Abstract
Purpose
Previous investigations analyzing resistance training’s influence on motor unit (MU) firing rates have yielded mixed results. These mixed results may be clarified by concurrently measuring changes in MU size. Thus, this study analyzed whether post-training strength gains were due to increases in MU firing rates and/or sizes as measured indirectly via action potential amplitudes.
Methods
Sixteen males (age = 20.7 ± 1.9 years) completed 8 weeks of resistance training, while eight males (age = 19.4 ± 2.5 years) served as controls. Vastus lateralis surface electromyography signals collected during submaximal isometric knee extensions were decomposed to yield an action potential amplitude (MUAPAMP), mean firing rate (MFR), and recruitment threshold (RT) for each MU. Each contraction’s average MFR and MUAPAMP, and coefficients of the linear (y-intercept and slope) MUAPAMP-RT, linear MFR-RT and exponential (A and B terms) MFR–MUAPAMP relationships were analyzed. Firing instances and action potentials were validated via reconstruct-and-test and spike-triggered averaging procedures. Vastus lateralis cross-sectional area (CSA) was analyzed with ultrasonography.
Results
Resistance training increased isometric strength from 204.6 ± 34.9 to 239.8 ± 36.3 Nm and vastus lateralis CSA from 28.7 ± 4.7 to 34.0 ± 5.0 cm2. Resistance training did not affect MFR–RT relationship parameters or average MFRs but did increase the slopes of the MUAPAMP–RT relationships (0.0067 ± 0.0041 to 0.0097 ± 0.0045 mV/%MVC) and average MUAPAMPs from 0.189 ± 0.093 to 0.249 ± 0.127 mV. MU hypertrophy altered the MFR–MUAPAMP relationships (B terms: − 3.63 ± 1.40 to − 2.66 ± 1.38 pps/mV).
Conclusion
Resistance training induced MU and muscle hypertrophy, but did not alter firing rates. Greater MU twitch forces resulting from larger MUs firing at pre-training rates likely explain resistance training strength gains.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increases in maximal strength following resistance training are attributed to both muscle hypertrophy (Balshaw et al. 2017; Erskine et al. 2014) and neuromuscular adaptations (Gabriel et al. 2006; Moritani and deVries 1979), including decreased agonist muscle coactivation (Balshaw et al. 2017; Carolan and Cafarelli 1992) and increased agonist muscle activity (Gabriel et al. 2006; Jenkins et al. 2017; Knight and Kamen 2001; Moritani and deVries 1979; Pucci et al. 2006). The increased agonist muscle activity is attributed to greater motor unit (MU) recruitment during submaximal contractions due to decreased recruitment thresholds (Del Vecchio et al. 2019; Van Cutsem et al. 1998), greater voluntary activation (Jenkins et al. 2017; Knight and Kamen 2001; Pucci et al. 2006) and alterations in rate coding, or the firing rates of MUs. Resistance training’s influence on MU firing rates during submaximal contractions is unclear due to conflicting findings and is further complicated by differences in training programs, the muscles measured, electromyography (EMG) methodology (intramuscular vs. surface EMG), and analysis procedures applied to MU firing rates. For example, increases (Del Vecchio et al. 2019; Vila-Cha et al. 2010), and no change (Kamen and Knight 2004; Pucci et al. 2006; Rich and Cafarelli 2000) in firing rates have been reported when MUs are pooled across contraction intensities and participants.
In addition, analysis of firing rates relative to recruitment thresholds calculated on a per participant and contraction basis demonstrate no alterations in the firing rate scheme of the muscle following resistance training (Beck et al. 2011; Stock and Thompson 2014). This approach minimizes the effects of measuring MU firing rates at different levels of neural drive, such as, pooling MU firing rates across contraction intensities. For example, recording a greater number of MUs during a lower-intensity contraction than a higher-intensity contraction will lower the average firing rate. In addition, differences or changes in the ratio of smaller (lower-threshold) to larger (higher-threshold) MUs recorded from pre- to post-treatment could arbitrarily change the average firing rate of pooled MUs as a result of differences in the recruitment thresholds and sizes of recorded MUs. In both instances, the firing rates could change pre- to post-treatment in absence of true changes in the excitability at the motoneuron level. The misinterpretation of firing rate changes due to MU grouping has been demonstrated by Contessa et al (2016). Previously, inter-individual differences in firing rate–recruitment threshold relationships exist as a function of myosin heavy chain isoform isoforms (Colquhoun et al. 2018; Trevino et al. 2016) and, thus, requiring analysis on a participant-by-participant basis as performed with regressing firing rates in relation to recruitment threshold.
In addition, regressing MU action potential amplitudes in relation to recruitment threshold is commonly used to analyze changes in MU sizes (Pope et al. 2016; Sterczala et al. 2017, 2018; Trevino et al. 2018). The action potential amplitude of the MU is considered an indirect marker of size (Hakansson 1956; Olson et al. 1968). Surface EMG-derived action potential amplitudes are influenced not only by the number and diameter of the muscle fibers that comprise the MUs, but also by factors unrelated to MU size, including distance from the electrode and the amount of subcutaneous fat (Farina et al. 2010). Therefore, the absolute MU action potential amplitude values analyzed irrespective of recruitment threshold may not yield much insight. However, MU action potential amplitudes analyzed in relation to recruitment thresholds provide information about characteristics of skeletal muscle regarding the increase in MU size along the recruitment threshold spectrum. Changes in the slopes of the MU action potential amplitude–recruitment threshold relationships are highly correlated with increase in the muscle cross-sectional area following resistance training (Pope et al. 2016). Furthermore, the slopes of the MU action potential amplitude–recruitment threshold relationships are correlated with myosin heavy chain isoform composition (Colquhoun et al. 2018; Herda et al. 2019) and explain sex-related differences in muscle cross-sectional area (Trevino et al. 2018; Herda et al. 2019) and muscular strength and power (Herda et al. 2019). To date, no investigation has concurrently examined changes in MU firing rates and action potential amplitudes following resistance training. In addition, resistance training-related alterations in MU firing rates expressed as a function of action potential amplitudes may provide valuable insight on mechanisms responsible for increase in maximal strength, such as whether strength increases as a function of greater MU firing rates and/or hypertrophy.
