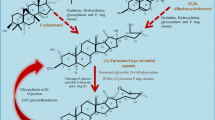
Main conclusion
This work reviews recent advances in the pathways and key enzymes of steroidal saponins biosynthesis and sets the foundation for the biotechnological production of these useful compounds through transformation of microorganisms.
Abstract
Steroidal saponins, due to their specific chemical structures and active effects, have long been important natural products and that are irreplaceable in hormone production and other pharmaceutical industries. This article comprehensively reviewed the previous and current research progress and summarized the biosynthesis pathways and key biosynthetic enzymes of steroidal saponins that have been discovered in plants and microoganisms. On the basis of the general biosynthetic pathway in plants, it was found that the starting components, intermediates and catalysing enzymes were diverse between plants and microorganisms; however, the functions of their related enzymes tended to be similar. The biosynthesis pathways of steroidal saponins in microorganisms and marine organisms have not been revealed as clearly as those in plants and need further investigation. The elucidation of biosynthetic pathways and key enzymes is essential for understanding the synthetic mechanisms of these compounds and provides researchers with important information to further develop and implement the massive production of steroidal saponins by biotechnological approaches and methodologies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Steroidal saponins are a class of oligosaccharides derived from spirostanes, which are formed by the condensation of steroidal sapogenin and sugars. They are mainly produced in Dioscoreaceae, Liliaceae, Smilaxaceae, Solanaceae, Dracaenaceae, Agaveaceae, etc. (Sidana et al. 2016; Sparg et al. 2004; Tian et al. 2017), and play a defensive role in plants (González-Lamothe et al. 2009; Osbourn 1996). A small amount of steroidal saponins are also found in certain marine animals and microorganisms (Iorrizzi et al. 2001; Tang et al. 2020; Tian et al. 2017).
The main use of steroidal saponins is as precursors of steroid drugs, which are second most in demanded in the global market after antibiotics (Fernández-Cabezón et al. 2018). Steroidal saponins have extensive pharmacological effects. Di’ao Xinxuekang capsule (steroidal saponins of Dioscorea panthaica or Dioscorea nipponica as the main components) has significant effects on cardiovascular and cerebrovascular diseases and was the first botanical drug approved for marketing in Europe (Dutch Medicines Evaluation Board 2012; Yu et al. 2014). Drugs with similar pharmacological effects include dioscornin tablets and Xinnaoshutong capsule, etc.. Steroidal saponins are also widely used to treat acute and chronic inflammation (Wang et al. 2015; Yang et al. 2017a, b; Yuan et al. 2016b; Zhu et al. 2017) and have therapeutic effects on a variety of high-incidence cancers and clinically difficult cancers (de Oliveira et al. 2019; Lu et al. 2018; Zhao et al. 2018; Zhu et al. 2018b), in addition to being used to solve problems stemming from multidrug resistance and poor prognosis that are difficult to overcome in cancer treatment (Song et al. 2019; Yuan et al. 2016). In addition, steroidal saponins can enhance the immune system, affect oxidative stress, and regulate lipid metabolism. Since saponins have foaming and emulsifying properties, they can be used as emulsifiers, foaming agents and preservatives in the food, cosmetic and pharmaceutical industries (Moses et al. 2014). Yucca extract is the main commercial source of steroidal saponins used as surfactants. It has been approved by the FDA as GRAS (generally recognized as safe) and has received widespread attention in various industries (Güçlü-Ustündağ and Mazza 2007; Tenon et al. 2017).
With the severe destruction of the natural environment, the natural materials from which steroidal saponins originate have been irreversibly decreased in number (Li et al. 2015; Shen et al. 2018). For example, Paris polyphylla, a famous natural medicine rich in steroidal saponins, has been listed as a vulnerable species by the IUCN (Chauhan 2020). Currently, the annual harvest of this natural medicine is becoming increasingly insufficient to meet the rising demand in the market (Cunningham et al. 2018). In addition, the extraction of steroidal saponins from plants also faces problems such as difficulty in separation and low abundance. For example, the highly anticipated OSW-1 possesses better anticancer effects than paclitaxel and camptothecin; however, it has the drawback of having high acquisition costs and limited supplies (Mimaki et al. 1997). To alleviate the dependence on plants for production of steroidal saponins and obtain the target products in a fast, effective and inexpensive way, it is necessary to develop in vitro technology to expand the sources of steroidal saponins.
In vitro plant culture is an important way for the production of biological active compounds (Espinosa-Leal et al. 2018). Currently, some biotechnological methods of plant cell and tissue culture, root culture and somatic embryogenesis to generate steroidal saponins have been investigated (Basu and Jha 2013; Chauhan et al. 2018; Nazir et al. 2021; Nikam et al. 2009; Puente-Garza et al. 2021; Rizvi et al. 2010). Using Agrobacterium tumefaciens-mediated transformation technology, a robust and reproducible method for transforming fenugreek hairy roots was established, and a promoter suitable for strong expression of transgenes was identified (Garagounis et al. 2020). Through the CRISPR/Cas system, a fast and effective new method for targeted gene editing of Dioscorea zingiberensis has been created (Feng et al. 2018; Zhu et al. 2009). However, these technologies are still at the laboratory level, and their transfer into industrial production will be difficult. In recent years, the heterogeneous expression of natural product biosynthetic pathways has become an important focus of bioactive compound production. Because the genetic manipulation of plant cells is very difficult, constructing engineered strains seems to be the most effective and economical way to produce natural medicines. A variety of engineered strains have been used to achieve in vitro production of natural and nonnatural active ingredients (Bai et al. 2016; Dai et al. 2014; Galanie et al. 2015; Nakagawa et al. 2016). For instance, the famous natural anti-malarial drug artemisinin, which is produced by semi-synthetic methods through engineered strains, has been put into industrial production as a precursor that meets the World Health Organization's requirements for drug quality and purity (Corsello and Garg 2015). However, to date, there have been no reports of strains engineering for the steroidal saponin.
An adequate understanding of natural product biosynthesis processes and deep mining of key enzymes are the basis for the utilization of various biotechnologies to produce natural medicines. At present, studies of the biosynthetic pathway of steroidal saponins mainly revolve around plants, and attention to the key enzymes required is focused on the upstream process. This article provides a comprehensive review of the biosynthetic pathways of steroidal saponins in plants and microorganisms and the key enzymes involved in the downstream biosynthesis process and summarizes the microorganisms involved in the transformation of steroidal saponins. The information supplied will benefit the development and application of biotechnology to promote the industrial production of steroidal saponins.
Biosynthetic pathway of steroidal saponins
With the advancement of research technology, such as high-throughput sequencing, gene expression analysis, and functional gene analysis of nonmodel plants, the biosynthetic pathways of steroidal saponins have been further improved and supplemented in recent years. Referencing and analysing the current research literature, provides a more comprehensive understanding of the biosynthesis process of steroidal saponins. The pathways related to the biosynthesis of steroidal saponins are shown in Fig. 1.
The biosynthesis process of steroidal saponins is divided into three stages: the production of sterols, the synthesis of sapogenins, and the connection of different sugars to synthesize steroidal saponins. The cytosolic mevalonate (MVA) pathway and the plastidial methylerythritol 4-phosphate (MEP) pathway initiate of steroidal saponin biosynthesis. These two pathways are very common in nature and are a common process for the biosynthesis of terpenes, steroids and other secondary metabolites, being carried out in the cytoplasm and plastids in plants (Liao et al. 2016; Newman and Chappell 1999). The two pathways can generate isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) from acetyl-CoA and pyruvate, respectively as starting points. Subsequently, geranyl diphosphate, farnesyl diphosphate, squalene and 2,3-oxidosqualene are synthesized. After further catalysis by various enzymes, different sterols are generated. Phytosterols are a major component of cell membranes and have important physiological functions in living organisms (Moreau et al. 2018). Although both pathways are involved in the biosynthesis of cholesterol and sitosterol, the precursors of steroidal saponins, the MVA pathway is thought to be the more dominant pathway (Sawai and Saito 2011).
Sitosterol is an important metabolite in plants, while cholesterol is the main sterol in animals. Although the cholesterol content in plants is very low, cholesterol is indispensable (Behrman and Gopalan 2005). The biosynthesis precursors of these two sterols are mainly lanosterol and cycloartenol. The production of cholesterol from lanosterol is the main process in animals and microorganisms; however, lanosterol synthase is only found in a few parts of dicotyledonous plants (Mehrafarin et al. 2010; Suzuki et al. 2006). Many studies have shown that in some plants, lanosterol can participate in the biosynthesis of plant sterols and steroid glycoalkaloids (Kumar et al. 2017; Ohyama et al. 2009). However, there is no direct evidence that lanosterol can be converted into steroidal saponins in plants. Conversion from cycloartenol to sitosterol and then to steroidal saponins is the most commonly accepted biosynthetic pathway for steroidal saponins. This process requires a series of methyltransferases, demethylases, reductases, dehydrogenases, and isomerases to produce sitosterol, followed by hydroxylase and glycosyltransferase to finally produce steroidal saponins (Singh et al. 2017; Upadhyay et al. 2014; Zhu et al. 2018a). Cycloartenol can also biosynthesize cholesterol and further form steroidal saponins, and this process is similar to that of phytosterols. One possibility is that the pathway lacks one-step methylation of cycloartenol. The other is the direct biosynthesis of cholesterol after cycloartenol produces isofucosterol along the phytosterol synthesis pathway (Ciura et al. 2017b; Sonawane et al. 2016). However, the second approach needs further verification (Yang et al. 2019). Comparing the changes in the contents of diosgenin, cycloartenol, cholesterol and sitosterol in Dioscorea nipponica before and after treatment with methyl jasmonate showed that both cholesterol and sitosterol in plants could participate in the biosynthesis of steroidal saponins, and the two approaches might exist simultaneously. The content of phytosterols in plants is much higher than that of cholesterol. Based on this, the phytosterol pathway may occupy a dominant position (Ciura et al. 2017a; Sun et al. 2017).
