Abstract
Background
Acidic soils with a pHwater below 5.5 occupy up to 40% of world’s arable land. Aluminum (Al) toxicity and magnesium (Mg) deficiency often coexist in acidic soils, limiting crop production. In this study, we investigated a role of Mg in alleviation of Al stress in maize (Zea mays) by characterizing the growth responses, Al accumulation and the antioxidant properties of plants cultured hydroponically.
Methods
Physiological and molecular analyses were used to investigate the mechanisms of Mg governing the alleviation of Al-induced ROS production and root growth inhibition.
Results
Aluminum (50 μM) decreased root growth and induced oxidative stress. Exogenously added millimolar concentrations of Mg significantly alleviated Al toxicity as evidenced by restoration of plant growth, suppression of Al uptake, and a decline in root H2O2 concentration. Furthermore, the addition of Mg to the Al treatment solution enhanced the activities and expression of genes encoding superoxide dismutase, catalase and peroxidase compared to the Al-only treatment.
Conclusions
The results indicate that Mg plays a role in alleviation of Al toxicity, reduction of Al accumulation and protection from Al-induced oxidative stress through activation of antioxidative enzymes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acid soils that limit crop production are found worldwide, occupying up to 40% of world’s arable land, particularly in the tropics and subtropics (Kochian et al. 2004). To produce a better crop yield on acid soils, farmers are recommended to apply alkaline materials such as CaCO3 to increase soil pH (Zheng 2010). Although liming of acid soils can ameliorate soil acidity, this is neither an economic option for poor farmers nor an effective strategy for alleviating subsoil acidity (Whitten et al. 2000). Aluminum (Al) toxicity is a major constraint to plant growth and production in acidic soils (Baligar et al. 1993; Rengel and Zhang 2003). Overproduction of ROS (reactive oxygen species) in plants is an early event in Al toxicity; ROS formation can induce oxidative stress, leading to cell membrane peroxidation, structural damage in cells, chromosomal aberration and programmed cell death, and poor growth and development of plant roots (Giannakoula et al. 2008). Compared with Al-sensitive plant cultivars, the Al-resistant cultivars have higher level of antioxidants, such as superoxide dismutase (SOD), peroxidase (POD), catalase (CAT) and glutathione S-transferase (GST), resulting in lower accumulation of ROS in their roots (Darkó et al. 2004; Mariano and Keltjens 2005; Silva et al. 2010; Sivaguru and Paliwal 1993). Furthermore, overexpression of genes encoding antioxidants, such as tobacco GST gene (parB) and Arabidopsis POD gene (AtPox), conferred transgenic Arabidopsis resistance to Al-induced oxidative stress (Ezaki et al. 2000, 2001).
Magnesium (Mg) is an essential nutrient for plant growth. In addition to being the central atom of the chlorophyll molecule, Mg is an activator of a large number of enzymes, such as RNA polymerases, protein kinases, phosphatases and carboxylases (Bose et al. 2011). Due to low cation exchange capacity, ease of leaching and the competition with Al for plant uptake, Mg deficiency often occurs in acidic soils (Guo et al. 2016; Rengel et al. 2015). In plants, the bacterial cobalt resistance A (CorA) protein homologues are the best studied transporters associated with Mg uptake (Knoop et al. 2005; Rengel et al. 2015). There are 10 members in the Mg transporter family in Arabidopsis (AtMRS2/AtMGT1; Schock et al. 2000; Li et al. 2001) and 9 members in rice (OsMRS2/OsMGT; Saito et al. 2013). In Arabidopsis, AtMGT1 is a high-affinity Mg2+ transporter, but its activity is inhibited by Al3+ (Li et al. 2001). However, overexpression of the AtMGT1 gene in Nicotiana benthamiana conferred Al tolerance (Deng et al. 2006). Additionally, the mutant lines lacking OsMGT1 with lower Mg content showed higher Al sensitivity than the wild type plants (Chen et al. 2012b), indicating that OsMGT1-mediated Mg uptake is required for rice Al resistance.
It has been shown that external application of Mg can alleviate plant toxicity of Al and heavy metals (Rengel et al. 2015). Application of dolomite (Mg-Ca carbonate) ameliorates soil acidity and thus alleviates Al toxicity in many soil-plant systems (eg. Kasongo et al. 2012; Cristancho et al. 2014). In dicotyledons such as faba bean (Chen et al. 2015), rice bean (Yang et al. 2007) and soybean (Silva et al. 2001), micromolar concentrations of Mg alleviate Al toxicity via enhancing exudation of citrate from plant roots. On the other hand, millimolar concentrations of Mg were found to alleviate Al toxicity in monocot plants (eg. wheat and rice), which might be related to Mg competing with Al for binding sites on the plasma membrane (Chen et al. 2012b; Ryan et al. 1997; Watanabe and Okada 2005). Additionally, Pandey et al. (2013) found that the application of 0.25 mM MgSO4 alleviated 0.5 mM AlCl3-induced oxidative stress in rice, but no information was provided on the Mg-Al interactions in influencing expression of genes coding for antioxidative enzymes.
