Abstract
With muscular training, the central nervous system may regulate motor unit firing rates to sustain force output and delay fatigue. The aims of this study were to investigate motor unit firing rates and patterns of the adductor pollicis (AdP) muscle in young, able-bodied adults throughout a sustained submaximal isometric fatiguing contraction and postactivation potentiation pre-post 4 weeks of muscular endurance training. Fifteen participants (training group: N = 10; control group: N = 5) performed maximal voluntary contractions (MVCs) and a sustained isometric 20 % MVC fatigue task pre-post training. Single-motor-unit potentials were recorded from the AdP during the fatigue task with intramuscular fine-wire electrodes. Twitch force potentiation was measured during single-pulse electrical stimulation of the ulnar nerve before and after MVCs. The training group endurance trained their AdP muscle at 20 % MVC for 4 weeks. Mean motor unit firing rates were calculated every 5 % of endurance time (ET). ET increased by 45.2 ± 8.7 % (p < 0.001) following muscular endurance training. Although ET increased, mean motor unit firing rates during the fatigue task did not change significantly with training. The general motor unit firing pattern consisted of an initial slowing followed by an increase in firing rate late in fatigue and remained consistent pre-post training. Potentiation did not change following training. These data suggest that the ability of the neuromuscular system to sustain motor unit firing rate may serve as a mechanism to augment the duration of submaximal muscle performance and delay muscular fatigue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The neuromuscular system is highly adaptable and quickly responds to new patterns of muscular activity. In the earliest phase of muscular training, changes in force production may be due to neuromuscular adaptations related to learning optimal muscle activation patterns (for review, see Griffin and Cafarelli 2005; Carroll et al. 2011). For example, increases in motor unit firing rate (Van Cutsem et al. 1998; Kamen and Knight 2004) and earlier motor unit recruitment (Van Cutsem et al. 1998; Keen et al. 1994) occur during the first few weeks of resistance training. Maximal H to M (H/M) wave ratios of the triceps surae muscles are higher in endurance-trained compared to power-trained and sedentary individuals (Rochcongar et al. 1979; Maffiuletti et al. 2001), suggesting that endurance training may increase excitability of the spinal cord. Further, motor unit firing rates during a brief non-fatiguing contraction at 30 % maximal voluntary contraction (MVC) were lower after 6 weeks of aerobic cycle ergometer training (Vila-Cha et al. 2010). In contrast, the same study also found that motor unit firing rates during brief non-fatiguing contractions were higher after resistance training (Vila-Cha et al. 2010). The study by Vila-Cha et al. (2010) and other studies cited above indicate that the specificity of training principle may play a role in training-related neuromuscular adaptations, namely single-motor-unit firing rate patterns. Therefore, it is also possible that altered motor unit firing rates and firing patterns contribute to delayed fatigue onset during prolonged submaximal force output following local muscular endurance training (i.e., low-intensity resistance training specifically designed to improve local muscular endurance). To our knowledge, however, no study has measured single-motor-unit firing rates and firing patterns throughout the time-course of muscle fatigue following local muscular endurance training. Throughout this paper, local muscular endurance training will often be referred to as muscular endurance training.
In untrained muscle, the typical pattern of most motor units recruited from the onset of submaximal isometric sustained fatiguing contractions consists of an early decrease in firing rate which increases later in fatigue (Garland et al. 1997; Adam and De Luca 2005). Additionally, Vila-Chã et al. (2012) reported a decline in motor unit firing rate from the first 10 s of muscle contraction to the 60–70-s time period of muscle contraction of a fatigue task in the quadriceps muscle, with no change in the rate of decline during this time segment following 6 weeks of cycle ergometer endurance training performed at 50–70 % of heart rate reserve. The present study expands on this study by examining firing rates and the pattern of firing rate of a single motor unit throughout the time-course of muscle fatigue following local muscular endurance training. The initial decline in motor unit firing rate during fatigue has been correlated with force potentiation magnitude (Klien et al. 2001). Because muscle fibers produce greater force in the potentiated state, firing rates may slow to avoid an overshoot of the target force during submaximal contraction. It is also possible that firing rates of active motor units fire at a higher rate and are maintained over a longer duration to maintain submaximal force output with recruitment of fewer motor units during fatigue. Our previous work has shown that 8-week muscular endurance training leads to greater postactivation potentiation during a submaximal fatigue task and following maximal effort conditioning contractions (Mettler and Griffin 2012). Endurance athletes have greater potentiation in the trained muscles following maximal voluntary conditioning contractions compared to untrained individuals (Hamada et al. 2000). Therefore, changes in force potentiation as a result of muscular endurance training may alter motor unit firing patterns and act as a mechanism to counteract muscular fatigue.