Microorganisms are a resource pool for the production of natural products. In particular, endophytes can produce the same metabolites as the host during their coexistence (Lata et al. 2018). In Paris polyphylla, more than 30 microbial strains, including fungi, bacteria and actinomycetes that can produce steroidal saponins and their analogue, have been isolated. Among them, the content of such components in the most capable strains was measured to reach 208.62 mg/L (Cao et al. 2005; Yang et al. 2018b; Zhang et al. 2007; Zhao et al. 2005; Zhou et al. 2004). Up to now, there is no direct research on the biosynthesis process of steroidal saponins in microorganisms. But the pathways of biosynthesizing a variety of sterols in microorganisms may be closely related to the production of steroidal saponins. For most fungi, ergosterol is the main sterol. A small number of fungi do not synthesize ergosterol, but can produce cholesterol, sitosterol, stigmasterol and other sterols (Fontaine et al. 2002; Grandmougin-Ferjani et al. 1999; Torres et al. 2017; Wang et al. 2012). The biosynthetic pathway of ergosterol has been clear. Compared with the biosynthetic process of sterols in plants, ergosterol is biosynthesized by lanosterol instead of cycloartenol through a series of reactions. The intermediate products of this process also include 4,4-dimethyl-cholesta-8,14,24-trienol, 4,4-dimethyl-zymosterol, zymosterol, feocosterol, episterol, ergosta5,7,24(28)-trienol, ergosta5,7,22,24(28)-tetraenol, etc. (Lees et al. 1995). Studies have shown that the way that microorganisms biosynthesize cholesterol is similar to that of animals, and the biosynthesis process before zymosterol is the same as that of ergosterol. The intermediates involved in the next biosynthesis process depend on the catalytic sequence of the enzyme, including cholest-8-enol, lathosterol, 7-dehydrocholesterol, cholesta-7,24-dienol, cholesta-5,7,24-trienol and desmosterol, etc. (Madoui et al. 2009; Weete et al. 2010). Studies on the biosynthetic pathway of phytosterols in microorganisms have shown that brassinosteroids can be biosynthesized through ergosta5,7,24(28)-trienol or ergosterol (Pereira et al. 2010; Visbal et al. 2003; Weete et al. 2010). However, the biosynthetic pathways of other phytosterols have not been reported so far. Microalgae are promising cell factories for the production of phytosterols and terpenes. Lanosterol, cycloartenol and cholesterol were found in some microalgae. The process of phytosterol synthesis in microalgae is similar to fungi and terrestrial plants, but there are some unique key genes involved (Fabris et al. 2014; Jaramillo-Madrid et al. 2019; Xie et al. 2017). Prokaryotes do not have cell membranes, and it is generally believed that they cannot produce sterols. However, there was evidence that a small number of bacteria could synthesize sterols. This phenomenon may be caused by the horizontal transfer of related genes from eukaryotes to prokaryotes during evolution (Bouvier et al. 1976; Desmond and Gribaldo 2009; Pearson et al. 2003). Studies have shown that the sterol biosynthesis pathways of some bacteria and the catalytic enzymes involved are very different from those of eukaryotes (Lee et al. 2018). Steroidal saponins also exist in marine organisms, but studies have mainly focused on the separation of compounds and the exploration of pharmacological effects. There is no relevant research on the in vivo steroidal saponin biosynthesis pathway, which deserves attention.
Although plants can produce steroidal saponins through different pathways, the enzymes involved in the synthesis are functionally consistent (Sonawane et al. 2016). Comparing of multiple high-throughput analysis results and systematic genomic analysis of eukaryotic steroid and sterol synthesis genes, it can be concluded that the enzymes that form sterols are homologous (Haubrich et al. 2014). During the continuous evolution of plants, some enzymes have continuously acquired new functions and the selection specificity has become stronger but still retains certain pre-evolution functions. The same enzyme may be able to choose multiple substrates to play a role. When the enzymes involved in cholesterol synthesis in plants are consistent with phytosterols, the phytosterol pathway may catalyse similar cholesterol substrates in a mixed manner. Plants and microorganisms synthesize sterols with different starting components. Although the intermediates and products produced are different, and the genes involved in regulating the necessary enzymes are diverse, the functions of these enzymes are very similar. Therefore, some enzymes with low selectivity can catalyse other similar substrates.
By exploring the mechanisms by which endophytes produce natural products, we believe that the endophytes can produce natural medicine either in the same way as in plants or in unique ways (Heinig et al. 2013; Venugopalan et al. 2016). Although some of the key enzymes of the biosynthesis process in endophytes are similar to the functions in plants, the amino acid sequence correlation between them is very low, proving that endophytes can independently evolve channels to produce natural products (Hedden et al. 2002). In addition, the amino acid sequences encoding key genes and transcription factors present in endophytes are highly homologous to those of the host. It has been proved that some genes can be transferred horizontally during the coexistence process. There is no report at the gene level of the mechanism of endophyte production and conversion of steroidal saponins, but it can be speculated that steroidal saponin-producing fungi also possess these mechanistic characteristics (Sah et al. 2017).
Key enzymes in the biosynthetic pathways of steroidal saponins in plants
The MVA and MEP pathways involved in the biosynthesis of steroidal saponins have been fully described and studied, and it has been found that the key genes involved can regulate the accumulation of sterols (Chaudhary et al. 2015; Suza and Chappell 2015). The key enzymes involved in the downstream biosynthetic pathways mainly include geranyl diphosphate synthase (GPPS), farnesyl diphosphate synthase (FPPS), squalene epoxidase (SQE), cycloartenol synthase (CAS), sterol 24-C-methyl transferase (SMT1), sterol 4α-methyl oxidase 1 (SMO1), cyclopropylsterol isomerase (CPI1), sterol C-14 demethylase (CYP51), sterol C-14 reductase (FK), sterol 8,7 isomerase (HYD1), sterol-C-24-methyl transferase 2 (SMT2), sterol 4α-methyl oxidase 2 (SMO2), sterol C-5(6) desaturase 1(DWF7), 7-dehydrocholesterol reductase (DWF5), delta24-sterol reductase(DWF1), CYPs, and UGTs. CPI, CYP51, FK and HYD1 are shared enzymes in the phytosterol and cholesterol pathways (Sonawane et al. 2016) (Fig. 1) At present, a variety of enzymes in plants related to steroidal saponins biosynthesis have been cloned and studied using gene silencing and other methods. The following content mainly describes some downstream genes that control the biosynthesis of steroidal saponins, reviews the latest research progress, and explores the regulatory roles of these genes.
Sterol methyltransferase
Sterol methyltransferase (SMT) is responsible for the methylation of C-24 alkyl sterols in different modes and is an important gene in the process of phytosterol biosynthesis. In plants, SMT1 and SMT2 are the two main types of SMT. SMT family sequences are highly similar and are considered to have a common ancestor. However, the specificity of the two types of SMT substrates is significantly different. At the same time, different SMTs may produce respective sterols when catalysing the same substrate (Guan et al. 2018; Nes 2005). SMT1 participates in the first step of sterol biosynthesis and is used to catalyse the conversion of cycloartenol to 24-methylenecycloenol. The biosynthesis of 24-methylenecycloenol is a common attribute of all photosynthetic plants. This step is also a bifurcation of phytosterol and cholesterol biosynthesis pathways. SMT1 has been characterized in model plants such as tobacco seed and Arabidopsis thaliana (Holmberg et al. 2002; Husselstein et al. 1996), and has been cloned and functionally identified in plants such as Tripterygium wilfordii (Guan et al. 2017), soybean (Neelakandan et al. 2009), upland cotton (Luo et al. 2008), and Paris polyphylla (Guan et al. 2018). This enzyme has different characteristics in the stereoscopic selection of substrates (Venkatramesh et al. 1996b), and differences in its expression are mainly reflected as changes in cholesterol levels. Based on studies of Withania somnifera, tobacco seed and Paris polyphylla, it was found that overexpression of SMT1 can result in undetectable cholesterol levels, but significant increases in other phytosterols (Guan et al. 2018; Holmberg et al. 2002; Pal et al. 2018). For plants lacking the SMT1 gene, the expression of genes related to the MVA and MEP processes and sterol biosynthesis process are affected, and various CYP450 genes are also suppressed to varying degrees, while cycloartenol and various transcription factors are significantly increased (Ciura et al. 2017a; Diener et al. 2000). By inhibiting the accumulation of cholesterol caused by SMT1, plant growth and diseases responses can be regulated (Chen et al. 2018a).
SMT2 is mainly involved in the biosynthesis of 24-ethylsterol and is responsible for converting 24-methylidenelophenol to 24-ethylidenelophenol. This enzyme plays a decisive role in regulating the contents of campesterol and sitosterol to meet plant growth and membrane integrity requirements. The study found that the initial appearance of SMT1 before SMT2 in phytosterol biosynthesis arose recently in plant evolution. A more primitive SMT gene may have been bifunctional and catalytically promiscuous (Neelakandan et al. 2009). Three SMTs were found in Arabidopsis, of which SMT2 and SMT3 both catalyse the biosynthesis of structural sterols and signal brassinosteroid derivatives and are highly regulated. By constructing SMT-deficient plants, it was found that the substrate specificity of SMTs is somewhat mixed. Although the gene sequence similarity of sterol methyltransferases in fungi and higher plants is not high, SMT2 and SMT3 in Arabidopsis can promote the addition of C1 and C2 in yeast Erg6p mutants blocked by SMTs (Diener et al. 2000).
Methyl oxidase and reductase
The phytosterol biosynthesis process is catalysed by SMO1 and SMO2 at the C4 position twice for demethylation. These two methyl oxidases have low sequence homology and functional differences (Darnet and Rahier 2004). SMO1 is responsible for converting 24-methylenecycloartanol to cycloeucalenol, while SMO2 converts 24-methylenelophenol and 24-ethylidenelophenol to episterol and delta7-avenasterol, and finally produces brassinosterol and sitosterol (Sonawane et al. 2016). DWF5 and DWF1 are the two main reductases in plant sterol biosynthesis. In the biosynthesis of stigmasterol, DWF5 first reduces delta-5,7-avenasterol to isofucosterol, and DWF1 further catalyses the production of stigmasterol. Moreover, these two enzymes are also involved in the biosynthesis of campesterol, which is considered to be the main precursor of brassinosteroids (Tsukagoshi et al. 2016; Youn et al. 2018b).These enzymes are key genes controlling auxin biosynthesis in plants and are related to embryonic development. The current research on these two enzymes mainly focuses on their regulation of plant growth hormones. SMO is a key gene controlling auxin synthesis in plants and is related to embryo development (Zhang et al. 2016b). The synthesis of bioactive brassinosteroids in mutants lacking DWF1 was reduced and led to dwarfism (Youn et al. 2018a). Studies on tomatoes have shown that DWF5 can cause dwarfing of tomato plants by affecting the synthesis of brassinosteroids (Rahim et al. 2018). However, there is currently no report on the regulation of steroidal saponin biosynthesis in plants by such reductases.