Therefore, the primary purpose of this investigation was to determine if increases in strength following resistance training are due to changes in MU firing rates and/or hypertrophy. MU firing rate–recruitment threshold, action potential amplitude–recruitment threshold and MU firing rate–action potential relationships were analyzed from surface EMG recordings of the vastus lateralis during submaximal isometric contractions of the knee extensors performed at 70% of maximal voluntary contraction (MVC) before and after an 8-week resistance training intervention. Panoramic ultrasound was included to confirm that the resistance training program resulted in significant muscle hypertrophy and there were no changes in subcutaneous fat thickness. Muscular cross-sectional area and strength will increase following the resistance training program and, therefore, the torque output needed to match the relative 70% MVC will increase pre- to post-intervention for the resistance training group. Additionally, it is hypothesized that the sizes of the MUs will increase accompanied by no changes in the firing rates. Therefore, the increase needed in torque to match the post-70% MVC (higher torque output) will primarily be generated via changes in strength and size (hypertrophy) and not increases in firing rates of MUs.
Methods
Participants
Thirty healthy, college-aged males were recruited for this investigation. Changes in motor unit activity as a function of exercise interventions have been reported with as few as ten participants (Pope et al. 2016; Vila-Cha et al. 2010). Twenty participants were assigned to the resistance training group, to ensure that fifteen completed the training. In addition, ten participants were recruited as controls to ensure changes in motor unit activity following the training program were not due to type I error. Sixteen participants completed the resistance training program (age: 20.7 ± 1.9 years; height: 178.4 ± 7.8 cm; weight: 75.9 ± 9.4 kg) and eight served as controls (age: 19.4 ± 2.5 years; height: 181.7 ± 7.3 cm; weight: 77.8 ± 12.0 kg). In the resistance training group, one participant voluntarily withdrew, one participant was injured outside of the study, and two were withdrawn by the study team due to noncompliance with the training program. Two control participants had unusable ultrasound scans (excessive subcutaneous adipose tissue) preventing cross-sectional area measurement and were removed from data analyses. Participants were physically active but had not participated in lower-body resistance training in the previous 6 months. Prior to participation, all participants completed a health history questionnaire and reported no neuromuscular conditions or musculoskeletal injuries that could impact the results of this investigation. Informed consent was obtained from all participants. The University of Kansas institutional review board for human participants approved this investigation. The investigation was performed in accordance with the ethical standards of the Declaration of Helsinki.
Experimental approach
Before and after an 8-week treatment period, longitudinal changes in maximal isometric knee extension torque, cross-sectional area (CSA), subcutaneous fat thickness, and MU properties of the vastus lateralis were measure in the training participants and controls. At each time point, participants first completed a familiarization visit, consisting of ultrasound images followed by a knee extension isometric maximal voluntary contraction (MVC) and several practice submaximal isometric contractions. Within 24–72 h from the familiarization visit, participants returned to the laboratory for the experimental visit, at which they performed MVCs and a submaximal contraction at 70% of that day’s peak MVC torque. Surface EMG signals were collected from the vastus lateralis during the MVCs and submaximal contraction, with the latter decomposed into MU action potential trains to measure firing rates, action potential amplitudes, and recruitment thresholds.
Resistance training program
Participants in the resistance training group completed three lower-body resistance training sessions per week for 8 weeks. At each visit, participants performed four lower-body exercises, including complex multi-joint movements and single-joint isolation exercises. The lower-body exercises included: back squats, front squats, Romanian deadlifts, knee extensions, leg presses, glute bridges, step ups, hamstring curls, and reverse hyperextensions. The training program was based on a linear periodization model with volume decreasing and intensity increasing over the course of the program. Participants performed 3 sets of 12 repetitions during weeks 1–3, 3 sets of 8 repetitions during weeks 4–6 and 4 sets of 5 repetitions during weeks 7–8. The intensity for each set was based on the repetitions in the reserve scale previously reported by Helms et al. (2016). The initial intensity for each exercise for the 1st day was relatively conservative due to participants’ limited lower-body resistance training experience. After each set, the participants reported their repetitions in reserve rating, and that rating along with the judgment of the trainer was used to determine the intensity of the following set. All training sessions were overseen by National Strength and Conditioning Association Certified Strength and Conditioning Specialists. Participants in the training group completed all exercise sessions, whereas participants in the control group did not perform any resistance training during the investigation.
Isometric strength testing
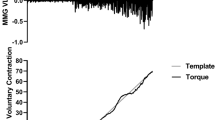
Participants performed isometric strength testing of the right knee extensors, regardless of leg dominance, with the knee positioned at 90° flexion. Testing was performed on a Biodex System 3 isometric dynamometer with participants seated and restraining straps positioned over the pelvis, trunk and contralateral thigh. The lateral condyle of the femur was aligned with the input axis of the dynamometer. The isometric strength was measured as the torque signal from the dynamometer. At each testing visit, participants completed two 3-s MVCs of the knee extensors with strong verbal encouragement. The highest observed MVC torque at each respective visit was used to determine the torque level for the subsequent submaximal contraction. Three minutes of rest was given between the MVC attempts and approximately 5 min of rest was given between the second MVC and the submaximal contraction. The submaximal contraction consisted of a linear torque increase, a 10 s steady torque plateau and a linear torque decrease. Torque increased and decreased at a rate of 20% MVC/s. Participants were provided with a visual template of the isometric trapezoidal contraction and real-time torque feedback. A second attempt was provided when participants were unable to adhere to the template during the initial attempt.
