Abstract
Purpose
The purpose of this study was to compare measurements of muscle strength, size, and activation of the forearm flexors in pre- and post-pubescent males and females.
Methods
Forty pre-pubescent (mean ± 95% confidence interval, age = 9.79 ± 0.35 years, n = 10 males, n = 10 females) and post-pubescent (age = 17.23 ± 0.58 years, n = 10 males, n = 10 females) youth participated. Subjects completed maximal voluntary isometric contractions (MVICs) of the forearm flexors, and submaximal isometric step muscle actions at 30, 50, and 70% of the peak MVIC. Percent voluntary activation (VA) was quantified during all isometric muscle actions. Forearm flexor (biceps brachii and brachialis) muscle cross-sectional area (CSA) was quantified from ultrasound images.
Results
MVIC strength was expressed in absolute terms and normalized to CSA. Post-pubertal males were 130% stronger, had 101% greater CSA, and 17% greater maximal VA than pre-pubertal males, while post-pubertal females were 72% stronger, had 54% greater CSA, and 23% greater maximal VA than pre-pubertal females. When MVIC strength was normalized to CSA, the post-pubertal males were still 15% stronger than the pre-pubertal males, while the post-pubertal females were only 12% stronger than the pre-pubertal females. The responses for VA across intensity reflected differences in muscle activation strategies between pre- and post-pubertal males and females.
Conclusion
These results suggest that muscle size may account for a greater proportion of the growth and development-related differences in strength among males, while females may be more affected by changes in muscle activation. Regardless of sex, changes in muscle size and neuromuscular function influence strength increases during growth and development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Muscular strength is an important determinant of physical performance as children grow and develop (Bergeron et al. 2015; Lloyd et al. 2016). In fact, Lloyd et al. (2016) recently stated, “Indeed, a real challenge for sport and exercise scientists and practitioners working with youth is to determine whether changes in performance are mediated from training-induced or growth-related adaptations” p. 1492. Thus, understanding the natural, biological mechanisms by which muscles get stronger during growth and development will provide practitioners with an appropriate framework for developing muscle-strengthening programs to promote long-term health. Much of the previous literature regarding the natural, biological mechanisms by which muscles get stronger have quantified and compared changes and/or differences in muscle size (Wood et al. 2004; Tonson et al. 2008; Pitcher et al. 2012; Fukunaga et al. 2014). Collectively, these studies concluded that increases in muscle size during growth and development may fully account for the growth-related increases in muscle strength. In contrast, other studies have suggested that muscle size alone may be unable to fully account for increases in muscle strength as children grow and develop (Kanehisa et al. 1995; Housh et al. 1995a, b, 1997; Neu et al. 2002; Wood et al. 2006; Gillen et al. 2019), concluding that augmentations in neuromuscular function may explain some of the variability in growth-related increases in strength, perhaps to a greater extent than increases in muscle size.
To examine the growth and development-related changes in neuromuscular function, several previous studies have quantified voluntary activation (O’Brien et al. 2009, 2010; Kluka et al. 2015, 2016; Martin et al. 2015; Chalchat et al. 2019), which compares stimulated or evoked muscle actions to voluntary muscle actions to assess the ability to maximally activate all available motor units. Collectively, these studies (O’Brien et al. 2009, 2010; Kluka et al. 2015, 2016; Martin et al. 2015; Chalchat et al. 2019) demonstrated differences in maximal and submaximal muscle activation, such that adults achieved greater voluntary activation during maximal muscle actions and lower voluntary activation during submaximal muscle actions (i.e., greater neuromuscular efficiency). Interestingly, only two of these studies included sex-specific comparisons between male and female children and adults. O’Brien et al. (2009) reported greater discrepancies in voluntary activation between child vs. adult females (67% vs. 87%) than child vs. adult males (75% vs. 86%). In a similar study, O’Brien et al. (2010) reported nearly identical findings for the differences in voluntary activation among child vs. adult males and females and suggested that muscle size accounted for 75% of the strength differences among males, but only 50% of the strength differences among females. Therefore, it is possible that females may have a greater aptitude for neuromuscular adaptations during growth and development compared to males, who are influenced to a greater degree by muscular hypertrophy. However, O’Brien et al. (2010) only compared children to adults. Less is known about the potential sex-specific mechanisms leading to increases in muscle strength occurring over the narrower window of puberty.
Although comparisons of children to adults provide some information regarding changes in muscle form and function during growth and development, comparisons of pre- vs. post-pubertal children may rule out developmental changes that occur from post-pubescence to adulthood, providing greater clarity regarding the growth-mediated changes in muscle strength during the pubertal process. Furthermore, as proposed by O’Brien et al. (2010), it is possible that the underlying mechanisms affecting the growth-mediated changes in muscle strength may be sex-specific, where males are more dependent on increases in muscle size and females are more dependent on increases in voluntary activation. However, to our knowledge, no previous studies have quantified the sex-specific differences in muscle strength, size, and activation among pre- and post-pubertal males and females (instead of children vs. adults). Therefore, the purpose of this study was to compare measurements of muscle strength, size, and activation of the forearm flexors in pre- and post-pubescent males and females. We hypothesized that post-pubescent males and females would be stronger, have greater muscle size, and greater voluntary activation than the pre-pubescent males and females, with the post-pubescent males having the greatest muscle strength and size. We also hypothesized that the differences in muscle size would be more pronounced among the males, while the differences in voluntary activation would be more pronounced among the females.
Methods
Subjects
Based on the results of previous studies (Wood et al. 2004; O’Brien et al. 2010), and the equation established by Gravetter and Wallnau (2017) and Vincent and Weir (2012), a priori calculations of sample sizes to detect differences in muscle strength, size, and voluntary activation at the statistical power of ≥ 0.80 were n = 8 per group. Based on an anticipated 20% attrition, at least n = 10 participants were recruited for each group. Furthermore, previous studies have used sample sizes ranging from n = 5 to n = 15 per cross-sectional group to examine differences in muscle strength, size, and voluntary activation in youth ranging from 7 to 16 years of age (Grosset et al. 2008; O’Brien et al. 2009, 2010; Pitcher et al. 2012; Kluka et al. 2015; Martin et al. 2015; Gillen et al. 2019). Forty-six pre-pubescent (n = 11 males, n = 13 females) and post-pubescent (n = 12 males, n = 10 females) subjects between the ages of 8–10 years and 15–18 years, respectively, were recruited. However, 1 pre-pubescent male and 3 pre-pubescent females withdrew from the study. Furthermore, 2 post-pubescent males did not meet the inclusion criteria for serum testosterone concentration. Therefore, data from 40 pre-pubescent (n = 10 males, n = 10 females) and post-pubescent (n = 10 males, n = 10 females) subjects were analyzed and reported herein.