As muscle contraction is maintained, metabolites accumulate that can activate Group III and IV afferents (Rotto and Kaufman 1988) which inhibit depolarization of the motor neuron. Ischemia, such as produced during sustained muscle contraction, increases the concentration of these metabolites in the active muscle tissue and can inhibit cortical excitability (Taylor and Gandevia 2008), reduce H-reflex amplitude (Garland 1991) and decrease single-motor-unit firing rates (Bigland-Ritchie et al. 1986). Endurance training effectively decreases the production of metabolites (Lucia et al. 2000) and increases capillarization and blood flow throughout the muscle to increase clearance of metabolites produced during muscle activity (Brodal et al. 1977; Lucia et al. 2000). Thus, muscular endurance training may reduce the inhibition from Group III and IV afferents to induce higher motor unit firing rates and to sustain firing frequencies over a longer duration and improve muscle endurance.
The purpose of this study was to determine single-motor-unit firing rates and patterns of the adductor pollicis (AdP) muscle throughout the time-course of a sustained submaximal fatigue task before and after 4 weeks of local muscular endurance training. We also aimed to determine the postactivation potentiation response to MVCs pre- can post- 4 weeks of muscular endurance training.
Materials and methods
Participants
Fifteen healthy males (N = 7) and females (N = 8) participated in this study. Participants were randomly assigned to training (N = 10) and control groups (N = 5). The training group consisted of four males and six females (25.1 ± 1.5 years old). The control group consisted of three males and two females (24.3 ± 1.8 years old). A control group was included in this study to ensure a methodologically sound design. Having a larger treatment group than control group does not present any special statistical problems for the analysis technique we used, other than the general truth that larger sample sizes yield greater power to detect effects that exist in the population from which the sample was drawn. In terms of both invasiveness and time, testing and analysis of each subject is not a trivial task. While the inclusion of a control group strengthens the design, there was of course no reason to suspect that the control group would differ from the treatment group at pretest. There was also little reason to suspect that the control group would change significantly over time, but in any event statistical power to detect change over time in the same individuals tends to be high. We suspected that a fairly minimal control group would therefore suffice. In contrast, capturing the change that was expected to occur in the training group was our primary concern. We considered the possibility of individual differences in both response to training and fidelity to the training regimen. These were obviously no concerns for the control group. Participants in this study were healthy, did not take medications known to affect nervous system function, and had no history of neurological disorder or injury to the non-dominant hand. Musicians and athletes with a highly trained non-dominant hand were also excluded from the study. Participants were instructed to abstain from caffeine consumption on experimental test days. All procedures were approved by The University of Texas at Austin Internal Review Board, and all individuals signed informed consent forms prior to participation in the study.
Experimental setup
Participants first attended an initial orientation session in which they were familiarized with the experimental setup, equipment and protocol. They also practiced performing MVCs and holding isometric contractions. They returned to the laboratory at least 48 h after the orientation session for the pretraining experimental session. Participants were seated with the non-dominant forearm supported in a splint. The wrist was placed in a pronated position, and the thumb was abducted and positioned against a metal strain-gauge force transducer. To determine maximal M-wave, a pair of pregelled, adhesive, Ag/AgCl disposable surface electromyography (EMG) electrodes (Danlee Products, Inc., Syracuse, NY) were placed on the palmer surface of the hand, over the AdP muscle, and a ground surface electrode was placed on the radial styloid process of the wrist. The skin surface was prepped with alcohol at the intramuscular electrode insertion site and the surface electrode placement sites. Intramuscular insulated stainless steel fine-wire (0.002 mm) electrodes (California Fine Wire Company, Grover Beach, CA) made of three fine wires were used to record single-motor-unit data. The intramuscular electrodes were then inserted with a thin hollow needle (25 g) just under the skin and into the muscle belly of the AdP of the non-dominant hand. A ground surface electrode was placed at the unlar styloid process of the wrist. A surface stimulating electrode was secured with a strap over the unlar nerve at the wrist. Straps at the wrist, forearm, upper arm and shoulder secured the limb and hand position. Therapeutic hand putty was also placed around the hand and fingers to prevent the hand from slipping. A visual display of the force and EMG was provided on a computer screen positioned in front of the individual.