Maize (Zea mays), rice (Oryza sativa) and wheat (Triticum aestivum) are the three most important cereals that provide more than half of all calories consumed by humans worldwide (Awika 2011; Ma et al. 2014). In this study, we focus on the role of Mg in alleviation of oxidative stress under Al exposure in maize plants. We characterized cellular stress responses, including root elongation, Al uptake, gene expression, and activity of antioxidant enzymes. The results suggested that addition of micromolar concentrations of Mg could efficiently alleviate plant growth inhibition, reduce Al accumulation, alleviate Al-induced oxidative stress, and activate the antioxidant enzyme system under Al exposure.
Materials and methods
Plant culture and treatments
Seeds of maize (Zea mays L. cv. YunRui-8) were obtained from the Food Crop Research Institute, Yunnan Academy of Agricultural Sciences (Kunming, Yunnan province, China). Undamaged seeds were selected and placed in a beaker, rinsed 5 times with deionized water, and soaked in deionized water in the dark at 25 °C in a cultivation cabinet for 12 h. Phosphorus (P) could interact with Al to form Al(PO4)3; hence, the MGRL medium [3.0 mM KNO3, 200 μM Ca(NO3)2, 1.5 mM MgSO4, 67 μM Na2EDTA, 8.6 μM FeSO4, 10.3 μM MnSO4, 30 μM H3BO3, 1.0 μM ZnSO4, 24 nM (NH4)6MO7O24, 130 nM CoCl2, and 1.0 μM CuSO4] without P has been used widely for Al toxicity research (eg. Iuchi et al. 2007; Li et al. 2019; Yang et al. 2014). We therefore used the similar nutrient solution in the present study. For germination, the seeds were sown in polypropylene containers filled with 1/5 MGRL without Mg (pH 5.8). The 3-day-old uniform seedlings with the main seminal root about 5 cm long were transferred to the treatment solutions (1/5 MGRL-Mg) containing 0, 0.01, 0.05, 0.15, 0.3, 2.0, or 10.0 mM MgSO4 with or without 50 μM AlCl3 (pH 4.2) for a short-term (24 h) or a long-term (7 day) treatment. The free activity of Al3+ was 22.98, 22.5, 20.79, 17.53, 14.34, 5.21 or 1.55 μM in 1/5 MGRL medium containing 0, 0.01, 0.05, 0.15, 0.3, 2.0 or 10 mM MgSO4, respectively, as calculated by GEOCHEM-EZ software (www.plantmineralnutrition.net/Geochem/geochem%20home.htm).
Plant growth measurements
After treatments, the plants were harvested for root length and biomass measurements. The root length was determined by measuring the length of the longest root of 20 plants randomly selected from each pot. For biomass measurement, shoots and roots were separated, rinsed with deionized water, dried to a constant value at 65 °C (48 h), and weighed.
Hematoxylin and morin staining of roots
Morin and hematoxylin are two Al-sensitive histochemical indicators widely used to indicate the presence of Al. After treatments, roots were rinsed for 10 min in each of three lots of distilled water. Then, the root tips (2–3 cm) were excised and transferred into new tubes containing 0.2% (w/v) hematoxylin and 0.02% (w/v) KIO3 for 1 h, or 100 μM morin hydrate (Sigma-Aldrich) for 15 min under dark. After rinsing for 10 min in distilled water 3–5 times to remove residual dye from the root surface, the root tips were observed and photographed under a stereomicroscope (Olympus, SZ61 equipped with a Nikon D600 digital SLR camera) for hematoxylin staining, or a fluorescence microscope (Olympus, BX60 equipped with a DP70 CCD camera) for morin staining (excitation at 440 nm and emission at 510 nm).
Measurement of magnesium and aluminum concentration
Quantitative assessments of Al and Mg concentration in tissues were done using inductively coupled plasma-atomic emission spectroscopy (ICP-AES, model PS-1000, Lowell, MA, USA) as we described recently (Li et al. 2019).
H2O2 detection
H2O2 was measured by the titanium sulfate method and a Micro Hydrogen Peroxide (H2O2) Assay Kit (BC3595, Solarbio Life Sciences, China).