Male subjects were initially screened for inclusion by calculating the age at peak height velocity with the Mirwald equation (Mirwald et al. 2002). To be included in the pre-pubescent group, males must have been at least 2 years prior to peak height velocity, while inclusion in the post-pubescent group required that males be at least 2 years after peak height velocity. Post hoc verification of pubertal status was assessed from capillary blood samples collected during the familiarization visit for serum testosterone concentration. Based on previous studies (Nielsen et al. 1986; Klein et al. 1996; Round et al. 1999; Khairullah et al. 2014; Pişkin et al. 2018), a pre-pubescent male must have had a serum testosterone concentration ≤ 2.03 nmol·L−1, while a post-pubescent male must have had a serum testosterone concentration ≥ 16.61 nmol·L−1 to be included in the statistical analyses.
Female subjects were initially screened for inclusion by asking if they had reached menarche. A female investigator (MES) asked each subject and their parent or guardian if they had reached the age of menarche. Females who had not yet reached menarche were considered pre-pubescent, while females who had passed the age of menarche were considered post-pubescent (Marshall and Tanner 1969; Zacharias and Rand 1983; Granados et al. 2015). Similar to the male subjects, to be included in the pre-pubescent group, females must have been at least 2 years prior to peak height velocity, while inclusion in the post-pubescent group required that females be at least 2 years after peak height velocity. Based on previous studies (Marshall and Tanner 1969, 1970; Zacharias and Rand 1983; Nielsen et al. 1986; Klein et al. 1996; Round et al. 1999; Khairullah et al. 2014; Pişkin et al. 2018), males and females who were 8–10 years old should have met the criteria for pre-pubescence, while males and females who were 15–18 years old should have met the criteria for post-pubescence. Thus, subjects in these age ranges were recruited.
This study was approved by the University of Nebraska-Lincoln’s Institutional Review Board (IRB) for the protection of human subjects (IRB Approval #: 20190719446FB, Title: Muscle Strength, Size, and Neuromuscular Function in Pre-pubescent and Post-pubescent Boys and Girls). Before any data collection, pre-pubescent subjects signed an approved child assent form, while post-pubescent subjects signed an approved youth assent form, and one parent or legal guardian signed an approved parental consent form. Each subject, with the assistance from their parent or legal guardian, completed the PAR-Q + 2015 (Warburton et al. 2011) and was included in this study if questions 1–7 were answered “no” or all of the follow-up questions of the PAR-Q + 2015 were answered “no.”
Research design
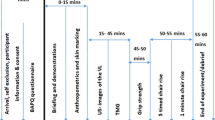
A cross-sectional factorial design was used to compare sexes and pubescent status groups on measurements recorded during the experimental visit only. Subjects visited the laboratory twice, once for familiarization and once for the experimental visit. The familiarization and experimental visits were separated by 2–5 days and occurred at the same time of day (± 2 h). The familiarization visit allowed subjects to experience and practice interacting with the testing equipment and procedures. During the familiarization visit, pubertal classification and separation into the pre-pubescent or post-pubescent group took place. Anthropometrics, ultrasound, and muscle strength assessments were performed during each visit.
Anthropometrics and body composition
Height, seated height, and body mass were measured using a digital scale and stadiometer (Seca 769, Hamburg, Germany). These variables were used to estimate maturity offset from peak height velocity based on the equations established by Mirwald et al. (2002). Percent body fat (BF%) and fat-free mass (FFM, kg) were assessed from skinfold measurements taken with a Lange caliper (Model 68902, Cambridge Scientific Industries, Inc., Cambridge, MD, USA). All skinfolds were taken on the right side of the body at the subscapular (diagonal fold immediately inferior to the interior angle of the scapula), triceps (vertical fold in the middle of the arm, midway between the acromion and olecranon process), and suprailiac (diagonal fold immediately superior to the anterior superior iliac spine) sites and were recorded to the nearest 0.5 mm (Jackson and Pollock 1985). Equations established by Housh et al. (1996, 2000) and Brozek et al. (1963) were used to estimate body density and BF%, respectively.
Serum testosterone
To ensure appropriate pubertal classification for the male subjects, capillary blood samples of 200 μL were collected in microvettes (Microvette® 200 μL, K3 EDTA, violet US code; 10.8 × 46.6 mm) to analyze for serum testosterone concentration (nmol·L−1). Enzyme-linked immunosorbent assay kits measured serum testosterone concentration (Testosterone ELISA kit (Serum), Crystal Chem USA, Elk Grove Village, IL, USA). Assay procedures were followed according to the manufacturer’s protocol (Crystal Chem USA, Elk Grove Village, IL, USA) and performed in duplicate for each blood sample. Before performing the assay, all reagents were brought to room temperature for 30 min before use and were mixed thoroughly by gentle agitation. In each microplate well, 10 μL of prepared sample, standard, or control was added, followed by 100 μL of testosterone-HRP conjugate which was then mixed by repeated pipetting. Then, 50 μL of anti-testosterone reagent was added to each well and mixed by repeated pipetting. The plate was then incubated for 90 min at 37 °C. After incubation, the well contents were aspirated, and the wells were washed 5 times using 300 μL of distilled water per well. After each wash, any remaining solution was removed by inverting and tapping the plate firmly on a clean paper towel. Following the wash, 100 μL of substrate solution was added to each well, followed by incubation for 20 min on a plate shaker at room temperature while the plate was covered. The reaction was stopped by adding 100 μL of stop solution to each well. The optical density was measured within 20 min using a plate reader (Varioskan LUX Multimode Microplate Reader, Thermo Fisher Scientific, Waltham, MA, USA) at 450 nm. Using computer software (SkanIt Microplate Reader Software, Thermo Fisher Scientific, Waltham, MA, USA), a testosterone calibration curve was created by plotting the mean optical density for each standard on the y-axis versus the corresponding testosterone concentration on the x-axis. Testosterone concentrations (nmol∙L−1) in the samples were interpolated using the calibration curve and mean optical density values for each sample or control. The average of the two samples was used as the representative serum testosterone concentration. The manufacturer (Crystal Chem USA, Elk Grove Village, IL, USA) reported an assay range from 0.348 to 62.409 nmol∙L−1, with a sensitivity of 0.0017 nmol∙L−1, and a coefficient of variation of ≤ 10% for the assay kit.
