2 ml of cold phosphate buffer (50 mM and pH 7.8). After being centrifuged at 10000 g for 15 min at 4 °C, the supernatant was collected for determination of MDA content and antioxidant enzyme activities. The 0.5 ml of supernatant and 1.0 ml of reaction solution (20% w/v trichloroacetic acid and 0.5% w/v thiobarbituric acid) were mixed evenly and then placed in boiling water bath for 10 min. After being cooled down in an ice bath, the solution was centrifuged at 8000 g for 10 min and the absorbance of supernatant was detected at 532 and 600 nm for the determination of MDA (Dhindsa et al. 1981). The SOD activity was detected based on the method of Giannopolitis and Ries (1977). The POD and CAT activities measured according to the method of Chance and Maehly (1955). The APX activity was determined by using the method of Nakano and Asada (1981). DHAR and MDHAR activities were detected according to the method of Cakmak et al. (1993).
Determination of osmotic potential and root vitality
For the determination of osmotic potential (OP), fresh roots were collected and cleaned carefully in distilled water. Roots were frozen in liquid nitrogen for 10 min and thawed at 4 °C. Root saps were pressed and 10 ml of sap was inserted into the osmometer (Wescor, Logan, UT) to detect osmolality (c). The OP was calculated based on MPa = −c × 2.58 × 10 −3 (Blum 1989). Root viability was estimated according to the method of McMichael and Burke (1994) with some modification. Fresh roots (0.2 g) were immersed in 5 ml of 0.4% triphenyltetrazolium chloride (TTC) and 5 ml of phosphate buffer (65 mM and pH 7.0) and then incubated at 37 °C for 1 h in the dark. After that, 2 ml of 1 M sulfuric acid was added into the mixture. Roots were then taken out from the mixture and put into a new centrifuge tube. A 20 ml of methanol was added into the tube and incubated at 40 °C for 5 h. The absorbance of the supernatant was measured at 280 nm.
Metabolomics analysis
For analysis of metabolite extraction and identification, the comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry (GC-TOFMS) was used. Assay method in details has been reported clearly in our previous study (Li et al. 2020). Briefly, fresh roots were freeze-dried and ground into fine powder. A total of 20 mg of powder was evenly mixed with 100 μL ddH2O and 500 μL of aqueous methanol, and then was sonicated for 20 min. The mixture was centrifuged at 12000 g for 10 min, and 300 μL of supernatant was mixed with 10 μl of ribitol solution. After being fully desiccated, sediments were re-dissolved in 80 μL of methoxyamine hydrochloride at 30 °C for 90 min, and the solution was trimethylsilylated with 80 μL N-methyl-N-(trimethylsily) trifluoroacetamide containing 1% trimethylchlorosilane for 60 min at 70 °C. Finally, the intermixture was used to analyze metabolites by using the GC-TOFMS. Metabolites were identified and quantified by using ChromaTOF software (v. 4.50.8.0, LECO, St. Joseph, MI, USA) coupled with commercially available compound libraries: NIST 2005, Wiley 7.0.
Statistical analysis
The General Linear Model procedure of SAS (version 9.1; SAS Institute, Cary, NC) was used to determine the significance for physiological parameters. The significance of differences among treatments was tested using the least significance test with P ≤ 0.05. Homogeneity of variance test was analyzed by using SPSS 20 (IBM, Armonk, NY, USA) based on Hartley’s Test.
Results
Oxidative damage and antioxidant metabolism regulated by GABA in roots
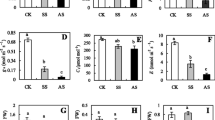
O2.- and H2O2 dyeing showed that heat stress induced a massive accumulation of O2.- or H2O2 in roots, but O2.- or H2O2 dyeing was darker in heat-stressed roots without the GABA pretreatment as compared to heat-stressed roots with the GABA pretreatment (Fig. 1A and B). O2.- or H2O2 content significantly increased in roots under heat stress, and the GABA-pretreated plants exhibited a 39% or 44% decrease in O2.- or H2O2 content in roots than the non-pretreated plants (Fig. 1C and D). Protein carbonyl content, MDA content, and EL significantly increased in all plants under heat stress, whereas TAC declined significantly in roots in response to heat stress (Fig. 2A-D). The GABA-pretreated plants maintained a 31%, 25%, or 18% decrease in protein carbonyl content, MDA content, or EL, but a 38% increase in the TAC in roots than the plants without the GABA pretreatment under heat stress (Fig. 2A-D). For changes in antioxidant enzyme activities, the GABA-pretreated plants exhibited significantly higher SDO, POD, CAT, APX, DHAR, and MDHAR activities in roots than the plants without GABA pretreatment in response to heat stress (Fig. 2E).
