Abstract
In the present study, a simple one medium formulation protocol for callus culture, somatic embryogenesis and in vitro production of β-carboline alkaloids and diosgenin in Tribulus terrestris L. was developed. Extensive callus induction and proliferation was obtained in leaf explant on Murashige and Skoog (MS) medium supplemented with 5.0 μM 6 benzyl adenine (BA) and 2.5 μM α-naphthaleneacetic acid (NAA). The embryogenic callus was maintained on subculture to fresh parental medium at 4-week intervals over a period of 28 months. The frequency of embryo formation was at a maximum (18.1 ± 0.9 per g of callus) on MS medium containing 5.0 μM BA and 2.5 μM NAA together with 75 mg l−1 casein hydrolysate. Globular embryo developed into torpedo stage embryo under the influence of starvation. The accumulation of β-carboline alkaloids (harmaline and harmine) and steroidal saponin (diosgenin) in non-embryogenic and embryogenic callus culture derived from leaf explant was compared with root, leaf, stem, and fruit of the mother plant. The embryogenic callus accumulated equivalent amounts of harmaline (66.4 ± 0.5 μg/g dry weight), harmine (82.7 ± 0.6 μg/g dry weight), and diosgenin (170.7 ± 1.0 μg/g dry weight) to that of the fruit of T. terrestris. The embryogenic callus culture of this species might offer a potential source for production of important pharmaceuticals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tribulus terrestris L. (Zygophyllaceae) is highly valued for the Indian and Chinese systems of medicine. It grows widely in most semi-arid and arid regions of the world. In India, the fruits have long been used as a tonic, a diuretic against kidney diseases and stones and for treating impotence (Chopra et al. 1956; Joshi et al. 2005), while in traditional Chinese medicine, the fruit has been used for treating high blood pressure and coronary heart disease (Phillips et al. 2005). This plant is extremely rich in substances having potential biological significance, including β-carboline alkaloids, saponin, flavonoids, phytosteroids, and other nutrients (Xu et al. 2001). The drug named ‘‘Xinnao Shutong’’ was manufactured from the crude saponin fraction of this plant having significant effects for the treatment of various cardiac diseases, including hypertension, coronary heart disease, myocardial infarction, cerebral arteriosclerosis, and thrombosis (Yang et al. 1991). It serves as a natural testosterone enhancer that invigorates and boosts strength, stamina, muscle growth, fat reduction, and libido (Drewes et al. 2003). The β-carboline alkaloids exert a wide range of pharmacological properties including anti-microbial, anti-leishmanial activity (Di Giorgio et al. 2004), bioinsecticidal (Rharrabe et al. 2007), and cytotoxic to cancer cells (Rivas et al. 1999).
Seed germination and seedling establishment in T. terrestris are vulnerable to environmental stress and produce very limited numbers of plants. The growth of plants is very slow and produces scanty biomass. Collecting naturally grown plants may be ecologically damaging and is usually controlled by state regulations. The establishment of a reliable in vitro regeneration protocol is a prerequisite for the application of genetic engineering techniques to enhance production of medicinally important metabolites. Somatic embryogenesis has often been considered to be a suitable system for large-scale plant propagation, conservation of germplasm, production of synthetic seeds, and genetic transformation. Plant regeneration via somatic embryogenesis has been reported in varieties of explants, plant genera, and species (Gopi and Ponmurugan 2006).
Tissue culture systems for a number of medicinal plants have been established, and this enables the analysis of callus and cell suspension for the presence of varieties of secondary metabolites (Rao and Ravishankar 2002; Komaraiah et al. 2003). Bioreactor cultures can produce plant material that is physiologically uniform and pathogen-free, as well as containing valuable secondary metabolites, and all with reduced labor. Field-grown plant material has generally been used for the preparation of drugs, but under different environmental conditions, pollutants and pathogens can alter productivity. The majority of plant-based alkaloids cannot be chemically synthesized. In view of this, attempts to produce pharmaceutically important secondary metabolites using tissue culture have been increasing. A review of the Tribulus literature revealed that in vitro plant regeneration from cotyledonary leaves of young seedlings (Ali et al. 1997) and somatic embryogenesis from stem-derived callus of T. terrestris (Mohan et al. 2000) required a few changes of media fortified with tested exogenous plant growth factors. The present study deals with the induction of somatic embryogenesis in leaf-derived callus, transfer of plantlets, and the detection and accumulation of harmaline, harmine, and diosgenin in non-embryogenic and embryogenic callus.