Electromyographic recording and decomposition
During the submaximal contractions, surface EMG signals were collected from the vastus lateralis via a 5-pin surface sensor array (Delsys, Inc, Natick, MA). The array consisted of five 0.5 mm pins arranged in a 5 × 5 mm square with the fifth pin positioned in the center. The sensor was positioned at approximately 60% of the distance between the greater trochanter and the lateral condyle of the femur via hypoallergenic tape. A reference electrode was placed over the left patella. Prior to the sensor and reference electrode placement, the sites were shaved, dry skin cells were removed via repeated application of adhesive tape, and the sites were sterilized with alcohol. At the pre-training testing visit, the sensor location was recorded and marked on the skin. The markings were maintained throughout training and used to ensure similar sensor placement during post-training testing. The EMG signals were sampled at 20 kHz and stored for subsequent decomposition and analysis. Using the previously detailed PDIII algorithm (version 4.1.1.0), the four channels of raw surface EMG data collected by the 5-pin sensor array were decomposed into their constituent MU action potential trains (De Luca et al. 2006; Chang et al. 2008; Nawab et al. 2010). The accuracy of the decomposed action potential trains was tested via the reconstruct-and-test procedure and only MUs that demonstrated ≥ 90% accuracy were included in the subsequent analyses (De Luca et al. 2006; Nawab et al. 2010).
A secondary validation of the accuracy of the MU firing times and action potential waveforms generated by the PDIII algorithm was performed via a spike triggered averaging (STA) protocol. Briefly, the PDIII algorithm-derived firing times were used as triggers for averaging the four raw EMG signals recorded from the surface sensor array (Hu et al. 2013b, a, c; McManus et al. 2015; Miller et al. 2019; Herda et al. 2019). Similar to Hu et al. (2013a), a window length of 4 s was shifted over the surface EMG signal using a 1 s step size. In addition to meeting the 90% accuracy criteria of the reconstruct-and-test procedure, the following criteria were required for a MU’s inclusion in the subsequent analyses: 1) a high correlation (r > 0.70) between the PDIII algorithm and STA-derived action potential waveforms and 2) a low coefficient of variation (< 0.30) of the STA-derived peak-to-peak action potential amplitudes across the contraction time (Hu et al. 2013b). It is possible to observe seemingly valid MU action potential waveforms from trigger events that do not correspond with MU discharges (Farina et al. 2014). To examine this possibility, we added Gaussian noise to the discharge times derived from the PDIII algorithm (Hu et al. 2013a; Thompson et al. 2018). The Gaussian noise added to the firing times was set at 1% of the standard deviation of the interspike interval for each MU (Hu et al. 2013a; Thompson et al. 2018). Correlations were performed on the MU action potential waveforms created from the raw EMG signals with a small amount of noise (< 2 ms shift in firing times) added to the firing times with the action potential waveforms that were triggered with the PDIII algorithm-derived firing times. In addition, the peak-to-peak amplitudes of the STA action potential waveforms were compared to the action potential waveforms derived from the firing times with the addition of Gaussian noise. A small amount of noise added to the firing times should reduce the correlation between action potential waveforms derived from the PDIII algorithm and the STA procedures. In addition, the peak-to-peak amplitudes derived from the firing times with Gaussian noise should decrease if no true action potential waveforms are consistently present (Fig. 1).
The individual action potential trains were analyzed to yield three parameters per MU: recruitment threshold (RT), mean firing rate (MFR) during steady torque and MU action potential amplitude (MUAPAMP). The RT was calculated as the average torque during the 0.01 s epoch following the first firing of the MU and was expressed relative to the MVC torque. The MFR was calculated as the inverse of the average interspike interval during the first 8 s of the steady force region of the isometric trapezoidal contraction. The MUAPAMP was calculated as the average peak-to-peak amplitude of each of the four unique action potential waveform templates derived from the PDIII algorithm and STA in accordance with previously reported methods (Hu et al. 2013b; Pope et al. 2016; Sterczala et al. 2018).
Ultrasound imaging
To measure muscle CSA and subcutaneous fat thickness, transverse ultrasound images of the right vastus lateralis were collected using a NextGen LOGIQ c ultrasound console (GE Healthcare UK, Ltd., Chalfont, Buckinghamshire, UK) with a multi-frequency linear array transducer (Model 12L-RS; 5–13 MHz; 38.4 mm field-of-view). Images were collected using brightness mode imaging with the equipment settings (depth: 6.0 cm; gain: 49 dB; frequency: 10 MHz) constant across all participants. Prior to imaging, participants rested supine for 10 min to allow fluid shifts to occur. After the rest period, transverse, panoramic images were collected at 50% of the distance from the anterior superior iliac spine to the superior patella. During image capture, a custom-made probe support composed of high-density foam padding was positioned perpendicular to the longitudinal axis of the thigh to ensure ultrasound probe movement in the transverse plane. Generous amounts of ultrasound gel were used to ensure minimal pressure on the skin. CSA and fat thickness were calculated using ImageJ software (National Institutes of Health, Bethesda, Maryland, USA). Images were scaled from pixels to centimeters using the straight-line function. To calculate CSA, the periphery of the vastus lateralis was outlined using the polygon function with care taken to exclude the surrounding fascia. Fat thickness was calculated as the straight-line distance from the bottom of the cutaneous layer to the top of the muscle fascia.