Ultrasound measurements
Panoramic cross-sectional ultrasound images of the forearm flexor muscles (biceps brachii and brachialis) were collected using a portable brightness mode (B-mode) ultrasound-imaging device (GE Logiq e, USA) interfaced with a multi-frequency linear-array probe (12L-RS; 5–13 MHz; 38.4 mm field-of-view). Subjects were positioned on a plinth in the supine position while lying with the right arm abducted, relaxed, and supported on the plinth with the forearm extended. All ultrasound images were taken at 66% of the distance between the acromion process and fossa cubit (Jenkins et al. 2016). Panoramic cross-sectional images were taken from the most lateral to the most medial aspect of the forearm flexors at a constant speed controlled by the investigator (ZMG). A generous amount of water-soluble transmission gel was applied to the skin to enhance acoustic coupling and reduce near field artifacts. Equipment settings were optimized for image quality with a gain of 58 dB and a frequency of 12 MHz. These settings were held constant across subjects. Image depth, however, was adjusted based on each subject’s arm size and was held constant for each subject. All images were taken by the same investigator (ZMG) prior to any exercise performed by this muscle. Images were taken until three images of acceptable quality, as determined by the investigator, were acquired at each measurement site. Images with the highest visual contrast were used for analysis.
Images were analyzed using Image-J Software (National Institutes of Health, USA, version 1.47v). Prior to analysis, images were scaled from pixels to cm using the Image-J straight-line function. Forearm flexor muscle cross-sectional area (CSA, cm2) was quantified from the panoramic images using the polygon function in Image-J to select the maximal region of interest that included as much of the forearm flexor muscles as possible while excluding the surrounding fascia (Jenkins et al. 2016). Intraclass correlation coefficients (ICCs) for CSA in the present study were ≥ 0.904.
Muscle strength and voluntary activation measurements
For isometric testing, subjects were seated with straps securing the trunk and pelvis on a Biodex System 3 (Biodex Medical Systems, Inc., Shirley, NY, USA) which was custom fitted with a load cell (Omegadyne, model LCHD-500, 0–500 lbs, Stamford, CT, USA) on an adjustable lever arm. All assessments were performed on the right arm. Each subject’s wrist was secured using a Velcro strap, the axis of rotation of the dynamometer head was aligned with the axis of rotation of the elbow joint, and the arm was abducted to 45° to better expose the musculocutaneous nerve for assessment of VA (%) (Jenkins et al. 2016). The arm and forearm were set at a joint angle of 90°.
Subjects completed two 3 s warm-up forearm flexion muscle actions at 50% and 75% of perceived effort with 30 s rest between each muscle action. Following the warm-up and 2 min of rest, subjects completed two, 4 s maximum voluntary isometric contractions (MVICs) of the forearm flexors with 2 min of rest between each muscle action. On each attempt, subjects were instructed to contract as hard and fast as possible when the investigator said “go.” Loud, verbal encouragement was given during each MVIC. Subjects then completed submaximal isometric step muscle actions at 30%, 50%, and 70% of the peak MVIC. For each submaximal muscle action, subjects were instructed to achieve the target intensity as rapidly as possible and maintain the contraction for 4 s.
The twitch interpolation procedure was used to estimate VA during all isometric muscle actions. Prior to completing the twitch interpolation procedure, all subjects were reminded that there was the possibility of mild discomfort. During the MVICs, doublet stimuli were transcutaneously superimposed and applied to the musculocutaneous nerve during a stable plateau in torque observed by the investigator (ZMG) (Allen et al. 1995, 1998; Jenkins et al. 2016). Three to 5 s after the MVIC, a potentiated twitch was evoked at rest with the same doublet stimuli. Peak torques of the MVIC, superimposed twitch, and potentiated twitch were used to calculate VA using the following equation (O’Brien et al. 2010; Kluka et al. 2015, 2016; Martin et al. 2015):
The twitch interpolation procedure was performed identically during the submaximal isometric step muscle actions (30%, 50%, and 70%).
Transcutaneous electrical stimuli were delivered via a high voltage (maximal voltage = 400 V), constant-current stimulator (Digitimer DS7AH, Hertfordshire, UK). The cathode was a hand-held probe (Digitimer Bipolar Felt Pad Electrodes, Hertfordshire, UK), covered in a saline-soaked sponge, and placed over the musculocutaneous nerve and the anode was a disposable, adhesive electrode (40 × 50 mm; Digitimer Ltd, Hertfordshire, UK) placed over the bicipital tendon based on previous descriptions (Allen et al. 1995, 1998). Optimal cathode location was determined by delivering single, low-amperage exploratory stimuli (20 mA) while visually monitoring the twitch torque and compound muscle action potential (M-wave) amplitudes displayed in real-time on an external computer screen. Once the location was determined, the skin was marked, and all further stimuli were delivered at that location. Maximal twitch torque and peak-to-peak M-wave amplitude were determined by successive single stimuli applied while systematically increasing the amperage in 10 mA increments until a plateau in twitch torque and peak-to-peak M-wave amplitude were observed after 3 consecutive amperage increases. To ensure a supramaximal stimulus, 120% of the stimulus amperage used to determine the plateau was used to evoke the forearm flexor muscles with a doublet stimulus (200 ms duration square-wave impulse at 100 Hz).
