Abstract
Main conclusion
This review summarizes the current understanding, future challenges and ongoing quest on sugar metabolic alterations that influence the outcome of plant–pathogen interactions.
Intricate cellular and molecular events occur during plant–pathogen interactions. They cause major metabolic perturbations in the host and alterations in sugar metabolism play a pivotal role in governing the outcome of various kinds of plant–pathogen interactions. Sugar metabolizing enzymes and transporters of both host and pathogen origin get differentially regulated during the interactions. Both plant and pathogen compete for utilizing the host sugar metabolic machinery and in turn promote resistant or susceptible responses. However, the kind of sugar metabolism alteration that is beneficial for the host or pathogen is yet to be properly understood. Recently developed tools and methodologies are facilitating research to understand the intricate dynamics of sugar metabolism during the interactions. The present review elaborates current understanding, future challenges and ongoing quest on sugar metabolism, mobilization and regulation during various plant–pathogen interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants are under constant exposure to various pathogens. Different pathogens have evolved different strategies to obtain nutrients and to propagate, while plants have elaborate counter attack strategies to defend themselves (Nimchuk et al. 2003; Jones and Dangl 2006; Pieterse et al. 2012). During this ongoing battle, physiology and cellular metabolism of both host and pathogen get perturbed. Metabolism is the total sum of various biochemical processes that occur within a living organism; therefore, manipulation of such machinery is an ideal battlefield during plant–pathogen interactions (Duan et al. 2013). Alteration in photosynthetic machinery is most common amongst various pathogenic responses (Berger et al. 2007b). Various photosynthetic parameters such as Fv/Fm (maximum quantum efficiency of photosystem II), ETR (linear electron transport rate), ØPSII (operating efficiency of photosystem II) and NPQ (non-photochemical quenching) are found altered during pathogenesis (Scholes and Rolfe 1996; Petit et al. 2006; Rolfe and Scholes 2010). Various transcriptome studies have revealed photosynthesis-associated genes to be downregulated while respiratory processes, i.e., glycolysis, tricarboxylic acid cycle (TCA cycle) and mitochondrial electron transport chain to be upregulated in the infected tissues (Doehlemann et al. 2008; Parker et al. 2009; Chandran et al. 2010; Voll et al. 2011; Teixeira et al. 2014; Xu et al. 2015). Also various host secondary metabolites get induced during plant–pathogen interactions (Piasecka et al. 2015; Pusztahelyi et al. 2015). Interestingly, some of the plant secondary metabolites are known precursor of various phytohormones (such as salicylic acid, jasmonates) and defense-related (including phytoalexins) compounds (VanEtten et al. 1994; Dixon and Paiva 1995; Bolton 2009; Wojakowska et al. 2013; Piasecka et al. 2015; Pusztahelyi et al. 2015). Overall, reprogramming of host metabolism has emerged as a common theme during plant–pathogen interactions.
Previously, Berger et al. (2007b) and Bolton (2009) had reviewed the association of host primary metabolic changes during pathogenesis. However, with recent technological advancement, significant progress has been achieved towards dissecting the involvement of primary metabolic changes during plant–pathogen interactions. As sugars are core of primary metabolism, in this review we deliberate on current understanding, opportunities as well as available knowledge gaps about their dynamics and role during pathogenesis. We also prospect whether these changes are beneficial for the plant or the pathogen. For involvement of non-sugar primary metabolites (such as nitrogen), we refer readers to some of the recent reviews (Fagard et al. 2014; Rojas et al. 2014).
Changes in plant sugar metabolism: a common response during pathogenesis
Photosynthesis plays a vital role in management of biosynthesis and mobilization of various sugars. Alterations in photosynthesis as well as sugar metabolism play an important role during plant interactions with various pathogens including fungi (Table 1). For example, decreased photosynthetic activity is observed upon biotrophic fungal (Albugo candida, Golovinomyces orontii, Erysiphe cichoracearum) pathogen infection in Arabidopsis (Chou et al. 2000; Zimmerli et al. 2004; Chandran et al. 2010). Similarly, severe inhibition of photosynthesis is observed during pathogenesis of Botrytis cinerea (a necrotrophic fungal pathogen) in plants like Arabidopsis, tomato and lettuce (Berger et al. 2004; Windram et al. 2012; De Cremer et al. 2013; Smith et al. 2014). Moreover, the hemibiotrophic fungal pathogens like Colletotrichum lindemuthianum and Mycosphaerella graminicola are also known to inhibit photosynthesis during necrotrophic phase of their pathogenesis on beans and wheat, respectively (Lopes and Berger 2001; Meyer et al. 2001; Scholes and Rolfe 2009).
Besides fungal pathogens, decrease in photosynthesis is also observed during bacterial and viral infections in plants (Suppl. Table S1). For example, photosynthesis is significantly reduced during pathogenesis of Pseudomonas syringae on different hosts (Zou et al. 2005; Bonfig et al. 2006; Berger et al. 2007a). In a recent study, it has been observed that P. syringae utilizes effector molecules to disrupt photosystem II, inhibit photosynthetic CO2 assimilation and reprogram nuclear encoded chloroplast-targeted genes (NECGs) expression (de Torres Zabala et al. 2015). Repression of photosynthesis-associated genes is also observed during Bean Common Mosaic Virus (BCMV) infection on common bean (Martin et al. 2016). Besides this, downregulation of photosynthesis is also observed during plant–herbivore interactions (Zangerl et al. 2002; Tang et al. 2006). For example, repression of photosynthesis-related genes is observed in Nicotiana attenuate upon the attack of Manduca sexta, a moth (Hui et al. 2003). However, mirid bugs (Tupiocoris notatus) attack is found to enhance photosynthetic activity in N. attenuate (Halitschke et al. 2011). The authors had speculated that elevated CO2 assimilation might balance net photosynthesis in the mirid bugs affected leaves. Interestingly, the elevated rate of photosynthesis is also observed in the adjoining area of A. candida and B. cinerea infected regions of the leaves of Arabidopsis and tomato, respectively (Chou et al. 2000; Berger et al. 2004). Based on various studies, it has become apparent that during necrotrophic interaction, alteration in photosynthesis is rapid while during biotrophic interaction, such alterations are delayed, being observed after visible appearance of disease symptoms (Rolfe and Scholes 2010).
Besides photosynthesis, the host carbohydrate metabolism is also modulated by both biotrophic and necrotrophic pathogens (Table 1; Suppl. Table S1). For example, biotrophic fungal pathogen Ustilago maydis causes alteration in soluble sugar content in the infected maize leaves (Doehlemann et al. 2008; Horst et al. 2008). Notably, the defects in sugar accumulation (id1: indeterminate1; increased accumulation of sucrose) or starch metabolism (su1: sugary1; altered starch metabolism) impart tolerance against U. maydis infections in maize (Kretschmer et al. 2017). The adjustment in concentration of various sugars seems to play a determinative role in plant defense during necrotrophic interaction of B. cinerea and Sclerotinia sclerotiorum with tomato (Lecompte et al. 2013). A recent study has also suggested that relative proportion (but not the absolute concentration) of fructose amongst the pool of sugars (sucrose, glucose and fructose) plays a decisive role during tomato defense against B. cinerea (Lecompte et al. 2017). Several recent studies have suggested that alteration in host sugar metabolism is also important for the pathogenesis of soil-borne pathogens, such as Verticillium dahlia, Fusarium oxysporum, Phytophthora infestans and Rhizoctonia solani (Gyetvai et al. 2012; Buhtz et al. 2015; Kumar et al. 2016; Copley et al. 2017; Ghosh et al. 2017; Witzel et al. 2017).
Sugar mobilization in the battlefield
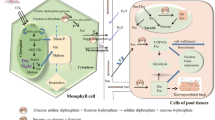
Plant tissues self-sufficient in producing sugars are known as source, while other tissues are called sink. Sink tissues require net sugar import (predominantly in the form of sucrose) via phloem and they are equipped to utilize sucrose as energy source (Kocal et al. 2008). When a pathogen attacks source tissues (such as leaves), a sink-type environment is created. Whereas when a pathogen colonizes sink tissues (such as developing leaves, meristems, etc.), the sink to source transition is arrested and sink status is retained at the site of infections (Teixeira et al. 2014; Dhandapani et al. 2017). The cascade of events including downregulation of photosynthetic genes, upregulation of respiratory genes and accumulation of hexose sugars facilitates creation of sink-type environment in the infected tissues (Fig. 1). Furthermore, sugar hydrolysis and uptake mechanism are modulated in the infected tissues (Fatima and Senthil-Kumar 2015; Oliva and Quibod 2017). Besides foliar pathogens, there are pathogens that infect non-aerial parts of the plants such as roots (sink tissue). The root knot nematode (Meloidogyne incognita) infection alters the primary metabolism during susceptible interaction but not during resistant interactions with tomato roots (Shukla et al. 2017). Recently, Zhao and colleagues have reported that root knot nematode (M. incognita) upregulates sugar transport-related genes and increases the sugar content in both roots as well as leaves of the infected tomato (Zhao et al. 2018). Overall, it seems that root pathogens can alter sugar mobilization in both foliar and non-foliar (root) tissues.