Effects of γ-aminobutyric acid (GABA) priming on (A) superoxide anion (O2.-) staining, (B) hydrogen peroxide (H2O2) staining, (C) O2.- content, and (D) H2O2 content in roots under normal condition and heat stress. Vertical bars indicate ±SE of mean (n = 4). Different letters above columns indicate significant differences based on LSD (P < 0.05). C, control; C + GABA, control+GABA; HS, heat stress; HS + GABA, heat stress+GABA
Effects of γ-aminobutyric acid (GABA) priming on (A) carbonyl content, (B) malondialdehyde content, (C) electrolyte leakage, (D) total antioxidant capacity, and (D) antioxidant enzyme activity in roots under normal condition and heat stress. Vertical bars indicate ±SE of mean (n = 4). Different letters above columns indicate significant differences based on LSD (P < 0.05). C, control; C + GABA, control+GABA; HS, heat stress; HS + GABA, heat stress+GABA
Root vitality and osmotic potential regulated by GABA in roots
Heat stress induced a significant decline in root vitality in all plants; however, the GABA-pretreated plants could maintain 16% increase in root vitality than those plants without the GABA pretreatment under heat stress (Fig. 3A). As compared to the control, the OP remained unchanged in roots of heat-stressed plants without the GABA pretreatment, but the OP significantly declined in roots of heat-stressed plants with the GABA pretreatment (Fig. 3B). Under normal condition, the GABA application had no significant effects on root vitality and OP (Fig. 3A and B).
Effects of γ-aminobutyric acid (GABA) priming on (A) root vitality and (B) osmotic potential in roots under normal condition and heat stress. Vertical bars indicate ±SE of mean (n = 4). Different letters above columns indicate significant differences based on LSD (P < 0.05). C, control; C + GABA, control+GABA; HS, heat stress; HS + GABA, heat stress+GABA
Metabolites identification and change in total metabolites regulated by GABA in roots
A total of 71 metabolites were detected in roots including 13 amino acids, 17 sugars, 26 organic acids, and 15 other metabolites (Fig. 4A). Heat map showed that these metabolites were differentially regulated by the exogenous GABA and heat stress (Fig. 4A). Most of metabolites were not significantly changed by exogenous GABA under normal condition (C + GABA Vs. C) (Fig. 4B). The GABA induced increases in almost all metabolites under heat stress (HS + GABA Vs. HS). In response to heat stress, the overwhelming majority of metabolites in roots decreased in the GABA-pretreated and untreated plants (HS Vs. C and HS + GABA Vs. C) (Fig. 4B). Heat stress induced significant declines in amino acids, sugars, organic acids, and other metabolites contents in roots of plants without the GABA application, but did not affect accumulation of sugars and other metabolites in roots of the GABA-pretreated plants (Fig. 4C). In addition, the GABA-pretreated plants also had significantly higher accumulation of amino acids and organic acids in roots than those plants without the GABA application under heat stress (Fig. 4C).
Changes in (A) heat map of all identified 71 metabolites, (B) percentage of up-regulated, down-regulated, and unchanged metabolites, and (C) relative content of amino acids, sugars, organic acids, and other metabolites in roots under normal condition and heat stress. Vertical bars indicate ±SE of mean (n = 4). Different letters above columns indicate significant differences based on LSD (P < 0.05). C, control; C + GABA, control+GABA; HS, heat stress; HS + GABA, heat stress+GABA
Differentially accumulated metabolites regulated by GABA in roots
Heat stress led to significant decreases in all amino acids in roots of plants without GABA application (Table 1). Exogenous GABA pretreatment effectively alleviated heat-induced declines in GABA, serine, valine, isoleucine, threonine, proline, aspartic acid, glutamine, asparagine, or phenylalanine content, and also significantly improved the glycine accumulation under heat stress (Table 1). For changes in various organic acids, heat stress significantly inhibited their accumulations in roots. However, the GABA application significantly alleviated inhibitory effects on heat-induced declines in all detected organic acids under heat stress, except for aconitic acid and glyceric acid (Table 1). Except for gentiobiose, melibiose, and glucose, the GABA-pretreated plants maintained significantly higher all detected sugars than those plants without GABA application under heat stress (Table 2). Similarly, contents of 15 other metabolites in roots were significantly decreased in response to heat stress. Exogenous GABA application did not affect ribitol content under heat stress, but significantly improved the accumulation of putrescine (Put), myo-inositol, urea, or glycerol. The GABA application also significantly ameliorated heat-induced declines in pyrrolidinone, arabitol, arbutin, maltitol, glycerol monostearate, campesterol, and stigamsterol contents in roots (Table 2).