Electromyography
To ensure appropriate stimulus, bipolar surface electromyography (EMG) signals were recorded from the biceps brachii with pre-gelled electrodes (Ag/AgCl, Accusensor, Lynn Medical, Wixom, MI, USA) and a center-to-center interelectrode distance of 30 mm to determine peak-to-peak M-wave amplitude during the recruitment curves. The EMG signals were amplified (gain = 1,000) using a differential amplifier (EMG 100, Biopac Systems, Inc., bandwidth 1–5000 Hz) with a common mode rejection ratio of 110 dB min and an input impedance of 2 MΩ. The electrodes for the biceps brachii were placed at 66% of the distance between the acromion process and fossa cubit, parallel to the muscle fibers (Hermens 1999; Gaudet et al. 2016). A single, pre-gelled reference electrode (Ag/AgCl, Accusensor, Lynn Medical, Wixom, MI, USA) was placed over the lateral epicondyle of the humerus. To reduce interelectrode impedance and increase the signal-to-noise ratio, the placement site for the electrodes was shaved, abraded, and cleaned with isopropyl alcohol prior to application.
Signal processing
The force and EMG signals were sampled simultaneously at 2 kHz with a Biopac data acquisition system (MP150WSW; Biopac Systems, Inc., Santa Barbara, CA, USA). All signals were stored on a personal computer and processed off-line with custom-written software (LabVIEW v. 18.0, National Instruments, Austin, TX). The force signal obtained from the load cell (N) was multiplied by the lever arm length (m) to calculate torque (Nm), but the force signal was not filtered. Voluntary peak torque was calculated using the highest consecutive 500 ms average torque value during the torque plateau, but before the superimposed twitch. Superimposed and potentiated twitch torques were the highest consecutive 5 ms average torque values during the evoked twitches.
Statistical analyses
As per the procedures described by Weir (2005), repeated measures analyses of variance (ANOVAs) were used on the raw values to determine test–retest reliability for the common measures between visits. The ICC used model “3,1” (Shrout and Fleiss 1979; Weir 2005):
where MSS is the mean square of the dependent variable, MSE is the mean square error, and k is the number of trials (k = 2). Weir (2005) has suggested that the ICCs from model 3,1 are specific to the study.
Means and 95% confidence intervals for each group were calculated and reported for all measurements. MVIC strength was expressed in absolute terms and normalized to CSA to examine the influence of muscle size on differences in strength between groups. An independent samples t test was used to analyze testosterone concentration between pre- and post-pubescent males. Two-way factorial ANOVAs [sex (male vs. female) × group (pre-pubescent vs. post-pubescent)] were used to analyze age, maturity offset, height, body mass, BF%, FFM, absolute MVIC strength, CSA, and MVIC strength normalized to CSA. A three-way mixed factorial ANOVA [sex (male vs. female) × group (pre-pubescent vs. post-pubescent) × intensity (30% vs. 50% vs. 70% vs. MVIC)] was used to analyze VA. Significant interactions were decomposed with follow-up lower order ANOVAs and Bonferroni-corrected dependent and/or independent samples t tests on the simple main effects. Equality of variances was tested using Levene’s Test for Equality of Variances. In cases where the homogeneity of variances assumption was not met, the error term and degrees of freedom were adjusted using the Welch–Satterthwaite method. Sphericity was tested for each mixed factorial ANOVA using Mauchly’s Test of Sphericity. In cases where the assumption of sphericity was not met, Greenhouse–Geisser corrections were applied (Greenhouse and Geisser 1959). Effect sizes were calculated as partial eta squared (\({\eta }_{p}^{2}\)) for each ANOVA. Cohen’s d effect sizes (d) were calculated for independent samples t tests as described previously (Cohen 1988). The d effect sizes for dependent samples t tests were corrected for dependence among means based on the correlation between means as described by Morris and DeShon (2002). All statistical analyses were performed in IBM SPSS v. 26 (Chicago, IL, USA). An alpha level of 0.05 was considered statistically significant for all F-tests and was used as the basis of all Bonferroni corrections.
Results
Reliability
Anthropometrics and measurements of growth (i.e., maturity offset, height, body mass, body fat %, fat-free mass) exhibited ICCs ≥ 0.967 for the pre-pubertal males, ICCs ≥ 0.953 for the pre-pubertal females, ICCs ≥ 0.943 for the post-pubertal males, and ICCs ≥ 0.935 for the post-pubertal females. CSA exhibited an ICC = 0.904 for the pre-pubertal males, ICC = 0.957 for the pre-pubertal females, ICC = 0.938 for the post-pubertal males, and ICC = 0.945 for the post-pubertal females. Measurements of maximal and submaximal strength exhibited ICCs ≥ 0.921 for the pre-pubertal males, ICCs ≥ 0.910 for the pre-pubertal females, ICCs ≥ 0.936 for the post-pubertal males, and ICCs ≥ 0.926 for the post-pubertal females.
Subjects
For the males, the pre-pubertal group had a lower testosterone concentration than the post-pubertal group (p < 0.001, d = 18.73, Table 1). Based on previous studies (Nielsen et al. 1986; Klein et al. 1996; Round et al. 1999; Khairullah et al. 2014; Pişkin et al. 2018), we are confident the males included in the statistical analyses had appropriate testosterone concentrations to be included in their respective group (i.e., pre- or post-pubertal). There was a group-related main effect for age such that age was greater for the post-pubertal group than the pre-pubertal group collapsed across sex (p < 0.001, \({\eta }_{p}^{2}\hspace{0.17em}\)= 0.937, Table 1). There were group- and sex-related main effects for maturity offset such that the females were greater than the males collapsed across group (p < 0.001, \({\eta }_{p}^{2}\) = 0.366, Table 1), and the post-pubertal group was greater than the pre-pubertal group collapsed across sex (p < 0.001, \({\eta }_{p}^{2}\) = 0.784, Table 1). There were group- and sex-related main effects for body mass such that the males were greater than the females collapsed across group (p = 0.011, \({\eta }_{p}^{2}\) = 0.847, Table 1), and the post-pubertal group was greater than the pre-pubertal group collapsed across sex (p < 0.001, \({\eta }_{p}^{2}\hspace{0.17em}\)= 0.929, Table 1). There were significant sex × group interactions for height, BF%, and FFM (p ≤ 0.016). For height, the post-pubertal females were greater than the pre-pubertal group (p < 0.001, d ≥ 5.93, Table 1), while the post-pubertal males were greater than all other groups (p < 0.001, d ≥ 2.13, Table 1). For BF%, the pre-pubertal males were greater than the pre-pubertal females and post-pubertal males (p ≤ 0.013, d ≥ 1.22, Table 1), while the post-pubertal females were greater than all other groups (p < 0.001, d ≥ 0.76, Table 1). For FFM, the post-pubertal females were greater than the pre-pubertal group (p < 0.001, d ≥ 4.04, Table 1), while the post-pubertal males were greater than all other groups (p ≤ 0.014, d ≥ 1.75, Table 1).