We observed that the in vitro developed embryos contained alkaloids and saponin similar to the seeds of naturally grown plants. By using a simple one medium formulation, the embryogenesis protocol reported here makes the production of secondary metabolites in bioreactors a possibility.
Materials and methods
Plant material and sterilization
Healthy plants of T. terrestris were collected from the natural population of Western Ghats, Pune District, Maharashtra, India, and used as a source of leaflet explants after establishment at the botanic garden, University of Pune. The plant specimens were identified and deposited at the Botanical Survey of India Regional Office, Western Circle, Pune, India (specimen voucher numbers-MAET2). The young leaflets were washed five times with sterilized distilled water then disinfected for 5 min in 0.1% HgCl2. Later explants were rinsed five times with sterile distilled water and inoculated on culture media after applying cuts at the edge of the leaflets.
Media preparation and culture conditions
For all experiments, MS (Murashige and Skoog 1962) medium with 3% sucrose and 100 mg l−1 myo-inositol was used. The pH was adjusted to 5.5–6.0 using 1 N HCl or 1 N NaOH before adding 0.8% (w/v) of agar, and was subsequently autoclaved under 105 kPa at a temperature 121°C for 15 min. The MS medium with different concentrations of BA (benzyl adenine) and Kn (Kinetin) (0.0–20.0 μM) individually, or with indoleacetic acid (IAA), NAA (α-naphthaleneacetic acid) and 2,4-D (2,4-dichlorophenoxyacetic acid) (0.0–10 μM), was investigated to optimize hormonal requirement for callus formation and somatic embryogenesis. All cultures were maintained at 25 ± 2°C in 8 h light/16 h dark photoperiod with irradiance (20–40 μmol m−2 s−1) by cool fluorescent tubes.
Maintenance of calluses, their growth kinetics, somatic embryogenesis and transplantation
The extensive callus proliferating media were selected during experimental trials and used for maintenance of callus (Table 1). The callus masses of 500 mg were subcultured at 4-week intervals in 300-ml glass bottles containing 25 ml fresh parental medium for maintenance and study of growth kinetic of each callus line. At every subculture after initiation, 10 cultures were put aside to allow further somatic embryo development. The calluses were subcultured on media containing various concentration of casein hydrolysate (0.0–100 mg l−1) and observed weekly for the development of embryos. The calluses were allowed to develop into somatic embryos for about 6 weeks. The callus from 10 bottles of each callus line were harvested every 7 days over a period of 42 days to determine each callus line's fresh and dry weight. The dried callus biomass was determined after the calluses were air-dried until constant weight was attained.
The well-developed cotyledonary embryos were transferred to MS medium without phytohormones for germination and development of the plantlets. The plantlets were then transferred to pots containing garden soil and placed in shady conditions. After 4 weeks of hardening, the plants were transferred to the field.
Detection of β-carboline alkaloids
The air-dried powdered callus collected on days 7, 14, 21, 28, and 35 of culture and natural plant material (fruit, stem and leaf) were used for obtaining the crude extract by soaking 1.0 g of the dried biomass in 50 ml methanol at 50°C in water bath for 1 h. The extracts were combined and evaporated to dryness. The residue was dissolved in 50 ml HCl (2%) then filtered through Whatman No. 1 filter paper. The filtrate was extracted twice with 20 ml petroleum ether. The aqueous acid layer was basified (pH 10) with NH4OH and extracted four times with 50 ml chloroform. The chloroform layer was combined and evaporated to dryness, and then the residues were dissolved in 25 ml methanol (Kartal et al. 2003). The solution of alkaloid extract was passed through a 0.45-μm filter and then 0.2 μl extract was directly injected into the HPTLC column. CAMAG analytical HPTLC system was used for estimation of β-carboline alkaloid (Anchrom lab, Mumbai, 400081, India). The results were obtained as a mean value of three separate injections. Harmine (H-8646; Sigma Chemicals) and harmaline (H-2256; Sigma Chemicals) were used as standards. Aluminum sheets of silica gel 60F254 (Merck) were used. The chromatograms were developed in the mobile phase chloroform:methanol:25% ammonia—5:4:1, dried and sprayed. Alkaloids were analyzed by using CAMG TLC Scanner 3 in UV-254 and UV-366 nm. The peak corresponding to harmine and harmaline were confirmed by comparison with the commercial standard and the crude extract samples with standard. The alkaloids content in the crude extract was determined by comparing the peak areas with those of standard harmine and harmaline.