Metabolic pathways associated with metabolites regulated by GABA in roots
A total of 32 metabolites were assigned into metabolic pathways involved in sugar and amino acid metabolism, TCA cycle, and GABA shunt (Fig. 5). Heat stress inhibited sugar and amino acid metabolism and transformation, TCA cycle, and GABA shunt in roots of the GABA-pretreated and untreated plants. As compared to heat-stressed plants without GABA application, the heat-stressed plants with GABA application could maintain higher accumulation of intermediates involved in TCA cycle (citrate, succinate, and malate) and also improved GABA metabolism into glutamine, proline, urea, and Put in roots (Fig. 5).
Discussion
Temperature optimization is more difficult than the regulation and control of other abiotic stresses such as drought and soil salinization in field (Aloni et al. 1992). Previous study has proved the important role of root systems in whole-plant adaptation to high temperature stress (Huang et al. 2012). Heat stress triggers ROS (O2.- and H2O2) accumulation leading to oxidative damage to cellular membrane, proteins, and nucleic acid (Belhadj Slimen et al. 2014). Creeping bentgrass is sensitive to heat stress and its root viability significantly declined when soil temperature increases above 25 °C (Huang et al. 2012). In response to a prolonged period of heat stress, the heat-tolerant rough bentgrass could activate stronger antioxidant scavenging systems to decrease overaccumulation of O2.- and H2O2 in roots than the heat-sensitive creeping bentgrass (Xu et al. 2015). In recent years, GABA has been used as stress-ameliorating agent to improve plant adaptability to heat stress associated with enhanced antioxidant defense system in leaves (Ansari et al. 2021). Our current study demonstrated that ROS level (O2.- and H2O2), protein carbonyl and MDA contents, and EL level significantly increased, but total antioxidant capacity significantly declined in roots of creeping bentgrass under heat stress. However, the application of GABA mitigated heat-induced oxidative damage to proteins and cell membranes through improving total antioxidant capacity and multiple antioxidant enzymes (SOD, POD, CAT, APX, DHAR, and MDHAR) activities in roots. In addition, heat stress induces premature root senescence due to imbalanced production of ROS, which significantly decreases root vitality (Li et al. 2016a). Improved root activity regulated by plant growth regulators including salicylic acid and 24-epibrassinolide has been reported under heat stress (Khan et al. 2014). GABA-pretreated creeping bentgrass exhibited significantly higher root vitality than untreated plants under heat stress in the present study, which will be propitious to uptake and transport of water and nutrition for metabolic balance under heat stress.
Heat stress disrupts metabolic activity leading to loss in physiological function of roots. Amino acids serve as the basis of protein biosynthesis and also act as protective substance for OA, osmoprotection, or antioxidant when plants respond to heat stress (Batista-Silva et al. 2019). The GABA, glutamine, and other metabolites such as urea and Put also play a critical role in nitrogen transform and utilization for growth regulation and stress tolerance (Rodrigues-Corrêa and Fett-Neto 2019; Valderrama-Martín et al. 2022; Witte 2011). Heat stress significantly hindered amino acid accumulation in roots, but this symptom could be effectively alleviated by the GABA application in our current study. As compared to untreated plants, the GABA-pretreated creeping bentgrass accumulated more endogenous GABA along with significant increases in proline, glutamine, urea, and Put contents in roots. Previous studies have showed that foliar application of GABA attenuated heat damage to wheat (Triticum aestivum) and creeping bentgrass by regulating amino acid homeostasis in leaves (Rossi et al. 2021; Wang et al. 2021). Multiple positive roles of proline as a free radical-scavenger, osmoregulator, or stress signal molecule have been reported in plants under stressful condition (Kaur and Asthir 2015). It has been found that exogenous application of urea and proline regulated the accumulation of diverse amino acids in leaves conferring creeping bentgrass tolerance against heat-induced senescence (Jespersen et al. 2015; Rossi et al. 2021). Put could alleviate heat damage to wheat in relation to improvement in root viability and antioxidant capacity in roots (Asthir and Deep 2011). These studies together with current findings indicate that the GABA-regulated accumulation of amino acids, urea, and Put enhanced heat tolerance in relation to nitrogen metabolism, antioxidant, and delayed senescence in roots of creeping bentgrass.