A simplified overview of source to sink transition during plant–pathogen interactions. During pathogen attack, source tissues (such as leaves) undergo extensive metabolic changes. The source-specific genes are repressed and sink-specific ones are induced. This leads to increase in hexose to sucrose ratio in the infected tissues and causes source to sink transition. Generally, sugars are transported from source tissues to various other parts of the plants (black arrow) while upon pathogen attack, sugars get translocated to infected zones (blue arrow). During pathogenesis, hexose transporters are upregulated in the infected tissues to facilitate sugar uptake from the host. RbcS ribulose-1,5-bisphosphate carboxylase, Cab chlorophyll a, b binding protein, INVs invertases, HXTs hexose transporters, SWEETs Sugars Will Eventually be Exported Transporters
Sink-related enzymes
In most cases, sucrose is not readily available to pathogen and it needs to be broken down into more accessible form, i.e., glucose for utilization (Paul et al. 2008). Invertases (INVs) present in sink tissue assist in hydrolyzing sucrose into glucose and fructose (reviewed in Tauzin and Giardina 2014). They influence sucrose level, sink strength as well as sucrose: hexose ratio. The host cell wall invertases upregulated during pathogen infection are known to increase the hexose to sucrose ratio in the infected tissues (Chou et al. 2000; Fotopoulos et al. 2003; Hayes et al. 2010). Also there are reports which suggest that some phytopathogens upregulate their own invertase(s) to promote host colonization (Voegele et al. 2006; Chang et al. 2017). Besides invertases, sucrose synthases (plant/pathogen origin) which are involved in breakdown of sucrose into fructose and UDP-glucose are also upregulated in some pathosystems (Hren et al. 2009; Brzin et al. 2011; Cabello et al. 2014). The upregulation of these sucrose synthases can also alter sucrose:hexose ratio in the infected tissues.
Sink-related transporters
Generally, membrane transporters are upregulated at the site of infection to promote uptake of sugars from the infected tissues (Table 2). For example, hexose transporters (HXTs) of either plant or pathogen origins are upregulated in various pathosystems and facilitate uptake of hexoses. Recently, the HXT1 of U. maydis has been shown to be required for its pathogenesis on maize (Schuler et al. 2015). Interestingly, different paralogs of HXT1 transporters of C. graminicola are differentially regulated during different phases of its pathogenesis on maize (Lingner et al. 2011). The CgHXT1 and CgHXT3 are induced during biotrophic phase while the CgHXT2 and CgHXT5 are induced during necrotrophic phase. Besides HXTs, induction of another hexose transporter, i.e., mfs1 (major facilitator superfamily), has been observed during necrotrophic phase of anthracnose disease causing fungal (C. lindemuthianum) infection in common bean (Pereira et al. 2013). In general, the HXTs are co-regulated with cell wall invertases, suggesting that they might be functioning to facilitate sugar uptake in a coordinated fashion (Sutton et al. 2007; Essmann et al. 2008). For example, both invertase (UfINV1) and hexose transporter (UfHXT1) are upregulated in the haustoria of Uromyces fabae (a biotrophic pathogen) and promote hexose uptake from the host during its pathogenesis on broad bean (Voegele et al. 2001, 2006).
It is noteworthy that some host origin hexose transporters such as sugar transport proteins (STPs) are also modulated during plant–pathogen interactions. Moore and colleagues had reported that a mutation in the wheat STP13 (lr67) gene imparts resistance against multiple biotrophic pathogens (Moore et al. 2015). Similarly, Arabidopsis STP13 is known to provide basal resistance against B. cinerea (Lemonnier et al. 2014). Upregulation of pathogen encoded sucrose transporters at infection site suggests that they might facilitate the pathogen to directly uptake sucrose from the host (Table 2). Srt1 is the first characterized pathogen origin sucrose transporter which is involved in the virulence of U. maydis on maize (Wahl et al. 2010). Interestingly, during the C. graminicola infection in maize another type of sucrose transporter, i.e., SUT1 has been upregulated (Vargas et al. 2012). In recent years, new types of sugar transporters (commonly referred to as SWEETs) have been found to facilitate glucose and sucrose efflux into the plant apoplast. These host origin SWEET transporters are induced upon pathogen invasion and it has been thought that pathogens induce them to promote uptake of sugars from their host (Chen et al. 2010). For example, Xanthomonas oryzae pv. oryzae utilizes transcriptional activator-like (TAL) effectors, i.e., PthXo1 and PthXo2 to induce rice OsSWEET11 (sucrose uniporter) and OsSWEET13 genes, respectively, during infection process (Chen et al. 2010; Zhou et al. 2015). Similarly, the pathogen (X. oryzae pv. oryzae) uses AvrXa7 and PthXo3 effectors to induce the OsSWEET14 (Os11N3) gene to promote susceptibility (Antony et al. 2010). Cassava sugar transporter MeSWEET10a is also induced by TAL20Xam668 effector from causal agent of bacterial blight disease, i.e., X. axonopodis (Cohn et al. 2014). Similarly, the X. citri ssp. citri was found to modulate the CsSWEET1 gene in citrus by TAL effectors (PthA4 and PthAw) (Hu et al. 2014).
Besides bacterial pathogens, fungal pathogens such as Golovinomyces cichoracearum and B. cinerea are also known to modulate host SWEET genes to promote pathogenesis (Ferrari et al. 2007; Chen et al. 2010). The host sweet gene (VvSWEET4) has been found upregulated during pathogenesis of B. cinerea in Vitis vinifera (Chong et al. 2014). Overall it seems that most of the foliar pathogens induce host SWEET transporters to facilitate infections (Chen et al. 2010; Cohn et al. 2014). However, the SWEET transporters seem to have different role during pathogenesis of soil-borne pathogens. For example, loss of host vacuolar SWEET2 transporter promotes enhanced susceptibility against a common root pathogen, Pythium irregulare infection in Arabidopsis (Chen et al. 2015). Similarly, overexpression of IbSWEET10 gene of sweet potato enhances host resistance to F. oxysporum infections (Li et al. 2017).
Sugars as regulators of plant defense—a stone unturned
In recent years, a pivotal role of various sugars like glucose, sucrose and trehalose in regulating the defense-related metabolic pathways has become apparent (Fig. 2) (Rolland et al. 2006; Wind et al. 2010; Bolouri Moghaddam and Van den Ende 2012). Glucose-mediated induction of defense-related secondary metabolites such as chalcone synthase and phenylalanine ammonia-lyase (Dao et al. 2011; Kim and Hwang 2014; Tonnessen et al. 2014) has been demonstrated (Xiao et al. 2000). Furthermore, sucrose can promote host defense response by enhancing the expression of anthocyanin biosynthesis genes and stimulating accumulation of isoflavonoids (Morkunas et al. 2005; Solfanelli et al. 2006).