Muscle strength and size
There was a significant sex × group interaction for absolute MVIC strength (p = 0.002) such that the post-pubertal females were greater than the pre-pubertal group (p < 0.001, d ≥ 2.64, Fig. 1A), while the post-pubertal males were greater than all other groups (p < 0.001, d ≥ 2.11, Fig. 1A). There was a significant sex × group interaction for CSA (p < 0.001) such that the post-pubertal females were greater than the pre-pubertal group (p < 0.001, d ≥ 6.03, Fig. 2), while the post-pubertal males were greater than all other groups (p < 0.001, d ≥ 3.88, Fig. 2). When MVIC strength was normalized to CSA, there was a main effect for group such that the post-pubertal group was greater than the pre-pubertal group collapsed across sex (p = 0.021, \({\eta }_{p}^{2}\)= 0.333, Fig. 1B).
Means ± 95% confidence intervals and individual data points for (A) absolute maximal voluntary isometric contraction (MVIC) strength, and B MVIC strength normalized to forearm flexor muscle cross-sectional area for pre-pubertal (PRE) and post-pubertal (POST) males and females. *Indicates greater than PRE group, **indicates greater than POST females (p ≤ 0.05)
Voluntary activation
There was a sex × group × intensity interaction for VA (p = 0.018). For the pre-pubertal males, VA increased systematically from 30% to MVIC (p ≤ 0.044, d ≥ 1.34, Fig. 3). For the pre-pubertal females, VA increased from 30 to 50% to 70% (p ≤ 0.048, d ≥ 1.58) and plateaued from 70% to MVIC (p = 0.386, d = 0.76, Fig. 3). For the post-pubertal group, VA increased systematically from 30% to MVIC collapsed across sex (p < 0.001, d ≥ 2.33, Fig. 3). VA was greater in the pre-pubertal males than the post-pubertal group at 30% (p ≤ 0.016, d ≥ 1.19, Fig. 3). At 70% and MVIC, VA was greater in the post-pubertal than the pre-pubertal males and females (p ≤ 0.018, d ≥ 1.13, Fig. 3). At MVIC, VA was greater in the pre-pubertal males than the pre-pubertal females (p = 0.042, d = 0.98, Fig. 3).
Means ± 95% confidence intervals for percent voluntary activation across percent maximum voluntary isometric contraction (% MVIC) for pre-pubertal (PRE) and post-pubertal (POST) males and females. *Indicates POST group greater than PRE group, **Indicates PRE males greater than POST group, ***indicates PRE males greater than PRE females, #indicates greater than 30% for all groups, ##indicates greater than 30 and 50% for all groups, ###indicates greater than 30, 50, and 70% for PRE males and POST group (p ≤ 0.05)
Discussion
The primary results of this study demonstrated greater differences in muscle strength and size among the males (Figs. 1 and 2), and greater differences in maximal VA among the females (Fig. 3). Specifically, the post-pubertal males were 130% stronger, had 101% greater CSA, and 17% greater maximal VA than the pre-pubertal males, while the post-pubertal females were 72% stronger, had 54% greater CSA, and 23% greater maximal VA than the pre-pubertal females. Additionally, normalizing MVIC strength to muscle size accounted for a slightly greater proportion of the difference in strength among the males than females (Fig. 1). Furthermore, the collective responses for VA across intensity may reflect changes in muscle activation strategies during growth and development for both males and females (Fig. 3). Thus, the results of the present study extend the hypothesis of O’Brien et al. (2010) that muscle size may account for at least a slightly greater proportion of the growth and development-related increases in strength among males, while females may be more affected by changes in muscle activation. However, regardless of sex, changes in muscle size and neuromuscular function both influence the growth and development-related increases in muscle strength. The findings of the present study add to the understanding of the natural mechanisms by which muscles get stronger during growth and development for young males and females, which may aid practitioners in designing muscle-strengthening programs to promote long-term health, particularly when taking into consideration the growth-related changes in muscle function that accompany the narrow window of puberty.
To our knowledge, only one previous study has attempted to determine the sex-specific influences of muscle size and voluntary activation on the growth and development-related differences in muscle strength by comparing male and female children to adults (O’Brien et al. 2010). In a well-controlled study, O’Brien et al. (2010) suggested that up to 75% of the difference in strength may be accounted for by muscle size in males, while only 50% of the difference in strength may be accounted for by muscle size in females. In the present study, normalizing MVIC strength to CSA reduced the magnitude of difference in strength from 130 to 15% for the males, and 72–12% for the females (Fig. 1). Therefore, based on the initial magnitudes of difference for males and females (130% and 72%, respectively), 89% of the difference in strength among males was accounted for by muscle size, while 83% of the difference in strength among females was accounted for by muscle size. Although these results suggest that a greater proportion of the difference in strength may be accounted for by muscle size compared to O’Brien et al. (2010), the present results still indicate that a slightly greater proportion of the difference in strength may be accounted for by muscle size in males than females. One possible explanation for a greater proportion of the difference in strength being accounted for by muscle size in the present study compared to O’Brien et al. (2010) may be the difference in muscle group examined, where O’Brien et al. (2010) examined the quadriceps femoris and the present study examined the forearm flexors. However, further research examining muscle specific differences for the influence of muscle size on increases in muscle strength between pre- and post-pubertal males and females, not simply children vs. adults, are needed to better understand the natural mechanisms by which muscles get stronger during the growth and development years in various muscle groups. Furthermore, although the magnitude of difference in strength was reduced for both males and females (Fig. 1), the present findings are in agreement with previous studies suggesting that muscle size is unable to fully account for increases in strength during growth and development (Kanehisa et al. 1995; Housh et al. 1995a, 1997; Neu et al. 2002; Wood et al. 2006; Gillen et al. 2019).
