Abstract
Purpose
The purpose was to examine the effects of 8-weeks (3 days/week) of linear periodization resistance exercise training (RET) on neuromuscular function in prepubescent youth.
Methods
Twenty-five healthy prepubescent youth (11 males, 14 females, age = 9.1 ± 0.8 years) completed the RET (n = 17) or served as controls (CON, n = 8). Isometric maximal voluntary contractions (MVCs) and trapezoidal submaximal contractions at 35 and 60% MVC of the right leg extensors were performed with surface electromyography (EMG) recorded from the leg extensors [vastus lateralis (VL), rectus femoris, and vastus medialis] and flexors (biceps femoris and semitendinosus). EMG amplitude of the leg extensors and flexors were calculated during the MVCs. Motor unit (MU) action potential trains were decomposed from the surface EMG of the VL for the 35 and 60% MVCs. MU firing rates and action potential amplitudes were regressed against recruitment threshold with the y-intercepts and slopes calculated for each contraction. Total leg extensor muscle cross-sectional area (CSA) was collected using ultrasound images. ANOVA models were used to examine potential differences.
Results
Isometric strength increased post-RET (P = 0.006) with no changes in leg extensor and flexor EMG amplitude. Furthermore, there were no changes in total CSA or the MU action potential amplitude vs. recruitment threshold relationships. However, there were increases in the firing rates of the higher-threshold MUs post-RET as indicated with greater y-intercepts (P = 0.003) from the 60% MVC and less negative slope (P = 0.004) of the firing rates vs. recruitment threshold relationships at 35% MVC.
Conclusions
MU adaptations contribute to strength increases following RET in prepubescent youth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well understood that significant increases in muscular strength following resistance exercise training (RET) can occur in prepubescent youth (Payne et al. 1997; Behm et al. 2008; Faigenbaum et al. 2009; Falk 2015). RET is becoming more prevalent in athletic development, physical education classes, and after-school fitness youth programs (Stricker et al. 2020), and is now recommended by The World Health Organization for all age groups (Bull et al. 2020). Several types of RET programing performed over various durations of time contribute to enhanced muscular strength. It remains unclear if strength gains in prepubescent youth equate to adults following RET (Faigenbaum et al. 2009), however, differences in programming and strength testing make it difficult for comparisons to be made between populations. Furthermore, it is unclear the effects of traditional linear periodization RET on muscular strength in prepubescent youth unlike in adults.
It is widely believed that RET-related increases in muscular strength in prepubescent youth are related to neural mechanisms with minimal contributions of hypertrophic factors (Behm et al. 2008; Faigenbaum et al. 2009). Differing hormone levels in prepubescent youth in comparison to older youth and young adults are often cited for the lack of whole muscle hypertrophy. There are, however, a few studies that suggest RET can increase muscle mass in prepubescent youth (Fukunaga et al. 1992; Faigenbaum et al. 2009). It is plausible that higher-intensity RET in combination with more direct imaging techniques and methods, such as ultrasound-derived muscle cross-sectional area (CSA), may provide insight into potential hypertrophy in prepubescent youth. Nonetheless, neural adaptations at the level of the motor unit (MU) are continually hypothesized to partially explain increases in strength in prepubescent youth following RET (Ramsay et al. 1990; Ozmun et al. 1994).
Remarkably, there is limited research that supports the consensus that neural adaptations primarily contribute to increases in muscular strength in prepubescent youth. A small number of studies demonstrate an increase in surface electromyography (EMG) amplitude of the leg extensors and/or forearm flexors following RET without increases in muscle CSA (Legerlotz et al. 2016). Surface EMG provides a global estimate of muscle activation but is limited in that several anatomical and technical variables can confound potential interpretations of neural mechanisms at the level of the MU (Farina et al. 2014). Ramsay et al. (1990) indicated no significant increase in voluntary activation of the leg extensors with the interpolated twitch technique (ITT) while also reporting no changes in CSA in prepubescent youth. The ITT may provide a theoretical estimation of MU activation. However, the technique has low sensitivity during high activation (Herbert and Gandevia 1999) that is likely particularly true for prepubescent youth because of the anticipation of electrical simulation in adults (Button and Behm 2008). Regardless, only indirect evidence suggests that changes in mechanisms that are neural in origin are occurring during RET in prepubescent youth.
Increases in MU firing rates and recruitment are routinely hypothesized as neural mechanisms that contribute to increases in muscular strength in prepubescent youth following RET (Faigenbaum et al. 2009; Legerlotz et al. 2016). The totality of firing rates of recruited MUs is the neural drive to the muscle (Farina et al. 2014; Martinez-Valdes et al. 2018) and can be indirectly quantified with the discharges of numerous recorded MUs regressed against recruitment thresholds (De Luca et al. 2006). The strong negative MU firing rates vs. recruitment threshold relationships demonstrate that earlier recruited lower-threshold MUs possess higher firing rates than the later recruited higher-threshold MUs. For most circumstances, increases in MU firing rates coincides with recruitment of inactive MUs (Miller et al. 2020). Our previous work indicates that MU firing rates of the vastus lateralis at various isometric contraction intensities is lower (~ 18%) for prepubescent youth in comparison to adults (Herda et al. 2018; Parra et al. 2020) and supports other studies that indirectly suggests lower MU firing rates and recruitment in youth with various other indirect methods (Dotan et al. 2012; Woods et al. 2022). Therefore, prepubescent youth may have the capacity to increase MU firing rates and recruitment during RET. This is in contrast to adults where our laboratory (Sterczala et al. 2020) and others (Beck et al. 2011; Stock and Thompson 2014) report no changes in MU firing rates of the vastus lateralis (VL) following RET when firing rates are regressed against recruitment threshold on a subject-by-subject basis.