Generalized overview of sugar signaling associated with metabolic reprogramming during plant–pathogen interactions. Repression of photosynthesis and activation of host cellular respiration and secondary metabolism are most common responses that occur during pathogen attack. Different sugars and sugar-related enzymes seem to control various aspects of these host metabolic changes to govern fate of interactions (green lines). SUS sucrose synthases, SPS sucrose-phosphate synthase, AGPase ADP-glucose pyrophosphorylase, SnRK1 SNF1-related kinase 1, bZIP11 basic region-leucine zipper transcription factor 11, TPS trehalose-6-phosphate synthase, TPP trehalose-6-phosphate phosphatase, TCA cycle tricarboxylic acid cycle
In addition, various sugar-related enzymes, transporters and signaling molecules that are induced during pathogen invasion can regulate the plant defense processes. For example, cell wall invertases can play a pivotal role in integrating sugar and defense signaling (Proels and Hückelhoven 2014). An increased invertase activity in the infected host tissue causes generation of sugar signals via modulation of sucrose/hexose ratio. Some sucrose transporters such as SUC2 and SUT1 might also function as sugar sensors (Lalonde et al. 1999). It is possible that sugar transporters that are upregulated during formation of secondary sink in the infected tissue might also be participating in defense response associated signaling processes (Sutton et al. 2007; Bezrutczyk et al. 2018). Similarly, various sugar signaling molecules such as hexokinase (HXK) and trehalose-6-phosphate (T6P) can also potentially regulate plant defense (Moore et al. 2003; Rolland et al. 2006; Paul et al. 2008; Sheen 2014). The HXKs are the best studied sugar sensors which are ascribed to be associated with glucose-mediated repression of photosynthetic genes (chlorophyll a/b binding protein and plastocyanin) (Sheen 1990; Moore et al. 2003; Cho et al. 2006). Also, HSKs potentially promote degradation of ETHYLENE-INSENSITIVE3 (EIN3), a key transcriptional regulator in ethylene signaling (Yanagisawa et al. 2003; Karve et al. 2012). The transcriptional de-repression of EIN3 is known to facilitate synergy between various plant defense hormone (jasmonate and ethylene) signaling pathways (Zhu et al. 2011). In addition, ethylene can also influence the photosynthesis and sugar partitioning (recently reviewed in Ceusters and Van de Poel 2018). Several other studies have also revealed interconnection between sugar and phytohormone signaling (León and Sheen 2003; Heil et al. 2012; Bolouri Moghaddam and Van den Ende 2012). Arabidopsis G-signaling protein AtRGS1 (regulator of G-protein signaling protein 1) has also been proven as a glucose sensor and it is known to influence the sugar-mediated gene regulation (Chen and Jones 2004; Grigston et al. 2008). Although the G-protein signaling has been known to play a pivotal role during plant–pathogen interactions (Urano et al. 2013), still the role of AtRGS1 in plant disease susceptibility or resistance remains to be analyzed. Establishing the links of sugar-hormone and sugar-G-protein signaling with plant pathogenesis is naive areas of research and largely remains unexplored.
Sucrose is known to translationally inhibit the expression of a particular group (S) of bZIP (basic region leucine zipper) transcription factor, i.e., ATB2/AtbZIP11 (Rook et al. 1998; Wiese et al. 2004, 2005). During sugar limiting condition, the bZIP11 is regulated by SnRK1 (SNF1-related kinase 1), a Ser/Thr kinase which acts as a metabolite sensor to regulate sugar and energy metabolism. Interestingly, the role of SnRK1 and bZIP transcription factors during plant–pathogen interactions has also been established (Alves et al. 2013; Morkunas and Ratajczak 2014; Hulsmans et al. 2016). Another sugar sensor, i.e., trehalose-6-phosphate (T6P, an intermediate of trehalose metabolism) is known to inhibit SnRK1 and influence bZIP11-SnRK1 regulatory pathway (Delatte et al. 2011; O’Hara et al. 2013; Nunes et al. 2013). T6P can also promote redox activation of ADP-glucose pyrophosphorylase (AGPase), which is involved in starch synthesis (Kolbe et al. 2005). Interestingly, the alterations of host starch metabolism (turnover) can also influence the outcome of the interaction (Engelsdorf et al. 2013). Besides host, trehalose biosynthetic pathway of the pathogens do play an important role during pathogenesis. For example, the T6P synthase (Tps1) deletion mutant of Magnaporthe oryzae exhibits reduced pathogenicity in rice (Foster et al. 2003; Wilson et al. 2007). The trehalose produced by X. citri subsp. citri acts as an important virulence determinant during pathogenesis in citrus (Piazza et al. 2015). Overall, it is being envisaged that by altering host SnRK1 activity, the pathogen origin trehalose as well as T6P modulates host metabolism and defense responses. In summary, the notion that sugars play a crucial role during plant defense response is emerging (Bolouri Moghaddam and Van den Ende 2012; Morkunas and Ratajczak 2014). A new term, sweet immunity has been coined to describe sugar-mediated induction of plant immune responses (Bolouri Moghaddam and Van Den Ende 2013). Various sugars like fructans and sucrose can also serve as damage-associated molecular patterns (DAMPs) which hallmark the pathogen infection (Duran-Flores and Heil 2016; Versluys et al. 2017) and are known to prime plant defense response against various pathogens (Duran-Flores and Heil 2016).
Metabolic shift—favoring plant or pathogen
It is apparent that metabolic shift occurs during both susceptible (compatible) and resistant (incompatible) interactions. The downregulation of photosynthesis and alteration in carbohydrate metabolism have been a common response during both types of interactions (Swarbrick et al. 2006; Fu et al. 2016; Li et al. 2016). However, the dynamics of gene expression has been found to be qualitatively similar but quantitatively different during compatible and incompatible interactions (Tao et al. 2003; Wang et al. 2010). For example, the photosynthesis and CO2 fixation-related genes are highly abundant amongst differentially regulated genes during compatible compared to incompatible interactions of P. infestans with potato (Gyetvai et al. 2012). The presence of high level of extracellular sugar at infection site is another common response during both susceptible and resistant interactions (Essmann et al. 2008; Siemens et al. 2011; Sun et al. 2014). However, a recent report has shown that bidirectional sugar transporters are upregulated only during colonization of a pathogenic isolate of F. oxysporum (Lanubile et al. 2015).
Interestingly, the timing of modulation of photosynthesis and sugar metabolism-related genes do vary between compatible and incompatible interactions (Fofana et al. 2007; Pérez-Bueno et al. 2015; Stare et al. 2015). The photosynthesis-related genes are transiently upregulated at early stage (before viral multiplication) of Potato Virus Y (PVY) infection in tolerant potato but subsequently they get downregulated (Stare et al. 2015). However, in case of the sensitive tomato (SA-deficient transgenic plants) the photosynthesis-related genes are consistently downregulated. How such temporal regulation of sugar metabolism as well as photosynthesis, influences the outcome of susceptible or resistant interaction, remain largely unanswered.
Recent updates and ongoing quest
With recent advent of transcriptomics, metabolomics, or proteomics-based approaches, exploring the complexity of metabolic alterations during plant–pathogen interactions has become feasible (Aliferis and Jabaji 2012; Hong et al. 2012; Yang et al. 2013; Teixeira et al. 2014; Aliferis et al. 2014). Nowadays, dual omics approaches are being adopted to solve this unfolded mystery. Studying interactions of M. oryzae and Oryza sativa (Kawahara et al. 2012), Hemileia vastatrix and Coffea arabica (Fernandez et al. 2012), Leptosphaeria maculans and Brassica napus (Lowe et al. 2014), Moniliophthora perniciosa and Theobroma cacao (Teixeira et al. 2014) are some of the recent examples wherein dual transcriptomics approach has been explored to understand the intricacies of plant–pathogen interactions. Also metabolomics/proteomics techniques, such as gas chromatography–mass spectrometry (GC–MS), liquid chromatography–mass spectrometry (LC–MS), and NMR spectroscopy, are being used to understand metabolic perturbations during pathogen infections (Botanga et al. 2012; Cevallos-Cevallos et al. 2012; Hong et al. 2012; Prezelj et al. 2016). In addition, targeted (having prior knowledge of the compounds of interest) or non-targeted MS analysis is also being explored (Heuberger et al. 2014). The limitation of distinguishing the plant or pathogen origin metabolites/proteins from the mixed pool is being resolved by in vitro co-culturing of the plant and pathogen cells and thereafter separating them to precisely identify the origin of metabolites/proteins (Allwood et al. 2010, 2012). However, such methodology is unsuitable for most of the pathosystems, as it is performed under in vitro condition and only partially mimics the changes that occur during pathogenesis in plants. The uses of the laser microdissection (LMD) to separate the host/pathogen cells in the infected tissues are a good alternative to understand the spatio-temporal regulation (Chandran et al. 2010). However, intimate association of the invading pathogen with the plant and presence of pathogen origin secreted proteins/metabolites in the host apoplast adds further complexity in data analysis. Considering such limitations, in recent years integrative approaches by combining more than one omics tools are being explored to unravel the complexity (Fig. 3). For example, simultaneous measurement of transcripts and/or proteins has been attempted to identify secreted effectors of Acyrthosiphon pisum (aphid) and P. infestans (fungus) during pathogenesis on pea and potato, respectively (Carolan et al. 2011; Ali et al. 2014). Similarly combined genome-wide RNAseq and global LC–MS and/or GC–MS-based metabolome analysis are being conducted to understand the metabolic alterations during plant–pathogen interactions (Rudd et al. 2015; Copley et al. 2017; Ghosh et al. 2017).