Experimental protocol
To test postactivation potentiation, maximal M-wave amplitude was determined by applying single-pulse (50 µs duration) surface electrical stimulation (Digitimer DS7A, Garden City, England) to the ulnar nerve at the wrist and increasing the stimulation intensity until the M-wave reached maximal amplitude and increasing the intensity did not increase the peak to peak M-wave amplitude. All stimulation was supramaximal (at a stimulation intensity 10 % higher than required to evoke a maximal M-wave). After the maximal M-wave was determined, five single twitches were evoked with single-pulse surface stimulation of the ulnar nerve at the wrist. Participants then performed three MVCs of the AdP muscle. Each MVC was held approximately 3.5 s, and participants were instructed to adduct the thumb against the metal strain-gauge bar as fast and forcefully as possible to MVC. Verbal encouragement was provided during each MVC. Immediately following the MVCs, five maximal twitches were again evoked to determine the potentiated twitch force.
Following a 7-min rest period, participants performed the fatigue task which consisted of holding a 20 % MVC isometrically until the endurance limit. Verbal encouragement was provided to participants during the fatigue task. The fatigue task force was calculated based on the largest MVC of each respective test day. The criterion for endurance time (ET) was determined post hoc and was defined as two force fluctuations of ≥10 % MVC within a 10-s time period. Previous single-motor-unit fatigue studies have used a similar criterion for ET (Adam and De Luca 2005; Garland et al. 1997; Contessa et al. 2009). The same experimental protocol was utilized for the pretest and posttest. The posttest was performed 2 days after the last training day. The control group did not participate in any training and performed only the pretest and posttest approximately 4 weeks apart.
Muscular endurance training
The training group trained the AdP muscle of the non-dominant thumb every other day for 4 weeks for a total of 14 training sessions. Muscular endurance training was performed using a portable, custom-designed and built thumb training device (University of Texas Mechanical Engineering Department, Austin, TX) which isolated thumb adduction. The muscular endurance training protocol consisted of performing three sets of seven 1-min isometric thumb adduction contractions at 20 % MVC. Each repetition was followed by a 5-s rest interval and each set by a 2-min rest interval. The training protocol in this study was based on The American College of Sports Medicine Position Stand that states that local muscular endurance can be most effectively improved by performing light resistance loads at less than 50 % of the 1-repetition maximum and high repetitions (≥15–25) (Ratamess et al. 2009; Garber et al. 2011).
Participants were provided with visual feedback of the force they were exerting via a gauge on the training device, which allowed them to maintain the target force level throughout the contraction. The first and every third training session were conducted in the laboratory under the supervision of the experimenter to ensure that training was performed correctly to measure MVC and to adjust training load accordingly. All other training sessions were performed at home, and participants were provided with a training device, a hand-held stopwatch and a training log on which to record their training sessions. Subjects reported 100 % training compliance and were able to complete each training session.
Data analysis
Surface EMG was high-pass-filtered at 13 Hz, gain 100 (Coulbourn Instruments, Allentown, PA), and digitized at 2000 Hz [Cambridge Electronic Design (CED), Cambridge, England]. Intramuscular EMG was preamplified, band-pass-filtered 10 Hz–3.12 kHz with a gain of 330 (B&L Engineering, Tustin, CA) and digitized at a sampling rate of 20 kHz. The force signal was low-pass-filtered at 1 kHz with a gain of 100 (World Precision Instruments, Sarasota, FL) and digitized at a sampling rate of 1 kHz. Force and intramuscular EMG were synchronously recorded (Fig. 1). All data were analyzed off-line using Spike2 for Windows (version 5) software package (CED, Cambridge, England). Individual motor unit potentials were analyzed off-line with the Spike2 waveform discrimination system (CED, Cambridge, England).
Mean single-motor-unit firing frequency was measured off-line during 5-s time bins every 5 % of ET. The first 5 % segment of the fatigue task represented the initial motor unit firing frequency, the last 5 % segment of the fatigue task represented the final motor unit firing frequency, and the segment with the lowest mean motor unit firing frequency represented the minimum motor unit firing frequency. Motor unit firing rates in absolute time and relative to ET (every 5 % ET) were compared. The slopes of firing rate over time during the fatigue task were compared before and after endurance training. Mean motor unit firing rates during the initial, minimum and final time bins of the fatigue task were also compared. During the fatigue task, a total of 31 motor units were recorded and analyzed throughout the fatigue task (14 pretraining and 17 posttraining). All interspike intervals (ISIs) ≤20 and ≥200 ms were excluded from analysis because they result in false-positive and false-negative ISIs, respectively, and, therefore, alter mean firing rate (Garland et al. 1994). Approximately 5 % of the identified action potentials were excluded from analysis.