Cytochrome P450 monooxygenase
Cytochrome P450 monooxygenase is a membrane-bound enzyme that originated in the prokaryotic era and is capable of various oxidation reactions such as C-H hydroxylation, epoxidation, heteroatom oxidation, aromatic epoxidation, and dealkylation. In the process of continuous evolution, enzymes exhibit different substrate specificity to adapt to different needs (Yoshida et al. 2000). In the process of steroid biosynthesis, CYP51 is the only family that appears in the fungal, mammalian and plant kingdoms and can convert obtusifoliol into delta 8,14-sterol. The P450 enzyme found in the downstream stage mainly plays a role in the hydroxylation of C-16, C-22 and C-26 (Ohnishi et al. 2009). CYP90B1, also known as DWF4, is a C-22 steroid hydroxylase that can catalyse C27/C28/C29 sterols. CYP90B1 has the strongest catalytic activity for cholesterol, followed by brassinosteroid, and the weakest activity for sitosterol, and is involved in the first step in cholesterol production of steroidal saponins (Ciura et al. 2017b; Fujita et al. 2006). PpCYP90B27 was the first P450 enzyme cloned from Paris polyphylla that participates in the biosynthesis of steroidal saponins, but its catalytic specificity and substrate specificity need further study (Yin et al. 2018). CYP734A1 and CYP18A1 are both 26-hydroxylase enzymes. CYP18A1 is mainly a key enzyme in the biosynthesis of plant ecdysis hormone, but the gene found in fenugreek has also been found to be involved in the biosynthesis of steroidal saponins through induction experiments (Ciura et al. 2017a). CYP734A1 is involved in the biosynthesis of brassinosteroids and auxin (Youn et al. 2015). Transcriptome analysis of allium plants was used to investigate the upregulation of CYP734A1 and CYP72B1 (Abdelrahman et al. 2017). There are few studies on C-16 hydroxylase. In exploring the biosynthesis of steroid alkaloids in potatoes, Masaru Nakayasu et al. discovered the 16DOX gene, which that can regulate the C-16 hydroxylation of sterols. This gene may be a suitable target for controlling the levels of toxic steroid alkaloids (Nakayasu et al. 2017).
Glycosyltransferase
Uridine diphosphate-dependent glycosyltransferases (UGTs) are the most common type of glycosyltransferases, and are responsible for transferring UDP-sugar donors to small molecule saponins and steroid receptors in the synthesis of steroidal saponins (Wilson and Tian 2019). Glucose, rhamnose, arabinose, xylose, glucuronic acid, fucose, apiose, etc. can be used as glycogen for UGTs, and multiple UGTs gradually catalyse the extension of oligosaccharides of saponins. UGTs are among the key enzymes that increases the diversity of secondary metabolites (Inoue and Ebizuka 1996a; Louveau et al. 2018). This type of enzyme has a catalytic effect on endogenous and exogenous substrates (Messner et al. 2003), and there is a certain evolutionary relationship between enzymes that catalyse monosaccharides and disaccharides (Huang et al. 2018). At the same time, these enzymes have a high degree of regioselectivity and stereoselectivity (Singh et al. 2018) and can identify a series of substrates with common characteristics , and its expression module shows a high tissue specificity (Song et al. 2015). SaGT4A, isolated from belladonna, was the first cloned functional glucosyltransferase involved in the biosynthesis of steroidal saponins (Moreau et al. 2018). Two rhamnosyltransferases, Dz3GT1 and Dz3GT2, identified from Dioscorea zingiberensis can glycosylate diosgenin and cholesterol (Zhu et al. 2018a). The UGT80 gene family mainly includes UGT80A2 and UGT80B1. UGT80B1 has been found to be involved in the biosynthesis of steroidal saponins (Singh et al. 2017), while UGT80A2 is mainly involved in the accumulation of minor sterol glycosides (SGs) and acyl sterol glycosides (ASGs) (Jiang et al. 2017). At present, the number of glycosyltransferases that can participate in the biosynthesis of steroidal saponins is limited. The identification and isolation of glycosyltransferases as well as the study of their characteristics and functions and use of protein modifications to construct enzymes with similar structures but more functions, will have a positive effect on expanding the source of saponins and enriching their types.
Glucosinase
A variety of glycosidic bonds exist in steroidal saponins. Among them, 26-O-β-glucosidase is a key enzyme in the formation of furosesteric saponins into spirosteroidal saponins. Kentaro et al. isolated and purified F26 from Costus speciosus Sm. Compared to common hydrolases, it was found that this enzyme had a high specificity for the recognition of furosteroid and spirosteroid saponins (Inoue and Ebizuka 1996a, b). Masaru Nakayasu et al. successfully cloned a 26-O-β-glucosidase in Dioscorea zingiberensis and conjectured that this physiological characteristic is a kind of defence mechanism of plants. Furosteroid saponins are the precursor forms of spirosteroid saponins. Under normal physiological conditions, these two types of metabolites are distributed in different regions. When encountering biological stress and other factors, the spatial and chemical relationships between the two are disrupted, and the substrate is transformed by the corresponding enzymes to resist unstable conditions (Nakayasu et al. 2015). The β-glycosidase purified from Solanum torvum also has the characteristic of recognizing natural products better than synthetic products (Arthan et al. 2006). The expression of this enzyme in yeast cells has been used for the hydrolysis of furanosyl glycoside (Suthangkornkul et al. 2016). This enzyme is the most widely studied glycosidase in the biosynthesis of steroidal saponins.
Enzymes related to the biosynthesis and transformation of steroidal saponins in microorganisms
The enzymes related to sterol biosynthesis in fungi and plants are quite different, but they possess similarities in function and homology. This is related to their co-evolution (Ohyama et al. 2009). The most important sterol in fungi is ergosterol. At present, metabolic engineering has been utilized to transform the key enzymes of fungal ergosterol synthesis to produce industrially relevant sterols and steroids using yeast-engineered strains (Wriessnegger and Pichler 2013). Using the synthetic pathway of microbial biosynthesis of ergosterol, an engineered yeast strain that can biosynthesize cholesterol with only a simple carbon source has been successfully constructed (Pompon et al. 2012; Souza et al. 2011). During its biosynthesis, a methyltransferase named ERG6, which is mainly responsible for converting cryptosterol to coprosterol was found. At present, SMT1 has been isolated from microorganisms such as Saccharomyces cerevisiae and Pseudomonas spp. (Desta et al. 2019; Venkatramesh et al. 1996a). The process of ergosterol biosynthesis also includes the methyl oxidase ERG25, and two reductases, ERG4 and ERG24. In contrast to the process in plants, the two demethylation processes are completed by ERG25 (Bard et al. 1996). By verifying the functional experiments of the SMO and SMT genes in Arabidopsis thaliana, the genes of the same family in fungi and plants showed low sequence homology. These genes in plants can express their function in mutant strains, but there are differences in their activities and ways of functioning (Darnet et al. 2001; Ganapathy et al. 2008). CYP51 is named ERG11 in fungi and is responsible for catalysing the formation of lanosterol 4-4-dimethylcholesterol-8,14,24-trienol. Although there are no relevant reports on the key enzymes for the direct synthesis of steroidal saponins by microorganisms, certain enzymes involved in the biosynthesis of ergosterol can participate in the synthesis of cholesterol, the precursor of steroidal saponins. UGTs isolated from microorganisms show high substrate flexibility and poor regional specificity, and can be used as effective biocatalysts for the glycation of natural products. The amino acid length of UGT sequences in fungi changes in different plants. The evolutionary conservation of sequence similarity of the two is up to 60–70%. This difference reflects the common evolution of fungi as part of plant systems (Singh et al. 2018). There are also various glycosidases in microorganisms. Ding et al. found that 26-O-β-glucosidase can be produced in an endophyte that can increase the content of dioscin (Ding et al. 2014). Pgase-1, which was isolated and purified from Aspergillus oryza can hydrolyse a variety of sugars at different positions, thereby participating in the biosynthesis of steroidal saponins (Liu et al. 2013b). Zhang et al. obtained β-galactosidase from Lactobacillus bulgaricus L3, which can make steroidal saponin galactose (Zhang et al. 2016a). GiGly obtained from Gibberella intermedia can specifically hydrolyse multiple-3-O-β-glycosides (Li et al. 2016).
The pharmacological effects of steroidal saponins are closely related to the configuration of steroidal sugar groups, and the number and mode of chain sugars (Podolak et al. 2010; Prawat et al. 2016; Yang et al. 2018a; Zhang et al. 2016c). Some microorganisms can use their own enzyme systems to biotransform steroidal saponins, which not only increases the types of steroidal saponins, but also promotes the production of target saponins and glycosides. The microorganisms found to promote the conversion of steroidal saponins are shown in Table 1. Enzymes that biotransform the natural products of microorganisms usually have strong catalytic ability and specificity, and their stereoselectivity can complement chemical synthesis. To date, the research on the transformation of steroidal saponins by these functional strains has mainly focused on the screening of target strains and elucidation of their transformation pathways. The regulatory mechanism of steroidal saponin biotransformation deserves further exploration.