Measurements of SOD, POD and CAT activities
The frozen roots (0.5 g) were homogenized in 1 mL of 50 mM potassium phosphate buffer (pH 7.8) containing 0.2 mM EDTA-Na2, 0.1 mM ascorbic acid and 1% w/v polyvinylpolypyrrolidone (PVPP) in an ice bath using a mortar and pestle. The homogenate was centrifuged for 20 min at 12,000 g at 4 °C, and the supernatant was used for enzyme analysis. SOD activity was measured according to the published method (Giannopolitis and Ries 1977). POD activity was measured according to the method of Maehly and Chance (Maehly and Chance 1954), and CAT activity was assayed by monitoring the consumption of H2O2 at 240 nm for 2 min (Aebi 1984). The protein content was measured by the Bradford method (Bradford 1976).
Real time RT-PCR analysis
Total RNA was isolated from seedlings using Trizol reagent (Invitrogen, USA). Reverse transcription was performed using a PrimeScript RT reagent kit with gDNA eraser (Takara, Dalian, China) according to the manufacturer’s instructions. The expression levels of genes were analyzed using SYBR Premix Ex Taq II (TaKaRa, Da Lian, China). PCR was performed using a Bio-Rad CFX-96 real-time PCR system. Actin was used as an internal control. All primers used for RT-PCR (listed in Table 1) were based on the literature (Li et al. 2016; Maschietto et al. 2016; Shi et al. 2018).
Statistical analysis
Experiments contained at least three replicates, and the data are expressed as means and S.D. SPSS 12.0 for Windows (SPSS, Chicago, IL, USA) software package was used to conduct the least significant difference (LSD) test to determine statistical significance at p < 0.05.
Results
Magnesium increases root growth and plant biomass under Al stress
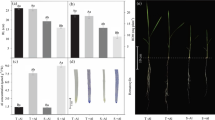
The most significant symptom of Al toxicity is the inhibition of root growth. Therefore, we firstly analyzed the effect of Mg on Al-induced root growth inhibition in maize in a short (24 h) and long-term (7 d) experiment. The results showed that the growth of maize primary roots was inhibited approximately 66% and 89% by the treatment with 50 μM Al for 24 h and 7 d, respectively, in comparison with the plants grown under control conditions (1/5 MGRL with 0.3 mM MgSO4, pH 4.2; Fig. 1a, b). However, exogenous application of Mg recovered root growth of the Al-treated seedlings, and the most optimal alleviation effect was observed at 0.3 and 2.0 mM Mg (Fig. 1a, b).
Root elongation of maize seedlings exposed to 0 or 50 μM Al with 0 to 10.0 mM Mg for 24 h (a) or 7 d (b). The 3-day-old uniform seedlings were transferred to the treatment solution (1/5 MGRL without Mg) containing 0 (control), 0.01, 0.05, 0.15, 0.3, 2.0 or 10.0 mM MgSO4 with 50 μM AlCl3 (pH 4.2) for the short-term (24 h) or the long-term (7 day) treatment. Date are presented as means ± standard deviation (S.D, n = 6). Different letters indicate significantly different values (p < 0.05) using LSD test
We further analyzed the biomass of maize plants under various treatments. The results showed that Al stress did not significantly affect shoot dry weight after 7-day treatments (Fig. 2a). However, the 50 μM Al treatment significantly decreased root dry weight and the root/shoot ratio, but these parameters were restored by the application of 2.0 and 10.0 mM Mg in the Al treatment solution (Fig. 2b, c).
Effect of external application of Mg on maize shoot dry weight (a), root dry weight (b) and shoot/root ratio (c) under Al stress. Three-day-old seedlings were treated by 0 or 50 μM Al with 0 to 10.0 mM Mg for 7 d (pH 4.2). The treatment solution was renewed every second day. The experiment was repeated three times, each treatment containing 12 independent biological samples. Values are means ± S.D. (n = 3). Different letters indicate significantly different values (p < 0.05) using LSD test
Effects of Mg on Al accumulation in maize root
As shown in Fig. 3a, the fluorescence intensity of morin was barely detectable in root tips not exposed to Al, whereas the root apex exhibited severe damage and strong Al accumulation after the plants were exposed to 50 μM Al for 24 h. Staining with hematoxylin also revealed substantial accumulation of Al in roots treated with 50 μM Al (Fig. 3b). The application of 2.0 mM Mg in the Al treatment solution lessened the staining of morin (Fig. 3a) and hematoxylin (Fig. 3b) and significantly decreased Al concentration in roots (Fig. 3c), but Mg concentration in roots (Fig. 3d) was significantly increased under Al stress.