Statistical analysis
MU variables (MFR, RT, MUAPAMP) from a single contraction were analyzed for each participant individually; data were neither pooled across multiple contractions nor multiple participants within a group. To examine the MFR–RT relationships, MFRs were linearly regressed against RT for each individual to yield a slope and y-intercept value for statistical analysis (Fig. 2). Additionally, a single average MFR was calculated for each individual at each time point for comparison with previous investigations (Del Vecchio et al. 2019; Kamen and Knight 2004; Pucci et al. 2006; Rich and Cafarelli 2000; Vila-Cha et al. 2010). MUAPAMP was also linearly regressed against RT for each contraction to yield a slope and y-intercept value. Previously, different relationships between MUAPAMP and RT have been reported for MUs of the vastus lateralis recruited before and after 30% MVC (Pope et al. 2016). Thus, a single relationship calculated for all MUs can result in negative y-intercept values, as observed in the present investigation (39 of 48 relationships). As limited MUs were observed below 20%, separate MUAPAMP–RT relationships were not calculated for MUs recruited between 0 and 30% MVC. Instead, to determine if the MUAPAMPS of the lower-threshold MUs recorded during the 70% MVC were altered by resistance training, we used the slope and y-intercept values of the individual MUAPAMP–RT relationships to calculate the estimated APAMP of a MU with a RT of 30% MVC for each individual at PRE and POST (Fig. 2). The RT of 30% MVC was based on the average lowest RT observed across contractions and the point at which the MUAPAMP–RT relationship is reported to change (Pope et al. 2016). The slopes of the relationships and estimated MUAPAMPS at 30% MVC were used for statistical analysis. In addition, an exponential model was applied to each individual’s MFR–MUAPAMP relationship to yield a B term and A term for statistical analysis (Fig. 2). In the MFR–MUAPAMP model, MFR = AeB(MUAPAMP), where the A term is the MFR scale factor, e is the natural constant, and the B term represents the rate of decay of MFR with increments in MUAPAMP.
a The normalized torque and individual motor unit (MU) firing instances during an isometric trapezoidal muscle action performed at 70% maximal voluntary contraction (%MVC). The lower-threshold MU (MU4) fires more frequently than the higher-threshold MU (MU18) throughout the contraction. b The four unique MU action potential waveforms of the highlighted MUs used to calculate MU action potential amplitude (MUAPAMP). c The MUAPAMP vs. recruitment threshold, mean firing rate vs. recruitment threshold and mean firing rate vs. MUAPAMP relationships for the individual
Eleven separate two-way mixed factorial repeated-measures ANOVAs [group (resistance trained vs. control) × time (PRE vs. POST)] were used to analyze possible differences in maximal peak torque, CSA, subcutaneous fat thickness, slopes and y-intercepts of the MFR–RT relationships, average MFRs, slopes of the MUAPAMP–RT relationships, average MUAPAMPS, estimated MUAPAMPS at 30% MVC, and the B and A terms of the MFR–MUAPAMP relationships. When appropriate, follow-up analyses were performed using paired and independent samples t tests with Bonferroni corrections. The correlations between the PDIII algorithm and STA action potential waveforms, with and without Gaussian noise, were collapsed across time and group, and analyzed using a dependent samples t test. In addition, dependent samples t tests were used to analyze differences in MUAPAMPS between the STA and STA with Gaussian noise-derived waveforms to further demonstrate deterioration of the action potentials with small errors introduced to the PDIII firing times. Finally, various correlations were performed on data of interest. Alpha was set at p ≤ 0.05. When significant interactions were observed, effect sizes were estimated using Cohen’s d. Cohen’s d effect size estimates were classified as trivial (0–0.2), small (0.2–0.5), moderate (0.5–0.8) or large (> 0.8). All statistical analyses were performed using SPSS version 25 (IBM Corp., Armonk, NY).
Results
Strength and muscle hypertrophy
Two-way mixed methods ANOVAs indicated group x time interactions for maximal isometric torque (p = 0.006) and muscle CSA (p = 0.001). The resistance training group demonstrated a large average maximal torque increase of 17.2% (PRE: 204.6 ± 34.9 Nm; POST: 239.8 ± 36.3 Nm; p < 0.001; d = 0.9), whereas a small, non-significant increase in strength was observed in the control group (PRE: 182.4 ± 42.0 Nm; POST: 190.9 ± 40.1 Nm; p = 0.079; d = 0.2). As a result, resistance training group was significantly stronger than the control group post-intervention (p = 0.006; d = 1.1), despite there being no significant maximal torque difference pre-intervention (p = 0.183; d = 0.6). Of note, the torque needed to match the steady segment of the 70% MVC increased from 143.3 ± 24.4 Nm to 167.9 ± 25.4 Nm (p = < 0.001; d = 0.9) for the resistance trained group. Similarly, a large muscle CSA increase was observed (~ 18.7%) following resistance training (PRE: 28.7 ± 4.7 cm2; POST: 34.0 ± 5.0 cm2; p < 0.001; d = 1.0), while a small, non-significant CSA increase was observed in the control participants (PRE: 30.0 ± 6.5 cm2; POST: 30.9 ± 5.1 cm2; p = 0.453; d = 0.2). There were, however, no differences in CSA between groups post-resistance training (p = 0.163; d = 0.7).
Motor unit decomposition
Surface EMG signal decomposition yielded a total of 865 MUs that met the 90% accuracy criteria of the reconstruct-and-test procedure. The secondary validation of the MU firing times and AP waveforms via STA resulted in the exclusion of 40 additional MUs. Therefore, a total of 825 MUs were used for analyses. Similar average MU counts were observed at both time points in the resistance training (PRE: 16.3 ± 3.7; POST: 16.8 ± 6.0) and control (PRE: 18.4 ± 4.4; POST: 18.5 ± 5.9) groups. Additionally, similar average RT ranges were observed at both times points, in both groups (TR: PRE 25.1–61.9%, POST: 30.2–62.6%; CON: PRE: 22.4–60.7%, POST: 25.6–59.0%). Significant MFR–RT (r = − 0.912 ± 0.069), MUAPAMP–RT (r = 0.818 ± 0.086), and MFR–MUAPAMP (r = 0.841 ± 0.067) relationships were observed for each contraction.
Numerous precautions were taken to ensure the validity of resistance training-related changes in MUAPAMPS and the MUAPAMP–RT relationships. Adipose tissue is thought to act as a filter on EMG amplitude and, thus, potentially arbitrarily change MUAPAMPS. There were no changes in subcutaneous fat thickness pre- and post-intervention or differences observed between groups (two-way interaction: p = 0.814; time: p = 0.359; group: p = 0.716). The lack of change in subcutaneous fat thickness in both the resistance training (PRE: 0.46 ± 0.24 cm; POST: 0.43 ± 0.21 cm) and control groups (PRE: 0.42 ± 0.24 cm; POST: 0.40 ± 0.23 cm) ensured that adipose tissue was not a confounding factor in the interpretation of MUAPAMPS.