Conclusion and perspectives
This article details the three pathways of steroidal saponin biosynthesis in different plants and the key enzymes required in downstream processes. The biosynthesis of steroidal saponins starts with MVA and MEP pathways, and then generates squalene, 2,3-oxysqualene, and cycloartenol. Through the action of a series of methyltransferases, oxidases, and reductases, sitosterol or cholesterol is biosynthesized. Finally, different steroidal saponins are produced under the catalysis of a variety of cytochrome P450 enzymes, glycosyltransferases and glycosidases. Although a pathway to synthesize cholesterol through lanosterol has been found in some plants, the ability to produce steroidal saponins through this pathway has not yet been discovered. In addition, it is believed that enzymes with the same function in these pathways may catalyse similar substrates in a mixed manner. Although the biosynthetic pathway of steroidal saponins is clearly understood, there is little research on how cholesterol and sitosterol are converted into steroidal saponins, differences in the biosynthetic pathways of saponins with different configurations, and biosynthetic pathways in nonplant organisms. It is necessary to further verify the pathway from lanosterol to cholesterol then to steroidal saponins and from sitosterol to steroidal saponins, to fully discover the biosynthesis of steroidal saponins in microorganisms and marine organisms, and to pay more attention to the effect of configurations on biosynthesis.
To date, some microorganisms have been found to have the ability to produce plant sterols, cholesterol and even steroidal saponins, and approximately 40 strains promote the conversion of steroidal saponins in the laboratory or in industrial production. Through studies on the biosynthetic pathway of microbial sterols and the mechanisms by which endophytes produce natural products, it can be speculated that the biosynthesis of steroidal saponins in microorganisms may utilize ergosterol biosynthetic enzymes or unique pathways. As genes from microorganisms are relatively easy to obtain and have a strong catalytic ability (Cheong et al. 2016; Fan et al. 2017), these functional strains are an important source of key enzymes in the construction of engineered strains that produce steroidal saponins.
At present, by integrating relevant genes in plants, animals and yeast, engineered strains for the production of diosgenin have been successfully constructed (Cheng et al. 2020). Regrettably, there is no report on the construction of engineering strains for steroidal saponin production, but the engineering strains for triterpene saponin production have not only improved the production capacity of ginsenosides but also achieved the acquisition of new saponins (Yang et al. 2015; Yu et al. 2017; Zhang et al. 2018). These successful practices provide ideas and promise for the construction of strains engineered to produce steroidal saponin. There are still many challenges in cloning and expressing biosynthetic gene clusters by heterologous enzymes in the host. Therefore, it is necessary to use genome mining technology to improve the understanding of the biosynthetic pathway of steroidal saponins, increase the discovery and utilization of biosynthetic gene clusters, and apply epigenetic modification and targeted genome modification to enhance gene silencing and certain specific gene activation mechanisms in steroidal saponin biosynthesis. Research achievements on the biosynthetic pathways of steroidal saponins and key enzymes will promote the industrial production of steroidal saponins by biotechnology.
Author contribution statement YYC compiled and wrote the manuscript and created the figures. XWD conceptualized, designed and edited the manuscript. JKW and DY provided scientific feedback and revised the content. All authors read and approved the manuscript.
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Abbreviations
- IPP:
-
Isopentenyl diphosphate
- CAS:
-
Cycloartenol synthase
- CPI1:
-
Cyclopropylsterol isomerase
- CYP51, ERG11:
-
Sterol C-14 demethylase
- DMAPP:
-
Dimethylallyl diphosphate
- DWF1, ERG4:
-
Delta 24-sterol reductase
- DWF5:
-
7-Dehydrocholesterol reductase
- DWF7, ERG3:
-
Sterol C-5 desaturase
- ERG5:
-
Sterol C-22 desaturase
- ERG6:
-
Sterol C-24 methylase
- FK, ERG24:
-
Sterol C-14 reductase
- FPPS:
-
Farnesyl diphosphate synthase
- GPPS:
-
Geranyl diphosphate synthase
- HYD1, ERG2:
-
Sterol 8,7 isomerase
- LAS:
-
Lanosterol synthetase
- SMO, ERG25:
-
Sterol 4α-methyl oxidase
- SMT:
-
Sterol C-24 methyltransferase
- SQE:
-
Squalene epoxidase
- UGTs:
-
Uridine diphosphate-dependent glycosyltransferases
References
Abdelrahman M et al (2017) RNA-sequencing-based transcriptome and biochemical analyses of steroidal saponin pathway in a complete set of Allium fistulosum—A. cepa monosomic addition lines. PLoS One 12:e0181784. https://doi.org/10.1371/journal.pone.0181784
Adham NZ, Zaki RA, Naim N (2009) Microbial transformation of diosgenin and its precursor furostanol glycosides. World J Microbiol Biotechnol 25:481–487. https://doi.org/10.1007/s11274-008-9913-1
Arthan D, Kittakoop P, Esen A, Svasti J (2006) Furostanol glycoside 26-O-β-glucosidase from the leaves of Solanum torvum. Phytochemistry 67:27–33. https://doi.org/10.1016/j.phytochem.2005.09.035
Bai Y, Yin H, Bi H, Zhuang Y, Liu T, Ma Y (2016) De novo biosynthesis of Gastrodin in Escherichia coli. Metab Eng 35:138–147. https://doi.org/10.1016/j.ymben.2016.01.002
Bard M et al (1996) Cloning and characterization of ERG25, the Saccharomyces cerevisiae gene encoding C-4 sterol methyl oxidase. Proc Natl Acad Sci USA 93:186–190. https://doi.org/10.1073/pnas.93.1.186
Basu S, Jha TB (2013) In vitro root culture : an alternative source of bioactives in the rare aphrodisiac herb Chlorophytum borivilianum Sant et Fern. Plant Tissue Cult Biotechnol 23:133–145. https://doi.org/10.3329/ptcb.v23i2.17505
Behrman E, Gopalan V (2005) Cholesterol and plants. J Chem Educ. https://doi.org/10.1021/ed082p1791
Blunden G, Patel AV, Crabb TA (1990) Microbiological transformation of hecogenin and diosgenin by Cunninghamella elegans. Phytochemistry 29:1771–1780. https://doi.org/10.1016/0031-9422(90)85013-6
Bouvier P, Rohmer M, Benveniste P, Ourisson G (1976) Delta 8(14)-steroids in the bacterium Methylococcus capsulatus. Biochem J 159:267–271. https://doi.org/10.1042/bj1590267
Cao XD, Zhou LG, Xu LJ, Tan ML (2005) Biological characters of the endophytic fungi from Paris polyphylla var.yunnanensis and their steroidal content. J Northwest A F Univ 033:125–128. https://doi.org/10.3321/j.issn:1671-9387.2005.z1.034
Chaudhary S et al (2015) Elicitation of diosgenin production in Trigonella foenum-graecum (Fenugreek) seedlings by Methyl Jasmonate. Int J Mol Sci 16:29889–29899. https://doi.org/10.3390/ijms161226208
Chauhan R, Keshavkant S, Quraishi A (2018) Enhanced production of diosgenin through elicitation in micro-tubers of Chlorophytum borivilianum Sant et Fernand. J Industrial Crops 113:234–239. https://doi.org/10.1016/j.indcrop.2018.01.029
Chauhan HK (2020) The IUCN Red List of Threatened Species 2020: e.T175617476A176257430. https://doi.org/10.2305/IUCN.UK.2020-3.RLTS.T175617476A176257430.en
Chen ND, Zhang J, Liu JH, Yu BY (2009) Microbial conversion of ruscogenin by Gliocladium deliquescens NRRL1086: glycosylation at C-1. Appl Microbiol Biotechnol 86:491–497. https://doi.org/10.1007/s00253-009-2315-y
Chen M, Chen JJ, Luo N, Qu RD, Guo ZF, Lu SY (2018a) Cholesterol accumulation by suppression of SMT1 leads to dwarfism and improved drought tolerance in herbaceous plants. Plant Cell Environ. https://doi.org/10.1111/pce.13168
Chen Y, Dong Y, Chi YL, He Q, Wu H, Ren Y (2018b) Eco-friendly microbial production of diosgenin from saponins in Dioscorea zingiberensis tubers in the presence of Aspergillus awamori. Steroids. https://doi.org/10.1016/j.steroids.2018.05.005
Cheng J, Chen J, Liu XN, Li XC, Ma YH (2020) The origin and evolution of diosgenin biosynthetic pathway in yam. Plant Commun 2:100079. https://doi.org/10.1016/j.xplc.2020.100079
Cheong S, Clomburg JM, Gonzalez R (2016) Energy- and carbon-efficient synthesis of functionalized small molecules in bacteria using non-decarboxylative Claisen condensation reactions. Nat Biotechnol 34:556–561. https://doi.org/10.1038/nbt.3505
Ciura J, Szeliga M, Grzesik M, Tyrka M (2017a) Changes in fenugreek transcriptome induced by methyl jasmonate and steroid precursors revealed by RNA-Seq. Genomics. https://doi.org/10.1016/j.ygeno.2017.10.006