The mean peak twitch force from the five single pulses measured before the three MVCs served as the control twitch force. The mean peak twitch force from the five single pulses after the MVCs served as the potentiated twitch force. Each participant’s ET and MVC are expressed as a percent change pre- and post-test.
Statistical analysis
A between–within general linear mixed model (GLMM) with group (training and control), training (pre and post) and fatigue time (initial, minimum and final) as the independent variables was used to test motor unit firing rate changes with training. A GLMM with group × training as the independent factors was also used to test pre- and post-training changes in time to minimum motor unit firing rate and training-related differences in overall mean motor unit firing rates at relative and absolute times during the fatigue task. The slopes of the motor unit firing rate changes at relative and absolute times from the start of the fatigue task were compared using a group × training × fatigue time GLMM.
Two-way repeated measures ANOVA with group × training as the independent factors was used to test pre-post training differences in ET and MVC. Postactivation potentiation was compared using a three-way repeated measures ANOVA with twitch force (control and potentiated), training and group as the independent factors. Bonferroni corrections were used for post hoc analysis of multiple comparisons. An alpha level p ≤ 0.05 was accepted as the level of statistical significance for all tests. All data are reported as mean ± standard error.
Results
Endurance time
There were a significant main effect for training (p < 0.001) and a significant group × training interaction (p = 0.030) for ET. Post hoc comparisons revealed that muscular endurance training resulted in a 45.2 ± 8.7 % (p < 0.001) (9.5 ± 1.7 and 13.8 ± 2.8 min, pre- and posttraining, respectively) increase in ET of the fatigue task for the training group and no significant change in ET for the control group (12.6 ± 6.8 %; p = 0.27) (12.0 ± 2.3 vs. 13.6 ± 2.8 min, pre- and posttraining, respectively).
Maximal voluntary contraction
There was a significant training main effect (p = 0.05) for an increase in MVC following training. There was no difference between groups (p = 0.09), and the training × group interaction (p = 0.22) was not significant for MVC (training group: 78.0 ± 10.7 vs. 91.3 ± 13.1 N; control group: 50.4 ± 6.6 vs. 53.9 ± 7.5 N, pre- and posttraining, respectively).
Postactivation potentiation
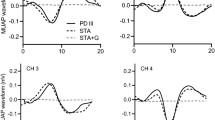
There was a significant main effect for potentiation (control vs. potentiated twitch force) (p < 0.001) which showed that twitch force increased significantly following the three 3.5-s MVCs. Compared to the control twitch force, the average potentiated twitch force increased by 3.9 N and 4.2 N pre- and posttraining, respectively, for the training group, and by 2.7 N and 2.9 N pre- and posttraining, respectively, for the control group. Data are displayed in Fig. 2. The main effect for training and the interactions were not significant.
Motor unit firing patterns
Thirty-one motor units were recorded throughout the course of the fatigue task. In the training group, nine motor units from eight subjects (one motor unit for seven subjects; two motor units for one subject) were analyzed pretraining and 12 motor units from 10 subjects (one motor unit for eight subjects; two motor units for two subjects) were analyzed posttraining. In the control group, five motor units from four subjects (one motor unit for three subjects; two motor units for one subject) were analyzed pretraining and five motor units from four subjects (one motor unit for three subjects; two motor units for one subject) were analyzed posttraining from four subjects. At least one motor unit for at least one test was recorded for all subjects included in the data analysis. Mean motor unit firing rate patterns did not change with muscular endurance training. Motor unit firing rates during the initial, minimum and final sections of the fatigue task were compared, and there was a significant main effect for fatigue time (p < 0.001). Post hoc results show that initial motor unit firing rates were significantly higher than minimum firing rates (p < 0.001) and final firing rates (p < 0.001) and that final firing rates were higher than minimum firing rates (p < 0.001) (Fig. 3). There was no significant main effect for training (p = 0.245) or group (p = 0.106), and no significant interactions.
Although the time from the start of the fatigue task to the point in the fatigue task where minimum motor unit firing rate occurred increased; the main effect for training and the training × group interaction were not statistically significant (Fig. 4). Additionally, the group × training and group × training × fatigue time interactions were not significantly different when mean motor unit firing rate of the 5-s time bins was compared at points relative to ET (Fig. 5) or when compared at absolute times (Fig. 6) during the fatigue task indicating no change in mean motor unit firing rate and no change in the slope of motor unit firing behavior, respectively, throughout the fatigue task (p > 0.05). Individual motor unit firing rate patterns during the fatigue task are displayed in Fig. 7. Additionally, newly recruited motor units were observed in some subjects during the fatigue task; however, they were not quantified or analyzed as the purpose of the present study was to examine a specific motor unit from the onset of muscle contraction throughout the time-course of muscle fatigue.