Detection of diosgenin
The dried powdered samples of 500 mg were added to 15 ml of 2 N H2SO4 in 70% isopropanol and boiled under reflux for 8 h. Fifteen ml of water was added and the aqueous phase extracted four times with hexane. The hexane extracts were combined and extracted twice with 10-ml portions of 1–2 N NaOH. The hexane phase was extracted once with 25 ml of water, then reduced to a volume that was convenient for analytical separation by HPTLC using CAMAG TLC scanner 3. The standard of diosgenin (Sigma) by dissolving 1 mg each of diosgenin in 1 ml methanol. The developing solvent was chloroform:acetic acid:methanol:water (6.4:3.2:1.2:0.8). TLC plates containing fluorescent indicator after derivatization with anisaldehyde sulphuric acid were examined for diosgenin content in UV-366 nm. Diosgenin content was calculated by area of standard and area of sample.
Statistical analysis
A completely randomized design was used in all experiments. The experiments were repeated thrice with 10 replicates each. The mean standard error and ANOVA were calculated, and mean separation were carried out using DMRT (Duncan 1955) at 5% level of significance.
Results and discussion
Callus initiation and maintenance
The leaflet explants of T. terrestris remained green and fresh for 2 weeks, but failed to morphogenically respond with callus formation on hormone-free MS medium. All leaflet explants cultured on MS medium fortified with phytohormones (1.0–10 μM) grew in size, and callus formation began from cut portions in some explants within 10–15 days of incubation. Of the various treatments (about 400) with MS medium, auxin:cytokinin ratios were generally recognized as a critical factor for proliferation of callus. Extensive callus formation was observed on medium containing an auxin:cytokinin ratio less than one or equal to one (Table 1). By the end of 4 weeks, the whole surface of explants was covered by callus. However, there was a trend toward a gradual decrease in proliferation of callus as the concentration of auxin or cytokinin increased to 15–20 μM. The frequency of callus varied depending on the hormonal combination used. 2,4-D was more effective than IAA and NAA for callus induction. The outcome of the present study, and earlier results of Erhun and Sofowora (1986), suggest that mostly auxin NAA or 2,4-D in combination with cytokinin, particularly BA, was most effective for initiation and growth of callus in T. terrestris. According to Mohan et al. (2000), MS supplemented with 0.5 mg l−1 2, 4 D and 0.5 mg l−1 Kn was best for callus induction in intermodal segments and maintenance of stem callus. Asghari and Lockwood (2002) obtained similar results in another member of Zygophyllaceae, Peganum harmala, where 2,4-D in combination with cytokinin was very effective for callus proliferation.
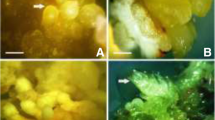
Initially, callus produced on all media was white, soft, and non-regenerative, except in media containing IAA (1.5–11.5 μM) or NAA (1.5–5.0 μM) where callus-mediated root formation was observed. In the fifth week, the calluses gradually turned dark yellow or faint red in color. However, after 3 weeks of subculturing on the same fresh medium over a period of 3–4 weeks, the white callus become translucent, greenish, and embryogenic on some media (Table 1, Fig. 1a), while in other media, it turned yellow in color without any morphogenic response. In the present study, the cultured leaf explants failed to respond for shoot regeneration. Similarly, there has been no report of shoot regeneration in stem explants (Mohan et al. 2000). However, Ali et al. (1997) reported the direct regeneration of shoots in cotyledonary explants of T. terrestris. This difference might be attributed to the differences in the shoot regeneration potentiality of various parts of T. terrestris.