To further validate the MUAP waveform, we constructed MUAP waveforms using the previously described STA methodology (Hu et al. 2013b, a, c) and analyzed the variation in MUAPAMPS for each MU throughout the contraction. For each individual MU, a separate average MUAPAMP coefficient of variation was calculated. Of the retained MUs, the average coefficient of variation of the MUAPAMPS collapsed across group and time was 0.113 ± 0.022%, indicating consistent MUAPAMP values. The correlations between the MUAP waveforms derived from the PDIII algorithm and STA procedure collapsed across group and time was 0.877 ± 0.027, indicating a high level of similarity between the action potential waveforms derived from the two methodologies. When MU firing times were slightly shifted by the addition of Gaussian noise, the STA action potential waveforms deteriorated, decreasing peak-to-peak amplitudes over 97% (p < 0.001; STA: 0.137 ± 0.066 mV; STA with Gaussian noise: 0.004 ± 0.005 mV) and reducing the average correlation with PDIII algorithm waveforms (p < 0.001) from 0.88 ± 0.03 to 0.77 ± 0.04.
Finally, slopes were calculated for the STA procedure-derived MUAPAMPS relative to RT and correlated with those of the PDIII algorithm. A high correlation was observed between the slopes of the PDIII- and STA-derived MUAPAMP–RT relationships at pre- (r = 0.858; p < 0.001) and post-intervention (r = 0.912; p < 0.001). Furthermore, the changes in the slopes from pre- to post-intervention from the two different methods were also highly correlated in both the resistance training (r = 0.870; p < 0.001) and control groups (r = 0.855; p = 0.007). As the slopes between the two methods were highly correlated, only the slopes of the MUAPAMP–RT relationships derived from the PDIII algorithm are reported.
Mean firing rates
For the slopes and y-intercepts from the MFR–RT relationships, there were no significant two-way interactions (p = 0.838, p = 0.949) or main effects for time (p = 0.576, p = 0.346) or group (p = 0.568, p = 0.458) (Fig. 3). Furthermore, resistance training did not alter the average MFR of the observed MUs (two-way interaction: p = 0.864; time: p = 0.619; group: p = 0.869) in either the resistance training or control groups (Fig. 3). The average interspike intervals at steady force (70% MVC) were 72 ± 8 ms, 70 ± 5 ms, 72 ± 11 ms, and 71 ± 7 ms for the resistance trained and controls pre- and post-intervention, respectively.
The slopes of the mean firing rate vs. recruitment threshold (expressed as percent of maximal voluntary contraction [%MVC]) relationship (a), y-intercepts (b), composite patterns for the resistance training and control groups (c) and average mean firing rates (d). Means (SDs) are displayed as bars, with individual values displayed as circles (resistance training) and triangles (control). The composite patterns are depicted for contractions performed pre-treatment (PRE) and post-treatment (POST) at 70% of the respective MVCs
MUAPAMPS
There were significant two-way interactions (p = 0.027, p = 0.025) for the slopes of the MUAPAMP–RT relationships and average MUAPAMPS. Moderate increases in the slopes of the MUAPAMP–RT relationships (p = 0.008; d = 0.6) and average MUAPAMPS (p = 0.002; d = 0.5) were observed following resistance training with trivial changes pre- to post-intervention for the control group (p = 0.483; d < 0.1, p = 0.816; d < 0.1) (Fig. 4). In addition, the resistance training group demonstrated significantly greater slopes than the control group post-intervention (p = 0.028; d = 0.9) despite there being no significant differences pre-intervention (p = 0.320; d = 0.4). There was a 32% increase in average MUAPAMP for the resistance training group, whereas there was no change for the control group. Although no significant differences in the average MUAPAMPS were observed between groups pre-intervention (p = 0.263; d = 0.5), the resistance training group demonstrated larger MUAPAMPS than the control group post-intervention (p = 0.045, d = 0.9).
The slopes of the motor unit action potential amplitude (MUAPAMP) vs. recruitment threshold (expressed as percent of maximal voluntary contraction [%MVC]) relationships (a), estimated MUAPAMPS for MUs recruited at 30% MVC (b), composite MUAPAMP vs. recruitment threshold patterns for the resistance training and control groups (c), and average MUAPAMPs (d). Means (SDs) are displayed as bars, with individual values displayed as circles (resistance training) and triangles (control). The composite patterns are depicted for contractions performed pre-treatment (PRE) and post-treatment (POST) at 70% of the respective MVCs. *Indicates that for the resistance training group, POST was greater than PRE. †Indicates that the resistance training group was greater than the control group post-treatment
Unlike the slopes, there was no significant two-way interaction (p = 0.802) or main effects for time (p = 0.995) or groups (p = 0.665) for MUAPAMPS recruited at 30% MVC. Therefore, the average MUAPAMP for a MU recruited at 30% MVC did not change as a function of resistance training (Fig. 4).
MFR–MUAPAMP relationship
There was a significant two-way interaction (p = 0.034) for the B terms from the MFR–MUAPAMP relationships. There was a moderate (p = 0.003; d = 0.7) attenuation of the rate of decay in MFR with increases in MUAPAMP following resistance training (Fig. 5). In contrast, there were trivial, non-significant changes in the B terms for the control group pre- to post-intervention (p = 0.878, d < 0.1). There were no differences between groups at pre-intervention (p = 0.376; d = 0.4), however, the resistance training group had significantly (p = 0.017; d = 1.0) lower B terms in comparison to the control group at post-intervention. There was no significant two-way interaction (p = 0.351) or main effects for time (p = 0.999) and group (p = 0.398) for the A terms from the MFR–MUAPAMP relationships.