The purpose of the present study was to examine MU firing rates and action potential amplitudes relative to recruitment threshold of the VL following 8 weeks (24 sessions) of linear periodization RET in prepubescent youth in a similar manner performed in adults (Sterczala et al. 2020). The strong positive relationship between MU action potential amplitude vs. recruitment threshold relationships demonstrates that the later recruited higher-threshold MUs are larger than the earlier recruited lower-threshold MUs. Isometric maximal voluntary contractions (MVC) and trapezoidal muscle actions were performed at 35 and 60% of MVC. A weakness of previous RET research that includes MU recordings is not utilizing moderate- and higher-intensity contractions to isolate potential non-uniform changes in firing rates (Herda 2022). Furthermore, prepubescent youth had difficulties achieving 70% MVC or higher for the leg extensors in our previous research and, subsequently, 60% MVC was chosen for the present study. We hypothesized that 8 weeks of RET would increase firing rates at 35 and 60% MVC and that this would be shown with higher y-intercepts from the MU firing rate vs. recruitment threshold relationships post-intervention. Thus, demonstrating increases in MU firing rates across recruitment thresholds contribute to greater isometric strength. Ultrasound images of the leg extensors [VL, rectus femoris (RF) and, vastus medialis (VM)] were taken to examine potential changes in CSA. Previous research in adults indicates increases in action potential amplitudes of higher-threshold MUs as indicated by greater slopes in the MU action potential amplitude vs. recruitment threshold relationships. The greater slope from these relationships corresponds with increases in CSA of VL (Pope et al. 2016; Sterczala et al. 2020; Jenkins et al. 2020). Thus, we hypothesized the slopes from the MU action potential vs. recruitment threshold relationships and CSA will not increase in the prepubescent youth since we do not expect hypertrophy.
Methods
Participants
Twenty-five healthy, prepubescent youth participants (11 males, 14 females, age = 9.1 ± 0.8 years) completed an 8-week (3 days/week) linear periodization RET program (n = 17) or served as non-exercise controls (CON, n = 8). There is no direct research that examines changes in MU firing rates or muscle lipids following RT in children. Therefore, we performed sample size estimates based on muscular strength data pre- and post-RET in children (Ramsay et al. 1990). Effect sizes for increase in muscular strength tend to be very large (ES = 1.12) (Behringer et al. 2011). Specifically for isometric leg extensor strength (Ramsay et al. 1990), with an alpha set at 0.05 and a power 0.80, six youth will yield actual power of 85% given an effect size of 0.83 (estimated from mid-program testing on Figure 5) based on sample size procedures (G*Power 3.1.9.7, Dusseldorf, Germany) described in Beck (2013). Height, body mass, and maturation offset (Moore et al. 2015) pre- and post-intervention are presented in Table 1. None of the participants reported any neuromuscular, metabolic diseases, or current/recent musculoskeletal injuries via a health history questionnaire completed by the parents. This study was approved by the university’s institutional review board for human subject research, with each parent and child providing written informed consent.
Research design
Prior to and following an 8-week intervention, longitudinal changes in maximal isometric leg extension and flexion torque along with CSA and subcutaneous fat thickness of the VL, RF, and VM were assessed. EMG amplitude of the VL, RF, VM, biceps femoris (BF), and semitendinosus (ST) muscles were recorded during the maximal isometric leg extensor and flexor MVCs. MU firing rates were recorded from the vastus lateralis during the 35 and 60% MVCs. Two visits were conducted during pre- and post-testing for a total of four testing visits. Standing and seated height, body mass, and ultrasound images were recorded along with participants practicing the isometric strength testing during visits 1 (pre) and 3 (post) with the experimental strength testing that included recordings of surface EMG signals occurring during visits 2 (pre) and 4 (post). The pre- and post-testing visits occurred within 1–5 days of each other.
RET
Participants in the RET group completed three sessions per week for 8 weeks (Table 2). At each visit, participants completed four lower- and two-upper body exercises. The lower-body exercises included, various squat movements, deadlifts, leg presses, step ups, hamstring curls, leg extensions, and sled drags and pushes. Upper body movements included, bench press, shoulder press, seated rows, and lat pull-downs. Participants completed a 1–3 min warm up that included various agility drills (ladder, short hurdles, etc.) and could elect to perform timed planks or prone isometric back extensions (Superman’s) at the end of the RET session. Participants performed 3 sets of 12 repetitions during weeks 1–3, 3 sets of 8 repetitions during weeks 4–6 and 4 sets of 5 repetitions during weeks 7–8. The initial loads for the 1st week were relatively conservative due to participants’ limited exercise training experience. The judgment of the trainer was used to determine the adjustments in load during and between training sessions. Participants in the RET group completed all exercise sessions while the CON group did not perform any resistance training during the investigation.
Isometric voluntary strength testing
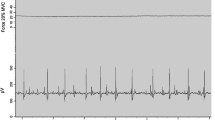
Participants performed isometric voluntary strength testing of the right leg extensors and flexors, regardless of leg dominance, with the leg at 90° flexion. Testing was performed on a knee dynamometer (OT Bioelectronica, Turin, Italy) with participants seated and restrained with a 4-point harness seatbelt. A pillow was placed behind the participant’s back if needed to accommodate for shorter limb length and height, to allow proper fit in the dynamometer, and was noted for post-testing consistency. The right leg was secured to the dynamometer using 3 Velcro straps on the shin to the ankle region with a foam pad placed under the Velcro straps for comfort. Participants were instructed to grip the chest straps during contractions. The non-contracting left leg remained relaxed for the duration of testing with the left foot rested on a box to the side of the dynamometer. Warm-up ranging from 20 to 80% of perceive maximal effort was performed followed by 3 to 4 MVCs to test isometric extension and flexion strength of the right leg limb. MVCs lasted 3–6 s in duration with 1–2 min rest in between each trial. Participants were provided strong verbal encouragement during each MVC. Force (N) was recorded with custom written software (LabVIEW 2015, National Instruments Corporation, Austin, Texas). The highest 0.25 s epoch of force (N) during 2–3 MVCs was determined as peak isometric force. Isometric trapezoidal muscle actions to 35 and 60% MVC were used to decompose MU action potential trains (Fig. 1). Submaximal contractions consisted of a linear torque increase, a 10 s steady torque plateau and a linear torque decrease. Force increased and decreased at a rate of 10% MVC/s. Participants were provided with a visual template of the isometric trapezoidal contraction and real-time torque feedback. A second attempt was provided when participants were unable to adhere to the template during the initial attempt.