A diagrammatic overview of dual or integrative omic approaches to understand the plant–pathogen interaction. Either entire tissues or laser-assisted precise sampling of infected tissues is analyzed to study the transcriptional dynamics during infection process. Through in silico analysis, the host and pathogen origin transcripts are filtered out from the mixed transcriptome and they are analyzed separately. The observed changes in the host as well as pathogen transcripts might influence individually or cumulatively the outcome of the interactions. Besides this, the proteomics and metabolomics approaches are being integrated with transcriptome studies nowadays to understand the intricacies of plant–pathogen interactions. The accolade (in blue) on the right side of the picture represents such integrative approaches
In conclusion, with the advent of new technologies, systematic and holistic understanding of metabolic perturbation during host–pathogen interactions is becoming possible. Sugar metabolism and mobilization have emerged as important players, which decide the fate of ongoing battle between plant and pathogen during infection process. However, in spite of various recent advancements, the metabolic signatures and their regulatory nodes, which decide the susceptible or resistant responses, remain poorly understood. The host metabolic signature that favors plant or pathogen remains a major ongoing quest for future research. In addition, metabolic signatures that are associated with diverse lifestyle (biotrophic, necrotrophic and hemibiotrophic) as well as different modes of colonization (foliar, soil-borne, etc.) of the pathogen are yet to be established. We have summarized the current understanding and important unanswered quests about sugar metabolic alterations during plant–pathogen interactions in Fig. 4.
Understanding sugar metabolic shift: an ongoing quest with future implications. A model showing the major contributions (blue arrow) and the open questions/information gap (green arrow) in understanding host sugar metabolic signature during plant–pathogen interaction. Also, it remains to be established how host sugar metabolism is affected by various modes of pathogen colonization (question marks). In conclusion, despite recent advances, it is still not clear how host metabolic changes (depicted as wheel in the figure) favor the plant or the pathogen (depicted as direction of wheel) during their interaction. If the metabolic signature is properly understood, one can exploit it to develop disease-resistant plants
Author contribution statement
PK and GJ designed the outline of the article. PK designed the figures and tables. PK and GJ have written and edited the manuscript.
References
Agudelo-Romero P, Erban A, Rego C et al (2015) Transcriptome and metabolome reprogramming in Vitis vinifera cv. Trincadeira berries upon infection with Botrytis cinerea. J Exp Bot 66:1769–1785. https://doi.org/10.1093/jxb/eru517
Ali A, Alexandersson E, Sandin M et al (2014) Quantitative proteomics and transcriptomics of potato in response to Phytophthora infestans in compatible and incompatible interactions. BMC Genom 15:497. https://doi.org/10.1186/1471-2164-15-497
Aliferis KA, Jabaji S (2012) FT-ICR/MS and GC-EI/MS metabolomics networking unravels global potato sprout’s responses to Rhizoctonia solani infection. PLoS One 7(8):e42576. https://doi.org/10.1371/journal.pone.0042576
Aliferis KA, Faubert D, Jabaji S (2014) A metabolic profiling strategy for the dissection of plant defense against fungal pathogens. PLoS One 9:e111930. https://doi.org/10.1371/journal.pone.0111930
Allwood JW, Clarke A, Goodacre R, Mur LAJ (2010) Dual metabolomics: a novel approach to understanding plant–pathogen interactions. Phytochemistry 71:590–597. https://doi.org/10.1016/j.phytochem.2010.01.006
Allwood JW, Heald J, Lloyd AJ et al (2012) Separating the inseparable: the metabolomic analysis of plant–pathogen interactions. Methods Mol Biol 860:31–49. https://doi.org/10.1007/978-1-61779-594-7_3
Alves MS, Dadalto SP, Gonçalves AB et al (2013) Plant bZIP transcription factors responsive to pathogens: a review. Int J Mol Sci 14:7815–7828. https://doi.org/10.3390/ijms14047815
Antony G, Zhou J, Huang S et al (2010) Rice xa13 recessive resistance to bacterial blight is defeated by induction of the disease susceptibility gene Os-11N3. Plant Cell 22:3864–3876. https://doi.org/10.1105/tpc.110.078964
Berger S, Papadopoulos M, Schreiber U et al (2004) Complex regulation of gene expression, photosynthesis and sugar levels by pathogen infection in tomato. Physiol Plant 122:419–428. https://doi.org/10.1111/j.1399-3054.2004.00433.x
Berger S, Benediktyová Z, Matous K et al (2007a) Visualization of dynamics of plant–pathogen interaction by novel combination of chlorophyll fluorescence imaging and statistical analysis: differential effects of virulent and avirulent strains of P. syringae and of oxylipins on A. thaliana. J Exp Bot 58:797–806. https://doi.org/10.1093/jxb/erl208
Berger S, Sinha AK, Roitsch T (2007b) Plant physiology meets phytopathology: plant primary metabolism and plant pathogen interactions. J Exp Bot 58:4019–4026. https://doi.org/10.1093/jxb/erm298
Bezrutczyk M, Yang J, Eom JS et al (2018) Sugar flux and signaling in plant–microbe interactions. Plant J 93(4):675–685. https://doi.org/10.1111/tpj.13775
Bolouri Moghaddam MR, Van den Ende W (2012) Sugars and plant innate immunity. J Exp Bot 63:3989–3998. https://doi.org/10.1093/jxb/ers129
Bolouri Moghaddam MR, Van Den Ende W (2013) Sweet immunity in the plant circadian regulatory network. J Exp Bot 64:1439–1449
Bolton MD (2009) Primary metabolism and plant defense—fuel for the fire. Mol Plant Microbe Interact 22:487–497. https://doi.org/10.1094/MPMI-22-5-0487
Bonfig KB, Schreiber U, Gabler A et al (2006) Infection with virulent and avirulent P. syringae strains differentially affects photosynthesis and sink metabolism in Arabidopsis leaves. Planta 225:1–12. https://doi.org/10.1007/s00425-006-0303-3
Botanga CJ, Bethke G, Chen Z et al (2012) Metabolite profiling of Arabidopsis inoculated with Alternaria brassicicola reveals that ascorbate reduces disease severity. Mol Plant Microbe Interact 25:1628–1638. https://doi.org/10.1094/MPMI-07-12-0179-R
Brzin J, Petrovič N, Ravnikar M, Kovač M (2011) Induction of sucrose synthase in the phloem of phytoplasma infected maize. Biol Plant 55:711–715. https://doi.org/10.1007/s10535-011-0173-9
Buhtz A, Witzel K, Strehmel N et al (2015) Perturbations in the primary metabolism of tomato and Arabidopsis thaliana plants infected with the soil-borne fungus Verticillium dahliae. PLoS One 10(9):e0138242. https://doi.org/10.1371/journal.pone.0138242
Cabello S, Lorenz C, Crespo S et al (2014) Altered sucrose synthase and invertase expression affects the local and systemic sugar metabolism of nematode-infected Arabidopsis thaliana plants. J Exp Bot 65:201–212. https://doi.org/10.1093/jxb/ert359
Carolan JC, Caragea D, Reardon KT et al (2011) Predicted effector molecules in the salivary secretome of the pea aphid (Acyrthosiphon pisum): a dual transcriptomic/proteomic approach. J Proteome Res 10:1505–1518. https://doi.org/10.1021/pr100881q
Ceusters J, Van de Poel B (2018) Update: ethylene exerts species-specific and age-dependent control of photosynthesis. Plant Physiol 176(4):2601–2612. https://doi.org/10.1104/pp.17.01706
Cevallos-Cevallos JM, Futch DB, Shilts T et al (2012) GC–MS metabolomic differentiation of selected citrus varieties with different sensitivity to citrus huanglongbing. Plant Physiol Biochem 53:69–76. https://doi.org/10.1016/j.plaphy.2012.01.010