It has been documented that the GABA could be metabolized to the tricarboxylic acid (TCA) cycle for energy supply in plants under normal condition, salt stress, or heat stress (Hijaz and Killiny 2019; Li et al. 2020; Li et al. 2016b). Metabolomic analysis found that heat stress significantly inhibited production of intermediates of TCA cycle including citric acid, aconitic acid, succinic acid, and malic acid in roots of creeping bentgrass, but the application of GABA effectively alleviated the heat-induced inhibitory effect of TCA cycle through improving the citric acid, succinic acid, and malic acid contents in roots. The study of Liu et al. (2020) also showed that a short-term salt stress (1 day) decreased sucrose accumulation, but increased the malic acid content in sugar beet roots, indicating that the regulation of intermediates of TCA cycle was an important adaptive response to the early phase of stress. In addition, both of malic acid and citric acid could significantly enhanced rose (Rosa × hybrida) rooting (Ghazijahani et al. 2017). It was noteworthy that the GABA also up-regulated the accumulation of other organic acids such as oxalic acid and pipecolic acid in roots of creeping bentgrass in response to heat stress. Oxalic acid could mitigate cadmium toxicity to roots of chickpea (Cicer arietinum) seedlings by regulating antioxidant defense to protect plasma membrane integrity (Sakouhi et al. 2022). Pipecolic acid is a critical endogenous plant immunity inducer in roots against microbial pathogen invasion and abiotic stress such as drought (Abeysekara et al. 2016; Caddell et al. 2020; Návarová et al. 2012). However, modulatory mechanism of heat tolerance induced by these diverse organic acids in roots deserves further investigation in the future.
Roots require adequate carbohydrates supply to maintain growth and renewal. Heat stress delays root development and also increases root mortality associated with a shortage of available carbohydrates (Xu and Huang 2000). Thermal rough bentgrass accelerated sugar biosynthesis than the heat-sensitive creeping bentgrass in roots under heat stress (Xu and Huang 2008). Drought preconditioning enhanced heat tolerance of Kentucky bluegrass (Poa pratensis) related to higher carbohydrates accumulation and OA in roots (Jiang and Huang 2001). Our current findings also showed that exogenous application of GABA could restore the accumulation of sugars inhibited by heat stress in roots of creeping bentgrass. Fructose-6-phosphate and glucose-6-phosphate are two critical intermediates of glycolysis for energy production (Fernie et al. 2004). The study of Sun et al. (2021) found that fucose synthesis was one of major responsive pathways in roots when thermotolerant pearl millet (Pennisetum glaucum) suffered from heat shock. Enhanced stress tolerance by increasing endogenous mannose content has been achieved in many plant species (He et al. 2017; Zhao et al. 2020). Raffinose and galactinol (a substrate of the raffinose biosynthesis) exhibiting stronger antioxidant properties could protect plants from oxidative damage induced by abiotic stress (ElSayed et al. 2014; Nishizawa et al. 2008). Overexpression of ZmHSFA2 in Arabidopsis increased the raffinose content contributing to enhanced heat tolerance (Gu et al. 2019). In addition, heat stress impeded carbohydrates metabolism into root cellular structures leading to decreases in amounts of root pectic substances such as arabinose, which hindered cell-wall maintenance (Huang et al. 2012). The GABA-induced accumulation of fructose-6-phosphate, glucose-6-phosphate, fucose, mannose, galactinol, and raffinose in roots of creeping bentgrass could be attributed to better energy metabolism, osmotic balance, antioxidant capacity, and cellular structures for root vitality and renewal under heat stress.