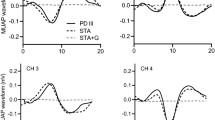
Top left: The motor unit (MU) action potential waveforms decomposed by the PD III algorithm, the user created waveforms from the raw electromyography signals by spike trigger averaging (STA), and the recreation of the waveforms via STA but with the inclusion of small errors in the firing times. The errors included in the firing times (circles) are overlaid onto the correct firing times (vertical line) for a segment of the action potential train below the waveforms. Bottom left: The force tracing from a 60% maximal voluntary contraction (MVC) overlaid the decomposed MU action potential trains. The selected segment of the MU action potential train was used to analyze firing rates of all MUs. Right: The plotted firing rate (pps) and action potential amplitude (mV) vs. recruitment threshold relationship (expressed as %MVC) for one participant. The slopes and y-intercepts from each relationship are used for statistical purposes. Of note, the rapid rise in MU action potential waveforms in relation to recruitment threshold can result in negative y-intercepts, particularly for the 60% MVCs
EMG recording
During contractions, surface EMG signals were collected from the VL, RF, VM, BF, and ST muscles. Sensors were placed at 40% of the distance from the lateral epicondyle of the femur to the anterior superior iliac spine (ASIS) for the VL, 50% of the distance from the superior line of the patella to the ASIS for the RF, and 20% of the distance starting from the medial epicondyle of the femur to the ASIS for the VM. For the BF and ST, sensors were placed at 50% of the distance on the line between the ischial tuberosity and lateral or medial epicondyle of the tibia. A reference electrode was placed over the left patella. The sites of sensor placements were shaved dry skin cells were removed via repeated application of adhesive tape, and the sites were sterilized with alcohol. The EMG signals were sampled at 20 kHz and stored for subsequent analyses.
EMG decomposition
For the VL, a 5-pin surface sensory array (Delsys, Inc, Natick, MA) was placed on the VL for MU decomposition purposes. The array consisted of five 0.5 mm pins arranged in a 5 × mm square with the fifth pin positioned in the center. The four channels of raw surface EMG data collected by 5-pin sensory array were decomposed into their constituent MU action potential trains with the PD III algorithm (version 4.1.1.0) (De Luca et al. 2006; Chang et al. 2008; Nawab et al. 2010).
Strict user driven procedures (Fig. 1) involving the recreation of the MU action potential waveforms from the raw EMG signals were performed to test the validity of the decomposed MU action potential trains (Hu et al. 2013; McManus et al. 2016; Thompson et al. 2018; Herda et al. 2020b) rather than solely relying on the reconstruct-and-test procedure because of perceived limitations of the procedure (Farina and Enoka 2011). These procedures that included spike trigger averaging (STA) are performed by various laboratories to ensure the accuracy of decomposed MU action potential trains (Hu et al. 2013; McManus et al. 2016; Thompson et al. 2018; Herda et al. 2020b).
First, the PD III-derived firing times were used to recreate the MU action potential waveforms from the raw EMG signal for each of the four channels (Fig. 1). A limitation of the STA is that it does not account for missed firing times that are not decomposed. Thus, 4 MU action potential waveforms were created with STA in a custom written LabVIEW (2015, National Instruments Corporation, Austin, Texas) program. Each action potential train was visually examined and statistically compared to the PD III algorithm-derived four waveforms. The average correlation between the STA and the PD III created waveforms was 0.88 ± 0.02. The user created MU action potential waveforms possessed the characteristics of the action potential waveforms from the PD III algorithm. Any MUs with correlations < 0.7 between the STA and PD III waveforms were removed from further analyses and was not used for statistical purposes in accordance with previous research (Hu et al. 2013; McManus et al. 2016). Second, small errors were applied to the PD III-derived firing times (Fig. 1), via Gaussian noise, to further demonstrate validity of the firing times and action potential waveforms. The Gaussian noise was set at 0.1 of the standard deviation of the interspike interval for each MU and, consequently, results in small errors of the firing times (~ 3 ms). Correlations were performed on the MU action potential waveforms created from the raw EMG signals with Gaussian noise with the PD III activation potential waveforms. As expected, the correlations were lower (r = 0.80 ± 0.03). Furthermore, visual inspection of the waveforms created with Gaussian noise applied to the firing times (Fig. 1) demonstrates a loss in the defining characteristics of the PD III-derived MU action potential waveforms. Subsequently, the PD III algorithm successfully decomposed surface EMG into valid firing times and waveforms.
EMG analyses
EMG amplitude was calculated during the highest 0.25 s epoch of force from the isometric leg extension MVCs. EMG amplitude was averaged across the leg extensors (VL, RF, and VM) and flexors (ST and BF) for statistical purposes. The individual action potential trains for the VL were analyzed to yield three parameters per MU: recruitment threshold (RT), firing rate (MFR) at steady force, and action potential amplitude (MUAPAMP). The RT was calculated as the average torque during the 0.01 s epoch following the first firing of the MU and was expressed relative to the peak isometric leg extension MVC. The MFR was calculated as the inverse of the average interspike interval during the selected submaximal steady forces. The MUAPAMP was calculated as the average peak-to-peak amplitude of each of the four unique action potential waveform templates derived from the PD III algorithm.
