Abstract
Botrytis cinerea is the causing agent of the grey mold disease in more than 200 crop species. While signaling pathways leading to the basal resistance against this fungus are well described, the role of the import of sugars into host cells remains to be investigated. In Arabidopsis thaliana, apoplastic hexose retrieval is mediated by the activity of sugar transport proteins (STPs). Expression analysis of the 14 STP genes revealed that only STP13 was induced in leaves challenged with B. cinerea. STP13-modified plants were produced and assayed for their resistance to B. cinerea and glucose transport activity. We report that STP13-deficient plants exhibited an enhanced susceptibility and a reduced rate of glucose uptake. Conversely, plants with a high constitutive level of STP13 protein displayed an improved capacity to absorb glucose and an enhanced resistance phenotype. The correlation between STP13 transcripts, protein accumulation, glucose uptake rate and resistance level indicates that STP13 contributes to the basal resistance to B. cinerea by limiting symptom development and points out the importance of the host intracellular sugar uptake in this process. We postulate that STP13 would participate in the active resorption of hexoses to support the increased energy demand to trigger plant defense reactions and to deprive the fungus by changing sugar fluxes toward host cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Botrytis cinerea is a necrotrophic ascomycete responsible for the grey mold disease (Williamson et al. 2007). This fungus kills host cells using a large arsenal of weapons that enables it to infect a broad range (>200) of host plants. Secretion of non-specific toxins, i.e. botrydial, and production of reactive oxygen species (ROS) are commonly associated features of the infection process (van Kan 2006; Choquer et al. 2007). B. cinerea also triggers an active oxidative burst produced by the host itself. In the case of necrotrophs, the subsequent plant cell death clearly promotes the pathogen growth (Govrin and Levine 2000). B. cinerea possesses multiple lytic enzymes, i.e. endopolygalacturonases, cutinases, cellulases, that degrade the cell wall and the cuticle (ten Have et al. 1998; van Kan 2006). Activities of cell wall-degrading enzymes allow the maceration of the plant biomass, generating an important source of nutrients for the fungus consumption.
Pathogens, whatever their type, develop at the expense of the sugars produced by plants and it is largely assumed that classical sources turn into sinks when colonized by pathogens (Biemelt and Sonnewald 2006; Lemoine et al. 2013). B. cinerea can infect either source or sink organs. The availability of carbohydrates may be an important issue for B. cinerea during every stage of the infection, e.g. germination, the penetration of the host surface, the killing of the host tissue and spreading. In most plants, sucrose is the main photo-assimilate translocated from sources to sinks. In the release phloem, sucrose can move simplistically or can be unloaded into the apoplast (van Bel 2003). Apoplastic sucrose enters the sink cells via sucrose transporters or alternatively, can be cleaved into hexoses (glucose and fructose) by the activity of cell wall invertases, consequently increasing the level of apoplastic free hexoses (Roitsch and Gonzalez 2004; Lemoine et al. 2013). Potentially, both the fungus and the host cells can exploit those sugars. In plant/biotrophic fungus interactions, the haustorial structure represents the site of nutrient exchanges where the pathogen can acquire sugars from the apoplast through the activity of fungal sugar transporters (Hall and Williams 2000; Voegele and Mendgen 2011; Voegele et al. 2001). Regarding B. cinerea, information on sugar acquisition mechanisms during pathogenesis is fragmentary. A fungal invertase has been detected in grape infected by B. cinerea (Ruiz and Ruffner 2002). A high affinity fructose transporter (BcFRT1) and a multigenic family of hexose transporter (BcHXTs) have been identified, suggesting that B. cinerea possesses a versatile hexose uptake system (Doehlemann et al. 2005; Dulermo et al. 2009).
Plant ability to retrieve extracellular sugars may be determinant for the final outcome of the interaction and may be part of the plant immune response to defend itself against pathogens (Roitsch et al. 2003). Plant innate immunity is triggered upon the perception of pathogen-associated molecular patterns (PAMPs), which are conserved components of pathogens, through pattern recognition receptors. The so-called PAMP-triggered immunity (PTI) has been extensively studied over the past years and excellent reviews have described the complex molecular perception and signaling mechanisms (Boller and Felix 2009; Monaghan and Zipfel 2012; Mengiste 2012; Thomma et al. 2011). PTI is characterized by common downstream signaling events, including ROS production, MAP kinases activation, hormone signaling and transcriptional reprogramming (Tsuda and Katagiri 2010). Hormone signaling mediates host resistance according to the pathogen lifestyle (Glazebrook 2005; Thomma et al. 1998, 2001). Classically, resistance to biotrophs is associated with the activation of a salicylic acid-dependent signaling pathway while jasmonic acid and ethylene are required for the basal resistance to necrotrophs, such as B. cinerea (Pieterse et al. 2012; Bari and Jones 2009). The roles of sugars in the PTI, via the coordinated activities of cell wall invertases and hexose transporters, have been postulated to satisfy the additional energy required to activate the cascade of defense reactions. Besides their roles as carbon and energy sources, sugars can modulate, as signaling molecules, many physiological processes including pathogenesis-related (PR) gene expression and can consequently participate actively in the basal resistance to pathogens (Roitsch 1999; Bolouri Moghaddam and Van den Ende 2012; Herbers et al. 1996).
Modulation of host glucose transport activity has been described in several pathosystems. For example, Pinus pinaster suspension cells showed an increase in glucose uptake after inoculation with B. cinerea (Azevedo et al. 2006) whereas Bourque et al. (2002) reported an immediate inhibition of glucose absorption in tobacco cells elicited by cryptogein, a proteinaceous elicitor secreted by Phytophtora cryptogea. Host hexose uptake is mediated by the activity of monosaccharide transporters. In Arabidopsis thaliana, monosaccharide transporter (-like) gene superfamily (MST) comprises 53 genes (Büttner 2007). The retrieval of hexoses from the apoplasm across the plasma membrane is mediated by the sugar transport protein (STP) family, which is encoded by 14 highly homologous genes. STP is the best characterized family among MSTs and their substrate specificities, gene expression patterns, transcriptional regulation and functional roles are well described (Büttner 2010; Slewinski 2011). To date, little is known concerning the involvement of pre-existing or pathogen-induced Arabidopsis STPs in the changes of glucose absorption upon pathogen challenge. During the biotrophic interaction between Erysiphe cichoracearum and A. thaliana, the up-regulation of the plant cell-wall invertase (cwINV) AtβFRUCT1 and the hexose transporter AtSTP4 is correlated with increased invertase activity and glucose uptake, supporting the functional coordination of STPs and cwINVs in order to supply host tissues with hexoses (Fotopoulos et al. 2003). STP4 is also transcriptionally induced by wounding, by treatments with bacterial elicitors from Pseudomonas syringae pv tabaci and after infection with necrotrophic fungi, e.g. Fusarium oxysporum and Alternaria brassicicola (Truernit et al. 1996). Global data collected from microarray experiments have showed that other STP genes are responsive to environmental stresses (Büttner 2010). For example, STP13 is differentially regulated by various external factors or stresses, i.e. light, pathogens, drought, or chemicals, and is also induced in tissues undergoing programmed cell death (PCD) (Norholm et al. 2006).