The B terms of the firing rate vs. motor unit action potential amplitude (MUAPAMP) relationships (a), A terms (b) and composite patterns for the resistance training and control groups (c). Means (SDs) are displayed as bars, with individual values displayed as circles (resistance training) and triangles (control). The composite patterns are depicted for contractions performed pre-treatment (PRE) and post-treatment (POST) at 70% of the respective MVCs. *Indicates that for the resistance training group, POST was greater than PRE. †Indicates that the resistance training group was greater than the control group post-treatment
Discussion
The 8-week, lower-body resistance training program elicited significant increases in knee extensor strength and vastus lateralis CSA. The ~ 18% CSA increase agrees with previous resistance training studies in untrained participants reporting 10–17% vastus lateralis CSA hypertrophy (Damas et al. 2018; Pope et al. 2016; Walker et al. 2013). The increased slopes of the MUAPAMP–RT indirectly demonstrate likely hypertrophy of the muscle fibers that comprise the higher-threshold MUs but not the lower-threshold MUs as evident with no changes in APAMPS of MUs recruited at 30% MVC. The MFR–RT relationships were unaltered by training and, thus, observed changes in the MFR–MUAPAMP relationships were primarily the result of changes in MU sizes. Thus, increases in isometric knee extension muscular strength that were necessary to sustain the higher torques at the post-resistance training 70% MVCs were primarily the result of MU hypertrophy and not increase in firing rates following 8 weeks of lower-body resistance training.
Conflicting reports of no change (Beck et al. 2011; Stock and Thompson 2014; Kamen and Knight 2004; Rich and Cafarelli 2000; Pucci et al. 2006) and increases in firing rates (Del Vecchio et al. 2019; Vila-Cha et al. 2010) following short-term resistance training interventions may be partially explained by methodological differences in the analysis of firing rates. As a result, we examined potential firing rate adaptations via the comparison of the slopes and y-intercepts of the MFR–RT relationships calculated for each participant and firing rates pooled across recruitment thresholds. Regardless of the analysis method, the results indicate that resistance training did not alter firing rates at 70% MVC. The lack of changes in the slopes or y-intercepts of the MFR-RT relationships agrees with both Beck et al. (2011) and Stock et al. (2014), both of which similarly measured firing rate activity in the vastus lateralis. Additionally, the lack of change in firing rates pooled across recruitment thresholds is in accordance with several previous investigations (Kamen and Knight 2004; Pucci et al. 2006; Rich and Cafarelli 2000), but in disagreement with two reports of firing rate increases following resistance training (Del Vecchio et al. 2019; Vila-Cha et al. 2010).
The firing rate increase reported by Vila Cha et al. (2010) may be the result of sampling bias, as the low MU yield per contraction (~ 4 MUs/contraction; as low as 1 MU) forced researchers to average the MU firing rates collapsed across participants. Given that resistance training may increase the observation of faster firing, lower-threshold MUs (Van Cutsem et al. 1998), the reported firing rate increase may be due to an increase in the number of lower-threshold MUs observed post-resistance training rather than changes in the properties of the motoneuron. More recently, Del Vecchio et al (2019) reported that tracking MUs from pre- to post-resistance training on the basis of action potential waveforms indicated increases in firing rates post-resistance training. Again, these findings were based on firing rates of MUs pooled across six different contractions at three different contraction intensities. This approach is particularly concerning, given that changes to force demands will alter the neural drive to the muscle and, thus, the firing rate of a MU (De Luca and Erim 1994; De Luca and Hostage 2010; Farina et al. 2014). Consequently, the same MU will discharge at different rates for each of the contract intensities, yet will be pooled together during the statistical procedures. In addition, at the reported average firing rate of ~ 23 pps, a MU would fire approximately 230 times during the 10 s steady force segment, however, the researchers calculated the average firing rate solely on the first ten firings of each MU (less than 5% of total MU discharges). Importantly, in the present study there was no observed change in firing rates pooled across recruitment thresholds, which is in agreement with the lack of changes in the MFR–RT relationships, and five other previous investigations reporting no change in firing rates during submaximal contractions following resistance training (Beck et al. 2011; Kamen and Knight 2004; Pucci et al. 2006; Rich and Cafarelli 2000; Stock and Thompson 2014).
Of note, Van Cutsem et al. (1998) also reported an increase in tibialis anterior firing rates following resistance training, however, the recordings were made during the onset of ballistic contractions, whereas the previously discussed studies measured firing rates during isometric contractions with controlled rates of torque/force development. Furthermore, firing rates were calculated using only the first three interspike intervals (four MU firings), which is more representative of the firing rates at recruitment than during a sustained contraction at a given force. Lastly, increases in maximal MU firing rates have been reported (Kamen and Knight 2004; Patten et al. 2001), although in both investigations the increases occurred between two pre-intervention testing visits with no further increases in firing rates following resistance training.
The peak-to-peak amplitude of the MU action potential waveform has been correlated with the motoneuron size (Olson et al. 1968) and the size of its component muscle fibers (Hakansson 1956), thus providing an indirect measure of MU size. When MUAPAMPS are expressed relative to RT, the slopes of the relationships indicate MU sizes across the torque/force spectrum and are sensitive to increases in MU sizes of the vastus lateralis following resistance training (Pope et al. 2016). Similar to the methods used to analyze firing rates, potential changes in MUAPAMPS were examined by comparison of the slopes of the MUAPAMP–RT relationships and the MUAPAMPS pooled across recruitment thresholds. Regardless of statistical method, the results indicated that MUAPAMPS were significantly larger following resistance training. The increase in the slopes of the MUAPAMP–RT relationships with no changes in the MUAPAMPS of the lowest recorded (lowest-threshold) MUs (RT = 30% MVC) suggests negligible hypertrophy in the smaller, lower-threshold MUs observed in the present study, but progressively greater MU hypertrophy with increments in RT (Fig. 4). Similar to Pope et al. (2016), the increase in APAMPS was isolated to MUs with RTs > 30% MVC. Unlike Pope et al. (2016), the absolute increases in CSAs were not significantly correlated with the absolute increases in the slopes from the MUAPAMP–RT relationships. The lack of correlation in the present investigation is likely the result of differences in the observed RT ranges and the contraction intensity (70% MVC). Specifically, Pope et al. (2016) observed larger RT ranges (PRE: 13.7–64.2%; POST: 20.1–67.6%) during a 90% MVC. Nonetheless, the increase in the slopes of the MUAPAMP–RT relationships, without changes in the MUAPAMPS of the lowest-threshold MUs, suggests that the observed increase in muscle size (~ 18.7% CSA increase) was due to hypertrophy of the higher-threshold MUs.