MU variables from a single contraction were analyzed for each participant individually; data were neither pooled across multiple contractions nor multiple participants within a group. MFRs and MUAPAMPS were linearly regressed against RT for each individual and contraction to yield a slope and y-intercept values for statistical purposes.
Ultrasound imaging
Ultrasound imaging was used to capture muscle CSA and quality of the right leg extensors in accordance with previous methods (Herda et al. 2020a). Imaging was performed using a LOGIQe ultrasound device (GE Healthcare, Buckinghamshire, United Kingdom) set in LOGIQ View®, to capture panoramic images for CSA, with the musculoskeletal preset, and a GE 12L-RS linear ultrasound transducer (5–13 MHz) with a 42 × 7-mm footprint. Scan depth was set at 6.0 cm with a gain of 68 dB and a frequency of 10 MHz. Participants laid on the table with a wedge pad placed under the head for support, the Velcro strap was removed from the ankles, and a foam roller was placed under the knees for comfort during ultrasound imaging. Imaging sites were then marked for the VL, RF, and VM. The VL was imaged at 40% of the distance from the lateral epicondyle of the femur to the anterior superior iliac spine (ASIS). The RF was imaged at 50% of the distance from the superior line of the patella to the ASIS. The VM was imaged at 20% of the distance starting from the medial epicondyle of the femur to the ASIS. All measurements originated at the knee joint and moved proximal towards the ASIS. For all scans, great care was taken to ensure that consistent and minimal pressure was applied with the probe to the skin to avoid any muscle compression. A generous amount of water-soluble transmission gel was applied to the skin to reduce possible near-field artifacts and enhance acoustic coupling. Muscles were imaged beginning at the lateral border and ending at the medial border. Ultrasound imaging analyses were performed using ImageJ software (version 1.46r, National Institutes of Health, Bethesda, MD). Each image was scaled from pixels to centimeters using the straight-line function. For CSA (cm2), the muscle was outlined using the polygon function, with care taken to exclude the surrounding fascia. Total CSA (cm2) of the three leg extensors was also calculated for each subject. The subcutaneous fat [cm, (sFAT)] of the three leg extensors was quantified as the distance between the skin and the superficial aponeurosis of the muscles at the midpoint and medial and lateral borders of the muscle and the average was used for statistical purposes.
Statistical analysis
Two-way mixed factorial ANOVAs [group (RET vs. CON) × time (pre- vs. post-intervention)] were used to measure potential changes in height, body mass, maturation offset, isometric leg extensor MVC strength, leg extensors and flexor peak EMG amplitude, total leg extensor CSA and sFAT. Three-way mixed factorial ANOVAs [group (RET vs. CON) × contraction intensity (35 vs. 60% MVC)× time (pre- vs. post-intervention)] were used to measure potential changes in the y-intercepts and slopes of the MFR– and MUAPAMP–RT relationships. Alpha was set at P < 0.05 with partial eta-squared (ηp2) and Cohen’s d was calculated for the ANOVAs and follow-up statistical tests.
Results
For height, there was a significant two-way interaction (P = 0.029; ηp2 = 0.183). Height increased pre- to post-intervention for the RET (P < 0.001; d = 2.332) and CON (P < 0.001; d = 1.957). There were no significant differences between RET and CON at pre- (P = 0.405; d = 0.103) or post-intervention (P = 0.335; d = 0.184) (Table 1). For body mass and maturation offset, there were no two-way interactions (P = 0.110 & 0.147; ηp2 = 0.103 & 0.086) or main effect for group (P = 0.504 & 0.661; ηp2 = 0.019 & 0.008), but there was a main effect for time (P < 0.001 & < 0.001; ηp2 = 0.449 & 0.741). Body mass and maturation offset increased pre- to post-intervention for the RET and CON (Table 1).
Isometric strength and EMG amplitude
For isometric leg extension MVC strength (Fig. 2), there was a significant two-way interaction (P = 0.006, ηp2 = 0.283). There was no significant increase (P = 0.742, d = 0.121) pre- to post-CON, however, there was a significant increase (P < 0.001, d = 0.285) pre- to post-RET. There were no differences between RET and CON at pre- (P = 0.213, d = 0.527) or post-intervention (P = 0.859, d = 0.077). For leg extensor MVC EMG amplitude, there was no significant two-interaction (P = 0.468, ηp2 = 0.024) or main effects for time (P = 0.661, ηp2 = 0.009) and group (P = 0.101, ηp2 = 0.118). There was a two-way interaction (P = 0.048, ηp2 = 0.166) for peak EMG amplitude of the leg flexors during the leg extension MVCs. There were, however, no significant differences at pre- (P = 0.399, d = 0.372) and post-testing (P = 0.504, d = 0.294) between groups or changes pre- to post-RET (P = 0.347, d = 0.243) and CON (P = 0.167, d = 0.545) with the follow-up analyses.
CSA and sFAT
For total leg extensor CSA (Fig. 2), there was an error in the scan location for the RF post-intervention for a participant in the CON group and, thus, data were removed from analyses. Nonetheless, there was no significant two-way interaction (P = 0.111, ηp2 = 0.111) or main effects for time (P = 0.939, ηp2 < 0.001) and group (P = 0.610, ηp2 = 0.012). For sFAT, there was no significant two-way interaction (P = 0.959, ηp2 < 0.001) or main effect for time (P = 0.386, ηp2 = 0.034). However, there was a main effect for the group (P = 0.019, ηp2 = 0.227). The RET (0.99 ± 0.34 cm) had greater sFAT than the CON (0.65 ± 0.19 cm). MUAPAMPS may be influenced by sFAT and, therefore, the lack of changes pre- to post-intervention limits sFAT as a confounding variable.