In this study, we identified the Sugar Transporter Protein STP13 as the only member of the A. thaliana STP family induced by the necrotrophic fungus B. cinerea isolate B05.10, at transcriptional and translational levels. Plants impaired in STP13 expression exhibit a reduced glucose uptake rate and increased disease symptoms, indicating that STP13 contributes to the basal resistance to B. cinerea by limiting the development of necrosis. High constitutive expression of STP13 protein, before challenge, associated with an increased glucose uptake activity, leads to an enhanced resistance phenotype by preventing and slowing down the development of disease symptoms caused by B. cinerea. Our results demonstrate that the modification of the host intracellular glucose uptake prior to infection affects B. cinerea resistance level. This work further suggests that the differential expression level of plant hexose transporters can probably change the balance of hexose fluxes toward either host living tissues or fungus cells, highlighting the importance of the competition for apoplastic hexoses at the plant/fungus interface.
Results
Expression of Arabidopsis STP genes upon Botrytis cinerea isolate B05.10 infection
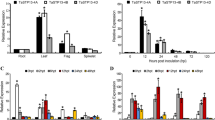
Our goal was to identify Arabidopsis Sugar Transport Proteins (STP), which may play a role in defense mechanisms against the necrotrophic fungus B cinerea. In order to find candidate genes with a B. cinerea-induced expression pattern, we monitored the expression of the 14 STP genes by qRT-PCR. The transcriptional-based screening of STP genes was carried out 48 h post-inoculation (hpi) when disease symptoms were visible and the JA-responsive marker gene PDF1.2 was up-regulated, being conscious that an earlier time point might have allowed the identification of other candidates. We performed inoculation experiments in the dark to prevent the known diurnal and light-regulated expression of STPs (Büttner 2010; Stadler et al. 2003). Firstly, transcripts of STP2, STP5, STP6, STP8, STP9, STP10, STP11 and STP12 were not detected before and after infection, suggesting that these genes were not expressed to a detectable level in the leaves (data not shown). A clear amplification signal was obtained for only 6 out of the 14 STP genes: STP1, STP3, STP4, STP7, STP13 and STP14. After inoculation of Arabidopsis leaves with B. cinerea isolate B05.10, the expression of STP4, STP7 and STP14 was not modified, while STP1 and STP3 were slightly down-regulated compared to mock-treated leaves. In contrast, the expression of STP13 was clearly induced by B. cinerea at 48 hpi (Fig. 1).
Fold change in expression of 6 STP genes upon B. cinerea infection at 48 h post-inoculation. Five-week-old wild-type plants were sprayed with mock solution or B. cinerea suspension (5 × 104 conidia ml−1) and at least three plants were harvested 48 h after treatment. STPs transcript levels were quantified by RT-qPCR. The gene expression of each STP was normalized to the plant reference gene At4g26410 (Czechowski et al. 2005) and is relative to the corresponding mock treatment. Data are the mean (±SE) of 3 independent experiments
Because of its unique expression pattern among STPs, we focused our study on the B. cinerea-responsive STP13, and monitored more precisely its kinetic of expression upon infection (Fig. 2). It appeared that the level of transcripts of STP13 increased weakly after 48 and 72 h in mock-treated leaves, suggesting that STP13 responded slightly to the stress caused by the experimental procedure, i.e. high humidity and extended darkness. However, after B. cinerea inoculation, STP13 transcript level was maximal within 48 h post inoculation and was approximately 4 times higher compared to mock treatment, confirming the results shown in Fig. 1. No significant induction of STP13 expression was observed 12 h (data not shown) and 24 h (Fig. 2) after B. cinerea inoculation while disease symptoms were absent.
STP13 mRNA accumulation in wild-type (WT), stp13-2 and STP13OE-6 plants. WT and stp13-2 plants were sprayed with mock solution or B. cinerea suspension (5 × 104 conidia ml−1). Healthy (0 hpi) or treated leaves from at least three plants were harvested at indicated time points. STP13 transcript levels were quantified by RT-qPCR. Data are expressed as normalized expression (no unit) to the plant reference gene At4g26410 expression level (Czechowski et al. 2005) and are the mean (± SE) of 12 (WT), 5 (stp13-2) and 3 (STP13OE-6) independent experiments. Different letters represent groups that were significantly different as determined by an ANOVA with general scores followed by a multiple comparison test (P < 0.05). B.c.: B. cinerea. hpi: hours post-inoculation
Collectively, these data showed that STP13 is the only STP positively regulated by B. cinerea during symptom development, suggesting that it may play a role in late plant defenses triggered in response to B. cinerea.
Characterization of STP13-modified plants
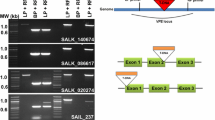
To determine the biological importance of STP13, the homozygous mutant line stp13-2 (salk_021204), with a T-DNA insertion in the first intron of the STP13 genomic sequence, was selected. We first monitored the expression of STP13 in this mutant by RT-qPCR. STP13 was not detected in healthy leaves and was no longer inducible after B. cinerea inoculation (Fig. 2). Two other homozygous insertion lines, with T-DNA in the third exon (stp13-1) and the promoter (stp13-3), were analyzed but not retained because both lines displayed a residual expression of STP13 (data not shown). Conversely, a transgenic line expressing the complete STP13 predicted coding sequence under the control of the CaMV35S promoter was produced (named STP13OE-6). As expected, STP13OE-6 line displayed a high constitutive expression of STP13 before and after infection (Fig. 2 and Fig. S1).
To verify the presence of the protein and to exclude a putative post-transcriptional regulation of STP13, we examined its expression profile at the protein level by immunodetection. A specific peptide containing the last 27 C-terminal amino-acids of STP13 was produced and used to immunize rabbits in order to obtain a polyclonal antibody raised against this protein. The purified serum detected a single protein corresponding to STP13 in the microsomal fraction of yeast expressing STP13, while no signal was detected in WT yeast (data not shown). The purified serum was then tested on the microsomal fraction of WT A. thaliana leaves (Fig. 3). STP13 protein was constitutively present in non-stimulated WT leaves and undetectable in stp13-2, while the STP13OE-6 line exhibited an elevated constitutive expression of STP13 (Fig. 3a). Kinetic studies established that the STP13 protein accumulated slightly in WT mock-treated leaves and was induced at a high level within 48 hpi in response to B. cinerea (Fig. 3b). No induction of the STP13 protein was detected at 24 hpi compared to the mock treatment, which is consistent with transcript accumulation data (Fig. 2). In contrast to WT plants, no STP13-related signal was visible in the stp13-2 mutant, neither in control nor in stimulated leaves, confirming the specificity of the antibody and supporting that this T-DNA insertion line is a full knockout (Fig. 3c).