Ciura J, Szeliga M, Grzesik M, Tyrka M (2017b) Next-generation sequencing of representational difference analysis products for identification of genes involved in diosgenin biosynthesis in fenugreek (Trigonella foenum-graecum). Planta 245:977–991. https://doi.org/10.1007/s00425-017-2657-0
Corsello MA, Garg NK (2015) Synthetic chemistry fuels interdisciplinary approaches to the production of artemisinin. Nat Prod Rep 32:359–366. https://doi.org/10.1039/c4np00113c
Cunningham AB, Brinckmann JA, Bi YF, Pei SJ, Schippmann U, Luo P (2018) Paris in the spring: a review of the trade, conservation and opportunities in the shift from wild harvest to cultivation of Paris polyphylla (Trilliaceae). J Ethnopharmacol 222:208–216. https://doi.org/10.1016/j.jep.2018.04.048
Dai Z et al (2014) Producing aglycons of ginsenosides in bakers’ yeast. Sci Rep 4:3698. https://doi.org/10.1038/srep03698
Darnet S, Rahier A (2004) Plant sterol biosynthesis: identification of two distinct families of sterol 4α-methyl oxidases. Biochem J 378:889–898. https://doi.org/10.1042/BJ20031572
Darnet S, Bard M, Rahier A (2001) Functional identification of sterol-4α-methyl oxidase cDNAs from Arabidopsis thaliana by complementation of a yeast erg25 mutant lacking sterol-4α-methyl oxidation. FEBS Lett 508:39–43. https://doi.org/10.1016/S0014-5793(01)03002-2
de Oliveira JVA et al (2019) Saponin-rich fraction from Agave sisalana: effect against malignant astrocytic cells and its chemical characterisation by ESI-MS/MS. Nat Prod Res 33:1769–1772. https://doi.org/10.1080/14786419.2018.1434633
Desmond E, Gribaldo S (2009) Phylogenomics of sterol synthesis: insights into the origin, evolution, and diversity of a key eukaryotic feature. Genome Biol Evol 1:364–381. https://doi.org/10.1093/gbe/evp036
Desta M, Wang WW, Zhang LG, Xu P, Tang HZ (2019) Isolation, characterization, and genomic analysis of Pseudomonas sp. strain SMT-1, an efficient fluorene-degrading bacterium. Evol Bioinform Online 15:1176934319843518. https://doi.org/10.1177/1176934319843518
Diener AC, Li H, Zhou W, Whoriskey WJ, Nes WD, Fink GR (2000) Sterol methyltransferase 1 controls the level of cholesterol in plants. Plant Cell 12:853–870. https://doi.org/10.1105/tpc.12.6.853
Ding CH et al (2014) Screening for differentially expressed genes in endophytic fungus strain 39 during co-culture with herbal extract of its host Dioscorea nipponica makino. Curr Microbiol 69:517–524. https://doi.org/10.1007/s00284-014-0615-7
Dong M, Feng XZ, Wang BX, Ikejima T, Wu LJ (2004) Microbial metabolism of pseudoprotodioscin. Planta Med 70:637–641. https://doi.org/10.1055/s-2004-827187
Dong XR, Gao ZH, Hu HX, Gao RR, Sun D (2016) Microbial transformation of pseudoprotodioscin by Chaetomium olivaceum. J Mol Catal B Enzym. https://doi.org/10.1016/j.molcatb.2016.05.001
Dutch Medicines Evaluation Board (2012) First Authorisation of Traditional Herbal Medicine from outside the European Union. http://www.cbg-meb.nl/CBG/en/human-medicines/actueel/First_Authorisation_of_Traditional_Herbal_Medicine_from_outside_the_European_Union/. Accessed 21 July 2013
Espinosa-Leal CA, Puente-Garza CA, Garcia-Lara S (2018) In vitro plant tissue culture: means for production of biological active compounds. Planta 248:1–18. https://doi.org/10.1007/s00425-018-2910-1
Fabris M et al (2014) Tracking the sterol biosynthesis pathway of the diatom Phaeodactylum tricornutum. New Phytol. https://doi.org/10.1111/nph.12917
Fan B, Chen TY, Zhang S, Wu B, He BF (2017) Mining of efficient microbial UDP-glycosyltransferases by motif evolution cross plant kingdom for application in biosynthesis of salidroside. Sci Rep 7:463. https://doi.org/10.1038/s41598-017-00568-z
Feng B, Ma BP, Kang LP, Xiong CQ, Wang SQJC (2005) The microbiological transformation of steroidal saponins by Curvularia Lunata. Tetrahedron 61:11758–11763
Feng S, Song W, Fu RR, Zhang H, Xu AR, Li JR (2018) Application of the CRISPR/Cas9 system in Dioscorea zingiberensis. Plant Cell Tissue Organ Cult (PCTOC) 135:133–141. https://doi.org/10.1007/s11240-018-1450-5
Fernández-Cabezón L, Galán B, García JL (2018) New insights on steroid biotechnology. Front Microbiol 9:958. https://doi.org/10.3389/fmicb.2018.00958
Fontaine J, Grandmougin-Ferjani A, Hartmann MA, Sancholle M (2002) Sterol biosynthesis by the arbuscular mycorrhizal fungus Glomus intraradices. Lipids 36:1357–1363. https://doi.org/10.1007/s11745-001-0852-z
Fujita S et al (2006) Arabidopsis CYP90B1 catalyses the early C-22 hydroxylation of C27, C28 and C29 sterols. Plant J 45:765–774. https://doi.org/10.1111/j.1365-313X.2005.02639.x
Galanie S, Thodey K, Trenchard IJ, Interrante MF, Smolke CD (2015) Complete biosynthesis of opioids in yeast. Science 349:1095–1100. https://doi.org/10.1126/science.aac9373
Ganapathy K, Jones CW, Stephens CM, Vatsyayan R, Marshall JA, Nes WD (2008) Molecular probing of the Saccharomyces cerevisiae sterol 24-C methyltransferase reveals multiple amino acid residues involved with C2-transfer activity. Biochem Biophys Acta 1781:344–351. https://doi.org/10.1016/j.bbalip.2008.04.015
Gao RR, Gao ZH, Dong XR, Hu HX, Qiao Y, Sun AD (2017) Microbial transformation of gracillin by Penicillium Lilacinum ACCC 31890. China Pharmacist 020:988–993
Garagounis C et al (2020) A hairy-root transformation protocol for Trigonella foenum-graecum L. as a tool for metabolic engineering and specialised metabolite pathway elucidation. Plant Physiol Biochem 154:451–462. https://doi.org/10.1016/j.plaphy.2020.06.011
González-Lamothe R, Mitchell G, Gattuso M, Diarra MS, Malouin F, Bouarab K (2009) Plant antimicrobial agents and their effects on plant and human pathogens. Int J Mol Sci 10:3400–3419. https://doi.org/10.3390/ijms10083400
Grandmougin-Ferjani A, Dalpé Y, Hartmann M-A, Laruelle F, Sancholle M (1999) Sterol distribution in arbuscular mycorrhizal fungi. Phytochemistry 50:1027–1031. https://doi.org/10.1016/S0031-9422(98)00636-0
Guan HY et al (2017) Molecular cloning and functional identification of sterol C24-methyltransferase gene from Tripterygium wilfordii. Acta Pharmaceutica Sin B 7:603–609. https://doi.org/10.1016/j.apsb.2017.07.001
Guan HY et al (2018) Cloning and functional analysis of two sterol-C24-methyltransferase 1 (SMT1) genes from Paris polyphylla. J Asian Nat Prod Res 20:595–604. https://doi.org/10.1080/10286020.2016.1271791
Güçlü-Ustündağ O, Mazza G (2007) Saponins: properties, applications and processing. Crit Rev Food Sci Nutr 47:231–258. https://doi.org/10.1080/10408390600698197
Haubrich BA et al (2014) Characterization, mutagenesis and mechanistic analysis of an ancient algal sterol C24-methyltransferase: implications for understanding sterol evolution in the green lineage. Phytochemistry 113:64–72. https://doi.org/10.1016/j.phytochem.2014.07.019
Hayakawa S, Sato Y (1963) Microbiological transformation of diosgenin. J Org Chem 28:2742–2743. https://doi.org/10.1021/jo01045a059
He XJ, Liu B, Wang GH, Wang XL, Su L, Qu GX, Yao XS (2006) Microbial metabolism of methyl protodioscin by Aspergillus niger culture—A new androstenedione producing way from steroid. J Steroid Biochem Mol Biol 100:87–94. https://doi.org/10.1016/j.jsbmb.2006.03.007
Hedden P, Phillips AL, Rojas MC, Carrera E, Tudzynski B (2002) Gibberellin biosynthesis in plants and fungi: a case of convergent evolution? J Plant Growth Regul 20:319–331. https://doi.org/10.1007/s003440010037
Heinig U, Scholz S, Jennewein S (2013) Getting to the bottom of Taxol biosynthesis by fungi. Fungal Div 60:161–170. https://doi.org/10.1007/s13225-013-0228-7
Holmberg N et al (2002) Sterol C-24 methyltransferase type 1 controls the flux of carbon into sterol biosynthesis in tobacco seed. Plant Physiol 130:303–311. https://doi.org/10.1104/pp.004226
Hu YM, Yu ZL, Fong WF (2011) Stereoselective biotransformation of timosaponin A-III by Saccharomyces cerevisiae. J Microbiol Biotechnol 21:582–589. https://doi.org/10.4014/jmb.1101.12041
Hu HX, Gao RR, Gao ZH, Qiao Y, Dong XR, Ding G, Sun DA (2018) Microbial transformation of pseudoprotodioscin by Gibberella fujikuroi. J Asian Nat Prod Res 20:1–9. https://doi.org/10.1080/10286020.2018.1468438
Huang FC, Giri A, Daniilidis M, Sun G, Hartl K, Hoffmann T, Schwab W (2018) Structural and functional analysis of UGT92G6 suggests an evolutionary link between mono- and disaccharide glycoside-forming transferases. Plant Cell Physiol 59:857–870. https://doi.org/10.1093/pcp/pcy028
Husselstein T, Gachotte D, Desprez T, Bard M, Benveniste P (1996) Transformation of Saccharomyces cerevisiae with a cDNA encoding a sterol C-methyltransferase from Arabidopsis thaliana results in the synthesis of 24-ethyl sterols. FEBS Lett 381:87–92. https://doi.org/10.1016/0014-5793(96)00089-0
Inoue K, Ebizuka Y (1996a) Purification and characterization of a β-glucosidase which converts furostanol glycosides to spirostanol glycosides from Costus speciosus. Adv Exp Med Biol 404:57–69. https://doi.org/10.1007/978-1-4899-1367-8_6