Motor unit relationships
There were (mean ± SD) 26.8 ± 7.2 and 22.9 ± 5.3 MUs decomposed from the 35 and 60% MVCs, respectively. The differences in sFAT between groups did not result in a difference in identified MUs (CON = 23.7 ± 8.0; RET = 25.4 ± 7.7 MUs). The RT ranges of the decomposed MUs were from 4.6 ± 5.5 to 23.1 ± 10.7 and from 16.4 ± 11.3 to 46.8 ± 17.0% for the 35 and 60% MVCs. The correlations were − 0.84 ± 0.06 and − 0.83 ± 0.10 for the individual MFR–RT relationships while the correlations for the individual MUAPAMP–RT relationships were 0.77 ± 0.10 and 0.77 ± 0.09 for the 35 and 60% MVCs, respectively.
For the MFR–RT relationships (Fig. 3), there was a significant three-way interaction (P = 0.017, ηp2 = 0.225) for the y-intercepts. There was a significant increase (P = 0.003, d = 0.837) in the y-intercepts at 60% MVC from pre- to post-RET, however, there were no other significant increases for the CON at either contraction intensity (35% MVC: P = 0.551, d = 0.222; 60% MVC: P = 0.757, d = 0.114) or at the 35% MVC (P = 0.815, d = 0.419) for the RET. There were no differences between groups at either contraction intensity pre- or post-intervention (P = 0.124–0.508, d = 0.288–0.685). For the slopes, there was a significant three-way interaction (P = 0.040, ηp2 = 0.172). There was a significantly less negative slope (P = 0.004, d = 0.812) pre- to post-intervention at 35% for the RET, however, there were no other significant changes for the CON at either contraction intensity (35% MVC: P = 0.619, d = 0.184; 60% MVC: P = 0.245, d = 0.449) or at 60% MVC for the RET (P = 0.127, d = 0.391). There were no differences between groups at either contraction intensity pre- or post-intervention (P = 0.129–0.497, d = 0.296–0.675).
The y-intercepts (top) and slopes (bottom) from the motor unit (MU) firing rate vs. recruitment threshold [expressed as percentage of maximal voluntary contraction (%MVC)] relationships pre- and post-resistance exercise training (RET) and control (CON) groups. * indicates significant increase pre- to post-RET
For the y-intercepts from the MUAPAMP–RT relationships (Fig. 4), there was no three-way interaction (P = 0.092, ηp2 = 0.119), no two-way interactions (P = 0.163–0.780, ηp2 = 0.083–0.481), or main effects for time (P = 0.275, ηp2 = 0.052) and group (P = 0.940, ηp2 < 0.001). There was, however, a main effect for intensity (P < 0.001, ηp2 = 0.481). The y-intercepts for the 35% MVC were greater than the 60% MVC, which was primarily because of differences in RT ranges rather than recording larger MUAPAMPS during the 35% MVC. For the slopes, there was no significant three-way interaction (P = 0.060, ηp2 = 0.146) and no two-way intensity × time (P = 0.738, ηp2 = 0.005) or time × group (P = 0.427, ηp2 = 0.028) interactions. There was a significant two-way intensity × group interaction (P = 0.033, ηp2 = 0.183). There was a significant difference between groups at 35% MVC (P = 0.023, d = 1.040), but not at 60% MVC (P = 0.865, d = 0.074). The slopes were significantly higher for the 60% MVC than 35% MVC for the RET (P < 0.001, d = 0.750), but not for the CON (P = 0.379, d = 0.332).
The y-intercepts (top) and slopes (bottom) from the motor unit (MU) action potential amplitude vs. recruitment threshold [expressed as percentage of maximal voluntary contraction (%MVC)] relationships pre- and post-resistance exercise training (RET) and control (CON) groups. There were no changes pre- to post-RET or CON
Discussion
The primary findings of the present study were that 8 weeks of RET increased MU firing rates in a non-uniform manner. Firing rates of the higher-threshold MUs increased, however, the lowest-threshold MUs measured during the 35% MVC did not. Total muscle CSA and slopes of the MUAPAMPS relative to RT did not increase with 8 weeks of RET suggesting there was no hypertrophy for higher-threshold MUs, a response that is different than previous studies in adults (Pope et al. 2016; Sterczala et al. 2020; Jenkins et al. 2020). Increases in isometric muscular strength in prepubescent youth who completed traditional linear periodization RET may be partially explained by changes in neural activation.
It is still being determined if prepubescent youth experience similar relative increases in strength as those observed in adults (Faigenbaum et al. 2009), however, direct comparisons are lacking because RET programs and methods to analyze strength vary greatly across studies. In the present study, isometric leg extensor muscular strength increased by 16.2% and compares favorably to the increases (17.2%) reported for the same RET program and testing in young adults (Sterczala et al. 2020). Prepubescent youth may experience similar increases in isometric strength in comparison to adults when RET programs are matched.
The level of firing rates can dictate the force output of the MU (Burke et al. 1970; Enoka and Duchateau 2017). In theory, increases in firing rates results in greater force output of the MU and, subsequently, increase strength of the muscle. The greater y-intercepts with no differences in the slopes of the MFR–RT relationships from the 60% MVC indicate greater firing rates across the recorded MU pool (RT = 16–47%) at post-RET (Fig. 5). Of note, the prepubescent youth may be close to maximal neural activation when completing the isometric trapezoidal muscle action at 60% MVC since they have difficulties reaching 70% MVC analogous to adults who struggle to complete isometric trapezoidal muscle actions at 80 and 90% MVC. Subsequently, the greater discharge rates of the MU pool led to higher force output of the muscle needed to match the higher force level at post-60% MVC (Fig. 5).