The difference in hypertrophy between lower- and higher-threshold MUs may be related to the muscle fiber type characteristics. Muscle fibers co-express type I and type II fiber characteristics resulting in a continuum of twitch force properties (Enoka and Duchateau 2015). However, lower-threshold MUs commonly exhibit twitch force properties associated with type I muscle fibers, whereas higher-threshold MUs exhibit twitch force properties associated with type II muscle fibers (Bottinelli et al. 1991; Burke et al. 1971; Eddinger and Moss 1987). Therefore, the greater hypertrophy observed in the higher-threshold MUs was likely the result of hypertrophy of muscle fibers that primarily express type II characteristics, in accordance with previous reports of greater hypertrophy in type II muscle fibers than type I muscle fibers (Damas et al. 2018; Snijders et al. 2016). In addition, researchers have correlated the slopes of the MUAPAMP–RT relationships with the myosin heavy chain composition of the muscle (Colquhoun et al. 2018; Trevino et al. 2018). Trevino et al. (2018) observed significant correlations between myosin heavy chain compositions and the slopes of the MUAPAMP–RT relationship of the vastus lateralis (Trevino et al. 2018). Similarly, Colquhoun et al. (2018) recently reported a non-significant positive relationship between type II myosin heavy chain areas and the slopes of the MUAPAMP-RT relationships in resistance trained males. However, it is unclear the role that myosin heavy chain isoforms and/or muscle fiber typing may have on muscular strength and power (Herda et al. 2019). Nonetheless, these findings provide further support that the greater slopes post-resistance training, in the present investigation, were related to hypertrophy of higher-threshold MUs comprised of muscle fibers primarily expressing type II characteristics.
The MFR–MUAPAMP relationship expresses firing rates relative to the sizes of the MU rather than RTs. In the present study, no changes were observed in the scale factor (A terms), however, the attenuated rates of decay (B terms) indicate an altered relationship between MU firing rate and size. Therefore, the associated firing rate was greater post-resistance training for a given MU action potential amplitude. Although the effect was minimal in the smaller MUs, the difference in firing rates between pre- and post-resistance training increased along the MU action potential amplitude spectrum. As a result, the biggest differences in firing rates were present for the largest recorded MUs (Fig. 5). The altered firing rates in relation to MU action potential amplitudes were likely not the result of adaptations in the firing properties of the motoneuron, as there were no changes in the MFR–RT relationships or firing rates pooled across recruitment thresholds following resistance training. Furthermore, firing rates are sensitive to changes in neural drive (Farina et al. 2014). Therefore, the similar range of observed MFRs at steady torque following resistance training (PRE: 9.4 ± 1.2 to 22.4 ± 2.5 pps; POST: 9.8 ± 1.5 to 23.1 ± 2.1 pps) tentatively suggests that neural drive was similar at the relative 70% MVCs despite the absolute torque output being different. Instead, the MFR–MUAPAMP relationships were altered as a function of MU hypertrophy (Fig. 6) as indicated by greater slopes of the MUAPAMP–RT relationships and the increased range of observed MUAPAMPS (PRE: 0.100 ± 0.056 to 0.357 ± 0.190 mV; POST: 0.132 ± 0.061 to 0.491 ± 0.270 mV). For the present study, the maintenance of pre-resistance training firing rates in combination with greater MU sizes (due to muscle fiber hypertrophy) suggests that each higher-threshold MU could generate more force, thus sustaining the higher absolute torque post-resistance training with similar neural drive.
Left graph: Lower- and higher-threshold motor units (MUs) recruited at similar relative torques (expressed as percentage of maximal voluntary contraction [%MVC]) pre- (dark circles) and post-resistance training (gray circles) overlaid on the linear segment of the isometric trapezoid template of the PRE and POST contractions for one participant. The MU firing rates (vertical lines) were similar pre- and post-resistance training. Right graphs: the MU action potential amplitude (MUAPAMP) was larger post-resistance training (dashed line) than pre-resistance training (solid line) for the higher-threshold MU, unlike for the lower-threshold MU
In the present investigation, specific MUs were not tracked longitudinally, as recently reported (Del Vecchio et al. 2019), however, researchers noted that the tracked and non-tracked MUs demonstrated similar changes in firing rates. Therefore, the MU tracking procedure does not appear to provide significant additional insight regarding adaptations to firing rates following resistance training. Furthermore, strong positive relationships were observed between MUAPAMP and RT in accordance with Henneman’s size principle (Henneman and Olson 1965) and in agreement with previous research (Hu et al. 2013b; Pope et al. 2016; Sterczala et al. 2017, 2018). Strong negative relationships were observed between firing rate and RT in agreement with previous research (De Luca and Hostage 2010; Person and Kudina 1972; Watanabe et al. 2016; Hu et al. 2013b). Of note, the negative MFR-RT relationship has been observed using the surface sensor array/PDIII algorithm (De Luca and Hostage 2010; Hu et al. 2013b), high-density surface EMG grid/CKC decomposition (Watanabe et al. 2016) and intramuscular needle electrodes (Person and Kudina 1972). The orderly MU recruitment indicated by these strong relationships suggests that, within a given contraction, MUs with similar RTs will demonstrate comparable characteristics. As a result, analyzing MU adaptations via the relationship-based approach does not require specific MU tracking.