Accumulation of STP13 protein in wild-type (WT), stp13-2 and STP13OE-6 plants. Leaves were harvested and proteins extracted from the microsomal fraction were submitted to protein immunoblot analysis with a polyclonal antibody raised against the STP13 protein (@STP13). a STP13 protein accumulation in healthy leaves from WT, stp13-2 and STP13OE-6 plants. Plants were placed and harvested in a controlled growth chamber with 60 % humidity on a 10-h photoperiod. Time course study of STP13 protein accumulation in WT (b) or stp13-2 (c) leaves sprayed with mock solution or B. cinerea suspension (5 × 104 conidia ml−1). During inoculation experiments (panel b and c), mock and B. cinerea-treated plants were kept in the dark under saturating humidity (for 3 days). Thirty micrograms of total microsomal proteins have been loaded in each lane except for STP13OE-6 (2 µg). To visualize protein loading, blots were stained with a Ponceau S solution. hpi hours post-inoculation, B.c. B. cinerea, WT lanes (mock and B. cinerea) are used as a positive control of the panel c
STP13 has been described as a high affinity H+/hexoses symporter (Norholm et al. 2006). To verify whether stp13-2 and STP13OE-6 plants had modified rates of sugar uptake, glucose absorption was assayed using discs from healthy mature leaves that were floated on [14C]-glucose-containing solution. As shown in Fig. 4, the total glucose uptake by the over-expressor line was approximately 3 times higher than in the WT, while the stp13-2 line had a rate of glucose transport that was reduced by 27 %. We used the protonophore carbonylcyanide m-chlorophenyl hydrazone (CCCP), which uncouples oxidative phosphorylation in mitochondria and collapses the transplasmalemma pH gradient, in order to discriminate the passive diffusion of radiolabelled glucose into the leaf discs and the active glucose uptake mediated by proton-driven glucose symporters. The total glucose uptake was inhibited by CCCP by 62, 59 and 71 % in WT, stp13-2 and STP13OE-6 respectively, which is consistent with a proton-driven glucose transport mechanism (Fig. 4). We noticed an increase in the CCCP-insensitive glucose uptake by the STP13OE-6 line compared to the WT. This increase is probably caused by a higher quantity of proteins in the plasma membrane of STP13OE-6, making it more permeable to glucose. Interestingly, a lack of STP13 in stp13-2 provokes a significant reduction (by approximately 30 %) of the active glucose uptake compared to the WT, indicating that STP13 contributes up to 30 % of the active glucose transport in WT healthy leaves. In agreement with the high level of STP13 protein detected in STP13OE-6 (Fig. 3a), the amount of glucose actively taken up in this line was more than 3 times higher than in WT (Fig. 4). This result demonstrates that the STP13 protein immuno-detected in the STP13OE-6 line was functional.
Glucose uptake into wild-type (WT; black bars), stp13-2 (light grey bars) and STP13OE-6 (dark grey bars) leaf discs. Discs were obtained from 5-week-old plants and total glucose uptake was measured after 45 min in equilibration buffer containing 0.2 mM of glucose (glucose buffer). Active glucose uptake results from the difference between total uptake and CCCP-insensitive uptake measured after incubation of leaf discs in glucose buffer containing CCCP (20 µM). Data are the mean (±SE) of 6 (WT) and 4 (stp13-2 and STP13OE-6) independent experiments. Statistical analyses were undertaken independently for total, active and CCCP-insensitive glucose uptake. Stars represent groups that were significantly different from WT as determined by a one-way ANOVA followed by a multiple comparison test (*P < 0.05; ***P < 0.001)
To check whether the STP13 induction in B. cinerea-treated leaves could correlate with an increase in glucose uptake, we tried to measure the STP13-mediated glucose uptake activity in leaf discs infected with B. cinerea. However, the accumulation of the STP13 protein is concomitant with the formation of necrosis. As a result, it was technically unachievable to obtain reliable results on such damaged tissues.
The STP13 protein level affects B. cinerea susceptibility
The availability of transgenic plants that exhibited differential expression of STP13 and altered glucose uptake rate prompted us to investigate the contribution of STP13 in the outcome of B. cinerea infection. To this end, STP13-modified plants were challenged with B. cinerea and tested for their susceptibility.
Plants were drop-inoculated on leaves with a solution of conidia, and symptoms were observed 72 h after inoculation. We first used the average of the lesion diameters (LD) as an indicator for the susceptibility of each genotype. As shown in Fig. 5a, WT plants were susceptible to B. cinerea isolate B05.10. Compared to WT plants, the stp13-2 mutant showed a significant increase in LD, suggesting that this mutant is more susceptible than the WT, whereas STP13OE-6 plants displayed lesions with reduced size (decrease of LD by 26 %), indicating that these plants exhibited an enhanced resistance phenotype (Fig. 5a). Then, we looked at the distribution of the lesions according to their size (Fig. 5b). We determined three classes of necrotic lesions: small (LD < WT first quartile), medium (LD = WT interquartile range) and large (LD > WT third quartile). This scoring reflects the visual severity of symptoms. Compared to the WT, an increased proportion of large lesions and fewer small lesions were observed in the stp13-2 mutant (Fig. 5b). Conversely, STP13OE-6 showed a twofold increase of the small lesion percentage, which represents 47 % of the total lesion number, whereas the number of large lesions was reduced (Fig. 5b). We introduced the cDNA corresponding to the STP13 mRNA into stp13-2 under the control of the CaMV35S promoter. In the resulting plants (named C2-7), the construct was able to complement stp13-2 mutation and to fully restore the WT resistance state, demonstrating that the susceptible phenotype observed in stp13-2 was attributable to STP13 (Fig. S2).