Myo-inositol is a versatile metabolite that generates diversiform derivatives such as phosphatidylinositols as membrane structural lipid and signaling molecule or galactinol and raffinose as antioxidants in response to abiotic stress in plants (Valluru and Van den Ende 2011). Myo-inositol could induce the galactinol synthase (MfGolS1) gene expression which played a positive role in enhancing tolerance to freezing, chilling, drought, or salt stress in plants (Zhuo et al. 2013). Similarly, overexpression of a myo-inositol-1-phosphate synthase 2 (TaMIPS2) could confer heat tolerance to Arabidopsis thaliana associated with significant increases in endogenous myo-inositol and raffinose contents under heat stress (Khurana et al. 2017). Arbutin has been identified as a strong antioxidative and membrane-stabilizing compound when plants respond to heat and drought stresses (Hincha et al. 1999; Ioku et al. 1992; Lawas et al. 2019). Sterols including campesterol and stigmasterol act as regulators of cell membrane stability and fluidity in plants (Saffan 2008). It has been found that heat-tolerant hard fescue (Festuca trachyphylla) cultivar ‘Reliant IV’ accumulated more stigmasterol than the heat-sensitive ‘Predator’ (Wang et al. 2017). Transgenic A. thaliana overexpressing an Atcyp710A1 which catalyzed conversion of sitosterol into stigmasterol exhibited better heat tolerance associated with improved cell membrane stability. On the contrary, the Atcyp710a1 mutant showed significantly lower heat tolerance than wild type (Senthil-Kumar et al. 2013). These findings suggest that the GABA-regulated adaptability to heat stress could be associated with improvement in accumulation of myo-inositol, arbutin, campesterol, and stigmasterol in roots of creeping bentgrass.
In conclusion, heat stress significantly induced ROS production resulting in oxidative damage to roots, but the GABA could effectively increase antioxidant capacity, root vitality, and OA associated with improved root adaptability to heat stress. Diverse metabolites regulated by heat stress or heat stress together with the GABA application were identified in roots. The GABA improved the accumulation of multiple amino acids, urea, and Put in relation to nitrogen metabolism, antioxidant, and delayed senescence in roots of creeping bentgrass. The GABA not only could be metabolized to the TCA cycle for energy supply, but also induced the accumulation of fructose-6-phosphate, glucose-6-phosphate, fucose, mannose, galactinol, and raffinose, which attributed to better energy metabolism, osmotic balance, antioxidant capacity, and cellular structures in roots under heat stress. In addition, the GABA-regulated adaptability to heat stress could be associated with improvement in accumulation of myo-inositol, arbutin, campesterol, and stigmasterol in roots, since these metabolites exhibited positive function of antioxidant and cell membrane stability. Current findings uncover regulatory mechanism of the GABA-induced thermotolerance when root grows under high temperature condition.
References
Abeysekara NS, Swaminathan S, Desai N, Guo L, Bhattacharyya MK (2016) The plant immunity inducer pipecolic acid accumulates in the xylem sap and leaves of soybean seedlings following fusarium virguliforme infection. Plant Sci 243:105–114
Aloni B, Karni L, Daie J (1992) Effect of heat stress on the growth, root sugars, acid invertase and protein profile of pepper seedlings following transplanting. J Horticult Sci 67:717–725
Ansari MI, Jalil SU, Ansari SA, Hasanuzzaman M (2021) GABA shunt: a key-player in mitigation of ROS during stress. Plant Growth Regul 94:131–149
Asthir B, Deep A (2011) Thermotolerance and antioxidant response induced by putrescine and heat acclimation in wheat seedlings. Seed Sci Biotechnol 5:42–46
Atkinson RR, Burrell MM, Osborne CP, Rose KE, Rees M (2012) A non-targeted metabolomics approach to quantifying differences in root storage between fast-and slow-growing plants. New Phytol 196:200–211