In the present investigation, calluses were maintained on subculture over a period of 28 months. The embryogenic and non-embryogenic response was consistent during the maintenance of callus (Table 1). The influence of auxin and cytokinin on callus proliferation was evaluated at different time intervals. The combination of 5.0 μM BA and 2.5 μM 2,4-D produced slightly more callus than the combinations of 5.0 μM BA/Kn with 2.5 μM NAA and 5.0 μM BA/Kn with 2.5 μM IAA tested at all the time intervals (Table 1). However, there was no significant difference in the fresh weight and dry weight of the calluses, but the calluses were classified on the basis of response as non-embryogenic and embryogenic calluses. The growth of callus was less on media fortified with auxin and cytokinin separately, suggesting that both auxin and cytokinin was necessary for extensive callus proliferation in T. terrestris. Similar to Erhun and Sofowora (1986), the fresh weight and dry weight of callus noticed in this study were higher on media containing auxin and cytokinin together. The callus grown in this medium was faint greenish in color, granular, and fast growing. The callus was subcultured at intervals of 4 weeks for further proliferation and induction of embryogenesis.
Embryogenesis and plant regeneration
Phytohormone concentrations in the MS medium greatly affected the embryogenesis in the callus (Table 1). Embryogenic calluses were snowy white to faint greenish in color and were highly friable after 4 weeks of culture (Fig. 1a). The embryogenetic callus induction with higher percentage frequency (>68%) was obtained in media containing 5.0 μM BA and 2.5 μM NAA. Appearance of starch in the callus cells was usually linked with an embryogenic nature of the cells (Profumo et al. 1987). Similarly, the embryogenic calluses of T. terrestris were composed of small greenish and densely cytoplasmic cells with starch. These calluses led to somatic embryogenesis from small greenish globular masses (Fig. 1b), passing through successive developmental stages, heart and cotyledonary, during 5–6 weeks of culture (Fig. 1c). The somatic embryos were easily detected, and about 90% of somatic embryos germinated and converted into plantlets. Plantlets were planted in pots containing a (1:1) soil:sand mixture and were transferred to the field where they grew until flowering as natural plants (Fig. 1d).
The frequency of formation of the mean number of embryos per culture was increased on incorporation of casein hydrolysate (CH) in the MS medium. Among the different concentrations of CH (0.0–100 mg l−1), addition of 75 mg l−1 CH was very effective, with the percent frequency of embryogenic callus (>84%) and mean number of embryos formation reaching a maximum (18.1 ± 0.9). In the present study, we observed that the supplementation of 2.5 μM NAA together with 5.0 μM BA and 75 mg l−1CH was sufficient for induction of embryogenic callus and development of somatic embryo from leaf explants. The embryogenic potentiality of the callus more or less remained constant on subculture to fresh parental medium at 4-week intervals over a period of 28 months. The callus, which did not show embryogenesis in induction and proliferation media, were subcultured on medium containing 5.0 μM BA and 2.5 μM NAA incorporated with 75 mg l−1 CH. It was noticed that the response for embryogenesis depended on the initial composition of the callus induction medium. It was observed that larger numbers of embryos were produced in callus induced in media containing 2–5 μM BA and 5–10 μM NAA together with 75 mg l−1 CH. This indicated that the ratio of cytokinin BA to auxin and the addition of CH (75 mg l−1) was important for embryo formation (Table 2). The maximum mean number of embryos per callus (18.1 ± 0.9) was recorded after 6 weeks of culture.
After subculturing for the first 3 weeks, callus proliferation was observed without organization of embryos. This might be because of the presence of auxin in the medium, as auxin is known to inhibit embryogenesis after the formation of embryogenic cell cultures (Komamine et al. 1992). The fourth, fifth and sixth weeks of culture might create the environmental stress, such as availability of less water and also nutrients and phytohormones. Finally, the starvation inhibits the proliferation of undifferentiated cells of callus and stimulates the formation of embryos. There have been several reports of increased somatic embryogenesis following long-term neglect and a subsequent reduction of nutrients, e.g., in Daucus carota (Lee et al. 1997) and Panax ginseng (Choi et al. 1998). It has also been shown that there is a correlation between increased somatic embryogenesis and stress: e.g., high levels of sucrose (Kamada et al. 1989) or low levels of macronutrients in the medium (Choi et al. 1998).