Disease phenotype of wild-type (WT), stp13-2 and STP13OE-6 plants infected with B. cinerea. Leaves of 5-week-old plants have been used for inoculation tests. a Lesion diameters observed on WT, stp13-2 and STP13OE-6 plants 3 days after inoculation with 6 µl droplets containing 104 conidia ml−1. Data represent the mean (±SE) lesion diameter from 18 (WT and stp13-2) and 6 (STP13OE-6) experiments. In each experiment, at least five plants per genotype were infected with B. cinerea. Stars represent groups that were significantly different from the WT as determined by a permutation with general scores test (*P < 0.025; **P < 0.01). b Lesion size distribution observed on WT, stp13-2 and STP13OE-6 plants infected with B. cinerea. Plants were drop-inoculated and diameters of necrotic lesions (LD) were measured after 3 days. Lesions were grouped into 3 classes according to their size: small (LD < WT first quartile), medium (LD = WT interquartile range) and large (LD > WT third quartile). The percentage of lesion size distribution from 18 (WT and stp13-2) and 6 (STP13OE-6) independent experiments is shown. Significant differences from the WT were determined by a Chi square test (*P < 0.025; **P < 0.01). c Percentage of outgrowing lesions observed on WT, stp13-2 and STP13OE-6 infected with B. cinerea. Plants were inoculated with 6 µl droplets containing 103 conidia ml−1. During 5 days, the number of necrotic lesions was counted and the ratio between outgrowing lesions and total inoculated spots was calculated. Data represent the mean (±SE) from six independent experiments. Statistical analyses have been realized independently for each day post-inoculation. Stars represent groups that were significantly different from WT as determined by a Kruskal–Wallis one-way analysis of variance followed by a multiple comparison test (P < 0.05)
Finally, we evaluated the capacity of B. cinerea to develop and provoke necrosis in each genotype. In these experiments, a very low density of conidia was drop-inoculated on leaves in order to extend the duration of the infection process. During 5 days, the number of necrotic lesions was counted and the ratio between visible necrosis and total inoculated spots was calculated in order to assess the percentage of outgrowing lesions. The appearance of new necrotic lesions represents the ability of B. cinerea to kill host cells and spread. In these experimental conditions, necrotic lesions started to be visible 3 days post-inoculation in WT plants (Fig. 5c). The infection process reaches its maximum within 4 days post inoculation, where approximately 80 % of successfully infected spots were recorded. The number of necrotic spots did not increase much after 4 days. In comparison with WT plants, the kinetic of the outgrowing lesion appearance did not change in the stp13-2 mutant (Fig. 5c), while the size of the necrotic lesions was previously shown to be larger in those plants (Fig. 5b). These results and the late induction of STP13 during B. cinerea infection suggest that STP13 may be involved in limiting the expansion of the symptoms but not in the formation of necrosis. By contrast, STP13OE-6 plants, which constitutively accumulated high amount of STP13 protein (Fig. 3a) and displayed a higher rate of glucose uptake (Fig. 4), were found to have a major reduction of the outgrowing lesion percentage (Fig. 5c). Compared to the WT, the overexpression of STP13 resulted in a drop of 50, 30 and 20 % in the number of visible necrotic spots at respectively 3, 4 and 5 days post-inoculation. These data indicated that the high expression of STP13 associated with an increased rate of glucose uptake prior to infection slowed down the formation of necrotic lesions and reduced the disease severity by limiting the symptom development.
To summarize, STP13-modified plants with altered resistance levels revealed that STP13 contributes to the basal resistance to B. cinerea in WT plants. Furthermore, we evidenced that a high constitutive level of glucose uptake in the host before infection has a clear protective effect against B. cinerea.
Expression of the jasmonic acid-dependent defense gene PDF1.2 and the camalexin biosynthetic gene PAD3 in STP13-modified plants
To explain the modified resistance phenotype of stp13-2 and STP13OE-6 mutants, we verified whether marker genes of the jasmonic acid-dependent signaling and the camalexin biosynthesis were affected in those transgenic lines. B. cinerea induces the expression of a subset of JA-dependent defense genes, including the antifungal defensin-like peptide PDF1.2, which are required for resistance (Glazebrook 2005). The role of camalexin in resistance to B. cinerea seems to be isolate-dependent (Kliebenstein et al. 2005; Ferrari et al. 2003; Rowe and Kliebenstein 2008). In response to several B. cinerea strains, including B05.10, the induction of CYP79B2, CYP71A13, and CYP71B15 (PAD3), involved in the camalexin biosynthesis, is highly correlated with the accumulation of camalexin (Stefanato et al. 2009; Schuhegger et al. 2006; Arbelet et al. 2010). By contrast, Rowe et al. (2010) evidenced a lack of correlation with some B. cinerea isolates. Moreover, pad3 mutant, which is deficient in camalexin accumulation, displays an increased sensitivity to the B. cinerea isolate B05.10, indicating that the amount of camalexin present in WT plants limits spreading (Stefanato et al. 2009).
The expression of PDF1.2 and PAD3 was tested after B. cinerea inoculation and used as specific markers of the JA signaling and camalexin biosynthetic pathways. In our experimental conditions, PDF1.2 and PAD3 were strongly induced at 48 hpi and are concomitantly expressed with STP13 (Figs. 2, 6). This result validates our initial assumption that specific defenses were fully active 48 hpi. In stp13-2-infected plants, the pattern of transcript accumulation of PAD3 was not affected in comparison with WT whereas the expression of PDF1.2 was reduced at 72 hpi, which is consistent with the higher susceptibility of this line (Fig. 6 and table S2).
PDF1.2 and PAD3 mRNA accumulation in wild-type (WT), stp13-2 and STP13OE-6 plants. Plants were sprayed with mock solution or B. cinerea suspension (5 × 104 conidia ml−1). Healthy (0 hpi) or treated leaves from at least three plants were harvested at indicated time points. PDF1.2 (a) and PAD3 (b) transcript levels were quantified by RT-qPCR. Data are expressed as normalized expression (no unit) to the plant reference gene At4g26410 expression level (Czechowski et al. 2005). Data are mean ± SE from two independent experiments. Stars represent groups that were significantly different from WT as determined by an ANOVA with general scores followed by a multiple comparison test (P < 0.05). B.c. B. cinerea, hpi hours post-inoculation
Because sugars are known to act as signaling molecules that can positively regulate gene expression (Herbers et al. 1996; Ehness et al. 1997; Roitsch et al. 2003; Bolouri Moghaddam and Van den Ende 2012; Rolland et al. 2006), we tested the hypothesis whether the improved resistance of STP13OE-6 line is attributable to a sugar-mediated stimulation of defense genes. To this end, we monitored the expression level of PDF1.2 and PAD3 in STP13OE-6 plants (Fig. 6). The basal expression level (0 hpi) of PDF1.2 and PAD3 in STP13OE-6 did not differ from the WT, indicating that the higher amount of glucose uptake in STP13OE-6 did not result in the priming of these two genes. Unexpectedly, we noticed that the expression level of PDF1.2 was reduced at 48 and 72 hpi compared to the WT (Fig. 6a and table S2), which could be explained by the limited expansion of the infection in the STP13OE-6 line (Fig. 5b). However, the expression of PDF1.2 and PAD3 was still highly inducible in response to B. cinerea, but did not display over-stimulation (Fig. 6a, b). According to these results, we can conclude that the enhanced resistance phenotype observed in the STP13OE-6 line cannot be supported by a higher induction of the expression of these defense genes.
Discussion
Our goal was to identify plant STPs that play a role in the basal resistance to the necrotrophic fungus B. cinerea isolate B05.10. In A. thaliana, the uptake of hexoses from the apoplasm is mediated by STPs, which are encoded by 14 highly homologous genes. All the characterized STPs are proton symporters of the plasma membrane, showing specificities for a wide range of substrate (Büttner 2010). Most of them display a sink-specific expression pattern which correlates with their potential function in supporting sink demand for hexoses (Büttner 2010).