Effect of Mg on Al accumulation in maize roots. Morin (a) and hematoxylin (b) staining were used for detecting Al accumulation in maize root tips. c and d, quantitative analysis of Al and Mg in maize rots. The 3-day-old seedlings were treated by 0 or 50 μM Al with or without 2 mM Mg for 24 h. The plants grown in the nutrient solution with the standard Mg concentration (0.3 mM) and without Al were used as the control (Con). White (A) and black (B) scale bars represent 500 μm. For C and D, values are means ± S.D. (n = 3). Different letters indicate significantly different values (p < 0.05) using LSD test
Magnesium restored Al-induced inhibition of the expression of Mg transporter genes
Given the MGT1 transporter family-mediated Mg uptake is involved in alleviation of Al toxicity in Arabidopsis and rice (Deng et al. 2006; Chen et al. 2012b), several AtMGT1 and OsMGT1 homologous genes, namely ZmMGT1, ZmMGT2, ZmMGT8, and ZmMGT9 were identified in maize genome (Fig. 4a) and the expression of these genes was analyzed by RT-PCR (Fig. 4b). The results showed that the expression of all these four genes was reduced by the application of Al; in contrast, Mg supply promoted the expression of these genes to even a higher level than that of the control treatment (0.3 mM Mg without Al) (Fig. 4b).
Expression levels of genes encoding putative Mg transporters in maize roots. a Evolutionary relationships of the amino acid sequence of AtMGT1 and OsMGT1 and its homologues in maize. The evolutionary history was inferred using the Neighbor-Joining method of MEGA7 program. b Relative expression of ZmMGT1, ZmMGT2, ZmMGT8, and ZmMGT9 in maize roots. The 3-day-old seedlings were treated by 50 μM Al with or without 2 mM Mg for 24 h. The plants grown in the nutrient solution with the standard Mg concentration (0.3 mM) and without Al were used as the control (Con). Values are means ± S.D. (n = 3). Different letters indicate significantly different values (p < 0.05) using LSD test
Magnesium decreased Al-induced H2O2 production
H2O2, a major ROS in plants, is an important indicator of oxidative stress. As shown in Fig. 5, the H2O2 concentration was significantly increased by Al, whereas the application of 0.05, 0.3, 2.0, and 10.0 mM Mg significantly diminished H2O2 production by, respectively, 18%, 60%, 55%, and 21% compared with the Al-only treatment.
Magnesium up-regulated activity of antioxidant enzymes under Al stress
The activities of antioxidant enzymes SOD, POD and CAT were determined (Fig. 6). As the result of Al stress, the activities of the SOD and CAT increased by 15% and 59%, respectively, whereas the activity of POD was decreased by 47% (Fig. 6a). The addition of Mg in the Al treatment solution not only further stimulated the activities of SOD and CAT, but also restored POD activity to the control level, compared with the Al-only treatment. Additionally, the expression of several genes, such as cytosolic SOD, MnSOD, POD1, POD3, CAT1, and CAT2 was also enhanced by Mg application (Fig. 6b), which fitted well with the changes in antioxidant enzyme activities (Fig. 6a). These results suggested that Mg efficiently increased the gene expression and activity of the antioxidant enzymes under Al stress.
Effect of external application of Mg on the activity (a) and gene expression (b) of superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT) in maize roots under Al stress. Three-day-old maize seedlings were treated by 0 or 50 μM Al supplemented with 0, 0.05, 0.3, 2.0, or 10.0 mM Mg for 24 h. Values are means ± S.D. (n = 3–6). Different letters indicate significantly different values (p < 0.05) using LSD test
Discussion
Aluminum toxicity is a major factor limiting plant growth and crop yield in acidic soils. It has been shown to induce oxidative stress and root cell death, thereby leading to significant reductions in water and nutrient uptake (Chen et al. 2011). In the present study, we elucidated amelioration of Al toxicity in maize roots by Mg. The results showed that Al-induced plant growth inhibition (Figs. 1 and 2), Al accumulation (Fig. 3) and oxidative stress (Fig. 5) were significantly ameliorated by exogenous application of Mg. Moreover, Mg significantly increased activity and gene expression of antioxidant enzymes (Fig. 6) as well the expression of Mg transporters (Fig. 4) under Al stress.
Uptake and translocation of Mg is mainly mediated by the homologues of bacterial Mg transporters in plants (bacterial cobalt resistance A, CoA; Knoop et al. 2005; Chen and Ma 2013). Ten and 9 members of the MGT transporter family are found in Arabidopsis and rice, respectively. Among them, only AtMGT6 (Mao et al. 2014) in Arabidopsis roots and OsMGT1 in rice roots (Zhang et al. 2019) are induced by Mg deficiency, indicating their role in Mg uptake in response to low Mg status. Although Al toxicity and Mg deficiency did not affect the expression of AtMGT1 in Arabidopsis, the AtMGT1-mediated Mg uptake was highly sensitive to Al toxicity (Li et al. 2001). In contrast to Arabidopsis, OsMGT1 was upregulated by Al toxicity (Chen et al. 2012b) and Mg deficiency (Zhang et al. 2019). Additionally, the upregulation of OsMGT1 increased Mg uptake in rice plants under Al stress (Chen et al. 2012b). In comparison with the wild type plants, the rice mutant lines lacking OsMGT1 and having low Mg concentration in tissues (Chen et al. 2012b) showed increased Al sensitivity, whereas transgenic tobacco plants overexpressing Arabidopsis AtMGT1 and having high tissue Mg concentration (Deng et al. 2006) exhibited increased Al resistance. These results indicated that MGT1-mediated Mg uptake is essential for plant Al resistance.