Mean motor unit firing rate averaged over 5-s bins every 5 % endurance time (20 time bins) pre- and posttraining. Each data point is the mean of all motor units analyzed. Training group: pretraining 12 motor units, posttraining nine motor units; control group: pretraining five motor units, posttraining five motor units. a Training group and b control group. There was no significant change between the pre- and post-tests (p = 0.09)
Motor unit firing rates in absolute time from the onset of the fatigue task for the training group. Each data point represents the mean firing rate over a 5-s time bin during the fatigue task. To demonstrate the mean motor unit firing rate and slope of the change in firing rate pattern throughout the course of fatigue, the data were fit with a second-order polynomial trendline. a Pretraining firing rates: y = 8E − 06x 2 − 0.0087x + 15.061; R 2 = 0.0442, and b posttraining firing rates: y = 2E − 06x 2 − 0.0045x + 15.662; R 2 = 0.0245. There were no significant changes in mean motor unit firing rate or the slope of the curve pre-post training
Individual single-motor-unit data are displayed during the time-course of muscle fatigue relative to endurance time. To demonstrate the individual single-motor-unit firing rate pattern throughout the course of fatigue, the mean motor unit firing rate of each motor unit during the 5-s time bins was fit with a second-order polynomial trendline. Motor units are numbered, with each number representing a specific subject a pretraining and b posttraining
Discussion
To our knowledge, this is the first study to examine motor unit firing rates and the pattern in which the firing rate of a single motor unit changes throughout the course of a sustained submaximal voluntary fatigue task before and after local muscular endurance training. Although ET increased, we found that the pattern of single-motor-unit firing rate activity during the fatigue task did not change with muscular endurance training. The general pattern of motor unit firing observed in the present study during a submaximal fatiguing contraction is consistent with our previous work (Garland et al. 1997; Griffin et al. 2000) and the work of others (Garland et al. 1994; Adam and De Luca 2005) in untrained individuals, whereby most initially recruited motor units display an initial decrease in firing rate and an increase late in fatigue. Our data are also in agreement with a cycle ergometer training study in which motor unit firing rates were lower during the 60–70-s time period of contraction compared to the first 10 s of the 30 % MVC with no training-related change (Vila-Chã et al. 2012). This decline in motor unit firing rate during fatigue has been attributed to the muscle wisdom hypothesis (Marsden et al. 1983), twitch force potentiation (Klien et al. 2001), decreased central drive (Gandevia 2001; Taylor and Gandevia 2008), motor neuron adaptation (Kernell and Monster 1982), decrease in Ia afferent excitatory input to the motor neuron (Macefield et al. 1991) and inhibition from Group III and IV afferents (Bigland-Ritchie et al. 1986; Garland 1991). The late increase in motor unit firing rate may be due to increased central drive and increased Ia afferent activity late in fatigue (Ljubisavlijecvic and Anastasijevic 1994). In the present study, this pattern was also maintained pre-post muscular endurance training and, therefore, may serve as the optimal central nervous system (CNS) strategy to postpone fatigue during sustained muscular activity.
The mean firing rate at the onset of contraction, at the end of the fatigue task, and the lowest firing frequency during the fatigue task did not change with training. Additionally, mean motor unit firing rates did not change with training when compared at points relative to ET or when compared in absolute time from the onset of muscle contraction. Yet, because muscular ET increased significantly with training, the CNS was able to sustain similar firing rates of a single motor unit over a longer period of time in the endurance-trained state compared to the untrained state.
Several neural and metabolic adaptations that occur in response to endurance training may have contributed to the maintenance of pretraining firing rates over the course of a longer posttraining fatigue task and thus contribute to the enhanced muscular endurance. Group III and IV small diameter afferents likely inhibit cortical motoneurons (Taylor and Gandevia 2008), and increased activation of Group III and IV afferents occurs in response to the accumulation of metabolites produced during prolonged contractile activity (Mense and Stahnke 1983; Rotto and Kaufman 1988). Trained individuals, however, deplete muscle glycogen stores more slowly than untrained individuals and in so doing may delay and/or reduce lactate production (Hermansen et al. 1967) which may reduce motor unit inhibition to allow the neuromuscular system to sustain a given motor unit firing frequency over a longer duration of submaximal muscle contraction.