In healthy source leaves, the analysis of transcript accumulation of the 14 STP genes revealed that 6 of them are expressed in leaves, namely STP1, STP3, STP4, STP7, STP13 and STP14. Our results confirmed the data obtained in different publications. It has been previously reported that STP1 was strongly expressed in guard cells of different leaf types but also in other organs, i.e. roots, seeds and seedlings, having a major contribution to the absorption of monosaccharides from the surrounding environment (Stadler et al. 2003; Sherson et al. 2000; Yamada et al. 2011). STP3 has been described as a low affinity monosaccharide transporter that is expressed in green tissues, and is up-regulated after wounding (Büttner et al. 2000). STP4 is expressed primarily in sink tissues, i.e. roots and flowers but is also induced in leaves by various stress conditions, e.g. wounding, elicitors and pathogens (Truernit et al. 1996; Fotopoulos et al. 2003). STP13 is found in source and sink tissues and is transcriptionally regulated by many abiotic or biotic stresses, like wounding, drought, high salinity, pathogens, or programmed cell death (Büttner 2010; Norholm et al. 2006; Yamada et al. 2011). STP13 mediates the uptake of glucose, mannose, galactose and fructose at a significant rate (Norholm et al. 2006). A role for STP13 in the uptake of monosaccharides in roots under high salinity has been shown (Yamada et al. 2011). STP14 was found to be expressed in different tissues, mainly in leaves and siliques, but unlike other STPs, it has been identified as a plasma membrane galactose-specific transporter (Poschet et al. 2010). Concerning STP7, no uptake activities have been found so far for the common hexoses, suggesting it may be a pseudogene (Büttner 2010; Yamada et al. 2011).
The expression of STP1, STP3, STP4, STP13 and STP14 is also known to display a diurnal regulation (Büttner 2010; Stadler et al. 2003; Poschet et al. 2010). In order to minimize this effect, we decided to perform the inoculation experiments in the dark. Profiling of the transcriptional activities of the 14 STPs in B. cinerea-infected leaves showed that the expression of many STPs was not modified by B. cinerea challenge. Interestingly, none of the undetected STPs in healthy leaves were induced by B. cinerea, confirming that they may be involved in other physiological processes, particularly in sink tissues. STP4, whose expression is correlated with glucose resorption during the interaction between the biotroph E. cichoracearum and A. thaliana leaves (Fotopoulos et al. 2003), is not affected by B. cinerea, indicating that different STPs could be activated depending on pathogens. We found that solely STP13 was induced upon B. cinerea infection. The detailed analysis of STP13 expression and the immunodetection of STP13 showed that transcripts and proteins accumulated in B. cinerea-inoculated leaves. They followed the expression of late defense genes and the formation of necrotic lesions. Such disease symptoms are the consequence of the cell death of the host. This phenomenon occurs through fungal toxin activities and an oxidative burst generated by the pathogen and the host itself, which triggers an active or programmed cell death (Choquer et al. 2007). Our observations are in agreement with previous findings showing that STP13 expression was upregulated in tissues undergoing PCD (Norholm et al. 2006).
The unique expression profile of STP13 among STPs led us to undertake the functional analysis of this gene. To investigate the impact of STP13 expression on B. cinerea resistance, we analyzed transgenic plants with modified levels of STP13. Under normal growth conditions on soil, a lack or an overexpression of STP13 did not result in any obvious developmental or morphological changes (Fig. S3). Interestingly, Schofield et al. (2009) showed that STP13-overexpressing seedlings grown on soil-less media supplemented with 55 mM glucose and 9 mM nitrate had an increased biomass, higher endogenous sucrose levels and an improved capacity to use nitrogen, but observed no difference when plants were grown on soil.
Upon infection by B. cinerea, KO and overexpressor lines displayed clear contrasting phenotypes. Plants that did not accumulate STP13 had an increased susceptibility to B. cinerea associated with a reduced basal capacity to absorb glucose. The significant residual glucose uptake activity observed in the stp13-2 mutant suggests that the probable activity of other STPs may limit the effect of the mutation of a single STP (Fig. 4). However, the stp13-2 line exhibited necrosis with increased size, providing genetic evidence that STP13 contributes to the basal resistance but does not act in the prevention of symptom development caused by the highly virulent B. cinerea isolate B05.10. Since STP13 is expressed simultaneously with necrosis, it would rather indicate that this protein plays a role in limiting the expansion of the symptoms. It should be noted that there is a large level of genetic diversity within B. cinerea strains (Kliebenstein et al. 2005; Rowe et al. 2010) and we can not exclude that the phenotype we observed could be different with other B. cinerea isolates. When challenged with the necrotroph A. brassicicola, no difference in susceptibility was observed in stp13 knockout mutants by Norholm et al. (2006) (data were not shown). It may be explained by the difference of virulence between these two necrotrophic fungi, A. brassicicola being poorly virulent compared to B. cinerea on A. thaliana leaves (Mukherjee et al. 2010).
In the present study, the importance of host hexose uptake has also been evidenced in STP13OE-6 plants. In this line, a high amount of STP13 prior to infection associated with a high level of glucose uptake rate clearly improved the plant resistance. It resulted in delaying the appearance and development of the symptoms. We showed that this protection against B. cinerea is not attributable to the priming of marker genes of the jasmonic acid (PDF1.2) and camalexin (PAD3) pathways, which are generally associated with basal levels of defense against necrotrophic fungi.
Collectively, our data suggest that STP13 could be involved in the resorption of monosaccharides released by damaged tissues in order to allow host living cells to compete with the pathogen for apoplastic hexoses. The role of the imported hexoses into host cells remains to be elucidated, but we hypothesize that host cells could use them as a fuel to support the increased energy demand to trigger plant defense reactions. The enhancement of host hexose resorption would imply a change in the direction of sugar fluxes toward host cells. It could consequently participate in the starvation of the fungus by limiting the availability of apoplastic hexoses. Such competition for apoplastic sugars is crucial for the final outcome of the interaction between the plant and the pathogen. B. cinerea has the functional equipment for apoplastic sugar uptake at the plant/pathogen interface (Lemoine et al. 2013). Dulermo et al. (2009) reported that B. cinerea possesses a multigenic flexible hexose uptake system (BcHXTs and BcFRT genes), allowing the fungus to re-allocate plant hexoses for subsequent conversion into mannitol through fungal metabolic pathways. The next challenge will be to understand the precise role of STP13 in this competition for nutrients between host cells and the invader, and more largely the regulatory role of sugar fluxes in the plant immunity.
In conclusion, we showed, using complementary approaches, a strong correlation between transcripts, protein detection, in planta glucose uptake rate and resistance level, which indicates that STP13 is involved in the basal resistance to B. cinerea. We and others have shown that STP13 contributes to the resorption of hexoses allowing plant cells to adapt to environmental changes, particularly during biotic and abiotic stresses. More investigations will be necessary to dissect the regulatory pathways leading to the expression of STP13 and the precise role of the sugar transport in the plant immunity and in the response to environmental stresses.