Inoue K, Ebizuka Y (1996b) Purification and characterization of furostanol glycoside 26-O-β-glucosidase from Costus speciosus rhizomes. FEBS Lett 378:157–160. https://doi.org/10.1016/0014-5793(95)01447-0
Iorrizzi M, De Marino S, Zollo F (2001) Steroidal oligoglycosides from the asteroidea. Curr Org Chem 5:951–973. https://doi.org/10.2174/1385272013374978
Jaramillo-Madrid AC, Ashworth J, Fabris M, Ralph PJ (2019) Phytosterol biosynthesis and production by diatoms (Bacillariophyceae). Phytochemistry 163:46–57. https://doi.org/10.1016/j.phytochem.2019.03.018
Jiang X et al (2017) Panax ginseng genome examination for ginsenoside biosynthesis. GigaScience. https://doi.org/10.1093/gigascience/gix093
Kim M et al (2017) Optimal fermentation conditions of hyaluronidase inhibition activity on Asparagus cochinchinensis merrill by Weissella cibaria. J Microbiol Biotechnol. https://doi.org/10.4014/jmb.1611.11051
Kumar A, Fogelman E, Weissberg M, Tanami Z, Veilleux RE, Ginzberg I (2017) Lanosterol synthase-like is involved with differential accumulation of steroidal glycoalkaloids in potato. Planta. https://doi.org/10.1007/s00425-017-2763-z
Lata R, Chowdhury S, Gond SK, White JF (2018) Induction of abiotic stress tolerance in plants by endophytic microbes. Lett Appl Microbiol. https://doi.org/10.1111/lam.12855
Lee AK, Banta AB, Wei JH, Kiemle DJ, Feng J, Giner J-L, Welander PV (2018) C-4 sterol demethylation enzymes distinguish bacterial and eukaryotic sterol synthesis. Proc Natl Acad Sci 115:201802930. https://doi.org/10.1073/pnas.1802930115
Lees ND, Skaggs B, Kirsch DR, Bard M (1995) Cloning of the late genes in the ergosterol biosynthetic pathway of Saccharomyces cerevisiae–a review. Lipids 30:221–226. https://doi.org/10.1007/bf02537824
Li PQ, Mao ZL, Lou JF, Mou Y, Lu SQ, Peng YL, Zhou LG (2011) Enhancement of diosgenin production in Dioscorea zingiberensis cell cultures by oligosaccharides from its endophytic fungus Fusarium oxysporum Dzf17. Molecules 16:10631–10644. https://doi.org/10.3390/molecules161210631
Li XW, Chen YN, Lai YF, Yang Q, Hu H, Wang YT (2015) Sustainable utilization of traditional Chinese medicine resources: systematic evaluation on different production modes. Evid Based Complement Altern Med 2015:218901. https://doi.org/10.1155/2015/218901
Li H, Wang XD, Ma Y, Yang NN, Zhang XJ, Xu ZH, Shi JS (2016) Purification and characterization of a glycosidase with hydrolyzing multi-3-O-glycosides of spirostanol saponin activity from Gibberella intermedia. J Mol Catal B Enzym. https://doi.org/10.1016/j.molcatb.2016.03.003
Li YB, Lin L, Liao QH, Yang SC, Liu T (2019) Screening of endophytic fungi for promote the accumulation of active components of saponins from Pairs polyphylla var yunnanensis. J Yunnan Agric Univ 34:132–137
Liao P, Hemmerlin A, Bach TJ, Chye ML (2016) The potential of the mevalonate pathway for enhanced isoprenoid production. Biotechnol Adv 34:697–713. https://doi.org/10.1016/j.biotechadv.2016.03.005
Liu L, Dong YS, Qi SS, Wang H, Xiu ZL (2010) Biotransformation of steriodal saponins in Dioscorea zingiberensis C. H. Wright to diosgenin by Trichoderma harzianum. Appl Microbiol Biotechnol 85:933–940. https://doi.org/10.1007/s00253-009-2098-1
Liu TQ et al (2013a) Preparation of progenin III from total steroidal saponins of Dioscorea nipponica Makino using a crude enzyme from Aspergillus oryzae strain. J Ind Microbiol Biotechnol 40:427–436. https://doi.org/10.1007/s10295-013-1246-x
Liu TQ et al (2013b) Protodioscin-glycosidase-1 hydrolyzing 26-O-β-D-glucoside and 3-O-(1 → 4)-α-L-rhamnoside of steroidal saponins from Aspergillus oryzae. Appl Microbiol Biotechnol 97:10035–10043. https://doi.org/10.1007/s00253-013-4791-3
Liu JY et al (2015) Selective glycosylation of steroidal saponins by Arthrobacter nitroguajacolicus. Carbohyd Res. https://doi.org/10.1016/j.carres.2014.07.006
Louveau T et al (2018) Analysis of two new arabinosyltransferases belonging to the carbohydrate-active enzyme (CAZY) glycosyl transferase family1 provides insights into disease resistance and sugar donor specificity. Plant Cell 30:3038–3057. https://doi.org/10.1105/tpc.18.00641
Lu Q et al (2018) Saponins from Paris forrestii (Takht) H. Li display potent activity against acute myeloid leukemia by suppressing the RNF6/AKT/mTOR signaling pathway. Front Pharmacol. https://doi.org/10.3389/fphar.2018.00673
Luo M, Tan KL, Xiao ZY, Hu MY, Liao P, Chen KJ (2008) Cloning and expression of two sterol C-24 methyltransferase genes from upland cotton (Gossypium hirsuturm L.). J Genet Genomics 35:357–363. https://doi.org/10.1016/s1673-8527(08)60052-1
Madoui MA, Bertrand-Michel J, Gaulin E, Dumas B (2009) Sterol metabolism in the oomycete Aphanomyces euteiches, a legume root pathogen. New Phytol 183:291–300. https://doi.org/10.1111/j.1469-8137.2009.02895.x
Mehrafarin A, Qaderi A, Rezazadeh S, Badi HN, Zand E (2010) Bioengineering of important secondary metabolites and metabolic pathways in fenugreek (Trigonella foenum-graecum L.). J Med Plants 9:1–18
Messner B, Thulke O, Schäffner AR (2003) Aradiposis glucosyltransferases with activities toward both endogenous and xenobiotic substrates. Planta 217:138–146. https://doi.org/10.1007/s00425-002-0969-0
Mimaki Y, Kuroda M, Kameyama A, Sashida Y, Hirano T, Oka K (1997) Cholestane glycosides with potent cytostatic activities on various tumor cells from Ornithogalum saundersiae bulbs. BioorgMedChemLett 7:633–636. https://doi.org/10.1016/S0960-894X(97)00071-1
Moreau RA, Nyström L, Whitaker BD, Winkler-Moser JK, Baer DJ, Gebauer SG, Hicks KB (2018) Phytosterols and their derivatives: structural diversity, distribution, metabolism, analysis, and health-promoting uses. Prog Lipid Res. https://doi.org/10.1016/j.plipres.2018.04.001
Moses T, Papadopoulou KK, Osbourn A (2014) Metabolic and functional diversity of saponins, biosynthetic intermediates and semi-synthetic derivatives. Crit Rev Biochem Mol Biol 49:1–24. https://doi.org/10.3109/10409238.2014.953628
Nakagawa A et al (2016) Total biosynthesis of opiates by stepwise fermentation using engineered Escherichia Coli. Nat Commun 7:10390. https://doi.org/10.1038/ncomms10390
Nakayasu M, Kawasaki T, Lee HJ, Sugimoto Y, Mizutani M (2015) Identification of furostanol glycoside 26-O-β-glucosidase involved in steroidal saponin biosynthesis from Dioscorea esculenta. Plant Biotechnol 32:299–308. https://doi.org/10.5511/plantbiotechnology.15.1023b
Nakayasu M et al (2017) A Dioxygenase catalyzes steroid 16α-hydroxylation in steroidal glycoalkaloid biosynthesis. Plant Physiol 175:120–133. https://doi.org/10.1104/pp.17.00501
Nazir R, Kumar V, Gupta S, Dwivedi P, Pandey DK, Dey A (2021) Biotechnological strategies for the sustainable production of diosgenin from Dioscorea spp. Appl Microbiol Biotechnol 105:569–585. https://doi.org/10.1007/s00253-020-11055-3
Neelakandan AK et al (2009) Cloning, functional expression and phylogenetic analysis of plant sterol 24C-methyltransferases involved in sitosterol biosynthesis. Phytochemistry 70:1982–1998. https://doi.org/10.1016/j.phytochem.2009.09.003
Nes WD (2005) Enzyme redesign and interactions of substrate analogues with sterol methyltransferase to understand phytosterol diversity, reaction mechanism and the nature of the active site. Biochem Soc Trans 33:1189–1196. https://doi.org/10.1042/BST20051189
Newman JD, Chappell J (1999) Isoprenoid biosynthesis in plants: carbon partitioning within the cytoplasmic pathway. Crit Rev Biochem Mol Biol 34:95–106. https://doi.org/10.1080/10409239991209228
Nikam TD, Ebrahimi MA, Patil VA (2009) Embryogenic callus culture of Tribulus terrestris L. a potential source of harmaline, harmine and diosgenin. Plant Biotechnol Rep 3:243–250. https://doi.org/10.1007/s11816-009-0096-5
Ohnishi T, Yokota T, Mizutani M (2009) Insights into the function and evolution of P450s in plant steroid metabolism. Phytochemistry 70:1918–1929. https://doi.org/10.1016/j.phytochem.2009.09.015
Ohyama K, Suzuki M, Kikuchi J, Saito K, Muranaka T (2009) Dual biosynthetic pathways to phytosterol via cycloartenol and lanosterol in Arabidopsis. Proc Natl Acad Sci USA 106:725–730. https://doi.org/10.1073/pnas.0807675106
Osbourn AE (1996) Preformed antimicrobial compounds and plant defense against fungal attack. Plant Cell 8:1821–1831. https://doi.org/10.1105/tpc.8.10.1821
Pal S, Rastogi S, Nagegowda DA, Gupta PM, Shasany AK, Chanotiya CS (2018) RNAi of sterol methyl transferase1 reveals its direct role in diverting intermediates towards withanolide/phytosterol biosynthesis in Withania somnifera. Plant Cell Physiol. https://doi.org/10.1093/pcp/pcy237