Mohan et al. (2000) reported the formation of somatic embryogenesis in stem-derived callus of T. terrestris and suggested the inclusion of 0.5 mg l−1 2,4-D in conjunction with 0.5 mg l−1 BA for callus induction, and later subculture of callus on medium containing 0.5 mg l−1 indolebutyric acid (IBA) together with 2.0 mg l−1 BA, 500 mg l−1 CH and 2.0 mg l−1 silver nitrate, for somatic embryo development. Plantlet formation from somatic embryos showed the necessity of transfer of somatic embryos on media fortified with 0.1 mg l−1NAA and 2.0 mg l−1 IBA. In this study, we report a simplified single medium formulation for somatic embryogenesis in T. terrestris which has importance in micropropagation and medicinally important secondary metabolite production, eventually in term of cost, time and efforts.
Secondary metabolites
The results of HPTLC and spectrophotometric analysis recorded in Table 1 and Fig. 2 showed significant differences on the accumulation of harmaline, harmine, and diosgenin in the callus harvested weekly on media supplemented with different phytohormones and their various concentrations.
HPTLC fingerprint profile and densitometric scanning of non-embryogenic and embryogenic callus culture of T. terrestris L. obtain on MS medium containing auxins and cytokinins. a Harmaline and harmine (Lane 1 5 μM Kn + 2.5 μM, 24-D, 2 5 μM Kn + 2.5 μM NAA, 3 5 μM Kn + 2.5 μM IAA, 4 Standard for harmaline, 5 Blank, 6 natural leaf; 7 natural fruits, 8 natural roots, 9 5 μM BA + 2.5 μM NAA, 10 5 μM BA + 2.5 μM NAA + 75 mg-1 CH, 11 standard Harmine, 12 blank, 13 5 μM BA + 2.5 μM 2,4-D, 14 5 μM BA + 2.5 μM NAA, 15 5 μM BA + 2.5 μM IAA. b Diosgenin in callus (Lane 1 5 μM BA + 2.5 μM NAA, 2 5 μM BA + 2.5 μM NAA + 75 mg-1 CH, 3 natural leaf, 4 natural roots, 5 natural fruits, 6–7 standard diosgenin, 8 5 μM BA + 2.5 μM 2,4-D, 9 5 μM Kn + 2.5 μM NAA, 10 5 μM Kn + 2.5 μM IAA, 11 5 μM BA + 2.5 μM 2,4-D, 12 5 μM BA + 2.5 μM NAA, 13 5 μM BA + 2.5 μM IAA). c Harmaline and harmine in embryogenic callus. d Diosgenin in embryogenic callus
Among the different media, harmaline accumulation was higher at the fifth week during the growth cycle of the callus obtained on MS medium fortified with 5.0 μM BA and 2.5 μM NAA together, while incorporation of Kn was more effective than BA along with auxins for accumulation of harmine. The harmine content was greater in the callus obtained on MS containing 5.0 μM Kn and 2.5 μM NAA. The content of these alkaloids was significantly less in the callus derived on MS medium with 2,4-D alone and 2,4-D in conjunction with Kn and BA. Similar results were reported by Berlin et al. (1994) with lower accumulation of β-carboline alkaloid on 2,4-D-containing media in Peganum harmala, another member of the Zygophyllaceae.
The diosgenin content of callus was comparatively less on the MS medium supplemented with 2,4-D, IAA and NAA alone and with the combination of IAA and NAA with BA or Kn. The influence of 2,4-D in combination with BA or Kn was considerable. Higher accumulation of diosgenin was observed at the fifth week during the growth cycle of callus on MS fortified with 5.0 μM Kn and 2.5 μM 2,4-D. These results suggest that the growth regulator in the medium influences the biosynthesis of harmaline, harmine, and diosgenin in callus of Tribulus terrestris.
It is well known that the synthesis and accumulation of many secondary plant metabolites is in some way associated with tissue differentiation and development. In the present study, we noted that, the accumulation of harmaline, harmine and diosgenin was higher in the embryogenic callus as compared to the non-embryogenic callus (Table 1). In agreement with this, Garve et al. (1980) observed lower levels of cardenolide in unorganized cells; however, when embryo formation was triggered, cardenolide levels were enhanced. During somatic embryogenesis in Aesculus hippocastanum, aescin content reached higher levels than in calluses (Profumo et al. 1991). Piovan et al. (2000) reported on the relationship between tissue differentiation and secondary metabolite biosynthesis; the biosynthetic capabilities (alkaloid production) of an embryogenic and two non-embryogenic cell lines of Catharanthus roseus were compared. Faster cell growth rate was observed to be associated with higher alkaloid production in the embryogenic cell line.