In the present study, there were unique, sex-specific differences in maximal VA among males and females. In the post-pubertal group, both males and females had similar VA during the MVIC (~ 90%), which were greater than the pre-pubertal group (67–73%, Fig. 3). However, within the pre-pubertal group, the males had greater maximal VA than the females (73% versus 67%, Fig. 3). To our knowledge, only two studies have quantified the sex-specific, growth and development-related differences in VA among males and females (O’Brien et al. 2009, 2010), and did so by comparing children to adults. Two studies by O’Brien et al. (2009, 2010) reported nearly identical findings to each other, with young males achieving a maximal VA of 75% and young females achieving a maximal VA of 67–68%. Based on their findings, there was an 11% difference in VA between child and adult males, and a 19–20% difference in VA between child and adult females (O’Brien et al. 2009, 2010), which was interpreted as females having a greater aptitude for neuromuscular adaptations during growth and development. Similarly, in the present study, there was a 23% difference in VA among the females versus a 17% difference in VA among the males. Thus, the lower maximal VA among the pre-pubertal females compared to the pre-pubertal males, in conjunction with the greater magnitude of difference in maximal VA among the females, may indeed reflect a greater aptitude for changes in muscle activation during growth and development for females (O’Brien et al. 2009, 2010). However, although females had a greater difference in maximal VA, the 17% difference in VA among the males indicates that significant changes in muscle activation capabilities still occur as young males grow and develop. Thus, in conjunction with the hypothesis of O’Brien et al. (2010), changes in muscle strength during growth and development may be more mediated by changes in neuromuscular function in females than males. However, regardless of sex, changes in muscle activation should be considered when examining the underlying mechanisms that increase strength during growth and development. Although these findings are similar to those reported in comparing children to adults (O’Brien et al. 2009, 2010), the present results provide clarity regarding the neurally mediated changes that promote increases in muscle strength across the narrower window of puberty.
In the present study, there were differences in the responses of VA across intensity between the pre- and post-pubertal groups (Fig. 3). During the 30% muscle action, the pre-pubertal males had greater VA than all other groups, while there were no differences at 50%, and the post-pubertal group had 5–11% greater VA during the 70% muscle action (Fig. 3). Interestingly, VA stayed the same from 70% to MVIC for the pre-pubertal females, but increased for all other groups, which may provide further evidence that growth and development-related increases in strength for females may be more influenced by changes in muscle activation. Few studies have quantified muscle activation across intensity in children and adolescents (Grosset et al. 2008; Chalchat et al. 2019), and none have compared these responses in pre- and post-pubescent males and females. Using the central activation ratio, Grosset et al. (2008) found that child males and females required greater muscle activation during submaximal muscle actions (25–75% of MVIC) than adult males. The authors hypothesized this may reflect less efficient muscular activation in children, meaning that children require a greater percentage of their muscle to be activated to achieve the same relative torque output. In contrast, using the interpolated twitch technique, Chalchat et al. (2019) found nearly identical responses for VA across intensity (20–90% of MVIC) in child and adult males. However, Grosset et al. (2008) and Chalchat et al. (2019) compared pre-pubertal children to adults with no comparisons of pre- versus post-pubertal males and females. In conjunction with the findings of Grosset et al. (2008), the present results suggest that pre-pubertal children, specifically males, may have less efficient muscle activation capabilities during low intensity muscle actions compared to post-pubertal males and females. However, the lack of differences at 50% is in agreement with Chalchat et al. (2019), indicating that there may be no differences in muscle activation capabilities between pre- and post-pubertal males and females during moderate intensity muscle actions. Furthermore, although it may be tempting to suggest that the greater VA at 70% represents less efficient muscle activation for the post-pubertal group, this more likely reflects an inhibition of muscle activation capabilities at higher intensities for pre-pubertal children (Grosset et al. 2008; O’Brien et al. 2009, 2010; Kluka et al. 2015, 2016; Martin et al. 2015), which is supported by the greater strength and VA during the MVIC in the post-pubertal group (Fig. 3). Therefore, pre-pubertal children may have less efficient muscle activation during low intensity muscle actions, similar muscle activation during moderate intensity muscle actions, and inhibited muscle activation during higher intensity muscle actions compared to post-pubertal children.
Although the present study provides novel information regarding the sex-specific differences in muscle strength, size, and activation between pre- and post-pubertal males and females, there are limitations. For example, although participants were determined to by physical healthy via completion of the PAR-Q + 2015 (Warburton et al. 2011), it is possible that prior training status (i.e., sport participation) may have influenced the present results, which was not controlled for in the present study. However, previous studies of a similar design also did not report controlling prior training status in youth participants (Wood et al. 2004; Grosset et al. 2008; O’Brien et al. 2010; Pitcher et al. 2012). Furthermore, future studies may consider controlling for hydration status, sleep, or arousal, which may influence measures of muscle function. Nevertheless, the present study provides unique information that may be used to guide future research studies of a similar nature.
In conclusion, the present results demonstrated sex-specific differences in muscle strength, size, and activation among pre- and post-pubertal males and females. In agreement with O’Brien et al. (2010), normalizing MVIC strength to muscle size accounted for a slightly greater proportion of the difference in strength among males than females. However, regardless of sex, muscle size was unable to fully account for the differences in strength. In addition, there were sex-specific differences in VA such that the pre-pubertal females had 6% lower maximal VA than the pre-pubertal males, while there was a 17% difference in maximal VA among the males and a 23% difference in maximal VA among the females. The lower maximal VA among the pre-pubertal females, in conjunction with the greater discrepancy in maximal VA between pre- and post-pubertal females, may reflect a greater aptitude for neuromuscular adaptations during growth and development for females. Furthermore, the responses of VA across intensity may reflect growth and development-related changes in the efficiency of muscle activation, changes in motor unit recruitment strategies, and potentially changes in muscle fiber type characteristics for both males and females. Based on the present results, and in support of the hypothesis of O’Brien et al. (2010), there may be sex-specific neural and morphological changes in skeletal muscle that impact the growth and development-related increases in muscle strength. Thus, the present study provides novel information regarding the natural mechanisms by which muscles get stronger during the pubertal process. Collectively, the results of the present study may provide a better framework by which practitioners can develop muscle-strengthening programs for young males and females.