Pang X, Wen D, Zhao Y, Xiong CQ, Wang XQ, Yu LY, Ma BP (2014) Steroidal saponins obtained by biotransformation of total furostanol glycosides from Dioscora zingiberensis with Absidia coerulea. Carbohyd Res 402:236–240. https://doi.org/10.1016/j.carres.2014.11.011
Pearson A, Budin M, Brocks JJ (2003) Phylogenetic and biochemical evidence for sterol synthesis in the bacterium Gemmata obscuriglobus. Proc Natl Acad Sci USA 100:15352–15357. https://doi.org/10.1073/pnas.2536559100
Pereira M et al (2010) Cloning, mechanistic and functional analysis of a fungal sterol C24-methyltransferase implicated in brassicasterol biosynthesis. Biochem Biophys Acta 1801:1163–1174. https://doi.org/10.1016/j.bbalip.2010.06.007
Podolak I, Galanty A, Sobolewska D (2010) Saponins as cytotoxic agents: a review. Phytochem Rev 9:425–474. https://doi.org/10.1007/s11101-010-9183-z
Pompon D, Dumas B, Spagnoli R (2012) Cholesterol-producing yeast strains and uses thereof. US Patent 8211676, 7.3
Prawat H, Mahidol C, Kaweetripob W, Intachote P, Pisutjaroenpong S, Ruchirawat S (2016) Cytotoxic steroidal glycosides from the whole plant of Calamus acanthophyllus. Planta Med 82:1117–1121. https://doi.org/10.1055/s-0042-106972
Puente-Garza CA, Espinosa-Leal CA, García-Lara S (2021) Effects of saline elicitors on saponin production in Agave Salmiana plants grown in vitro. Plant Physiol Biochem 162:476–482. https://doi.org/10.1016/j.plaphy.2021.03.017
Rahim MA, Jung HJ, Afrin KS, Lee J-H, Nou I-S (2018) Comparative transcriptome analysis provides insights into dwarfism in cherry tomato (Solanum lycopersicum var. Cerasiforme). PLoS One 13:e0208770. https://doi.org/10.1371/journal.pone.0208770
Rizvi MZ, Kukreja AK, Bisht NS (2010) In vitro propagation of an endangered medicinal herb Chlorophytum borivilianum Sant. et Fernand. through somatic embryogenesis. Physiol Mol Biol Plants 16:249–257. https://doi.org/10.1007/s12298-010-0026-6
Sah B, Subban K, Chelliah J (2017) Cloning and sequence analysis of 10-deacetylbaccatin III-10-O-acetyl transferase gene and WRKY1 transcription factor from taxol producing endophytic fungus Lasiodiplodia theobromea. FEMS Microbiol Lett. https://doi.org/10.1093/femsle/fnx253
Saunders R, Cheetham PSJ, Hardman R (1986) Microbial transformation of crude fenugreek steroids. Enzyme Microb Technol 8:549–555. https://doi.org/10.1016/0141-0229(86)90040-2
Sawai S, Saito K (2011) Triterpenoid biosynthesis and engineering in plants. Front Plant Sci. https://doi.org/10.3389/fpls.2011.00025
Shen L, Xu J, Luo L, Hu H, Meng XX, Li XW, Chen SL (2018) Predicting the potential global distribution of diosgenin-contained Dioscorea species. Chin Med 13:58. https://doi.org/10.1186/s13020-018-0215-8
Sidana J, Singh B, Sharma OP (2016) Saponins of agave: chemistry and bioactivity. Phytochemistry 130:22–46. https://doi.org/10.1016/j.phytochem.2016.06.010
Singh P, Singh G, Bhandawat A, Singh G, Parmar R, Seth R, Sharma RK (2017) Spatial transcriptome analysis provides insights of key gene(s) involved in steroidal saponin biosynthesis in medicinally important herb Trillium govanianum. Sci Rep 7:45295. https://doi.org/10.1038/srep45295
Singh G, Dhar YV, Asif MH, Misra P (2018) Exploring the functional significance of sterol glycosyltransferase enzymes. Prog Lipid Res 69:1–10. https://doi.org/10.1016/j.plipres.2017.11.001
Sonawane PD et al (2016) Plant cholesterol biosynthetic pathway overlaps with phytosterol metabolism. Nat Plants 3:16205. https://doi.org/10.1038/nplants.2016.205
Song CK, Gu L, Liu JY, Zhao S, Hong XT, Schulenburg T, Schwab W (2015) Functional characterization and substrate promiscuity of UGT71 glycosyltransferases from strawberry (Fragaria × ananassa). Plant Cell Physiol. https://doi.org/10.1093/pcp/pcv151
Song XY, Han FY, Chen JJ, Wang W, Zhang Y, Yao GD, Song SJ (2019) Timosaponin AIII, a steroidal saponin, exhibits anti-tumor effect on taxol-resistant cells in vitro and in vivo. Steroids 146:57–64. https://doi.org/10.1016/j.steroids.2019.03.009
Souza CM et al (2011) A stable yeast strain efficiently producing cholesterol instead of ergosterol is functional for tryptophan uptake, but not weak organic acid, resistance. Metab Eng 13:555–569. https://doi.org/10.1016/j.ymben.2011.06.006
Sparg SG, Light ME, van Staden J (2004) Biological activities and distribution of plant saponins. J Ethnopharmacol 94:219–243. https://doi.org/10.1016/j.jep.2004.05.016
Sun W et al (2017) Weighted gene co-expression network analysis of the dioscin rich medicinal plant Dioscorea nipponica. Front Plant Sci. https://doi.org/10.3389/fpls.2017.00789
Suthangkornkul R et al (2016) A Solanum torvum GH3 β-glucosidase expressed in Pichia pastoris catalyzes the hydrolysis of furostanol glycoside. Phytochemistry. https://doi.org/10.1016/j.phytochem.2016.03.015
Suza WP, Chappell J (2015) Spatial and temporal regulation of sterol biosynthesis in Nicotiana benthamiana. Physiol Plant 157:120–134. https://doi.org/10.1111/ppl.12413
Suzuki M et al (2006) Lanosterol synthase in dicotyledonous plants. Plant Cell Physiol 47:565–571. https://doi.org/10.1093/pcp/pcj031
Tang GH, Lu N, Li W, Wu M, Chen YY, Zhang HY, He SY (2020) Mannosylxylarinolide, a new 3,4-seco-ergostane-type steroidal saponin featuring a β-d-mannose from the endophytic fungus Xylaria sp. J Asian Nat Prod Res 22:397–403. https://doi.org/10.1080/10286020.2018.1563075
Tenon M, Feuillère N, Roller M, Birtić S (2017) Rapid, cost-effective and accurate quantification of Yucca schidigera Roezl. steroidal saponins using HPLC-ELSD method. Food Chem 221:1245–1252. https://doi.org/10.1016/j.foodchem.2016.11.033
Tian LW, Zhang Z, Long HL, Zhang YJ (2017) Steroidal saponins from the genus smilax and their biological activities. Nat Prod Bioprospect 7:283–298. https://doi.org/10.1007/s13659-017-0139-5
Torres S et al (2017) Steroidal composition and cytotoxic activity from fruiting body of Cortinarius xiphidipus. Nat Prod Res 31:473–476. https://doi.org/10.1080/14786419.2016.1185717
Tsukagoshi Y, Suzuki H, Seki H, Muranaka T, Ohyama K, Fujimoto Y (2016) Ajuga Δ24-sterol reductase catalyzes the direct reductive conversion of 24-methylenecholesterol to campesterol. J Biol Chem 291:8189–8198. https://doi.org/10.1074/jbc.M115.703470
Upadhyay S, Phukan UJ, Mishra DS, Shukla RK (2014) De novo leaf and root transcriptome analysis identified novel genes involved in Steroidal sapogenin biosynthesis in Asparagus racemosus. BMC Genomics. https://doi.org/10.1186/1471-2164-15-746
Venkatramesh M, Guo DA, Harman JG, Nes WD (1996a) Sterol specificity of the Saccharomyces cerevisiae ERG6 gene product expressed in Escherichia coli. Lipids 31:373–377. https://doi.org/10.1007/BF02522922
Venkatramesh M, Guo DA, Jia ZH, Nes WD (1996b) Mechanism and structural requirements for transformation of substrates by the (S)-adenosyl-L-methionine:Δ24(25)sterol methyl transferase from Saccharomyces cerevisiae. Biochem Biophys Acta 1299:313–324. https://doi.org/10.1016/0005-2760(95)00218-9
Venugopalan A, Potunuru UR, Dixit M, Srivastava S (2016) Effect of fermentation parameters, elicitors and precursors on camptothecin production from the endophyte Fusarium solani. Biores Technol 206:104–111. https://doi.org/10.1016/j.biortech.2016.01.079
Visbal G, Alvarez A, Moreno B, San-Blas G (2003) S-Adenosyl-L-methionine inhibitors delta(24)-sterol methyltransferase and delta(24(28))-sterol methylreductase as possible agents against Paracoccidioides brasiliensis. Antimicrob Agents Chemother 47:2966–2970. https://doi.org/10.1128/aac.47.9.2966-2970.2003
Wang FQ, Li B, Wang Y, Zhang CG, Wei DZ (2007) Biotransformation of diosgenin to nuatigenin-type steroid by a newly isolated strain, Streptomyces virginiae IBL14. Appl Microbiol Biotechnol 77:771–777. https://doi.org/10.1007/s00253-007-1216-1
Wang LW, Xu BG, Wang JY, Su ZZ, Lin FC, Zhang CL, Kubicek CP (2012) Bioactive metabolites from Phoma species, an endophytic fungus from the Chinese medicinal plant Arisaema erubescens. Appl Microbiol Biotechnol 93:1231–1239. https://doi.org/10.1007/s00253-011-3472-3
Wang YC, Li X, Sun H, Yi KX, Zheng JL, Zhang J, Hao ZB (2014) Biotransformation of steroidal saponins in sisal ( Agave sisalana Perrine) to tigogenin by a newly isolated strain from a karst area of Guilin, China. Biotechnol Biotechnol Equip 28:1024–1033. https://doi.org/10.1080/13102818.2014.978199
Wang LS, Ma T, Zheng YP, Lv SQ, Li Y, Liu SX (2015) Diosgenin inhibits IL-1beta-induced expression of inflammatory mediators in human osteoarthritis chondrocytes. Int J Clinic Exp Pathol 8:4830–4836
Wang Q, Huiju Z, Min Y, Hua Z, Zhou N, Li Y (2018) Effects of 28 species of AM fungi on diosgenin contents in Paris polyphylla var. yunnanensis. J Dali Univ 2:22–25. https://doi.org/10.3969/j.issn.2096-2266.2018.10.005