There are 12 Mg transporter genes in the maize genome. Among them, ZmMGT1 and ZmMGT2 showed, respectively, 68% and 67% identity to AtMGT1, and ZmMGT8 and ZmMGT9 had, respectively, 55% and 55% identity to OsMGT1 (Fig. 4a). We found that the expression of AtMGT1 and OsMGT1 homologues, including ZmMGT1, ZmMGT2, ZmMGT8, and ZmMGT9, was decreased by Al toxicity after the 24-h treatment (Fig. 4b). The difference in the expression of MGT genes in Arabidopsis (Li et al. 2001), rice (Chen et al. 2012b) and maize (Fig. 4b) might be due to different plant species responding differently to Al stress. Additionally, the results presented here indicated that inhibition of Mg uptake by Al might be attributed, at least partly, to decreased expression of the Mg transporter genes in maize. However, the application of Mg significantly alleviated Al-mediated inhibition of the expression of these genes (Fig. 4b). The functions of these Mg transporter genes in Mg uptake and Al toxicity warrant further research.
Magnesium ion (Mg2+) has the smallest ionic radius (0.072 nm) and the largest hydrated radius among the major biological cations (Bose et al. 2011; Rengel et al. 2015). The hydrated radii of Al3+ (0.480 nm) and Mg2+ (0.476 nm) are similar. Therefore, Al3+ and Mg2+ could compete for the membrane transporters (Rengel et al. 2015). For example, Mg blocks non-selective slow vacuole (SV) channels and non-selective cation channels (NSCC) that are associated with Al transport to the vacuole and passive uptake by roots, respectively (Bose et al. 2013; Pérez et al. 2008; Wherrett et al. 2005). Relatively low (micromolar) concentrations of Mg significantly alleviated Al-induced root growth inhibition in legumes, including soybean (Silva et al. 2001), rice bean (Yang et al. 2007) and faba bean (Chen et al. 2015) through enhancement of Al-induced citrate exudation. However, relatively high concentrations (millimolar) of Mg were needed to alleviate Al toxicity in Poaceae species, eg. applicaiton of 3 mM and 0.45 mM Mg alleviated Al toxicity in wheat (Ryan et al. 1997) and maize (Watanabe and Okada 2005), respectively. Similarly, we found exogenous application of Mg efficiently ameliorated Al-induced root elongation and plant growth in this study (Figs. 1 and 2). In addition, 2 mM Mg significantly reduced Al accumulation in maize roots, further confirming millimolar concentrations of Mg could effectively alleviate Al toxicity in Poaceae species. The alleviation of Al toxicity and reduction of Al accumulation in maize by millimolar concentrations of Mg might be due to 1) a decrease in extracellular free Al3+ activity and 2) blocking of Al uptake by competition for transporters. The differences in concentrations of Mg (eg. millimolar vs micromolar) needed for alleviation of Al toxicity in monots vs dicots might be due to differential cell wall chemistry, Mg uptake efficiency and cellular Mg concentration (White et al. 2018).
The balance between generation and scavenging of ROS (such as superoxide anion and H2O2) was disturbed under a range of environmental stresses, including Al toxicity (Mittler 2002; Richards et al. 1998). Overproduction of ROS contributes to Al toxicity through causing cell membrane peroxidation, structural damage in cells, chromosomal aberration, and programmed cell death (Yi et al. 2010; Yoko et al. 2001). To alleviate such oxidative stress, plants have evolved mechanisms to scavenge ROS via the up-regulation of genes encoding antioxidant enzymes and thereby enhance the activities of antioxidant enzymes, such as SOD, POD and CAT (Cakmak and Kirkby 2008; Chen et al. 2012a). For example, Al exposure induced an efficient antioxidant system in Al-tolerant maize, but not in the Al-sensitive genotype (Giannakoula et al. 2010). Overexpression of a peroxidase gene from Arabidopsis elevated Al tolerance in transgenic tobacco plants (Wu et al. 2017). In addition, enhanced activities of the antioxidant enzymes due to external application of salicylic acid, nitric oxide, melatonin, and Mg were involved in alleviation of Al toxicity in Cassia tora (Wang and Yang 2005; Wang et al. 2004), soybean (Zhang et al. 2017) and rice (Pandey et al. 2013). In the present study, we also found that the application of Mg caused a significant decrease in H2O2 accumulation (Fig. 5) that had been induced by Al toxicity. Furthermore, higher activities and gene expression levels of SOD, POD and CAT were observed after the application of Mg in the Al-treatment solutions (Fig. 6).