During sustained MVC, motor unit firing rates have been found to continue to decline under ischemic conditions until blood flow was re-established (Woods et al. 1987). Motor unit firing rates, however, are maintained during dynamic fatiguing submaximal contractions (Miller et al. 1996; Griffin et al. 1998), perhaps due to greater blood flow during dynamic contractions which increases metabolite removal from the muscle. Aerobic endurance training (running) produces adaptations such as a reduction in metabolite production (Lucia et al. 2000), increased capillarization and increased blood flow to the muscle to aid in the clearance of metabolic byproducts (Brodal et al. 1977; Lucia et al. 2000). Indeed, increased blood flow and decreased metabolite production may decrease Group III and IV afferent inhibition to the motor neuron pool in the muscle trained for local muscular endurance and allow for prolonged maintenance of motor unit firing rate. In the present study, these physiological adaptations may explain why the neuromuscular system sustains pretraining firing frequencies over a longer period of time after training to defer muscle fatigue. Furthermore, a trend was observed where the minimum firing rate occurred later in the fatiguing contraction after training, also suggesting that higher firing rates were maintained over a longer duration in the endurance-trained muscle.
Central fatigue may also play a role in the performance of submaximal sustained contractions as excitability of the motor neuron has been found to decrease during submaximal fatiguing contractions (McNeil et al. 2011). Motor unit firing rates decrease as pain increases (Farina et al. 2004) in part due to stimulation of Group III and IV afferents which inhibit motor unit activation. Additionally, rating of perceived effort during a sustained 15 % MVC increased by almost four times during the course of a 40-min contraction (Sogaard et al. 2006). It is possible that perception of effort is reduced after muscular endurance training. Increases in central drive may allow for firing rate to be maintained over a longer duration, thereby sustaining force longer following muscular endurance training. Further, we have previously found that cortical excitability increases with short-term resistance training (Griffin and Cafarelli 2007) and the excitability of the spinal cord (H/M ratio) is greater in endurance-trained compared to sedentary individuals (Rochcongar et al. 1979; Maffiuletti et al. 2001). These factors may also allow for more efficient neural activation to assist in sustaining motor unit firing rates longer. Our data support previous findings that mean motor unit firing rate did not change following 6 weeks of cycle ergometer endurance training during unfatigued, 10-s contractions at 10 % MVC (Vila-Cha et al. 2010), but are in contrast to the same study that reported lower motor unit firing rates during an unfatigued, 10-s contraction performed at 30 % MVC after 6 weeks of cycle ergometer endurance training (Vila-Cha et al. 2010). Differences in training mode and the short duration of muscular activity may account for the discrepancy between results observed in the present study and those of Vila-Cha et al. (2010).
We did not find a muscular endurance training-related change in twitch force potentiation magnitude when MVCs were used as conditioning contractions. Our previous work shows that 8 weeks of similar muscular endurance training in the AdP muscle increased maximal muscle twitch force potentiation following MVC and during a submaximal fatiguing contraction (Mettler and Griffin 2012). Endurance athletes have also been found to have greater potentiation in the trained muscles compared to untrained individuals (Hamada et al. 2000). It is, however, likely that 4-week training was not long enough to induce significant changes in potentiation. Additionally, we did not evoke electrically induced contractions to test twitch force potentiation during the fatigue task in the present study as that may have altered motor unit behavior, and therefore, it is not clear whether potentiation influenced motor unit firing rates during the submaximal fatigue task.
Study limitations
Results obtained in the predominately slow twitch AdP muscle may not generalize to all muscles, and future study is needed to examine muscular endurance training responses during a fatiguing contraction in larger muscles of the lower and upper extremities and in muscles of different fiber type composition. Also, although a small sample of single motor units was followed throughout the fatigue task, the sample size in the present study is consistent with previous studies in which the same motor unit was followed throughout the entire course of the fatigue task (Garland et al. 1997; Adam and De Luca 2005). These studies followed seven motor units (Garland et al. 1997) and eight motor units (Adam and De Luca 2005) that were followed during the entire fatigue task. In addition, an inherent limitation to single motor unit recording using intramuscular EMG is that it is not possible to ensure that the same motor unit is recorded during different experimental sessions. The firing rates of motor units vary within a given motor pool. It is therefore possible that the firing rate of motor units recorded during pretraining may have changed with training; however, since different motor units were recorded posttraining, these physiological changes may have been obscured. Similarly, motor unit firing rates vary across subjects, and motor units from several subjects were pooled in this study which also may have potentially obscured training-related changes in motor unit firing patterns. Additionally, a control group was included in this study to ensure a methodologically sound design. Although the control group was smaller than the training group, having a larger treatment group than control group does not present any special statistical problems for the analysis technique we used, other than the general truth that larger sample sizes yield greater power to detect effects that exist in the population from which the sample was drawn.