The supplementation of CH at the concentration 25–100 mg l−1 stimulated the growth of callus and induction of somatic embryogenesis (Table 2). The harmaline (72.4 ± 0.6 μg/g dry weight), harmine (88.2 ± 0.8 μg/g dry weight), and diosgenin (181.3 ± 1.1 μg/g dry weight) content of callus showed about 9–18% increase when casein hydrolysate was added up to 75 mg l−1 to the medium as compared to control at the fifth week of culture. Similar results of increase of growth and secondary metabolites accumulation with addition of CH in culture media was reported in Artemisia annua (Woerdenbag et al. 1993), Coleus forskohlii (Mukherjee et al. 2000), Zanthoxylum stenophyllum (Biondi et al. 2004), and Panax ginseng (Wu et al. 2005).
Tribulus terrestris embryogenic calluses showed considerable increase in embryo differentiation and in the accumulation of the pharmaceuticals, harmaline, harmine, and diosgenin, equivalent to the fruits of the naturally grown plants when they were cultured in the presence of CH. These observations demonstrate why somatic embryos are becoming popular as model systems to verify the capabilities of different plant species. Experiments using this model system can provide us with new insights into the application of biotechnological advances and production of β-carboline alkaloids and diosgenin.
References
Ali G, Mughal MH, Srivastava PS, Iqbal M (1997) Micropropagation of Tribulus terrestris L., an important medicinal plant. J Plant Biol 40:202–205
Asghari G, Lockwood BG (2002) Stereospecific biotransformation of (±) phenylethyl propionate by cell cultures of Peganum harmala L. Iran Biomed J 6:43–46
Berlin JC, Rügenhagen C, Kuzovkina IN, Fecker LF, Sasse F (1994) Are tissue cultures of Peganum harmala a useful model system for studying how to manipulate the formation of secondary metabolites? Plant Cell Tissue Organ Cult 38:289–297
Biondi S, Antognoni F, Crespi PN, Sacchetti G, Minghetti A, Poli F (2004) Medium composition and methyl jasmonate influence the amount and spectrum of secondary metabolites in callus cultures of Zanthoxylum stenophyllum. Hemsl Plant Biosys 138:117–124
Choi YE, Yang DC, Choi KT (1998) Induction of somatic embryos by macro salt stress from mature zygotic embryos of Panax ginseng. Plant Cell Tissue Organ Cult 52:177–181
Chopra RN, Nayar SL, Chopra IC (1956) Glossary of Indian medicinal plants. Council of Scientist and Industrial Research, New Delhi, pp 246–247
Di Giorgio C, Delmas F, Ollivier E, Elias R, Balansard G, Timon-Davida P (2004) In vitro activity of the β-carboline alkaloids harmane, harmine, and harmaline toward parasites of the species Leishmania infantum. Exp Parasitol 106:67–74
Drewes SD, George J, Fatima KF (2003) Recent findings on natural products with erectile-dysfunction activity. Phytochemistry 62:1019–1025
Duncan DB (1955) Multiple range and multiple F-test. Biometrics 11:1–42
Erhun WO, Sofowora A (1986) Callus induction and detection of metabolites in Tribulus terrestris. J Plant Physiol 123:181–186
Garve R, Luckner M, Vogel E, Tewes A, Nover L (1980) Growth, morphogenesis and cardenolide formation in long term cultures of Digitalis lanata. Planta Med 40:92–103
Gopi C, Ponmurugan P (2006) Somatic embryogenesis and plant regeneration from leaf callus of Ocimum basilicum L. J Biotechnol 126:260–264
Joshi VS, Parekh BB, Joshi MJ, Vaidya AB (2005) Herbal extracts of Tribulus terrestris and Bergenia ligulata inhibit of calcium oxalate monohydrate crystals in vitro. J Cryst Growth 275:1403–1408
Kamada H, Kobayashi K, Kiyosue T, Harada H (1989) Stress induced somatic embryogenesis in carrot and its application to synthetic seed production. In Vitro Cell Dev Biol Plant 25:1163–1166