The data presented here showed that application of millimolar concentrations of Mg effectively alleviated Al-induced root elongation inhibition, Al accumulation and oxidative stress in maize plants. Additionally, increased expression of Mg transporter genes as well as enhancement of the gene expression and activity of antioxidant enzymes play a role in Mg alleviation of Al-induced oxidative stress in maize plants.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Awika J M (2011) Major cereal grains production and use around the world. pp. 1-13 in Cereal Science: Implications to Food Processing and Health Promotion, edited by Awika, J. M., V. Piironen, and S. Bean. American Chemical Society, Washington, DC.
Baligar VC, Schaffert RE, Dossantos HL, Pitta GVE, Filho AFDB (1993) Growth and nutrient-uptake parameters in sorghum as influenced by aluminum. Agron J 85:1068–1074. https://doi.org/10.2134/agronj1993.00021962008500050021x
Bose J, Babourina O, Rengel Z (2011) Role of magnesium in alleviation of aluminium toxicity in plants. J Exp Bot 62:2251–2264
Bose J, Babourina O, Shabala S, Rengel Z (2013) Low pH and aluminum resistance in Arabidopsis correlates with high cytosolic magnesium content and increased magnesium uptake by plant roots. Plant Cell Physiol 54:1093–1104
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cakmak I, Kirkby EA (2008) Role of magnesium in carbon partitioning and alleviating photooxidative damage. Physiol Plant 133:692–704
Chen ZC, Ma JF (2013) Magnesium transporters and their role in Al tolerance in plants. Plant Soil 368(1–2):51–56
Chen Q, Zhang X, Wang S, Wang Q, Wang G, Nian H, Li K, Yu Y, Chen L (2011) Transcriptional and physiological changes of alfalfa in response to aluminium stress. J Agric Sci 149:737–751
Chen Q, Wu KH, Zhang YN, Phan XH, Li KZ, Yu YX, Chen LM (2012a) Physiological and molecular responses of broad bean (Vicia faba L.) to aluminum stress. Acta Physiol Plant 34:2251–2263
Chen ZC, Yamaji N, Motoyama R, Nagamura Y, Ma JF (2012b) Up-regulation of a magnesium transporter gene OsMGT1 is required for conferring aluminum tolerance in rice. Plant Physiol 159:1624–1633
Chen Q, Kan Q, Wang P, Yu W, Yu Y, Zhao Y, Yu Y, Li K, Chen L (2015) Phosphorylation and interaction with the 14-3-3 protein of the plasma membrane H+-ATPase are involved in the regulation of magnesium-mediated increases in aluminum-induced citrate exudation in broad bean (Vicia faba. L), Plant Cell Physiol 56(6):1144–1153
Cristancho RJA, Hanafi MM, Syed OSR, Rafii MY (2014) Aluminium speciation of amended acid tropical soil and its effects on plant root growth. J Plant Nutr 37(6):811–827. https://doi.org/10.1080/01904167.2014.881856
Darkó É, Ambrusa H, Stefanovits-Bányaib É, Fodorc J, Bakosa F, Barnabása B (2004) Aluminium toxicity, Al tolerance and oxidative stress in an Al-sensitive wheat genotype and in Al-tolerant lines developed by in vitro microspore selection. Plant Sci 166:583–591
Deng W, Luo K, Li D, Zheng X, Wei X, Smith W, Thammina C, Lu L, Li Y, Pei Y (2006) Overexpression of an Arabidopsis magnesium transport gene, AtMGT1, in Nicotiana benthamiana confers Al tolerance. J Exp Bot 57:4235–4243
Ezaki B, Gardner RC, Ezaki Y, Matsumoto H (2000) Expression of aluminum-induced genes in transgenic arabidopsis plants can ameliorate aluminum stress and/or oxidative stress. Plant Physiol 122:657–665
Ezaki B, Katsuhara M, Kawamura M, Matsumoto H (2001) Different mechanisms of four aluminum (Al)-resistant transgenes for Al toxicity in Arabidopsis. Plant Physiol 127:918–927
Guo W, Nazim H, Liang Z, Yang D (2016) Magnesium deficiency in plants: An urgent problem. Crop Journal 4(2): 83-91Guo W, Nazim H, Liang Z, Yang D (2016). Magnesium deficiency in plants: An urgent problem. Crop Journal 4(2):83–91
Giannakoula A, Moustakas M, Mylona P, Papadakis I, Yupsanis T (2008) Aluminum tolerance in maize is correlated with increased levels of mineral nutrients, carbohydrates and proline, and decreased levels of lipid peroxidation and Al accumulation. J Plant Physiol 165:385–396