Weete JD, Abril M, Blackwell M (2010) Phylogenetic distribution of fungal sterols. PLoS One 5:e10899. https://doi.org/10.1371/journal.pone.0010899
Wei M, Bai Y, Ao MZ, Jin WW, Yu PP, Zhu M, Yu LJ (2013) Novel method utilizing microbial treatment for cleaner production of diosgenin from Dioscorea zingiberensis CH Wright (DZW). Bioresour Technol 146C:549–555. https://doi.org/10.1016/j.biortech.2013.07.090
Wilson AE, Tian L (2019) Phylogenomic analysis of UDP-dependent glycosyltransferases provides insights into the evolutionary landscape of glycosylation in plant metabolism. Plant J. https://doi.org/10.1111/tpj.14514
Wriessnegger T, Pichler H (2013) Yeast metabolic engineering–targeting sterol metabolism and terpenoid formation. Prog Lipid Res 52:277–293. https://doi.org/10.1016/j.plipres.2013.03.001
Wu GW, Gao JM, Shi XW, Zhang Q, Wei SP, Ding K (2011) Microbial transformations of diosgenin by the white-rot basidiomycete Coriolus versicolor. J Nat Prod 74:2095–2101. https://doi.org/10.1021/np2003484
Xiao X, Liu XK, Fu SB, Sun DA (2011) Microbial transformation of diosgenin by filamentous fungus Cunninghamella echinulata. J Asian Nat Prod Res 13:270–275. https://doi.org/10.1080/10286020.2010.551117
Xie YX, Sen B, Wang GY (2017) Mining terpenoids production and biosynthetic pathway in thraustochytrids. Bioresour Technol 244:1269–1280. https://doi.org/10.1016/j.biortech.2017.05.002
Xu M et al (2015) Microbial transformation of diosgenin by Cunninghamella blakesleana AS 3.970 and potential inhibitory effects on P-glycoprotein of its metabolites. RSC Adv 5:78081–78089. https://doi.org/10.1039/c5ra12253h
Yang XD, Yang YY, Ouyang DS, Yang GP (2015) A review of biotransformation and pharmacology of ginsenoside compound K. Fitoterapia. https://doi.org/10.1016/j.fitote.2014.11.019
Yang JC et al (2017a) Anti-trachea inflammatory effects of diosgenin from Dioscorea nipponica through interactions with glucocorticoid receptor α. Journal of International Medical Research 45:101–103. https://doi.org/10.1177/0300060516676724
Yang R et al (2017b) Dioscin relieves endotoxemia induced acute neuro-inflammation and protect neurogenesis via improving 5-HT metabolism. Scientific Reports 7:40035. https://doi.org/10.1038/srep40035
Yang BY, Yin X, Liu Y, Zhao DY, Kuang HX (2018a) New steroidal saponins from the roots of Solanum melongena L. Fitoterapia 128:12–19. https://doi.org/10.1016/j.fitote.2018.04.021
Yang XC, Li YB, Liao QH, Yang SC, Tao L (2018b) Isolation and screening of endophytic fungi that might produce saponins in Paris polyphylla var. yunnanensis. Mol Plant Breed 16:665–669. https://doi.org/10.13271/j.mpb.016.000665
Yang ZY, Yang LF, Liu CK, Qin XJ, Liu HY, Chen JH, Ji YH (2019) Transcriptome analyses of Paris polyphylla var. chinensis, Ypsilandra thibetica, and Polygonatum kingianum characterize their steroidal saponin biosynthesis pathway. Fitoterapia 135:52–63. https://doi.org/10.1016/j.fitote.2019.04.008
Yin Y, Gao LH, Zhang XN, Gao W (2018) A cytochrome P450 monooxygenase responsible for the C-22 hydroxylation step in the Paris polyphylla steroidal saponin biosynthesis pathway. Phytochemistry 156:116–123. https://doi.org/10.1016/j.phytochem.2018.09.005
Yoshida Y, Aoyama Y, Noshiro M, Gotoh O (2000) Sterol 14-demethylase P450 (CYP51) provides a breakthrough for the discussion on the evolution of cytochrome P450 gene superfamily. Biochem Biophys Res Commun 273:799–804. https://doi.org/10.1006/bbrc.2000.3030
Youn JH et al (2015) ARF7 increases the endogenous contents of castasterone through suppression of BAS1 expression in Arabidopsis thaliana. Phytochemistry. https://doi.org/10.1016/j.phytochem.2015.11.006
Youn JH et al (2018a) DWF1 functions as a C-24 reductase to convert 24-methylene brassinosteroids to 24-methyl brassinosteroids in Arabidopsis thaliana. J Exp Bot. https://doi.org/10.1093/jxb/ery038
Youn JH et al (2018b) Function and molecular regulation of DWARF1 as a C-24 reductase in brassinosteroid biosynthesis in Arabidopsis. J Exp Bot 69:1873–1886. https://doi.org/10.1093/jxb/ery038
Yu YN et al (2014) Comparative effectiveness of Di’ao Xin Xue Kang capsule and compound danshen tablet in patients with symptomatic chronic stable angina. Sci Rep 4:7058. https://doi.org/10.1038/srep07058
Yu SS et al (2017) Microbial transformation of ginsenoside Rb1, Re and Rg1 and its contribution to the improved anti-inflammatory activity of ginseng. Sci Rep 7:138. https://doi.org/10.1038/s41598-017-00262-0
Yuan WQ, Zhang RR, Wang J, Ma Y, Li WX, Jiang RW, Cai SH (2016) Asclepiasterol, a novel C21 steroidal glycoside derived from Asclepias curassavica, reverses tumor multidrug resistance by down-regulating P-glycoprotein expression. Oncotarget 7:31466–31483. https://doi.org/10.18632/oncotarget.8965
Yuan YL et al (2016b) Timosaponin B-II Ameliorates Palmitate-Induced Insulin Resistance and Inflammation via IRS-1/PI3K/Akt and IKK/NF-κB Pathways. The American Journal of Chinese Medicine 44:1–15. https://doi.org/10.1142/S0192415X16500415
Zhang XJ, Tang LL, Wang YD (2007) Isolating and screening of steroidal saponins-producing entophytic fungi, actinomycete from Paris Polyphylla var. Chinensis Franch. Prog Modern Biomed 7:358–360. https://doi.org/10.13241/j.cnki.pmb.2007.03.013
Zhang J et al (2016a) Galactosylation of steroidal saponins by β-galactosidase from Lactobacillus bulgaricus L3. Glycoconj J 33:53–62. https://doi.org/10.1007/s10719-015-9632-4
Zhang X et al (2016b) Sterol methyl oxidases affect embryo development via auxin-associated mechanisms. Plant Physiol 171:468–482. https://doi.org/10.1104/pp.15.01814
Zhang Y et al (2016c) Anti-inflammatory steroids from the rhizomes of Dioscorea septemloba Thunb. Steroids 112:95–102. https://doi.org/10.1016/j.steroids.2016.05.007
Zhang TT, Gong T, Hu ZF, Gu AD, Yang JL, Zhu P (2018) Enzymatic synthesis of unnatural ginsenosides using a promiscuous UDP-glucosyltransferase from Bacillus subtilis. Molecules. https://doi.org/10.3390/molecules23112797
Zhao M, He SR, Chen XJ, Huang CP, Wang YD (2005) Screening and identification of steroidal saponins-producing endophytes from Paris polyphylla var. Chinensis Franch. Acta Microbiol Sin 045:776–779
Zhao Y, Sun LM, Wang XN, Shen T, Ji M, Li X, Lou HX (2009) Microbial transformation of diosgenin by Syncephalastrum racemosum (Cohn) Schroeter. Chin Chem Lett 21:76–80. https://doi.org/10.1016/j.cclet.2009.08.015
Zhao Y, Jiang TC, Han BQ, Lu L, Feng B (2015) Preparation of some metabolites of timosaponin BII by biotransformation in vitro. Process Biochem 50:2182–2187. https://doi.org/10.1016/j.procbio.2015.09.022
Zhao YZ et al (2018) Advances in the antitumor activities and mechanisms of action of steroidal saponins. Chin J Nat Med 16:732–748. https://doi.org/10.1016/s1875-5364(18)30113-4
Zhou LG, Cao XD, Yang CD, Wu XH, Zhang LQ (2004) Endophytic fungi of Paris polyphylla var. yunnanensis and steroid analysis in the fungi. Nat Product Res Dev 16:198–200. https://doi.org/10.16333/j.1001-6880.2004.03.003
Zhou WB et al (2010) Hydrolysis of timosaponin BII by the crude enzyme from Aspergillus niger AS 3.0739. J Asian Nat Prod Res 12:955–961. https://doi.org/10.1080/10286020.2010.510470
Zhu Q, Wu FT, Ding F, Ye D, Chen YQ, Li Y, Zhifan Y (2009) Agrobacterium-mediated transformation of Dioscorea zingiberensis Wright, an important pharmaceutical crop. Plant Cell Tissue Organ Cult 96:317–324. https://doi.org/10.1007/s11240-008-9489-3
Zhu SL, Tang SY, Su F (2017) Dioscin inhibits ischemic stroke-induced inflammation through inhibition of the TLR4/MyD88/NF-κB signaling pathway in a rat model. Molecular Medicine Reports 17:660–666. https://doi.org/10.3892/mmr.2017.7900
Zhu JH, Li HL, Guo D, Wang Y, Dai HF, Mei WL, Peng SQ (2018a) Identification, characterization and expression analysis of genes involved in steroidal saponin biosynthesis in Dracaena cambodiana. J Plant Res 131:555–562. https://doi.org/10.1007/s10265-017-1004-7
Zhu X, Wang K, Zhang K, Pan Y, Zhou FF, Zhu L (2018b) Polyphyllin I induces cell cycle arrest and cell apoptosis in human retinoblastoma Y-79 cells through targeting p53. Anticancer Agents Med Chem 18:875–881. https://doi.org/10.2174/1871520618666180108095148
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81872967).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no confict of interest.
Additional information
Communicated by Gerhard Leubner.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, Y., Wu, J., Yu, D. et al. Advances in steroidal saponins biosynthesis. Planta 254, 91 (2021). https://doi.org/10.1007/s00425-021-03732-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-021-03732-y