The plotted mean (circles) and standard deviations (solid lines) from the composite motor unit (MU) firing rates vs. recruitment threshold [RT, expressed as percentage of maximal voluntary contraction (%MVC)] relationships (top: 35% MVC; bottom: 60% MVC) pre- and post-resistance exercise training (RET) and control (CON) groups. The slope and y-intercept from each contraction were used to calculate the MU firing rates for the observed average recruitment threshold ranges
In contrast, there were no increases in the y-intercepts, rather there was a rise in the negative slopes of the MFR–RT relationships from the 35% MVC post-RET (Fig. 5). The less negative slopes with no changes in the y-intercepts indicate that the later recruited higher-threshold MUs had elevated firing rates post-RET. The firing rates of the lower-threshold MUs did not increase to the same extent pre- to post-RET unlike the higher-threshold MUs recorded during the 35 and 60% MVCs. Of importance, excitability of the MU pool is far less during the 35 than 60% MVC. Lower-threshold MUs were not decomposed or observed during the 60% MVC and, subsequently, it is unknown if the lowest-threshold MUs recorded during the 35% MVC would achieve greater firing rates during the post-60% MVC. Previous research report that lower-threshold MUs may reach a point (saturation) where firing rates do not continuingly increase with greater excitation to the MU pool (Hu et al. 2014; Fuglevand et al. 2015). There is potential the lower-threshold MUs met a “saturation point” that limited increases in firing rates post-RET during the 35% MVC. Nonetheless, an impactful finding of the present study was that non-uniform changes occurred to the MU firing rate patterns, which is rarely addressed in RET research that includes MU recordings (Herda 2022).
The simplest explanation for the increase in isometric strength and firing rates for much of the MU pool is that voluntary effort of the youth increased as they became more familiar and comfortable with strength training and isometric testing, i.e., a learning effect (Patten and Kamen 2000; Kamen and Knight 2004). Nonetheless, the greater firing rates for the prepubescent youth may be indicative of greater overall excitation to the motoneuron pool to complete tasks at the higher absolute force levels. Woods et al. (2022), Gillen et al. (2021), and our labs (Herda et al. 2018; Parra et al. 2020) suggests that voluntary activation via the ITT and MU firing rates are lower for prepubescent youth in comparison to adolescent youth and adults for the leg extensors. Therefore, previous research supports the notion that prepubescent youth may have the capacity to increase neural activation of the leg extensors. Linear periodization RET may be an effective method to increase neural activation. Although speculative, increased neural activation in prepubescent youth could improve physical and metabolic function (i.e., muscle insulin sensitivity (DeFronzo et al. 1981) along with sport performance. Lastly, the overall scheme used by the nervous system to regulate MU recruitment and firing rates was not altered by RET.
There were no significant increases in leg extensor CSA pre- to post-RET. The same RET program in adults resulted in an 18% increase in VL CSA (Sterczala et al. 2020), whereas there was roughly a 3% increase for the VL in the present study. In adults (Pope et al. 2016; Sterczala et al. 2020; Jenkins et al. 2020), the slopes of the MUAPAMP–RT relationships increase pre- to post-RET in the presence of hypertrophy. Other studies also report associations among the slopes of the MUAPAMP–RT relationships, CSA, and type II fiber area for the VL. Subsequently, no changes in CSA or the MUAPAMP–RT relationships pre- to post-RET provides compelling evidence that increases in the diameters of fibers within higher-threshold MUs were not significantly contributing to increases in strength following RET for the prepubescent youth.
Potentially, increases in maximal strength may be the result of lower antagonist muscle activation. Surface EMG amplitude of the leg extensors and flexors did not change in the present study and tentatively suggests that lower muscle activation of the leg flexors was not the primary contributor to increases in isometric muscular strength. Our results do contrast reported increases in EMG amplitude for the agonist muscles pre- to post-RET in prepubescent youth. Ozmun et al. (1994) reported increases in strength and EMG with no changes in limb circumference following 8 weeks of RET of the biceps brachii. Fewer subjects (8 vs. 17 participants), slightly older youth with less variability in age (10.5 ± 0.5 vs. 9.1 ± 0.8 years), and/or testing of a different muscle group (forearm flexors vs. leg extensors) might explain the contrast in findings from Ozmun et al. (1994).
It should be noted that there are limitations in EMG decomposition to quantify MU action potential trains. First, there are likely errors in the firing times despite utilizing reconstruct-and-test and STA procedures to ensure that decomposed action potential trains are accurately reflecting ongoing MU firings. As mentioned in the methods, the STA procedure does not account for missed firing times by EMG decomposition. However, research demonstrates that errors in the action potential trains does not alter the calculated relationships (Herda et al. 2020b; Hernandez-Sarabia et al. 2020; Parra et al. 2021). Thus, the interpretation of the MU firing rate patterns remains intact despite errors in the action potential trains. Another limitation of this study is that MU firing rates were not recorded during the MVCs. MU firing rates relative to action potential amplitude (Herda 2022) may better explain the role of increased neural activation on strength improvements post-RET in prepubescent youth who did not undergo significant increases in CSA.
In conclusion, 8-weeks (3 days/week) of linear periodization RET of the leg extensors led to increases in isometric strength that is comparable to the same training program in adults. Furthermore, non-uniform changes in MU firing rates were observed that indicate increases in firing rates of the higher-threshold MUs unlike for the lower-threshold MUs recorded during the moderate-intensity contraction. The lack of changes in total CSA and MUAPAMPS would suggest hypertrophy was not a primary contributor to increases in isometric strength. Linear periodization RET is an effective intervention to improve neural activation and, subsequently, increase overall physical and metabolic function in prepubescent youth. Future research should examine potential changes in common input since MU firing rates did change pre- to post-RET and it may account for improvements in strength. Furthermore, examining changes in MU firing rates during MVCs may provide better insight on the effects of RET on neural activation.
Data availability
Data is available upon a resonable request.