Chandran D, Inada N, Hather G et al (2010) Laser microdissection of Arabidopsis cells at the powdery mildew infection site reveals site-specific processes and regulators. Proc Natl Acad Sci USA 107:460–465. https://doi.org/10.1073/pnas.0912492107
Chang Q, Liu J, Lin X et al (2017) A unique invertase is important for sugar absorption of an obligate biotrophic pathogen during infection. New Phytol 215:1548–1561. https://doi.org/10.1111/nph.14666
Chen J-G, Jones AM (2004) AtRGS1 function in Arabidopsis thaliana. Methods Enzymol 389:338–350. https://doi.org/10.1016/S0076-6879(04)89020-7
Chen L-Q, Hou B-H, Lalonde S et al (2010) Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 468:527–532. https://doi.org/10.1038/nature09606
Chen HY, Huh JH, Yu YC et al (2015) The Arabidopsis vacuolar sugar transporter SWEET2 limits carbon sequestration from roots and restricts Pythium infection. Plant J 83:1046–1058. https://doi.org/10.1111/tpj.12948
Cho Y-H, Yoo S-D, Sheen J (2006) Regulatory functions of nuclear hexokinase1 complex in glucose signaling. Cell 127:579–589. https://doi.org/10.1016/j.cell.2006.09.028
Chong J, Piron M-C, Meyer S et al (2014) The SWEET family of sugar transporters in grapevine: VvSWEET4 is involved in the interaction with Botrytis cinerea. J Exp Bot 65:6589–6601. https://doi.org/10.1093/jxb/eru375
Chou HM, Bundock N, Rolfe S, Scholes JD (2000) Infection of Arabidopsis thaliana leaves with Albugo candida (white blister rust) causes a reprogramming of host metabolism. Mol Plant Pathol 1:99–113. https://doi.org/10.1046/j.1364-3703.2000.00013.x
Chu Z, Yuan M, Yao J et al (2006) Promoter mutations of an essential gene for pollen development result in disease resistance in rice. Genes Dev 20:1250–1255. https://doi.org/10.1101/gad.1416306
Cohn M, Bart RS, Shybut M et al (2014) Xanthomonas axonopodis virulence is promoted by a transcription activator-like effector-mediated induction of a SWEET sugar transporter in cassava. Mol Plant Microbe Interact 27:1186–1198. https://doi.org/10.1094/MPMI-06-14-0161-R
Copley TR, Aliferis KA, Kliebenstein DJ, Jabaji SH (2017) An integrated RNAseq-1H NMR metabolomics approach to understand soybean primary metabolism regulation in response to Rhizoctonia foliar blight disease. BMC Plant Biol 17:84. https://doi.org/10.1186/s12870-017-1020-8
Dao TTH, Linthorst HJM, Verpoorte R (2011) Chalcone synthase and its functions in plant resistance. Phytochem Rev 10:397–412. https://doi.org/10.1007/s11101-011-9211-7
De Cremer K, Mathys J, Vos C et al (2013) RNAseq-based transcriptome analysis of Lactuca sativa infected by the fungal necrotroph Botrytis cinerea. Plant, Cell Environ 36:1992–2007. https://doi.org/10.1111/pce.12106
de Torres Zabala M, Littlejohn G, Jayaraman S et al (2015) Chloroplasts play a central role in plant defence and are targeted by pathogen effectors. Nat Plants 1:15074. https://doi.org/10.1038/nplants.2015.74
Delatte TL, Sedijani P, Kondou Y et al (2011) Growth arrest by trehalose-6-phosphate: an astonishing case of primary metabolite control over growth by way of the SnRK1 signaling pathway. Plant Physiol 157:160–174. https://doi.org/10.1104/pp.111.180422
Dhandapani P, Song J, Novak O, Jameson PE (2017) Infection by Rhodococcus fascians maintains cotyledons as a sink tissue for the pathogen. Ann Bot 119(5):841–852. https://doi.org/10.1093/aob/mcw202
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant cell 7:1085–1097. https://doi.org/10.1105/tpc.7.7.1085
Doehlemann G, Molitor F, Hahn M (2005) Molecular and functional characterization of a fructose specific transporter from the gray mold fungus Botrytis cinerea. Fungal Genet Biol 42:601–610. https://doi.org/10.1016/j.fgb.2005.03.001
Doehlemann G, Wahl R, Horst RJ et al (2008) Reprogramming a maize plant: transcriptional and metabolic changes induced by the fungal biotroph Ustilago maydis. Plant J 56:181–195. https://doi.org/10.1111/j.1365-313X.2008.03590.x
Duan G, Christian N, Schwachtje J et al (2013) The metabolic interplay between plants and phytopathogens. Metabolites 3:1–23. https://doi.org/10.3390/metabo3010001
Duran-Flores D, Heil M (2016) Sources of specificity in plant damaged-self recognition. Curr Opin Plant Biol 32:77–87
Engelsdorf T, Horst RJ, Pröls R et al (2013) Reduced carbohydrate availability enhances the susceptibility of Arabidopsis toward Colletotrichum higginsianum. Plant Physiol 162:225–238. https://doi.org/10.1104/pp.112.209676
Essmann J, Schmitz-Thom I, Schon H et al (2008) RNA interference-mediated repression of cell wall invertase impairs defense in source leaves of tobacco. Plant Physiol 147(3):1288–1299. https://doi.org/10.1104/pp.108.121418
Fagard M, Launay A, Clément G et al (2014) Nitrogen metabolism meets phytopathology. J Exp Bot 65:5643–5656. https://doi.org/10.1093/jxb/eru323
Fatima U, Senthil-Kumar M (2015) Plant and pathogen nutrient acquisition strategies. Front Plant Sci 6:750. https://doi.org/10.3389/fpls.2015.00750
Fernandez D, Tisserant E, Talhinhas P et al (2012) 454-pyrosequencing of Coffea arabica leaves infected by the rust fungus Hemileia vastatrix reveals in planta-expressed pathogen-secreted proteins and plant functions in a late compatible plant–rust interaction. Mol Plant Pathol 13:17–37. https://doi.org/10.1111/j.1364-3703.2011.00723.x
Ferrari S, Galletti R, Denoux C et al (2007) Resistance to Botrytis cinerea induced in Arabidopsis by elicitors is independent of salicylic acid, ethylene, or jasmonate signaling but requires PHYTOALEXIN DEFICIENT3. Plant Physiol 144:367–379. https://doi.org/10.1104/pp.107.095596
Fofana B, Banks TW, McCallum B et al (2007) Temporal gene expression profiling of the wheat leaf rust pathosystem using cDNA microarray reveals differences in compatible and incompatible defence pathways. Int J Plant Genom 2007:17542. https://doi.org/10.1155/2007/17542
Foster AJ, Jenkinson JM, Talbot NJ (2003) Trehalose synthesis and metabolism are required at different stages of plant infection by Magnaporthe grisea. EMBO J 22:225–235. https://doi.org/10.1093/emboj/cdg018
Fotopoulos V, Gilbert MJ, Pittman JK et al (2003) The monosaccharide transporter gene, AtSTP4, and the cell-wall invertase, Atbetafruct1, are induced in Arabidopsis during infection with the fungal biotroph Erysiphe cichoracearum. Plant Physiol 132:821–829. https://doi.org/10.1104/pp.103.021428
Fu Y, Zhang H, Mandal SN et al (2016) Quantitative proteomics reveals the central changes of wheat in response to powdery mildew. J Proteom 130:108–119. https://doi.org/10.1016/j.jprot.2015.09.006
Ghosh S, Kanwar P, Jha G (2017) Alterations in rice chloroplast integrity, photosynthesis and metabolome associated with pathogenesis of Rhizoctonia solani. Sci Rep 7:41610. https://doi.org/10.1038/srep41610
Grigston JC, Osuna D, Scheible W-R et al (2008) D-Glucose sensing by a plasma membrane regulator of G signaling protein, AtRGS1. FEBS Lett 582:3577–3584. https://doi.org/10.1016/j.febslet.2008.08.038
Gyetvai G, Sønderkær M, Göbel U et al (2012) The transcriptome of compatible and incompatible interactions of potato (Solanum tuberosum) with Phytophthora infestans revealed by DeepSAGE analysis. PLoS One 7:e31526. https://doi.org/10.1371/journal.pone.0031526
Halitschke R, Hamilton JG, Kessler A (2011) Herbivore-specific elicitation of photosynthesis by mirid bug salivary secretions in the wild tobacco Nicotiana attenuata. New Phytol 191:528–535. https://doi.org/10.1111/j.1469-8137.2011.03701.x
Hayes MA, Feechan A, Dry IB (2010) Involvement of abscisic acid in the coordinated regulation of a stress-inducible hexose transporter (VvHT5) and a cell wall invertase in grapevine in response to biotrophic fungal infection. Plant Physiol 153:211–221. https://doi.org/10.1104/pp.110.154765