Availability of data and material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
The custom-written software of this study is available from the corresponding author upon reasonable request.
Abbreviations
- ANOVA:
-
Analysis of variance
- BF%:
-
Percent body fat
- CSA:
-
Cross-sectional area
- FFM:
-
Fat-free mass
- MVIC:
-
Maximum voluntary isometric contraction
- PAR-Q:
-
Physical activity readiness questionnaire
- VA:
-
Voluntary activation
References
Allen G, Gandevia S, McKenzie D (1995) Reliability of measurements of muscle strength and voluntary activation using twitch interpolation. Muscle Nerve 18:593–600. https://doi.org/10.1002/mus.880180605
Allen GM, McKenzie DK, Gandevia SC (1998) Twitch interpolation of the elbow flexor muscles at high forces. Muscle Nerve 21:318–328
Bergeron M, Mountjoy M, Armstrong N et al (2015) International Olympic Committee consensus statement on youth athletic development. Br J Sports Med 49:843–851. https://doi.org/10.1136/bjsports-2015-094962
Brozek J, Grande F, Anderson J, Keys A (1963) Densiometric analysis of body composition: revision of some quantitative assumptions. Ann NY Acad Sci 110:113–140
Chalchat E, Piponnier E, Bontemps B et al (2019) Characteristics of motor unit recruitment in boys and men at maximal and submaximal force levels. Exp Brain Res 237:1289–1302
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Lawrence Erlbaum Associates, Hillsdale, NJ
Fukunaga Y, Takai Y, Yoshimoto T et al (2014) Effect of maturation on muscle quality of the lower limb muscles in adolescent boys. J Physiol Anthopol 33:30. https://doi.org/10.1186/1880-6805-33-30
Gaudet G, Raison M, Maso FD et al (2016) Intra- and intersession reliability of surface electromyography on muscles actuating the forearm during maximum voluntary contractions. J Appl Biomech 32:558–570. https://doi.org/10.1123/jab.2015-0214
Gillen ZM, Shoemaker ME, McKay BD et al (2019) Muscle strength, size, and neuromuscular function before and during adolescence. Eur J Appl Physiol 119:1619–1632. https://doi.org/10.1007/s00421-019-04151-4
Granados A, Gebremariam A, Lee JM (2015) Relationship between timing of peak height velocity and pubertal staging in boys and girls. J Clin Res Pediatr Endocrinol 7:235–237. https://doi.org/10.4274/jcrpe.2007
Gravetter FJ, Wallnau LB (2017) Statistics for the behavioral sciences, 10th edn. Cengage Learning, Boston, MA
Greenhouse SW, Geisser S (1959) On methods in the analysis of profile data. Psychometrika 24:95–112
Grosset JF, Mora I, Lambertz D, Pérot C (2008) Voluntary activation of the triceps surae in prepubertal children. J Electromyogr Kinesiol 18:455–465. https://doi.org/10.1016/j.jelekin.2006.11.002
Hermens HJ (1999) SENIAM 8: European recommendations for surface electromyography, 2nd edn. Roessingh Research and Development, Enschede, the Netherlands
Housh TJ, Stout JR, Housh DJ, Johnson GO (1995a) The covariate influence of muscle mass on isokinetic peak torque in high school wrestlers. Pediatr Exerc Sci 7:176–182. https://doi.org/10.1123/pes.7.2.176
Housh TJ, Stout JR, Weir JP et al (1995b) Relationships of age and muscle mass to peak torque in high school wrestlers. Res Q Exerc Sport 66:256–261. https://doi.org/10.1080/02701367.1995.10608840
Housh TJ, Johnson GO, Housh DJ et al (1996) Validity of skinfold estimates of percent fat in high school female gymnasts. Med Sci Sports Exerc 28:1331–1335. https://doi.org/10.1097/00005768-199610000-00019
Housh TJ, Johnson GO, Housh DJ et al (1997) Isokinetic peak torque and estimated muscle cross-sectional area in high school wrestlers. J Strength Cond Res 11:45–49. https://doi.org/10.1519/00124278-199702000-00010
Housh TJ, Johnson GO, Housh DJ et al (2000) Estimation of body density in young wrestlers. J Strength Cond Res 14:477–482
Jackson AS, Pollock ML (1985) Practical assessment of body composition. Phys Sportsmed 13:76–90. https://doi.org/10.1080/00913847.1985.11708790
Jenkins N, Housh T, Buckner S et al (2016) Neuromuscular adaptations after 2 and 4 weeks of 80% versus 30% 1 repetition maximum resistance training to failure. J Strength Cond Res 30:2174–2185. https://doi.org/10.1519/JSC.0000000000001308
Kanehisa H, Ikegawa S, Tsunoda N, Fukunaga T (1995) Strength and cross-sectional areas of reciprocal muscle groups in the upper arm and thigh during adolescence. Int J Sports Med 16:54–60
Khairullah A, Klein LC, Ingle SM et al (2014) Testosterone trajectories and reference ranges in a large longitudinal sample of male adolescents. PLoS ONE 9:e108838. https://doi.org/10.1371/journal.pone.0108838
Klein KO, Martha PM Jr, Blizzard RM et al (1996) A longitudinal assessment of hormonal and physical alterations during normal puberty in boys. II. Estrogen levels as determined by an ultrasensitive bioassay. J Clin Endocrinol Metab 81:3203–3207. https://doi.org/10.1210/jc.81.9.3203
Kluka V, Martin V, Vicencio SG et al (2015) Effect of muscle length on voluntary activation level in children and adults. Med Sci Sports Exerc 47:718–724. https://doi.org/10.1249/MSS.0000000000000463
Kluka V, Martin V, Vicencio SG et al (2016) Effect of muscle length on voluntary activation of the plantar flexors in boys and men. Eur J Appl Physiol 116:1043–1051. https://doi.org/10.1007/s00421-016-3362-6
Lloyd R, Cronin J, Faigenbaum A et al (2016) National strength and conditioning association position statement on long-term athletic development. J Strength Cond Res 30:1491–1509. https://doi.org/10.1519/JSC.0000000000001387
Marshall WA, Tanner JM (1969) Variations in pattern of pubertal changes in girls. Arch Dis Child 44:291–303. https://doi.org/10.1136/adc.44.235.291
Marshall WA, Tanner JM (1970) Variations in the pattern of pubertal changes in boys. Arch Dis Child 45:13–23. https://doi.org/10.1136/adc.45.239.13