Batista-Silva W, Heinemann B, Rugen N, Nunes-Nesi A, Araújo WL, Braun HP, Hildebrandt TM (2019) The role of amino acid metabolism during abiotic stress release. Plant Cell Environ 42:1630–1644
Belhadj Slimen I, Najar T, Ghram A, Dabbebi H, Ben Mrad M, Abdrabbah M (2014) Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int J Hyperth 30:513–523
Blum A (1989) Osmotic adjustment and growth of barley genotypes under drought stress. Crop Sci 29:230–233
Blum A, Ebercon A (1981) Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci 21:43–47
Caddell DF, Louie K, Bowen B, Sievert JA, Hollingsworth J, Dahlberg J, Purdom E, Northen T, Coleman-Derr D (2020) Drought shifts sorghum root metabolite and microbiome profiles and enriches the stress response factor pipecolic acid. bioRxiv preprint. https://doi.org/10.1101/2020.11.08.373399
Cakmak I, Strbac D, Marschner H (1993) Activities of hydrogen peroxide-scavenging enzymes in germinating wheat seeds. J Exp Bot 44:127–132
Chance B, Maehly AC (1955) Assay of catalase and peroxidases. Methods Enzymol 2:764–775
Dhindsa R, Plumb-Dhindsa P, Thorpe T (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Dunand C, Crèvecoeur M, Penel C (2007) Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: possible interaction with peroxidases. New Phytol 174:332–341
ElSayed AI, Rafudeen MS, Golldack D (2014) Physiological aspects of raffinose family oligosaccharides in plants: protection against abiotic stress. Plant Biol 16:1–8
Elstner EF, Heupel A (1976) Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal Biochem 70:616–620
Fahad S, Bajwa AA, Nazir U, Anjum SA, Farooq A, Zohaib A, Sadia S, Nasim W, Adkins S, Saud S (2017) Crop production under drought and heat stress: plant responses and management options. Front Plant Sci 1147
Fernie AR, Carrari F, Sweetlove LJ (2004) Respiratory metabolism: glycolysis, the TCA cycle and mitochondrial electron transport. Curr Opin Plant Biol 7:254–261
Fry J, Huang BR (2004) Applied turfgrass science and physiology. Wiley, Hoboken
Ghazijahani N, Hadavi E, Hwang CH, Jeong BR (2017) Regulating the rooting process of rose softwood cuttings by foliar citric and malic acid spray on stock plants. Folia Horticult 29:155
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. occurrence in higher plants. Plant Physiol 59:309–314
Gu L, Jiang T, Zhang C, Li X, Wang C, Zhang Y, Li T, Dirk LM, Downie AB, Zhao T (2019) Maize HSFA 2 and HSBP 2 antagonistically modulate raffinose biosynthesis and heat tolerance in Arabidopsis. Plant J 100:128–142
He C, Yu Z, Teixeira da Silva JA, Zhang J, Liu X, Wang X, Zhang X, Zeng S, Wu K, Tan J (2017) DoGMP1 from Dendrobium officinale contributes to mannose content of water-soluble polysaccharides and plays a role in salt stress response. Sci Rep 7:1–13
Hijaz F, Killiny N (2019) Exogenous GABA is quickly metabolized to succinic acid and fed into the plant TCA cycle. Plant Signal Behav 14:e1573096
Hincha DK, Oliver AE, Crowe JH (1999) Lipid composition determines the effects of arbutin on the stability of membranes. Biophys J 77:2024–2034
Hoagland DR, Arnon DI (1950) The solution-culture method for growing plants without soil. University of California, Berkeley, CA
Hu Z, Fu Q, Zheng J, Zhang A, Wang H (2020) Transcriptomic and metabolomic analyses reveal that melatonin promotes melon root development under copper stress by inhibiting jasmonic acid biosynthesis. Horticult Res 7:79
Huang B, Rachmilevitch S, Xu J (2012) Root carbon and protein metabolism associated with heat tolerance. J Exp Bot 63:3455–3465
Ioku K, Terao J, Nakatani N (1992) Antioxidative activity of arbutin in a solution and liposomal suspension. Biosci Biotechnol Biochem 56:1658–1659
Jespersen D, Yu J, Huang B (2015) Metabolite responses to exogenous application of nitrogen, cytokinin, and ethylene inhibitors in relation to heat-induced senescence in creeping bentgrass. PLoS One 10:e0123744
Jiang Y, Huang B (2001) Osmotic adjustment and root growth associated with drought preconditioning-enhanced heat tolerance in Kentucky bluegrass. Crop Sci 41:1168–1173