Materials and methods
Plant material and growth conditions
Seeds of A. thaliana (Columbia ecotype) were sown in an autoclaved mix of compost/vermiculite (3/1). Plants were grown in a controlled growth chamber at 23 °C (18 °C during the night), with 60 % humidity on a 10-h photoperiod. The stp13-2 homozygous mutant line (salk_021204) was obtained from the SALK T-DNA insertion mutant collection (Alonso et al. 2003).
Culture of Botrytis cinerea, infection methods and disease scoring
B. cinerea isolate B05.10 (Staats and van Kan 2012) was grown on Difco potato dextrose agar (Becton–Dickinson). Conidia were harvested in sterile water, filtered through miracloth (EMD Chemicals) and were diluted in quarter-strength potato dextrose broth (PDB; Becton–Dickinson) for infection.
For the evaluation of plant susceptibility, 6 µl of conidia suspension (104 conidia ml−1) were spotted on leaves of 5-week-old plants and lesion diameters (LD) were measured after 3 days. Three categories of necrotic lesions were determined. Small lesions : LD < 2,63 mm. Medium lesions : 2,63 mm < LD < 5,58 mm. Large lesions : LD > 5,58 mm. This range has been determined according to the lesion size distribution observed in the WT. Small, medium and large lesions corresponded to LD < WT first quartile, LD = WT interquartile range and LD > WT third quartile, respectively. For the analysis of the formation of necrosis, 6 µl of conidia suspension (103 conidia ml−1) were deposited on leaves and the percentage of outgrowing lesions was recorded every day for 5 days. For the analysis of gene expression by RT-qPCR, the entire rosette was sprayed with the conidia suspension (5 × 104 conidia ml−1) and mock-treated plants with quarter-strength PDB. Source leaves were harvested at the indicated time points. The inoculated plants were kept in the dark and under saturating humidity in a tray closed with a water-sprayed lid.
RNA extraction and RT-qPCR analysis
Total RNA was isolated using TRIzol reagent (Invitrogen) and treated with DNAse I (Sigma-Aldrich) prior to the retrotranscription. The cDNA was synthetized using M-MLV reverse transcriptase (Promega). Real-time quantitative PCR was performed using Gotaq PCR Mastermix (Promega). Target gene expression was normalized to the expression of the plant gene At4g26410, previously described as a stable reference gene (Czechowski et al. 2005; La Camera et al. 2011). The primers have been tested for their efficiency and their specificity. The primers are listed in Table S1.
Cloning procedures and plant transformation
The cDNA made from RNA extracted from B. cinerea-treated Arabidopsis plants were used to amplify STP13 complete coding sequence (Phusion Taq Polymerase, Thermo Scientific). The following primers were used for the amplification: 5′-CACCATGACCGGAGGAGGA-3′ and 5′-CACTTATTCACAAACGCAG-3′. PCR products were cloned into the pENTR-D-TOPO vector (Invitrogen), sequenced and LR-recombined with pB2GW7 destination vector (Karimi et al. 2002) to express STP13 under the control of the CaMV35S promoter. This construct was electroporated into Agrobacterium tumefaciens strain GV3101 (pMp90). Wild-type and stp13-2 plants were transformed using the floral dipping method (Clough and Bent 1998) and transgenic homozygous T2 plant lines were selected according to their Basta resistance.
Glucose uptake into leaf discs
Discs of eight-millimeter diameter were cut from leaves of 5-week-old plants and placed lower epidermis down in petri dishes containing equilibration buffer (20 mM MES-KOH pH 5.8 and 1 mM CaCl2) and left for 1 h under agitation. The discs were transferred in glucose buffer (20 mM MES pH 5.8, 1 mM CaCl2, 0.2 mM glucose containing 0.1 µCi ml−1 of [14C]-glucose) and incubated for 45 min. To measure CCCP-insensitive glucose uptake, CCCP (20 µM) was added in glucose buffer. After incubation, the discs were removed from glucose buffer, washed three times for 2 min in equilibration buffer, and dried on filter paper. The discs were incubated overnight in digestion buffer containing 36,4 % perchloric acid (w/v), 0,017 % triton X-100 (w/v) and 8,1 % hydrogen peroxide (w/v) at 55 °C. Samples were counted by liquid scintillation counting (Tri-Carb 2910 PR, PerkinElmer).
Western blot analysis
A microsomal fraction was prepared from A. thaliana leaves. Ground leaves were resuspended in 4 volumes of extraction buffer (50 mM HEPES pH 7.5, 500 mM sorbitol, 5 mM EDTA, 0.6 % PVPP 1 mM PMSF and 2 mM DTT), filtered through miracloth (EMD Chemicals) and centrifuged at 10,000g for 15 min at 4 °C. The supernatant was then centrifuged at 50,000g for 35 min at 4 °C. The pellet containing the microsomal fraction was resuspended in phosphate-sorbitol buffer (5 mM K phosphate pH 7.8, 330 mM sorbitol, 5 mM KCl, 1 mM EDTA, 0,5 mM DTT). Total microsomal proteins were separated by SDS-PAGE (10 % acrylamide) and blotted on nitrocellulose membrane (Hybond ECL, GE Healthcare) for western blot analysis. To visualize protein loading, blots were stained with a Ponceau S solution (Sigma-Aldrich).
A specific peptide containing the last 27 C-terminal amino-acids of STP13 (DDHNDHEFVNGEKSNGKSNGFDPSTRL) was synthesized (Proteogenix). After immunization of rabbits, the serum containing a polyclonal antibody raised against the STP13 protein was obtained and purified with the initial peptide (Proteogenix). Blots of total microsomal proteins were incubated with the purified STP13 antiserum and detection was realized using ECL Plus Western blotting Detection Reagents (GE Healthcare).