Heil M, Ibarra-Laclette E, Adame-Álvarez RM et al (2012) How plants sense wounds: damaged-self recognition is based on plant-derived elicitors and induces octadecanoid signaling. PLoS One 7:e30537. https://doi.org/10.1371/journal.pone.0030537
Heuberger AL, Robison FM, Lyons SMA et al (2014) Evaluating plant immunity using mass spectrometry-based metabolomics workflows. Front Plant Sci 5:291. https://doi.org/10.3389/fpls.2014.00291
Hong Y-S, Martinez A, Liger-Belair G et al (2012) Metabolomics reveals simultaneous influences of plant defence system and fungal growth in Botrytis cinerea-infected Vitis vinifera cv. Chardonnay berries. J Exp Bot 63:5773–5785. https://doi.org/10.1093/jxb/ers228
Horst RJ, Engelsdorf T, Sonnewald U, Voll LM (2008) Infection of maize leaves with Ustilago maydis prevents establishment of C4 photosynthesis. J Plant Physiol 165:19–28. https://doi.org/10.1016/j.jplph.2007.05.008
Hren M, Ravnikar M, Brzin J et al (2009) Induced expression of sucrose synthase and alcohol dehydrogenase I genes in phytoplasma-infected grapevine plants grown in the field. Plant Pathol 58:170–180. https://doi.org/10.1111/j.1365-3059.2008.01904.x
Hu Y, Zhang J, Jia H et al (2014) Lateral organ boundaries 1 is a disease susceptibility gene for citrus bacterial canker disease. Proc Natl Acad Sci USA 111:E521–E529. https://doi.org/10.1073/pnas.1313271111
Hui D, Iqbal J, Lehmann K et al (2003) Molecular interactions between the specialist herbivore Manduca sexta (lepidoptera, sphingidae) and its natural host Nicotiana attenuata: V. microarray analysis and further characterization of large-scale changes in herbivore-induced mRNAs. Plant Physiol 131:1877–1893. https://doi.org/10.1104/pp.102.018176
Hulsmans S, Rodriguez M, De Coninck B, Rolland F (2016) The SnRK1 energy sensor in plant biotic interactions. Trends Plant Sci 21:648–661. https://doi.org/10.1016/j.tplants.2016.04.008
Jones JDG, Dangl JL (2006) The plant immune system. Nature 444:323–329. https://doi.org/10.1038/nature05286
Karve A, Xia X, Moore BD (2012) Arabidopsis Hexokinase-Like1 and Hexokinase1 form a critical node in mediating plant glucose and ethylene responses. Plant Physiol 158:1965–1975. https://doi.org/10.1104/pp.112.195636
Kawahara Y, Oono Y, Kanamori H et al (2012) Simultaneous RNA-seq analysis of a mixed transcriptome of rice and blast fungus interaction. PLoS One 7:e49423. https://doi.org/10.1371/journal.pone.0049423
Kim DS, Hwang BK (2014) An important role of the pepper phenylalanine ammonia-lyase gene (PAL1) in salicylic acid-dependent signalling of the defence response to microbial pathogens. J Exp Bot 65:2295–2306. https://doi.org/10.1093/jxb/eru109
Kocal N, Sonnewald U, Sonnewald S (2008) Cell wall-bound invertase limits sucrose export and is involved in symptom development and inhibition of photosynthesis during compatible interaction between tomato and Xanthomonas campestris pv vesicatoria. Plant Physiol 148:1523–1536. https://doi.org/10.1104/pp.108.127977
Kolbe A, Tiessen A, Schluepmann H et al (2005) Trehalose 6-phosphate regulates starch synthesis via posttranslational redox activation of ADP-glucose pyrophosphorylase. Proc Natl Acad Sci USA 102:11118–11123. https://doi.org/10.1073/pnas.0503410102
Kretschmer M, Croll D, Kronstad JW (2017) Maize susceptibility to Ustilago maydis is influenced by genetic and chemical perturbation of carbohydrate allocation. Mol Plant Pathol 18:1222–1237. https://doi.org/10.1111/mpp.12486
Kumar Y, Zhang L, Panigrahi P et al (2016) Fusarium oxysporum mediates systems metabolic reprogramming of chickpea roots as revealed by a combination of proteomics and metabolomics. Plant Biotechnol J 14:1589–1603. https://doi.org/10.1111/pbi.12522
Lalonde S, Boles E, Hellmann H et al (1999) The dual function of sugar carriers. Transport and sugar sensing. Plant Cell 11:707–726. https://doi.org/10.1105/tpc.11.4.707
Lanubile A, Muppirala UK, Severin AJ et al (2015) Transcriptome profiling of soybean (Glycine max) roots challenged with pathogenic and non-pathogenic isolates of Fusarium oxysporum. BMC Genom 16:1089. https://doi.org/10.1186/s12864-015-2318-2
Lecompte F, Abro MA, Nicot PC (2013) Can plant sugars mediate the effect of nitrogen fertilization on lettuce susceptibility to two necrotrophic pathogens: Botrytis cinerea and Sclerotinia sclerotiorum? Plant Soil 369:387–401. https://doi.org/10.1007/s11104-012-1577-9
Lecompte F, Nicot PC, Ripoll J et al (2017) Reduced susceptibility of tomato stem to the necrotrophic fungus Botrytis cinerea is associated with a specific adjustment of fructose content in the host sugar pool. Ann Bot 119:931–943. https://doi.org/10.1093/aob/mcw240
Lemonnier P, Gaillard C, Veillet F et al (2014) Expression of Arabidopsis sugar transport protein STP13 differentially affects glucose transport activity and basal resistance to Botrytis cinerea. Plant Mol Biol 85:473–484. https://doi.org/10.1007/s11103-014-0198-5
León P, Sheen J (2003) Sugar and hormone connections. Trends Plant Sci 8:110–116. https://doi.org/10.1016/S1360-1385(03)00011-6
Li J, Yang X, Liu X et al (2016) Proteomic analysis of the compatible interaction of wheat and powdery mildew (Blumeria graminis f. sp. tritici). Plant Physiol Biochem 111:234–243. https://doi.org/10.1016/j.plaphy.2016.12.006
Li Y, Wang Y, Zhang H et al (2017) The plasma membrane-localized sucrose transporter IbSWEET10 contributes to the resistance of sweet potato to Fusarium oxysporum. Front Plant Sci 8:197. https://doi.org/10.3389/fpls.2017.00197
Lingner U, Münch S, Deising HB, Sauer N (2011) Hexose transporters of a hemibiotrophic plant pathogen: functional variations and regulatory differences at different stages of infection. J Biol Chem 286:20913–20922. https://doi.org/10.1074/jbc.M110.213678
Liu Q, Yuan M, Zhou Y et al (2011) A paralog of the MtN3/saliva family recessively confers race-specific resistance to Xanthomonas oryzae in rice. Plant Cell Environ 34:1958–1969. https://doi.org/10.1111/j.1365-3040.2011.02391.x
Lopes DB, Berger RD (2001) The effects of rust and anthracnose on the photosynthetic competence of diseased bean leaves. Phytopathology 91:212–220. https://doi.org/10.1094/PHYTO.2001.91.2.212
Lowe RGT, Cassin A, Grandaubert J et al (2014) Genomes and transcriptomes of partners in plant-fungal-interactions between canola (Brassica napus) and two Leptosphaeria species. PLoS One 9:e103098. https://doi.org/10.1371/journal.pone.0103098
Martin K, Singh J, Hill JH et al (2016) Dynamic transcriptome profiling of Bean Common Mosaic Virus (BCMV) infection in common bean (Phaseolus vulgaris L.). BMC Genom 17:613. https://doi.org/10.1186/s12864-016-2976-8
Meyer S, Saccardy-Adji K, Rizza F, Genty B (2001) Inhibition of photosynthesis by Colletotrichum lindemuthianum in bean leaves determined by chlorophyll fluorescence imaging. Plant Cell Environ 24:947–956. https://doi.org/10.1046/j.0016-8025.2001.00737.x
Moore B, Zhou L, Rolland F et al (2003) Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 300:332–336. https://doi.org/10.1126/science.1080585
Moore JW, Herrera-Foessel S, Lan C et al (2015) A recently evolved hexose transporter variant confers resistance to multiple pathogens in wheat. Nat Genet 47:1494–1498. https://doi.org/10.1038/ng.3439
Morkunas I, Ratajczak L (2014) The role of sugar signaling in plant defense responses against fungal pathogens. Acta Physiol Plant 36:1607–1619. https://doi.org/10.1007/s11738-014-1559-z
Morkunas I, Marczak Ł, Stachowiak J, Stobiecki M (2005) Sucrose-induced lupine defense against Fusarium oxysporum. Sucrose-stimulated accumulation of isoflavonoids as a defense response of lupine to Fusarium oxysporum. Plant Physiol Biochem 43:363–373. https://doi.org/10.1016/j.plaphy.2005.02.011