Giannakoula A, Moustakas M, Syros T, Yupsanis T (2010) Aluminum stress induces up-regulation of an efficient antioxidant system in the Al-tolerant maize line but not in the Al-sensitive line. Environ Exp Bot 67(3):487–494
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol 59:309–314
Iuchi S, Koyama H, Iuchi A, Kobayashi Y, Kitabayashi S, Kobayashi Y, Ikka T, Hirayama T, Shinozaki K, Kobayashi M (2007) Zinc finger protein STOP1 is critical for proton tolerance in Arabidopsis and coregulates a key gene in aluminum tolerance. Proc Natl Acad Sci USA 104(23):9900–9905
Kasongo RK, Ranst EV, Kanyankogote P, Verdoodt A, Baert G (2012) Response of soybean (Glycine max ) to Kanzi rock phosphate and Kimpese pink dolomite application on a sandy soil in DR Congo. Can J Soil Sci 92:905–916
Knoop V, Groth-Malonek M, Gebert M, Eifler K, Weyand K (2005) Transport of magnesium and other divalent cations: evolution of the 2-TM-GxN proteins in the MIT superfamily. Mol Gen Genomics 274(3):205–216
Kochian LV, Hoekenga OA, Pineros MA (2004) How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu Rev Plant Biol 55:459–493
Li L, Tutone AF, Drummond RS, Gardner RC, Luan S (2001) A novel family of magnesium transport genes in Arabidopsis. Plant Cell 13(12):2761–2775
Li H, Du H, Huang K et al (2016) Identification, and functional and expression analyses of the CorA/MRS2/MGT-type magnesium transporter family in maize. Plant Cell Physiol 57(6):1153–1168
Li D, Ma W, Wei J, Mao Y, Peng Z, Zhang J, Kong X, Han Q, Fan W, Yang Y, Chen J, Wu L, Rengel Z, Cui X, Chen Q (2019) Magnesium promotes root growth and increases aluminum tolerance via modulation of nitric oxide production in Arabidopsis. Plant Soil. https://doi.org/10.1007/s11104-019-04274-9
Ma JF, Chen ZC, Shen RF (2014) Molecular mechanisms of Al tolerance in gramineous plants. Plant Soil 381(1–2):1–12
Maehly AC, Chance B (1954) The assay of catalases and peroxidases. Methods Biochem Anal 1:357–424
Mao DD, Chen J, Tian LF, Liu Z, Yang L, Tang R, Li J, Lu CQ, Yang YH, Shi JS, Chen LB, Li DP, Luan S (2014) Arabidopsis transporter MGT6 mediates magnesium uptake and is required for growth under magnesium limitation. Plant Cell 26:2234–2248
Mariano ED, Keltjens WG (2005) Long-term effects of aluminum exposure on nutrient uptake by maize genotypes differing in aluminum resistance. J Plant Nutr 28:323–333
Maschietto V, Lanubile A, Leonardis SD (2016) Constitutive expression of pathogenesis-related proteins and antioxydant enzyme activities triggers maize resistance towards Fusarium verticillioides. J Plant Physiol 200:53–61
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Pandey P, Srivastava RK, Dubey RS (2013) Salicylic acid alleviates aluminum toxicity in rice seedlings better than magnesium and calcium by reducing aluminum uptake, suppressing oxidative damage and increasing antioxidative defense. Ecotoxicology 22(4):656–670
Pérez V, Wherrett T, Shabala S, Muniz J, Dobrovinskaya O, Pottosin I (2008) Homeostatic control of slow vacuolar channels by luminal cations and evaluation of the channel-mediated tonoplast Ca2+ fluxes in situ. J Exp Bot 59(14):3845–3855
Rengel Z, Zhang WH (2003) Role of dynamics of intracellular calcium in aluminium toxicity syndrome. New Phytol 159:295–314
Rengel Z, Bose J, Chen Q, Tripathi BN (2015) Magnesium alleviates plant toxicity of aluminium and heavy metals. Crop Pasture Sci 66:1298–1307
Richards KD, Schott EJ, Sharma YK, Davis KR, Gardner RC (1998) Aluminum induces oxidative stress genes in Arabidopsis thaliana. Plant Physiol 116:409–418
Ryan PR, Reid RJ, Smith FA (1997) Direct evaluation of the Ca2+-displacement hypothesis for Al toxicity. Plant Physiol 113:1351–1357
Saito T, Kobayashi NI, Tanoi K, Iwata N, Suzuki H, Iwata R, Nakanishi TM (2013) Expression and functional analysis of the CorA-MRS2-ALR-type magnesium transporter family in rice. Plant Cell Physiol 54(10):1673–1683
Schock I, Gregan J, Steinhauser S, Schweyen R, Brennicke A, Knoop V (2000) A member of a novel Arabidopsis thaliana gene family of candidate Mg2+ ion transporters complements a yeast mitochondrial group II intron-splicing mutant. Plant J 24(4):489–501