Kaur G, Asthir B (2015) Proline: a key player in plant abiotic stress tolerance. Biol Plant 59:609–619
Khan AR, Cheng Z, Ghazanfar B, Khan MA, Yongxing Z (2014) Acetyl salicylic acid and 24-epibrassinolide enhance root activity and improve root morphological features in tomato plants under heat stress. Acta Agric Scandinavica, Sect B—Soil Plant Sci 64:304–311
Khan MIR, Jalil SU, Chopra P, Chhillar H, Ferrante A, Khan NA, Ansari MI (2021) Role of GABA in plant growth, development and senescence. Plant Gene 26:100283
Khurana N, Sharma N, Khurana P (2017) Overexpression of a heat stress inducible, wheat myo-inositol-1-phosphate synthase 2 (TaMIPS2) confers tolerance to various abiotic stresses in Arabidopsis thaliana. Agri Gene 6:24–30
Lawas LMF, Li X, Erban A, Kopka J, Jagadish SK, Zuther E, Hincha DK (2019) Metabolic responses of rice cultivars with different tolerance to combined drought and heat stress under field conditions. GigaScience 8:giz050
Li H, Ahammed GJ, Zhou G, Xia X, Zhou J, Shi K, Yu J, Zhou Y (2016a) Unraveling main limiting sites of photosynthesis under below-and above-ground heat stress in cucumber and the alleviatory role of luffa rootstock. Front Plant Sci 7:746
Li Z, Yu J, Peng Y, Huang B (2016b) Metabolic pathways regulated by γ-aminobutyric acid (GABA) contributing to heat tolerance in creeping bentgrass (Agrostis stolonifera). Sci Rep 6:30338
Li Z, Cheng B, Zeng W, Zhang X, Peng Y (2020) Proteomic and metabolomic profilings reveal crucial functions of γ-aminobutyric acid in regulating ionic, water, and metabolic homeostasis in creeping bentgrass under salt stress. J Proteome Res 19:769–780
Liu X, Huang B (2000) Heat stress injury in relation to membrane lipid peroxidation in creeping bentgrass. Crop Sci 40:503–510
Liu T, Liu Z, Li Z, Peng Y, Zhang X, Ma X, Huang L, Liu W, Nie G, He L (2019) Regulation of heat shock factor pathways by γ-aminobutyric acid (GABA) associated with thermotolerance of creeping bentgrass. Int J Mol Sci 20:4713
Liu L, Wang B, Liu D, Zou C, Wu P, Wang Z, Wang Y, Li C (2020) Transcriptomic and metabolomic analyses reveal mechanisms of adaptation to salinity in which carbon and nitrogen metabolism is altered in sugar beet roots. BMC Plant Biol 20:1–21
McMichael BL, Burke JJ (1994) Metabolic activity of cotton roots in response to temperature. Environ Exp Bot 34:201–206
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Návarová H, Bernsdorff F, Döring A-C, Zeier J (2012) Pipecolic acid, an endogenous mediator of defense amplification and priming, is a critical regulator of inducible plant immunity. Plant Cell 24:5123–5141
Nishizawa A, Yabuta Y, Shigeoka S (2008) Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol 147:1251–1263
Ren T, Zheng P, Zhang K, Liao J, Xiong F, Shen Q, Ma Y, Fang W, Zhu X (2021) Effects of GABA on the polyphenol accumulation and antioxidant activities in tea plants (Camellia sinensis L.) under heat-stress conditions. Plant Physiol Biochem 159:363–371
Rodrigues-Corrêa KCS, Fett-Neto AG (2019) Abiotic stresses and non-protein amino acids in plants. Crit Rev Plant Sci 38:411–430
Rossi S, Chapman C, Yuan B, Huang B (2021) Improved heat tolerance in creeping bentgrass by γ-aminobutyric acid, proline, and inorganic nitrogen associated with differential regulation of amino acid metabolism. Plant Growth Regul 93:231–242. https://doi.org/10.1007/s10725-020-00681-6
Saffan SE-S (2008) Effect of heat stress on phytochemical composition of peanut seedlings. Res J Agric Biol Sci 4:167–174
Sakouhi L, Kharbech O, Ben Massoud M, Munemasa S, Murata Y, Chaoui A (2022) Exogenous oxalic acid protects germinating chickpea seeds against cadmium injury. J Soil Sci Plant Nutr 22:647–659
Senthil-Kumar M, Wang K, Mysore KS (2013) AtCYP710A1 gene-mediated stigmasterol production plays a role in imparting temperature stress tolerance in Arabidopsis thaliana. Plant Signal Behav 8:e23142