Nimchuk Z, Eulgem T, Holt BF, Dangl JL (2003) Recognition and response in the plant immune system. Annu Rev Genet 37:579–609. https://doi.org/10.1146/annurev.genet.37.110801.142628
Nunes C, O’Hara LE, Primavesi LF et al (2013) The trehalose 6-phosphate/SnRK1 signaling pathway primes growth recovery following relief of sink limitation. Plant Physiol 162:1720–1732. https://doi.org/10.1104/pp.113.220657
O’Hara LE, Paul MJ, Wingler A (2013) How do sugars regulate plant growth and development? New insight into the role of trehalose-6-phosphate. Mol Plant 6:261–274. https://doi.org/10.1093/mp/sss120
Oliva R, Quibod IL (2017) Immunity and starvation: new opportunities to elevate disease resistance in crops. Curr Opin Plant Biol 38:84–91. https://doi.org/10.1016/j.pbi.2017.04.020
Parker D, Beckmann M, Zubair H et al (2009) Metabolomic analysis reveals a common pattern of metabolic re-programming during invasion of three host plant species by Magnaporthe grisea. Plant J 59:723–737. https://doi.org/10.1111/j.1365-313X.2009.03912.x
Paul MJ, Primavesi LF, Jhurreea D, Zhang Y (2008) Trehalose metabolism and signaling. Annu Rev Plant Biol 59:417–441. https://doi.org/10.1146/annurev.arplant.59.032607.092945
Pereira MF, de Araújo Dos Santos CM, de Araújo EF et al (2013) Beginning to understand the role of sugar carriers in Colletotrichum lindemuthianum: the function of the gene mfs1. J Microbiol 51:70–81. https://doi.org/10.1007/s12275-013-2393-5
Pérez-Bueno ML, Pineda M, Díaz-Casado E, Barón M (2015) Spatial and temporal dynamics of primary and secondary metabolism in Phaseolus vulgaris challenged by Pseudomonas syringae. Physiol Plant 153:161–174. https://doi.org/10.1111/ppl.12237
Petit A-N, Vaillant N, Boulay M et al (2006) Alteration of photosynthesis in grapevines affected by esca. Phytopathology 96:1060–1066. https://doi.org/10.1094/PHYTO-96-1060
Piasecka A, Jedrzejczak-Rey N, Bednarek P (2015) Secondary metabolites in plant innate immunity: conserved function of divergent chemicals. New Phytol 206:948–964
Piazza A, Zimaro T, Garavaglia BS et al (2015) The dual nature of trehalose in citrus canker disease: a virulence factor for Xanthomonas citri subsp. citri and a trigger for plant defence responses. J Exp Bot 66:2795–2811. https://doi.org/10.1093/jxb/erv095
Pieterse CMJ, Van der Does D, Zamioudis C et al (2012) Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol 28:489–521. https://doi.org/10.1146/annurev-cellbio-092910-154055
Prezelj N, Covington E, Roitsch T et al (2016) Metabolic consequences of infection of grapevine (Vitis vinifera L.) cv. “Modra frankinja” with Flavescence Dorée phytoplasma. Front Plant Sci 7:711. https://doi.org/10.3389/fpls.2016.00711
Proels RK, Hückelhoven R (2014) Cell-wall invertases, key enzymes in the modulation of plant metabolism during defence responses. Mol Plant Pathol 15:858–864. https://doi.org/10.1111/mpp.12139
Pusztahelyi T, Holb IJ, Pócsi I (2015) Secondary metabolites in fungus–plant interactions. Front Plant Sci 6:573. https://doi.org/10.3389/fpls.2015.00573
Rojas CM, Senthil-Kumar M, Tzin V, Mysore KS (2014) Regulation of primary plant metabolism during plant–pathogen interactions and its contribution to plant defense. Front Plant Sci 5:17. https://doi.org/10.3389/fpls.2014.00017
Rolfe SA, Scholes JD (2010) Chlorophyll fluorescence imaging of plant–pathogen interactions. Protoplasma 247:163–175. https://doi.org/10.1007/s00709-010-0203-z
Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57:675–709. https://doi.org/10.1146/annurev.arplant.57.032905.105441
Rook F, Weisbeek P, Smeekens S (1998) The light-regulated Arabidopsis bZIP transcription factor gene ATB2 encodes a protein with an unusually long leucine zipper domain. Plant Mol Biol 37:171–178. https://doi.org/10.1023/A:1005964327725
Rudd JJ, Kanyuka K, Hassani-Pak K et al (2015) Transcriptome and metabolite profiling of the infection cycle of Zymoseptoria tritici on wheat reveals a biphasic interaction with plant immunity involving differential pathogen chromosomal contributions and a variation on the hemibiotrophic lifestyle def. Plant Physiol 167:1158–1185. https://doi.org/10.1104/pp.114.255927
Scholes J, Rolfe S (1996) Photosynthesis in localised regions of oat leaves infected with crown rust (Puccinia coronata): quantitative imaging of chlorophyll fluorescence. Planta 199:573–582. https://doi.org/10.1007/BF00195189
Scholes J, Rolfe SA (2009) Chlorophyll fluorescence imaging as tool for understanding the impact of fungal diseases on plant performance: a phenomics perspective. Funct Plant Biol 36:880–892. https://doi.org/10.1071/FP09145
Schuler D, Wahl R, Wippel K et al (2015) Hxt1, a monosaccharide transporter and sensor required for virulence of the maize pathogen Ustilago maydis. New Phytol 206:1086–1100. https://doi.org/10.1111/nph.13314
Sheen J (1990) Metabolic repression of transcription in higher plants. Plant Cell 2:1027–1038. https://doi.org/10.1105/tpc.2.10.1027
Sheen J (2014) Master regulators in plant glucose signaling networks. J Plant Biol 57:67–79. https://doi.org/10.1007/s12374-014-0902-7
Shukla N, Yadav R, Kaur P et al (2017) Transcriptome analysis of root-knot nematode (Meloidogyne incognita)-infected tomato (Solanum lycopersicum) roots reveals complex gene expression profiles and metabolic networks of both host and nematode during susceptible and resistance responses. Mol Plant Pathol 19:615–633. https://doi.org/10.1111/mpp.12547
Siemens J, González M-C, Wolf S et al (2011) Extracellular invertase is involved in the regulation of clubroot disease in Arabidopsis thaliana. Mol Plant Pathol 12:247–262. https://doi.org/10.1111/j.1364-3703.2010.00667.x
Smith JE, Mengesha B, Tang H et al (2014) Resistance to Botrytis cinerea in Solanum lycopersicoides involves widespread transcriptional reprogramming. BMC Genom 15:334. https://doi.org/10.1186/1471-2164-15-334
Solfanelli C, Poggi A, Loreti E et al (2006) Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol 140:637–646. https://doi.org/10.1104/pp.105.072579
Stare T, Ramsak Z, Blejec A et al (2015) Bimodal dynamics of primary metabolism-related responses in tolerant potato–potato virus Y interaction. BMC Genom 16:716. https://doi.org/10.1186/s12864-015-1925-2
Streubel J, Pesce C, Hutin M et al (2013) Five phylogenetically close rice SWEET genes confer TAL effector-mediated susceptibility to Xanthomonas oryzae pv. oryzae. New Phytol 200:808–819. https://doi.org/10.1111/nph.12411
Sun L, Yang D, Kong Y et al (2014) Sugar homeostasis mediated by cell wall invertase GRAIN INCOMPLETE FILLING 1 (GIF1) plays a role in pre-existing and induced defence in rice. Mol Plant Pathol 15:161–173. https://doi.org/10.1111/mpp.12078
Sutton PN, Gilbert MJ, Williams LE, Hall JL (2007) Powdery mildew infection of wheat leaves changes host solute transport and invertase activity. Physiol Plant 129:787–795. https://doi.org/10.1111/j.1399-3054.2007.00863.x
Swarbrick PJ, Schulze-Lefert P, Scholes JD (2006) Metabolic consequences of susceptibility and resistance (race-specific and broad-spectrum) in barley leaves challenged with powdery mildew. Plant Cell Environ 29:1061–1076
Tang JY, Zielinski RE, Zangerl AR et al (2006) The differential effects of herbivory by first and fourth instars of Trichoplusia ni (Lepidoptera: Noctuidae) on photosynthesis in Arabidopsis thaliana. J Exp Bot 57:527–536. https://doi.org/10.1093/jxb/erj032
Tao Y, Xie Z, Chen W et al (2003) Quantitative nature of Arabidopsis responses during compatible and incompatible interactions with the bacterial pathogen Pseudomonas syringae. Plant Cell 15:317–330