Shi F, Zhang Y, Wang K, Meng Q, Liu X, Ma L, Li Y, Liu J, Ma L (2018) Expression profile analysis of maize in response to Setosphaeria turcica. Gene 659:100–108
Silva IR, Smyth TJ, Israel DW, Raper CD, Rufty TW (2001) Magnesium ameliorates aluminum rhizotoxicity in soybean by increasing citric acid production and exudation by roots. Plant Cell Physiol 42:546–554
Silva S, Pinto-Carnide O, Martins-Lopes P, Matos M, Guedes-Pinto H, Santos C (2010) Differential aluminium changes on nutrient accumulation and root differentiation in an Al sensitive vs. tolerant wheat. Environ Exp Bot 68:91–98
Sivaguru M, Paliwal K (1993) Differential aluminum tolerance in some tropical rice cultivars. 2. Mechanism of aluminum tolerance. J Plant Nutr 16:1717–1732
Wang YS, Yang ZM (2005) Nitric oxide reduces aluminum toxicity by preventing oxidative stress in the roots of Cassia tora L. Plant Cell Physiol 46:1915–1923
Wang YS, Wang J, Yang ZM, Wang QY, Lu B, Li SQ, Lu YP, Wang SH, Sun X (2004) Salicylic acid modulates aluminum-induced oxidative stress in roots of Cassia tora. Acat Bot Sin 46:819–828. http://www.chineseplantscience.com
Watanabe T, Okada K (2005) Interactive effects of Al, Ca and other cations on root elongation of rice cultivars under low pH. Ann Bot 95:379–385
Wherrett T, Shabala S, Pottosin I (2005) Different properties of SV channels in root vacuoles from near isogenic Al-tolerant and Al-sensitive wheat cultivars. FEBS Lett 579(30):6890–6894
White PJ, Broadley MR, El-Serehy HA, George TS, NeugebauerK (2018) Linear relationships between shoot magnesium and calcium concentrations among angiosperm species are associated with cell wall chemistry. Ann Bot 122(2):221–226
Whitten MG, Wong MTF, Rate AW (2000) Amelioration of subsurface acidity in the south-west of Western Australia: downward movement and mass balance of surface-incorporated lime after 2-15 years. Aust J Soil Res 38:711–728
Wu Y, Yang Z, How J, Xu H, Chen L, Li K (2017) Overexpression of a peroxidase gene (AtPrx64) of Arabidopsis thaliana in tobacco improves plant’s tolerance to aluminum stress. Plant Mol Biol 95(1–2):157–168
Yang JL, You JF, Li YY, Wu P, Zheng SJ (2007) Magnesium enhances aluminum-induced citrate secretion in rice bean roots (Vigna umbellata) by restoring plasma membrane H+-ATPase activity. Plant Cell Physiol 48:66–73
Yang ZB, Geng X, He C, Zhang F, Wang R, Horst WJ, Ding Z (2014) TAA1-regulated local auxin biosynthesis in the root-apex transition zone mediates the aluminum-induced inhibition of root growth in Arabidopsis. Plant Cell 26(7):2889–2904
Yi HL, Yi M, Li HH, Wu LH (2010) Aluminum induces chromosome aberrations, micronuclei, and cell cycle dysfunction in root cells of Vicia faba. Environ Toxicol 25:124–129
Yoko Y, Yukiko K, Hideaki M (2001) Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol 125:199–208. https://doi.org/10.1104/pp.125.1.199
Zhang J, Zeng B, Mao Y, Kong X, Xu J, Rengel Z, Chen Q (2017) Melatonin alleviates aluminium toxicity through modulating antioxidative enzymes and enhancing organic acid anion exudation in soybean. Funct Plant Biol 44:961–968
Zhang L, Peng Y, Li J, Tian X, Chen Z (2019) OsMGT1 confers resistance to magnesium deficiency by enhancing the import of Mg in rice. Int J Mol Sci 20:207
Zheng SJ (2010) Crop production on acidic soils: overcoming aluminium toxicity and phosphorus deficiency. Ann Bot 106:183–184
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31960624, 31660595, 31501832 and 31360340), Science and Technology Project of Yunnan province (2017FB063), and Natural Science Foundation of Guangdong province (2018A030310192). Zed Rengel was supported by Australian Research Council (DP160104434).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Responsible Editor: Ismail Cakmak.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kong, X., Peng, Z., Li, D. et al. Magnesium decreases aluminum accumulation and plays a role in protecting maize from aluminum-induced oxidative stress. Plant Soil 457, 71–81 (2020). https://doi.org/10.1007/s11104-020-04605-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-020-04605-1