Shen Q, Yu J, Fu L, Wu L, Dai F, Jiang L, Wu D, Zhang G (2018) Ionomic, metabolomic and proteomic analyses reveal molecular mechanisms of root adaption to salt stress in Tibetan wild barley. Plant Physiol Biochem 123:319–330
Song T, Xu H, Sun N, Jiang L, Tian P, Yong Y, Yang W, Cai H, Cui G (2017) Metabolomic analysis of alfalfa (Medicago sativa L.) root-symbiotic rhizobia responses under alkali stress. Front Plant Sci 8:1208
Sun M, Lin C, Zhang A, Wang X, Yan H, Khan I, Wu B, Feng G, Nie G, Zhang X (2021) Transcriptome sequencing revealed the molecular mechanism of response of pearl millet root to heat stress. J Agron Crop Sci 207:768–773
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley—powdery mildew interaction. Plant J 11:1187–1194
Valderrama-Martín JM, Ortigosa F, Ávila C, Cánovas FM, Hirel B, Cantón FR, Cañas RA (2022) A revised view on the evolution of glutamine synthetase isoenzymes in plants. Plant J 110:946–960
Valluru R, Van den Ende W (2011) Myo-inositol and beyond–emerging networks under stress. Plant Sci 181:387–400
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants : protective role of exogenous polyamines. Plant Sci 151:59–66
Wang J, Juliani HR, Jespersen D, Huang B (2017) Differential profiles of membrane proteins, fatty acids, and sterols associated with genetic variations in heat tolerance for a perennial grass species, hard fescue (Festuca trachyphylla). Environ Exp Bot 140:65–75
Wang X, Wang X, Peng C, Shi H, Yang J, He M, Zhang M, Zhou Y, Duan L (2022) Exogenous gamma-aminobutyric acid coordinates active oxygen and amino acid homeostasis to enhance heat tolerance in wheat seedlings. J Plant Growth Regul 41:2787–2797
Witte C-P (2011) Urea metabolism in plants. Plant Sci 180:431–438
Xu Q, Huang B (2000) Growth and physiological responses of creeping bentgrass to changes in air and soil temperatures. Crop Sci 40:1363–1368
Xu C, Huang B (2008) Root proteomic responses to heat stress in two Agrostis grass species contrasting in heat tolerance. J Exp Bot 59:4183–4194
Xu Y, Burgess P, Huang B (2015) Root antioxidant mechanisms in relation to root thermotolerance in perennial grass species contrasting in heat tolerance. PLoS One 10:e0138268
Xu Y, Burgess P, Zhang X, Huang B (2016) Enhancing cytokinin synthesis by overexpressing ipt alleviated drought inhibition of root growth through activating ROS-scavenging systems in Agrostis stolonifera. J Exp Bot 67:1979–1992
Yuan L, Liu S, Zhu S, Chen G, Liu F, Zou M, Wang C (2016) Comparative response of two wucai (Brassica campestris L.) genotypes to heat stress on antioxidative system and cell ultrastructure in root. Acta Physiol Plant 38:1–8
Zhao S, Zeng W, Li Z, Peng Y (2020) Mannose regulates water balance, leaf senescence, and genes related to stress tolerance in white clover under osmotic stress. Biol Plant 64:406–416
Zhao Y, Liu Y, Ji X, Sun J, Lv S, Yang H, Zhao X, Hu X (2021) Physiological and proteomic analyses reveal cAMP-regulated key factors in maize root tolerance to heat stress. Food Energy Secur 10:e309
Zhuo C, Wang T, Lu S, Zhao Y, Li X, Guo Z (2013) A cold responsive galactinol synthase gene from Medicago falcata (MfGolS1) is induced by myo-inositol and confers multiple tolerances to abiotic stresses. Physiol Plant 149:67–78
Acknowledgements
This research was supported by the National Natural Science Foundation of China (32171684).
Author information
Authors and Affiliations
Contributions
ZL conceived and designed the experiments; MZ and WZ performed experiments; ZL, WZ, and MZ analyzed the data; ZL wrote the paper; YZ, LL, WL, and YP improved the paper. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Responsible Editor: Manuel T. Oliveira.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Z., Zhou, M., Zeng, W. et al. Root metabolites remodeling regulated by γ-aminobutyric acid (GABA) improves adaptability to high temperature in creeping bentgrass. Plant Soil 500, 181–195 (2024). https://doi.org/10.1007/s11104-023-05905-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-05905-y