Postfatigue MVCs and postfatigue twitch forces may have provided physiological information to assist with interpretation of the motor unit data. During pilot testing, however, we determined that it would not be technically feasible to obtain postfatigue twitch forces due to difficulty in maintaining electrode placement during the fatigue task and this would be necessary in order to elicit maximal twitch force immediately postfatigue task. In addition, subjects reported pain at the wrist from the pressure of securing the stimulating electrode during the fatigue task. Motor units are inhibited by pain, and we did not want to compromise the motor unit data; therefore, we elected to forfeit the postfatigue twitch data. Postfatigue MVCs were performed; however, because the fatigue criterion was determined post hoc, the postfatigue MVCs were not reported as they did not provide meaningful information regarding the degree of muscle fatigue. Motor unit recruitment thresholds were not measured in the present study. It is possible that altered recruitment threshold may have contributed to prolonged muscular endurance and future research is warranted to investigate recruitment threshold changes with muscular endurance training.
In conclusion, we found that 4 weeks of local muscular endurance training resulted in increased local muscular endurance. The mean motor unit firing rates of a sustained submaximal fatigue task did not change with 4 weeks of muscular endurance training. ET significantly increased posttraining; therefore, mean firing rates were sustained longer, and the pattern of single-motor-unit firing rate changes during the course of muscle fatigue occurred over a longer duration in the trained muscle. These findings suggest that preservation of firing rates during the course of sustained submaximal muscle contraction may be a mechanism that contributes to increased muscular endurance and delayed fatigue onset following short-term local muscular endurance training.
References
Adam A, De Luca CJ (2005) Firing rates of motor units in human vastus lateralis muscle during fatiguing isometric contractions. J Appl Physiol 99:268–280
Bigland-Ritchie BR, Dawson NJ, Johansson RS, Lippold OCJ (1986) Reflex origin for the slowing of motoneurone firing rates in fatigue of human voluntary contractions. J Physiol 379:451–459
Brodal P, Ingjer F, Hermansen L (1977) Capillary supply of skeletal muscle fibers in untrained and endurance-trained men. Am J Physiol 232:H705–H712
Carroll TJ, Selvanayagam VS, Riek S, Semmler JG (2011) Neural adaptations to strength training: moving beyond transcranial magnetic stimulation and reflex studies. Acta Physiol 202:119–140
Contessa P, Adam A, De Luca CJ (2009) Motor unit control and force fluctuation during fatigue. J Appl Physiol 107:235–243
Farina D, Arendt-Nielsen L, Merletti R, Graven-Nielsen T (2004) Effect of experimental muscle pain on motor unit firing rate and conduction velocity. J Neurophysiol 91:1250–1259
Gandevia SC (2001) Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81:1725–1789
Garber C, Blissmer B, Deschenes M et al (2011) American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 43:1334–1359
Garland SJ (1991) Role of small diameter afferents in reflex inhibition during human muscle fatigue. J Physiol 435:547–558
Garland SJ, Enoka RM, Serrano LP, Robinson GA (1994) Behaviour of motor units in human biceps brachii during a submaximal fatiguing contraction. J Appl Physiol 76:2411–2419
Garland SJ, Griffin L, Ivanova T (1997) Motor unit discharge rate is not associated with muscle relaxation time in sustained submaximal contractions in humans. Neurosci Lett 239:25–28
Griffin L, Cafarelli E (2005) Resistance training: cortical, spinal, and motor unit adaptations. Can J Appl Physiol 30:328–340
Griffin L, Cafarelli E (2007) Transcranial magnetic stimulation during resistance training of tibialis anterior muscle. J Electromyogr Kinesiol 17:446–452
Griffin L, Garland SJ, Ivanova T (1998) Discharge patterns in human motor units during fatiguing arm movements. J Appl Physiol 85:1684–1692
Griffin L, Ivanova T, Garland SJ (2000) Role of limb movement in the modulation of motor unit discharge rate during fatiguing contractions. Exp Brain Res 130:392–400
Hamada T, Sale DG, MacDougall JD (2000) Postactivation potentiation in endurance-trained male athletes. Med Sci Sport Exerc 32:403–411
Hermansen L, Hultman E, Saltin B (1967) Muscle glycogen during prolonged severe exercise. Acta Physiol Scand 71:129–139