Martin V, Kluka V, Vicencio SG et al (2015) Children have a reduced maximal voluntary activation level of the adductor pollicis muscle compared to adults. Eur J Appl Physiol 115:1485–1491. https://doi.org/10.1007/s00421-015-3132-x
Mirwald RL, Baxter-Jones ADG, Bailey DA, Beunen GP (2002) An assessment of maturity from anthropometric measurements. Med Sci Sports Exerc 34:689–694. https://doi.org/10.1097/00005768-200204000-00020
Morris SB, DeShon RP (2002) Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol Methods 7:105–125. https://doi.org/10.1037/1082-989x.7.1.105
Neu CM, Rauch F, Rittweger J et al (2002) Influence of puberty on muscle development at the forearm. Am J Physiol Endocrinol Metab 283:103–107. https://doi.org/10.1152/ajpendo.00445.2001
Nielsen CT, Skakkebaek NE, Darling JA et al (1986) Longitudinal study of testosterone and luteinizing hormone (LH) in relation to spermarche, pubic hair, height and sitting height in normal boys. Acta Endocrinol Suppl 279:98–106
O’Brien TD, Reeves ND, Baltzopoulos V et al (2009) The effects of agonist and antagonist muscle activation on the knee extension moment–angle relationship in adults and children. Eur J Appl Physiol 106:849–856. https://doi.org/10.1007/s00421-009-1088-4
O’Brien TD, Reeves ND, Baltzopoulos V, Jones DA (2010) In vivo measurements of muscle specific tension in adults and children. Exp Physiol 95:202–210. https://doi.org/10.1113/expphysiol.2009.048967
Pişkin İE, Gümüş M, Bayraktaroğlu T et al (2018) Growth and pubertal development in adolescent male wrestlers. J Sports Med Phys Fit 58:852–856
Pitcher CA, Elliott CM, Williams SA et al (2012) Childhood muscle morphology and strength: alterations over six months of growth. Muscle Nerve 46:360–366. https://doi.org/10.1002/mus.23326
Round JM, Jones DA, Honour JW, Nevill AM (1999) Hormonal factors in the development of differences in strength between boys and girls during adolescence: a longitudinal study. Ann Hum Biol 26:49–62. https://doi.org/10.1080/030144699282976
Shrout PE, Fleiss JL (1979) Intraclass correlations: uses in assessing rater reliability. Psychol Bull 86:420–428
Tonson A, Ratel S, Fur YL et al (2008) Effect of maturation on the relationship between muscle size and force production. Med Sci Sports Exerc 40:918–925. https://doi.org/10.1249/MSS.0b013e3181641bed
Vincent WJ, Weir JP (2012) Statistics in kinesiology, 4th edn. Human Kinetics, Champaign, IL
Warburton DER, Jamnik VK, Bredin SSD, Gledhill N (2011) The physical activity readiness questionnaire for everyone (PAR-Q+) and electronic physical activity readiness medical examination (ePARmed-X+). Health Fit J Can 4:3–23
Weir JP (2005) Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res 19:231–240
Wood LE, Dixon S, Grant C, Armstrong N (2004) Elbow flexion and extension strength relative to body or muscle size in children. Med Sci Sports Exerc 36:1977–1984. https://doi.org/10.1249/01.MSS.0000145453.02598.7E
Wood LE, Dixon S, Grant C, Armstrong N (2006) Elbow flexor strength, muscle size, and moment arms in prepubertal boys and girls. Pediatr Exerc Sci 18:457–469. https://doi.org/10.1123/pes.18.4.457
Zacharias L, Rand WM (1983) Adolescent growth in height and its relation to menarche in contemporary American girls. Ann Hum Biol 10:209–222. https://doi.org/10.1080/03014468300006381
Acknowledgements
We would like to thank the National Strength and Conditioning Association, the University of Nebraska Agriculture Research Division, and Abbott Nutrition for funding this study. We would also like to thank Nicholas Bohannon and Sydney Gibson for their help conducting the study, as well as each of the subjects for their participation.
Funding
Efforts for this study were funded, in part, by a National Strength and Conditioning Association Foundation (NSCAF) Graduate Research Doctoral Grant, the University of Nebraska Agriculture Research Division with funds provided by the Hatch Act (Agency: U.S. Department of Agriculture, National Institute of Food and Agriculture; Accession No: 1000080; Project No: NEB-36-078), and a grant from Abbott Nutrition, Columbus, OH.
Author information
Authors and Affiliations
Contributions
ZMG was a substantial contributor to the study concept and design, carried out data acquisition, analysis, and interpretation, and wrote the manuscript. TJH, RJS, TJH, and RJDA contributed to the study concept and design, and manuscript revision. MES contributed to data acquisition and manuscript revision. JTC was the primary manuscript reviser and a substantial contributor to the study concept, design, and interpretation. All authors provided critical feedback and approved the final version of the manuscript. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed. All the authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to report.
Ethical approval
This study was approved by the University of Nebraska-Lincoln’s Institutional Review Board (IRB) for the protection of human subjects (IRB Approval #: 20190719446FB, Title: Muscle Strength, Size, and Neuromuscular Function in Pre-pubescent and Post-pubescent Boys and Girls).
Informed consent
Before any data collection, pre-pubescent subjects signed an approved child assent form, while post-pubescent subjects signed an approved youth assent form, and one parent or legal guardian signed an approved parental consent form. Each subject, with the assistance from their parent or legal guardian, completed the PAR-Q + 2015 and was included in this study if questions 1–7 were answered “no” or all of the follow-up questions of the PAR-Q + 2015 were answered “no.”
Additional information
Communicated by William J. Kraemer.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gillen, Z.M., Housh, T.J., Schmidt, R.J. et al. Comparisons of muscle strength, size, and voluntary activation in pre- and post-pubescent males and females. Eur J Appl Physiol 121, 2487–2497 (2021). https://doi.org/10.1007/s00421-021-04717-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-021-04717-1