Abbreviations
- ANOVA:
-
Analysis of variance
- ASIS:
-
Anterior superior iliac spine
- BF:
-
Biceps femoris
- CON:
-
Control
- CSA:
-
Cross-sectional area
- d :
-
Cohn’s d
- EMG:
-
Electromyography
- ES:
-
Effect size
- ITT:
-
Interpolated twitch technique
- MFR:
-
Mean firing rate
- MVC:
-
Maximal voluntary contraction
- MU:
-
Motor unit
- MUAPAMPS :
-
Motor unit action potential amplitudes
- η p 2 :
-
Partial eta-squared
- PD:
-
Precision decomposition
- RET:
-
Resistance exercise training
- RF:
-
Rectus femoris
- RT:
-
Recruitment threshold
- ST:
-
Semitendinosus
- STA:
-
Spike trigger averaging
- sFAT:
-
Subcutaneous fat
- VL:
-
Vastus lateralis
- VM:
-
Vastus medialis
References
Beck TW (2013) The importance of a priori sample size estimation in strength and conditioning research. J Strength Cond Res 27:2323–2337. https://doi.org/10.1519/JSC.0b013e318278eea0
Beck TW, DeFreitas JM, Stock MS (2011) The effects of a resistance training program on average motor unit firing rates. Clin Kinesiol 65:9
Behm DG, Faigenbaum AD, Falk B, Klentrou P (2008) Canadian Society for Exercise Physiology position paper: resistance training in children and adolescents. Appl Physiol Nutr Metab 33:547–561. https://doi.org/10.1139/H08-020
Behringer M, Matthews M, Mester J (2011) Effects of strength training on motor performance skills in children and adolescents: a meta-analysis. Pediatr Exerc Sci 23:21
Bull FC, Al-Ansari SS, Biddle S et al (2020) World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 54:1451–1462. https://doi.org/10.1136/bjsports-2020-102955
Burke RE, Rudomin P, Zajac FE (1970) Catch property in single mammalian motor units. Science 168:122–124. https://doi.org/10.1126/science.168.3927.122
Button DC, Behm DG (2008) The effect of stimulus anticipation on the interpolated twitch technique. J Sports Sci Med 7:520–524
Chang S-S, De Luca C, Nawab H (2008) Aliasing rejection in precision decomposition of EMG signals. In: 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. pp 4972–4975
De Luca CJ, Adam A, Wotiz R et al (2006) Decomposition of surface EMG signals. J Neurophysiol 96:1646–1657. https://doi.org/10.1152/jn.00009.2006
DeFronzo RA, Jacot E, Jequier E et al (1981) The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 30:1000–1007. https://doi.org/10.2337/diab.30.12.1000
Dotan R, Mitchell C, Cohen R et al (2012) Child—Adult differences in muscle activation—a review. Pediatr Exerc Sci 24:2–21. https://doi.org/10.1123/pes.24.1.2
Enoka RM, Duchateau J (2017) Rate coding and the control of muscle force. Cold Spring Harb Perspect Med 7:a029702. https://doi.org/10.1101/cshperspect.a029702
Faigenbaum AD, Kraemer WJ, Blimkie CJR et al (2009) Youth resistance training: updated position statement paper from the national strength and conditioning association. J Strength Cond Res 23:S60-79. https://doi.org/10.1519/JSC.0b013e31819df407
Falk B (2015) Resistance training in children. Pediatr Exerc Sci 27:13–17
Farina D, Enoka RM (2011) Surface EMG decomposition requires an appropriate validation. J Neurophysiol 105:981–982; author reply 983–984. https://doi.org/10.1152/jn.00855.2010
Farina D, Merletti R, Enoka RM (2014) The extraction of neural strategies from the surface EMG: an update. J Appl Physiol 117:1215–1230. https://doi.org/10.1152/japplphysiol.00162.2014
Fuglevand AJ, Lester RA, Johns RK (2015) Distinguishing intrinsic from extrinsic factors underlying firing rate saturation in human motor units. J Neurophysiol 113:1310–1322. https://doi.org/10.1152/jn.00777.2014
Fukunaga T, Funato K, Ikegawa S (1992) The effects of resistance training on muscle area and strength in prepubescent age. Ann Physiol Anthropol Seiri Jinruigaku Kenkyukai Kaishi 11:357–364. https://doi.org/10.2114/ahs1983.11.357
Gillen ZM, Housh TJ, Schmidt RJ et al (2021) Comparisons of muscle strength, size, and voluntary activation in pre- and post-pubescent males and females. Eur J Appl Physiol 121:2487–2497. https://doi.org/10.1007/s00421-021-04717-1
Herbert RD, Gandevia SC (1999) Twitch interpolation in human muscles: mechanisms and implications for measurement of voluntary activation. J Neurophysiol 82:2271–2283. https://doi.org/10.1152/jn.1999.82.5.2271
Herda TJ (2022) Resistance exercise training and the motor unit. Eur J Appl Physiol 122:2019–2035. https://doi.org/10.1007/s00421-022-04983-7
Herda TJ, Ryan ED, Kohlmeier M et al (2018) Examination of muscle morphology and neuromuscular function in normal weight and overfat children aged 7–10 years. Scand J Med Sci Sports 28:2310–2321. https://doi.org/10.1111/sms.13256
Herda TJ, Gallagher P, Miller J et al (2020a) Skeletal muscle composition and glucose levels in children who are overweight and obese. Pediatr Exerc Sci 32:157–164
Herda TJ, Parra ME, Miller JD et al (2020b) Measuring the accuracies of motor unit firing times and action potential waveforms derived from surface electromyographic decomposition. J Electromyogr Kinesiol 52:102421. https://doi.org/10.1016/j.jelekin.2020.102421
Hernandez-Sarabia JA, Luera MJ, Barrera-Curiel A et al (2020) Does strict validation criteria for individual motor units alter population-based regression models of the motor unit pool? Exp Brain Res 238:2475–2485. https://doi.org/10.1007/s00221-020-05906-8
Hu X, Rymer WZ, Suresh NL (2013) Motor unit pool organization examined via spike-triggered averaging of the surface electromyogram. J Neurophysiol 110:1205–1220. https://doi.org/10.1152/jn.00301.2012