References
Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, Gadrinab C, Heller C, Jeske A, Koesema E, Meyers CC, Parker H, Prednis L, Ansari Y, Choy N, Deen H, Geralt M, Hazari N, Hom E, Karnes M, Mulholland C, Ndubaku R, Schmidt I, Guzman P, Aguilar-Henonin L, Schmid M, Weigel D, Carter DE, Marchand T, Risseeuw E, Brogden D, Zeko A, Crosby WL, Berry CC, Ecker JR (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301(5633):653–657
Arbelet D, Malfatti P, Simond-Côte E, Fontaine T, Desquilbet L, Expert D, Kunz C, Soulié M-C (2010) Disruption of the Bcchs3a Chitin Synthase gene in Botrytis cinerea is responsible for altered adhesion and overstimulation of host plant immunity. Mol Plant Microbe Interact 23(10):1324–1334
Azevedo H, Conde C, Geros H, Tavares RM (2006) The non-host pathogen Botrytis cinerea enhances glucose transport in Pinus pinaster suspension-cultured cells. Plant Cell Physiol 47(2):290–298
Bari R, Jones JG (2009) Role of plant hormones in plant defence responses. Plant Mol Biol 69(4):473–488
Biemelt S, Sonnewald U (2006) Plant-microbe interactions to probe regulation of plant carbon metabolism. J Plant Physiol 163(3):307–318
Boller T, Felix G (2009) A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Ann Rev Plant Biol 60(1):379–406
Bolouri Moghaddam MR, Van den Ende W (2012) Sugars and plant innate immunity. J Exp Bot 63(11):3989–3998
Bourque S, Lemoine R, Sequeira-Legrand A, Fayolle L, Delrot S, Pugin A (2002) The elicitor cryptogein blocks glucose transport in tobacco cells. Plant Physiol 130(4):2177–2187
Büttner M (2007) The monosaccharide transporter(-like) gene family in Arabidopsis. FEBS Lett 581(12):2318–2324
Büttner M (2010) The Arabidopsis sugar transporter (AtSTP) family: an update. Plant Biol (Stuttg) 12(Suppl 1):35–41
Büttner M, Truernit E, Baier K, Scholz-Starke J, Sontheim M, Lauterbach C, Huss VAR, Sauer N (2000) AtSTP3, a green leaf-specific, low affinity monosaccharide-H + symporter of Arabidopsis thaliana. Plant, Cell Environ 23(2):175–184
Choquer M, Fournier E, Kunz C, Levis C, Pradier J-M, Simon A, Viaud M (2007) Botrytis cinerea virulence factors: new insights into a necrotrophic and polyphageous pathogen. FEMS Microbiol Lett 277(1):1–10
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16(6):735–743
Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible W-R (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139(1):5–17
Doehlemann G, Molitor F, Hahn M (2005) Molecular and functional characterization of a fructose specific transporter from the gray mold fungus Botrytis cinerea. Fungal Genet Biol 42(7):601–610
Dulermo T, Rascle C, Chinnici G, Gout E, Bligny R, Cotton P (2009) Dynamic carbon transfer during pathogenesis of sunflower by the necrotrophic fungus Botrytis cinerea: from plant hexoses to mannitol. New Phytol 183(4):1149–1162
Ehness R, Ecker M, Godt DE, Roitsch T (1997) Glucose and stress independently regulate source and sink metabolism and defense mechanisms via signal transduction pathways involving protein phosphorylation. Plant Cell 9(10):1825–1841
Ferrari S, Plotnikova JM, De Lorenzo G, Ausubel FM (2003) Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2, but not SID2, EDS5 or PAD4. Plant J 35(2):193–205
Fotopoulos V, Gilbert MJ, Pittman JK, Marvier AC, Buchanan AJ, Sauer N, Hall JL, Williams LE (2003) The monosaccharide transporter gene, AtSTP4, and the cell-wall invertase, Atbetafruct1, are induced in Arabidopsis during infection with the fungal biotroph Erysiphe cichoracearum. Plant Physiol 132(2):821–829
Glazebrook J (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Ann Rev Phytopathol 43(1):205–227
Govrin EM, Levine A (2000) The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr Biol 10(13):751–757
Hall JL, Williams LE (2000) Assimilate transport and partitioning in fungal biotrophic interactions. Funct Plant Biol 27(6):549–560
Herbers K, Meuwly P, Frommer WB, Metraux JP, Sonnewald U (1996) Systemic acquired resistance mediated by the ectopic expression of invertase: possible hexose sensing in the secretory pathway. Plant Cell 8(5):793–803
Karimi M, Inzé D, Depicker A (2002) GATEWAY™ vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7(5):193–195
Kliebenstein DJ, Rowe HC, Denby KJ (2005) Secondary metabolites influence Arabidopsis/Botrytis interactions: variation in host production and pathogen sensitivity. Plant J 44(1):25–36
La Camera S, L’Haridon F, Astier J, Zander M, Abou-Mansour E, Page G, Thurow C, Wendehenne D, Gatz C, Metraux JP, Lamotte O (2011) The glutaredoxin ATGRXS13 is required to facilitate Botrytis cinerea infection of Arabidopsis thaliana plants. Plant J 68(3):507–519
Lemoine R, La Camera S, Atanassova R, Dédaldéchamp F, Allario T, Pourtau N, Bonnemain J-L, Laloi M, Coutos-Thévenot P, Maurousset L, Faucher M, Girousse C, Lemonnier P, Parrilla J, Durand M (2013) Source to sink transport and regulation by environmental factors. Front Plant Sci 4:272
Mengiste T (2012) Plant immunity to necrotrophs. Ann Rev Phytopathol 50(1):267–294
Monaghan J, Zipfel C (2012) Plant pattern recognition receptor complexes at the plasma membrane. Curr Opin Plant Biol 15(4):349–357
Mukherjee AK, Carp M-J, Zuchman R, Ziv T, Horwitz BA, Gepstein S (2010) Proteomics of the response of Arabidopsis thaliana to infection with Alternaria brassicicola. J Proteomics 73(4):709–720
Norholm MH, Nour-Eldin HH, Brodersen P, Mundy J, Halkier BA (2006) Expression of the Arabidopsis high-affinity hexose transporter STP13 correlates with programmed cell death. FEBS Lett 580(9):2381–2387
Pieterse CMJ, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SCM (2012) Hormonal modulation of plant immunity. Ann Rev Cell Dev Biol 28(1):489–521
Poschet G, Hannich B, Buttner M (2010) Identification and characterization of AtSTP14, a novel galactose transporter from Arabidopsis. Plant Cell Physiol 51(9):1571–1580
Roitsch T (1999) Source-sink regulation by sugar and stress. Curr Opin Plant Biol 2(3):198–206
Roitsch T, Gonzalez MC (2004) Function and regulation of plant invertases: sweet sensations. Trends Plant Sci 9(12):606–613
Roitsch T, Balibrea ME, Hofmann M, Proels R, Sinha AK (2003) Extracellular invertase: key metabolic enzyme and PR protein. J Exp Bot 54(382):513–524
Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signalling in plants: conserved and novel mechanisms. Ann Rev Plant Biol 57:675–709
Rowe HC, Kliebenstein DJ (2008) Complex genetics control natural variation in Arabidopsis thaliana resistance to Botrytis cinerea. Genetics 180(4):2237–2250
Rowe HC, Walley JW, Corwin J, Chan EKF, Dehesh K, Kliebenstein DJ (2010) Deficiencies in Jasmonate-mediated plant defense reveal quantitative variation in Botrytis cinerea pathogenesis. PLoS Pathog 6(4):e1000861