Kartal M, Altun ML, Kurucu S (2003) HPLC method for the analysis of harmol, harmalol, harmine and harmaline in the seeds of Peganum harmala L. J Pharm Biomed Anal 31:263–269
Komamine A, Kawahara R, Matsumoto M, Sunabari S, Fujwara A (1992) Mechanisms of somatic embryogenesis in cell cultures: physiology, biochemistry and molecular biology. In Vitro Cell Dev Biol Plant 28:11–14
Komaraiah P, Jogeswar G, Ramakrishna SV, Kavi kishor PB (2003) Acetylsalicylic acid and ammonium-induced somatic embryogenesis and enhanced plumbagin production in suspension cultures of Plumbago rosea. In Vitro Cell Dev Biol Plant 40:230–234
Lee EK, Cho DY, Soh WY (1997) Effect of humidity on somatic embryogenesis in cotyledon explant culture of carrot. J Plant Biol 40:89–94
Mohan J, Kumar VV, Aparna V, Vaidya RP (2000) Somatic embryogenesis and plant regeneration in Tribulus terrestris L. Phytomorphology 50:307–311
Mukherjee S, Ghosh B, Jha S (2000) Enhanced forskolin production in genetically transformed cultures of Coleus forskohlii by casein hydrolysate and studies on growth and organization. Biotechnol Lett 22:133–136
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Phillips OA, Mathew KT, Oriowo MA (2005) Antihypertensive and vasodilator effects of methanolic and aqueous extracts of Tribulus terrestris in rats. J Ethnopharmacol 3:351–355
Piovan A, Filippini R, Caniato R, Vecchia ED, Innocenti G, Cappelletti EM, Puricelli L (2000) Somatic embryogenesis in Catharanthus roseus and indole alkaloid production. Plant Biosyst 134:179–184
Profumo P, Gastaldo P, Rascio N (1987) Ultrastructural study of different types of callus from leaf explants of Aesculus hippocastanum L. Protoplasma 138:89–97
Profumo P, Caviglia AM, Gastaldo P, Dameri RM (1991) Aescin content in embryogenic callus and in embryoids from leaf explants of Aesculus hippocastanum. Planta Med 57:50–52
Rao SR, Ravishankar GA (2002) Plant cell cultures: chemical factories of secondary metabolites. Biotechnol Adv 20:101–153
Rharrabe K, Bakrim A, Ghailani N, Sayah F (2007) Pesticide biochemistry and physiology bioinsecticidal effect of harmaline on Plodia interpunctella development (Lepidoptera: Pyralidae). Pestic Biochem Physiol 98:137–145
Rivas P, Cassels BK, Morello A, Repetto Y (1999) Effects of some β-carboline alkaloids on intact Trypanosoma cruzi epimastigotes. Comp Biochem Physiol 122:27–31
Woerdenbag HJ, Lüers JFJ, Uden WV, Pras N, Malingré TM, Alfermann AW (1993) Production of the new antimalarial drug artemisinin in shoot cultures of Artemisia annua L. Plant Cell Tissue Organ Cult 32:247–257
Wu JY, Wong K, Ho KP, Zhou LG (2005) Enhancement of saponin production in Panax ginseng cell cultures by osmotic stress a nutrient feeding. Enzyme Microb Technol 36:133–138
Xu Y, Xie S, Zhao H, Han D, Xu T (2001) Studies on chemical constituents of Tribulus terrestris. Yao Xue Xue Bao 36:750–753
Yang XY, Hang BF, Jiang R, Yao YM, Chen HZ (1991) Xinnao shutong therapy in 50 patients with cerebral arteriosclerosis and the sequelae of cerebral thrombosis. New Drugs Clin Remedies 10:92–95
Acknowledgment
The authors would like to thank the UGC (DRS-SAP-II and ASIST) and DST (FIST) programme of Govt. of India for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nikam, T.D., Ebrahimi, M.A. & Patil, V.A. Embryogenic callus culture of Tribulus terrestris L. a potential source of harmaline, harmine and diosgenin. Plant Biotechnol Rep 3, 243–250 (2009). https://doi.org/10.1007/s11816-009-0096-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-009-0096-5