Tauzin AS, Giardina T (2014) Sucrose and invertases, a part of the plant defense response to the biotic stresses. Front Plant Sci 5:293. https://doi.org/10.3389/fpls.2014.00293
Teixeira PJPL, de Thomazella DP, Reis O et al (2014) High-resolution transcript profiling of the atypical biotrophic interaction between Theobroma cacao and the fungal pathogen Moniliophthora perniciosa. Plant Cell 26:4245–4269. https://doi.org/10.1105/tpc.114.130807
Tonnessen BW, Manosalva P, Lang JM et al (2014) Rice phenylalanine ammonia-lyase gene OsPAL4 is associated with broad spectrum disease resistance. Plant Mol Biol 87:273–286. https://doi.org/10.1007/s11103-014-0275-9
Urano D, Chen J-G, Botella JR, Jones AM (2013) Heterotrimeric G protein signalling in the plant kingdom. Open Biol 3:120186. https://doi.org/10.1098/rsob.120186
VanEtten HD, Mansfield JW, Bailey JA, Farmer EE (1994) Two classes of plant antibiotics: phytoalexins versus “phytoanticipins”. Plant Cell 6:1191–1192. https://doi.org/10.1105/tpc.6.9.1191
Vargas WA, Martín JMS, Rech GE et al (2012) Plant defense mechanisms are activated during biotrophic and necrotrophic development of Colletotrichum graminicola in maize. Plant Physiol 158:1342–1358. https://doi.org/10.1104/pp.111.190397
Versluys M, Tarkowski ŁP, Van den Ende W (2017) Fructans as DAMPs or MAMPs: evolutionary prospects, cross-tolerance, and multistress resistance potential. Front Plant Sci 7:2061. https://doi.org/10.3389/fpls.2016.02061
Voegele RT, Struck C, Hahn M, Mendgen K (2001) The role of haustoria in sugar supply during infection of broad bean by the rust fungus Uromyces fabae. Proc Natl Acad Sci USA 98:8133–8138. https://doi.org/10.1073/pnas.131186798
Voegele RT, Wirsel S, Möll U et al (2006) Cloning and characterization of a novel invertase from the obligate biotroph Uromyces fabae and analysis of expression patterns of host and pathogen invertases in the course of infection. Mol Plant Microbe Interact 19:625–634. https://doi.org/10.1094/MPMI-19-0625
Voll LM, Horst RJ, Voitsik A-M et al (2011) Common motifs in the response of cereal primary metabolism to fungal pathogens are not based on similar transcriptional reprogramming. Front Plant Sci 2:39. https://doi.org/10.3389/fpls.2011.00039
Wahl R, Wippel K, Goos S et al (2010) A novel high-affinity sucrose transporter is required for virulence of the plant pathogen Ustilago maydis. PLoS Biol 8:e1000303. https://doi.org/10.1371/journal.pbio.1000303
Wang X, Liu W, Chen X et al (2010) Differential gene expression in incompatible interaction between wheat and stripe rust fungus revealed by cDNA-AFLP and comparison to compatible interaction. BMC Plant Biol 10:9. https://doi.org/10.1186/1471-2229-10-9
Wiese A, Elzinga N, Wobbes B, Smeekens S (2004) A conserved upstream open reading frame mediates sucrose-induced repression of translation. Plant Cell 16:1717–1729. https://doi.org/10.1105/tpc.019349
Wiese A, Elzinga N, Wobbes B, Smeekens S (2005) Sucrose-induced translational repression of plant bZIP-type transcription factors. Biochem Soc Trans 33:272–275. https://doi.org/10.1042/BST0330272
Wilson RA, Jenkinson JM, Gibson RP et al (2007) Tps1 regulates the pentose phosphate pathway, nitrogen metabolism and fungal virulence. EMBO J 26:3673–3685. https://doi.org/10.1038/sj.emboj.7601795
Wind J, Smeekens S, Hanson J (2010) Sucrose: metabolite and signaling molecule. Phytochemistry 71:1610–1614. https://doi.org/10.1016/j.phytochem.2010.07.007
Windram O, Madhou P, McHattie S et al (2012) Arabidopsis defense against Botrytis cinerea: chronology and regulation deciphered by high-resolution temporal transcriptomic analysis. Plant Cell 24:3530–3557. https://doi.org/10.1105/tpc.112.102046
Witzel K, Buhtz A, Grosch R (2017) Temporal impact of the vascular wilt pathogen Verticillium dahliae on tomato root proteome. J. Proteom 169:215–224. https://doi.org/10.1016/j.jprot.2017.04.008
Wojakowska A, Muth D, Narożna D et al (2013) Changes of phenolic secondary metabolite profiles in the reaction of narrow leaf lupin (Lupinus angustifolius) plants to infections with Colletotrichum lupini fungus or treatment with its toxin. Metabolomics 9:575–589. https://doi.org/10.1007/s11306-012-0475-8
Xiao W, Sheen J, Jang JC (2000) The role of hexokinase in plant sugar signal transduction and growth and development. Plant Mol Biol 44:451–461
Xu X-H, Wang C, Li S-X et al (2015) Friend or foe: differential responses of rice to invasion by mutualistic or pathogenic fungi revealed by RNAseq and metabolite profiling. Sci Rep 5:13624. https://doi.org/10.1038/srep13624
Yanagisawa S, Yoo S-D, Sheen J (2003) Differential regulation of EIN3 stability by glucose and ethylene signalling in plants. Nature 425:521–525. https://doi.org/10.1038/nature01984
Yang B, Sugio A, White FF (2006) Os8N3 is a host disease-susceptibility gene for bacterial blight of rice. Proc Natl Acad Sci USA 103:10503–10508. https://doi.org/10.1073/pnas.0604088103
Yang F, Melo-Braga MN, Larsen MR et al (2013) Battle through signaling between wheat and the fungal pathogen Septoria tritici revealed by proteomics and phosphoproteomics. Mol Cell Proteom 12:2497–2508. https://doi.org/10.1074/mcp.M113.027532
Yu Y, Streubel J, Balzergue S et al (2011) Colonization of rice leaf blades by an African strain of Xanthomonas oryzae pv. oryzae depends on a new TAL effector that induces the rice nodulin-3 Os11N3 gene. Mol Plant Microbe Interact 24:1102–1113. https://doi.org/10.1094/MPMI-11-10-0254
Yuan M, Chu Z, Li X et al (2009) Pathogen-induced expressional loss of function is the key factor in race-specific bacterial resistance conferred by a recessive R gene xa13 in rice. Plant Cell Physiol 50:947–955. https://doi.org/10.1093/pcp/pcp046
Zangerl AR, Hamilton JG, Miller TJ et al (2002) Impact of folivory on photosynthesis is greater than the sum of its holes. Proc Natl Acad Sci USA 99:1088–1091. https://doi.org/10.1073/pnas.022647099
Zhao D, You Y, Fan H et al (2018) The role of sugar transporter genes during early infection by root-knot nematodes. Int J Mol Sci 19:302. https://doi.org/10.3390/ijms19010302
Zhou J, Peng Z, Long J et al (2015) Gene targeting by the TAL effector PthXo2 reveals cryptic resistance gene for bacterial blight of rice. Plant J Cell Mol Biol 82:632–643. https://doi.org/10.1111/tpj.12838
Zhu Z, An F, Feng Y et al (2011) Derepression of ethylene-stabilized transcription factors (EIN3/EIL1) mediates jasmonate and ethylene signaling synergy in Arabidopsis. Proc Natl Acad Sci USA 108:12539–12544. https://doi.org/10.1073/pnas.1103959108
Zimmerli L, Stein M, Lipka V et al (2004) Host and non-host pathogens elicit different jasmonate/ethylene responses in Arabidopsis. Plant J Cell Mol Biol 40:633–646. https://doi.org/10.1111/j.1365-313X.2004.02236.x
Zou J, Rodriguez-Zas S, Aldea M et al (2005) Expression profiling soybean response to Pseudomonas syringae reveals new defense-related genes and rapid HR-specific downregulation of photosynthesis. Mol Plant Microbe Interact 18:1161–1174. https://doi.org/10.1094/MPMI-18-1161
Acknowledgements
P.K. acknowledges the post-doctoral research fellowship from Department of Biotechnology (DBT, Govt. of India). The research in GJ lab is supported by research funding from DBT, Govt. of India and core research grant of National Institute of Plant Genome Research (NIPGR), New Delhi.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kanwar, P., Jha, G. Alterations in plant sugar metabolism: signatory of pathogen attack. Planta 249, 305–318 (2019). https://doi.org/10.1007/s00425-018-3018-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-018-3018-3