Hu X, Rymer WZ, Suresh NL (2014) Control of motor unit firing during step-like increases in voluntary force. Front Hum Neurosci 8:721
Jenkins NDM, Rogers EM, Banks NF et al (2020) Increases in motor unit action potential amplitudes are related to muscle hypertrophy following eight weeks of high-intensity exercise training in females. Eur J Sport Sci 21:1–11. https://doi.org/10.1080/17461391.2020.1836262
Kamen G, Knight CA (2004) Training-related adaptations in motor unit discharge rate in young and older adults. J Gerontol Ser A 59:1334–1338. https://doi.org/10.1093/gerona/59.12.1334
Legerlotz K, Marzilger R, Bohm S, Arampatzis A (2016) Physiological adaptations following resistance training in youth athletes—a narrative review. Pediatr Exerc Sci 28:501–520. https://doi.org/10.1123/pes.2016-0023
Martinez-Valdes E, Negro F, Falla D et al (2018) Surface electromyographic amplitude does not identify differences in neural drive to synergistic muscles. J Appl Physiol 124:1071–1079. https://doi.org/10.1152/japplphysiol.01115.2017
McManus L, Hu X, Rymer WZ et al (2016) Muscle fatigue increases beta-band coherence between the firing times of simultaneously active motor units in the first dorsal interosseous muscle. J Neurophysiol 115:2830–2839. https://doi.org/10.1152/jn.00097.2016
Miller JD, Lippman JD, Trevino MA, Herda TJ (2020) Neural drive is greater for a high-intensity contraction than for moderate-intensity contractions performed to fatigue. J Strength Cond Res 34:3013–3021. https://doi.org/10.1519/JSC.0000000000003694
Moore SA, McKay HA, Macdonald H et al (2015) Enhancing a somatic maturity prediction model. Med Sci Sports Exerc 47:1755–1764. https://doi.org/10.1249/MSS.0000000000000588
Nawab SH, Chang SS, De Luca CJ (2010) High-yield decomposition of surface EMG signals. Clin Neurophysiol 121:1602–1615. https://doi.org/10.1016/j.clinph.2009.11.092
Ozmun JC, Mikesky AE, Surburg PR (1994) Neuromuscular adaptations following prepubescent strength training. Med Sci Sports Exerc 26:510–514
Parra ME, Miller JD, Sterczala AJ et al (2020) Differences in the firing rate versus recruitment threshold relationships of the vastus lateralis in children ages 7–10 years and adults. Hum Mov Sci 72:102650. https://doi.org/10.1016/j.humov.2020.102650
Parra ME, Miller JD, Sterczala AJ et al (2021) The reliability of the slopes and y-intercepts of the motor unit firing times and action potential waveforms versus recruitment threshold relationships derived from surface electromyography signal decomposition. Eur J Appl Physiol 121:3389–3398. https://doi.org/10.1007/s00421-021-04790-6
Patten C, Kamen G (2000) Adaptations in motor unit discharge activity with force control training in young and older human adults. Eur J Appl Physiol 83:128–143. https://doi.org/10.1007/s004210000271
Payne VG, Morrow JR, Johnson L, Dalton SN (1997) Resistance training in children and youth: a meta-analysis. Res Q Exerc Sport 68:80–88. https://doi.org/10.1080/02701367.1997.10608869
Pope ZK, Hester GM, Benik FM, DeFreitas JM (2016) Action potential amplitude as a noninvasive indicator of motor unit-specific hypertrophy. J Neurophysiol 115:2608–2614. https://doi.org/10.1152/jn.00039.2016
Ramsay JA, Blimkie CJ, Smith K et al (1990) Strength training effects in prepubescent boys. Med Sci Sports Exerc 22:605–614
Sterczala AJ, Miller JD, Dimmick HL et al (2020) Eight weeks of resistance training increases strength, muscle cross-sectional area and motor unit size, but does not alter firing rates in the vastus lateralis. Eur J Appl Physiol 120:281–294. https://doi.org/10.1007/s00421-019-04273-9
Stock MS, Thompson BJ (2014) Effects of barbell deadlift training on submaximal motor unit firing rates for the vastus lateralis and rectus femoris. PLoS ONE 9:e115567. https://doi.org/10.1371/journal.pone.0115567
Stricker PR, Faigenbaum AD, McCambridge TM, Fitness C on SMA (2020) Resistance training for children and adolescents. Pediatrics 145: e20201011. https://doi.org/10.1542/peds.2020-1011
Thompson CK, Negro F, Johnson MD et al (2018) Robust and accurate decoding of motoneuron behaviour and prediction of the resulting force output. J Physiol 596:2643–2659. https://doi.org/10.1113/JP276153
Woods S, O’Mahoney C, Maynard J et al (2022) Increase in volitional muscle activation from childhood to adulthood: a systematic review and meta-analysis. Med Sci Sports Exerc 54:789–799. https://doi.org/10.1249/MSS.0000000000002853
Acknowledgements
We would like to acknowledge all children and parents for their time commitment to this study.
Funding
This study was funded by the National Strength and Conditioning Association Foundation.
Author information
Authors and Affiliations
Contributions
TH conceived and designed research. TH, EH, CC, KM, and AH conducted experiments. JT, RS, CC, and AH assisted in the development of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author has no financial or non-financial interests that are directly or indirectly related to the work. There was no funding for this paper.
Additional information
Communicated by Andrew Cresswell.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Herda, T.J., Holmes, E.A., Cleary, C.J. et al. Motor unit firing rates increase in prepubescent youth following linear periodization resistance exercise training. Eur J Appl Physiol 124, 2675–2686 (2024). https://doi.org/10.1007/s00421-024-05455-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-024-05455-w