Kamen G, Knight CA (2004) Training-related adaptations in motor unit discharge rate in young and older adults. J Gerontol A Biol Sci Med Sci 59:1334–1338
Keen DA, Yue GH, Enoka RM (1994) Training-related enhancement in the control of motor output in elderly humans. J Appl Physiol 77:2648–2658
Kernell D, Monster AW (1982) Time course and properties of late adaptation in spinal motoneurons of the cat. Exp Brain Res 46:191–196
Klien CS, Ivanova TD, Rice CL, Garland SJ (2001) Motor unit discharge rate following twitch potentiation in human triceps muscle. Neurosci Lett 316:153–156
Ljubisavlijecvic M, Anastasijevic R (1994) Fusimotor-induced changes in muscle spindle outflow and responsiveness in muscle fatigue in decerebrate cats. Neuroscience 63:339–348
Lucia A, Hoyos J, Pardo J, Chicharro JL (2000) Metabolic and neuromuscular adaptations to endurance training in professional cyclists: a longitudinal study. Jpn J Physiol 50:381–388
Macefield G, Hagbarth K, Gorman R, Gandevia SC, Burke D (1991) Decline in spindle support to alpha-motoneurones during sustained voluntary contractions. J Physiol 440:497–512
Maffiuletti NA, Martin A, Babault N, Pensini M, Lucas B, Schieppati M (2001) Electrical and mechanical Hmax-to-Mmax ratio in power-and endurance-trained athletes. J Appl Physiol 90:3–9
Marsden CD, Meadows JC, Merton PA (1983) “Muscular wisdom” that minimized fatigue during prolonged effort in man: peak ratios of motoneuron discharge and slowing of discharge during fatigue. Adv Neurol 39:169–211
McNeil CJ, Giesebrecht S, Gandevia SC, Taylor JL (2011) Behavior of the motorneurone pool in a fatiguing submaximal contraction. J Physiol 589:3533–3544
Mense S, Stahnke M (1983) Response in muscle afferent fibres of slow conduction velocity to contractions and ischaemia in the cat. J Physiol 342:383–397
Mettler JA, Griffin L (2012) Post-activation potentiation and muscular endurance training. Muscle Nerve 45:416–425
Miller KJ, Garland SJ, Ivanova T, Ohtsuki T (1996) Motor unit behavior in humans during fatiguing arm movements. J Neurophysiol 75:1629–1636
Ratamess NA, Alyar BA, Evetoch TK, Housh TJ, Kibler WB, Kraemer WJ, Triplett NT (2009) Progression models in resistance training for healthy adults: position Stand. Med Sci Sport Exerc 41:687–708
Rochcongar P, Dassonville J, Le Bars R (1979) Modification of the Hoffman reflex in function of athletic training. Eur J Appl Physiol Occup Physiol 40:165–170
Rotto DM, Kaufman MP (1988) Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol 64:2306–2313
Sogaard K, Gandevia SC, Todd G, Petersen NT, Taylor JL (2006) The effect of sustained low-intensity contractions on supraspinal fatigue in human elbow flexor muscles. J Physiol 573:511–523
Taylor JL, Gandevia SC (2008) A comparison of central aspects of fatigue in submaximal and maximal voluntary contractions. J Appl Physiol 104:542–550
Van Cutsem M, Duchateau J, Hainaut K (1998) Changes in single motor unit behavior contribute to the increase in contraction speed after dynamic training in humans. J Physiol 513:295–305
Vila-Cha C, Falla D, Farina D (2010) Motor unit behavior during submaximal contractions following six weeks of either endurance or strength training. J Appl Physiol 109:1455–1466
Vila-Chã C, Falla D, Correia MV, Farina D (2012) Adjustments in motor unit properties during fatiguing contractions after training. Med Sci Sport Exerc 44:616–624
Woods JJ, Furbush F, Bigland-Ritchie B (1987) Evidence for a fatigue-induced reflex inhibition of motoneuron firing rates. J Neurophysiol 58:125–137
Acknowledgments
The authors would like to thank statistician, Michael Mahometa, Ph.D., for consulting on the statistical analyses and Brian Huynh for his time working on this project. We would also like to thank all of the study participants for their time and dedication to this project.
Author contributions
Joni A. Mettler involved in experimental design, training protocol design, data acquisition and analysis, interpretation of data and writing of manuscript. Lisa Griffin involved in conception, experimental design, interpretation of data and editing of manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mettler, J.A., Griffin, L. Muscular endurance training and motor unit firing patterns during fatigue. Exp Brain Res 234, 267–276 (2016). https://doi.org/10.1007/s00221-015-4455-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-015-4455-x