Ruiz E, Ruffner HP (2002) Immunodetection of Botrytis-specific invertase in infected grapes. J Phytopathol 150(2):76–85
Schofield RA, Bi YM, Kant S, Rothstein SJ (2009) Over-expression of STP13, a hexose transporter, improves plant growth and nitrogen use in Arabidopsis thaliana seedlings. Plant, Cell Environ 32(3):271–285
Schuhegger R, Nafisi M, Mansourova M, Petersen BL, Olsen CE, Svatos A, Halkier BA, Glawischnig E (2006) CYP71B15 (PAD3) catalyzes the final step in camalexin biosynthesis. Plant Physiol 141(4):1248–1254
Sherson SM, Hemmann G, Wallace G, Forbes S, Germain V, Stadler R, Bechtold N, Sauer N, Smith SM (2000) Monosaccharide/proton symporter AtSTP1 plays a major role in uptake and response of Arabidopsis seeds and seedlings to sugars. Plant J 24(6):849–857
Slewinski TL (2011) Diverse functional roles of monosaccharide transporters and their homologs in vascular plants: a physiological perspective. Mol Plant 4(4):641–662
Staats M, van Kan JAL (2012) Genome Update of Botrytis cinerea Strains B05.10 and T4. Eukaryot Cell 11(11):1413–1414
Stadler R, Büttner M, Ache P, Hedrich R, Ivashikina N, Melzer M, Shearson SM, Smith SM, Sauer N (2003) Diurnal and light-regulated expression of AtSTP1 in guard cells of Arabidopsis. Plant Physiol 133(2):528–537
Stefanato FL, Abou-Mansour E, Buchala A, Kretschmer M, Mosbach A, Hahn M, Bochet CG, Métraux J-P, Schoonbeek H-J (2009) The ABC transporter BcatrB from Botrytis cinerea exports camalexin and is a virulence factor on Arabidopsis thaliana. Plant J 58(3):499–510
ten Have A, Mulder W, Visser J, van Kan JA (1998) The endopolygalacturonase gene Bcpg1 is required for full virulence of Botrytis cinerea. Mol Plant Microbe Interact 11(10):1009–1016
Thomma BPHJ, Eggermont K, Penninckx IAMA, Mauch-Mani B, Vogelsang R, Cammue BPA, Broekaert WF (1998) Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA 95(25):15107–15111
Thomma BPHJ, Penninckx IAMA, Cammue BPA, Broekaert WF (2001) The complexity of disease signalling in Arabidopsis. Curr Opin Immunol 13(1):63–68
Thomma BPHJ, Nürnberger T, Joosten MHAJ (2011) Of PAMPs and effectors: the blurred PTI-ETI dichotomy. Plant Cell 23(1):4–15
Truernit E, Schmid J, Epple P, Illig J, Sauer N (1996) The sink-specific and stress-regulated Arabidopsis STP4 gene: enhanced expression of a gene encoding a monosaccharide transporter by wounding, elicitors, and pathogen challenge. Plant Cell 8(12):2169–2182
Tsuda K, Katagiri F (2010) Comparing signalling mechanisms engaged in pattern-triggered and effector-triggered immunity. Curr Opin Plant Biol 13(4):459–465
van Bel AJE (2003) The phloem, a miracle of ingenuity. Plant, Cell Environ 26(1):125–149
van Kan JAL (2006) Licensed to kill: the lifestyle of a necrotrophic plant pathogen. Trends Plant Sci 11(5):247–253
Voegele R, Mendgen K (2011) Nutrient uptake in rust fungi: how sweet is parasitic life? Euphytica 179(1):41–55
Voegele RT, Struck C, Hahn M, Mendgen K (2001) The role of haustoria in sugar supply during infection of broad bean by the rust fungus Uromyces fabae. Proc Natl Acad Sci USA 98(14):8133–8138
Williamson B, Tudzynski B, Tudzynski P, Van Kan JAL (2007) Botrytis cinerea: the cause of grey mould disease. Mol Plant Pathol 8(5):561–580
Yamada K, Kanai M, Osakabe Y, Ohiraki H, Shinozaki K, Yamaguchi-Shinozaki K (2011) Monosaccharide absorption activity of Arabidopsis roots depends on expression profiles of transporter genes under high salinity conditions. J Biol Chem 286(50):43577–43586
Acknowledgments
Pauline Lemonnier and Florian Veillet are supported by Grants from the French Ministry of Higher Education and Research, and the Région Poitou–Charentes, respectively. We are grateful to Vincent Lebeurre and Bruno Faure for helping us producing numerous plants used in this study. Antoine Plasseraud Desgranges is acknowledged for his help in the correction of the manuscript. We would like to thank all our colleagues for inspiring discussions. The CNRS, the University of Poitiers and the Région Poitou–Charentes are gratefully acknowledged for their financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11103_2014_198_MOESM1_ESM.tif
Fig. S1. STP13 mRNA accumulation in STP13OE-6 plants. Plants were sprayed with mock solution or B. cinerea suspension (5.104 conidia.ml−1). Healthy (0 hpi) or treated leaves from at least 3 plants were harvested at indicated time points. The STP13 transcript levels were quantified by RT-qPCR. Data are expressed as normalized expression (no unit) to the plant reference gene At4g26410 expression level (Czechowski et al. 2005). Data are mean ± SE from 2 independent experiments. STP13 expression level of B. cinerea infected WT plants (48 hpi) is indicated. B.c.: B. cinerea. hpi: hours post-inoculation (TIFF 7185 kb)
11103_2014_198_MOESM2_ESM.tif
Fig. S2. Disease phenotype of wild-type (WT) and C2-7 plants infected with B. cinerea. A construct including the cDNA corresponding to STP13 mRNA driven by the CaMV35S promoter was introduced into stp13-2. In the resulting plants (named C2-7), leaves of five-week-old plants have been used for inoculation tests. A. Lesion diameters observed on WT and C2-7 plants 3 days after inoculation with 6 µl droplets containing 104 conidia.ml−1. Data represent the mean (± SE) lesion diameter from 4 independent experiments. In each experiment, at least 5 plants per genotype were infected with B. cinerea. No significant difference was determined between WT and C2-7 plants according to a permutation with general scores test (P < 0,05). B. Lesion size distribution observed on WT and C2-7 plants infected with B. cinerea. Plants were drop-inoculated and diameters of necrotic lesions (LD) were measured after 3 days. Lesions were grouped into 3 classes according to their size: small (LD < WT first quartile), medium (LD = WT interquartile range) and large (LD > WT third quartile). The percentage of lesion size distribution from 4 independent experiments is shown. No significant difference between WT and C2-7 was determined by a Chi square test (P < 0,05) (TIFF 5987 kb)
11103_2014_198_MOESM4_ESM.pdf
Table S2 Table reporting the results of the three-way ANOVA test of PDF1.2 and PAD3 expression. Genotype: wild-type, stp13-2 or STP13OE-6 plants. Time: 0, 24, 48 or 72 h post-treatment. Treatment: mock or B. cinerea (**P < 0,01; ***P < 0,001; nsd: not statistically different)(PDF 18 kb)
Rights and permissions
About this article
Cite this article
Lemonnier, P., Gaillard, C., Veillet, F. et al. Expression of Arabidopsis sugar transport protein STP13 differentially affects glucose transport activity and basal resistance to Botrytis cinerea . Plant Mol Biol 85, 473–484 (2014). https://doi.org/10.1007/s11103-014-0198-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-014-0198-5