MU size was measured indirectly via the MUAPAMPS derived from the decomposition of surface EMG signals. Given that several factors can influence the magnitude of MUAPAMPS measured via surface EMG, this investigation employed several safeguards to ensure the validity of the MUAPAMPS. Neither the control nor the resistance training group demonstrated a significant change in subcutaneous fat thickness. In addition to the reconstruct-and-test validation procedure, MU waveforms were further validated via a previously reported STA (Hu et al. 2013b, a, c; McManus et al. 2015; Miller et al. 2019) method that reconstructs the action potential waveforms from the raw EMG signals via the firing times derived from the PDIII algorithm. Importantly, only MUs which demonstrated little variance in STA-derived action potential waveforms across the contraction and a strong correlation between the STA and PDIII algorithm-derived action potential waveforms were included in our analyses. Furthermore, to demonstrate the validity of the PDIII firing times, we reanalyzed the correlation between the PDIII and STA action potential waveforms and observed significantly weaker relationships and non-existent action potential amplitudes after introducing minor Gaussian noise (~ 2 ms) in the firing times, in agreement with previous investigations (Hu et al. 2013a; Thompson et al. 2018). Had the PDIII-derived firing times been erroneous, the introduction of additional error would have had little impact on the correlation and action potential amplitudes. Since the small error introduced by the Gaussian noise significantly altered the STA calculated MU action potential waveforms and, thus, the correlation with the PDIII waveforms, our data suggest that the PDIII algorithm successfully and correctly identified the firing instances of the individual MUs. In further support, the interspike intervals in the present study closely match the findings of Martinez-Valdes et al. (2016) who examined the same muscle and contraction intensity with a different decomposition method.
The action potential waveforms derived from both the PDIII algorithm and STA were also used to construct MUAPAMP–RT relationships, with high correlations observed between the slopes at pre-intervention, post-intervention, and changes in slopes from pre- to post-intervention. Thus, we are confident that greater slopes of the MUAPAMP–RT relationships observed post-resistance training were due to hypertrophy of higher-threshold MUs.
Lastly, this investigation measured adaptations of MUs of the vastus lateralis in response to high-intensity resistance training. Previous reports of increases in MU firing rates were reported in the tibialis anterior, which possesses different muscle fiber type distribution and architecture, in response to a mix of high velocity ballistic and isometric contractions. Evidence of the exercise training modality’s influence on MU adaptations is provided by Dimmick et al. (2018), who observed significantly greater MUAPAMPS in lower-threshold MUs (< 25% MVC) in highly endurance-trained (runners) females in comparison to sedentary female controls. The authors proposed that the differences in firing rates between the chronic distance runners versus sedentary females may have been the result of altered recruitment patterns that arbitrarily changed the firing rate scheme of the muscle at a lower-intensity torque output. In the present study, the high-intensity resistance training primarily stressed the higher-threshold MUs, whereas the lower-intensity distance running would not activate the higher-threshold MUs and instead applied chronic stress to the lower-threshold MUs. Nonetheless, changes in firing rates of a large limb lower-body muscle were not observed after 8 weeks of resistance training, but the effects of long-term resistance training (> 1 year) on firing rates are largely unknown.
In conclusion, 8 weeks of high-intensity, lower-body resistance training induced significant increases in muscular strength and CSA. In accordance with the increase in muscle CSA, the increases in MUAPAMP suggest that the short-term, high-intensity resistance training program increased the muscle fiber diameters of the higher-threshold MUs. In the resistance training group, the greater absolute torques produced during the post-resistance training 70% MVCs were not achieved via increased firing rates, but likely due to increased force production of the hypertrophied higher-threshold MUs. Thus, this study demonstrates that in vastus lateralis, strength increases observed after 8 weeks of resistance training were due to MU hypertrophy and not adaptations to firing rates.
Abbreviations
- ANOVA:
-
Analysis of variance
- CSA:
-
Cross-sectional area
- EMG:
-
Electromyography
- MFR:
-
Mean firing rate
- MU:
-
Motor unit
- MUAPAMP :
-
Motor unit action potential amplitude
- MVC:
-
Maximal voluntary contraction
- PDIII:
-
Precision decomposition III
- RT:
-
Recruitment threshold
- STA:
-
Spike-triggered averaging
References
Balshaw TG, Massey GJ, Maden-Wilkinson TM, Morales-Artacho AJ, McKeown A, Appleby CL, Folland JP (2017) Changes in agonist neural drive, hypertrophy and pre-training strength all contribute to the individual strength gains after resistance training. Eur J Appl Physiol 117(4):631–640. https://doi.org/10.1007/s00421-017-3560-x
Beck TW, J.M. D, M.S. S (2011) The effects of a resistance training program on average motor unit firing rates. Clin Kinesiol (65):1–8
Bottinelli R, Schiaffino S, Reggiani C (1991) Force-velocity relations and myosin heavy chain isoform compositions of skinned fibres from rat skeletal muscle. J Physiol 437:655–672
Burke RE, Levine DN, Zajac FE 3rd (1971) Mammalian motor units: physiological-histochemical correlation in three types in cat gastrocnemius. Science 174(4010):709–712
Carolan B, Cafarelli E (1992) Adaptations in coactivation after isometric resistance training. J Appl Physiol 73(3):911–917. https://doi.org/10.1152/jappl.1992.73.3.911
Chang SS, De Luca CJ, Nawab S (2008) Aliasing rejection in precision decomposition of EMG signals. Conf Proc IEEE Eng Med Biol Soc 2008:4972–4975. https://doi.org/10.1109/IEMBS.2008.4650330
Colquhoun RJ, Magrini MA, Haun CT, Muddle TWD, Tomko PM, Luera MJ, Mackey CS, Vann CG, Martin JS, Young KC, DeFreitas JM, Roberts MD, Jenkins NDM (2018) Muscle phenotype is related to motor unit behavior of the vastus lateralis during maximal isometric contractions. Physiol Rep 6(5). https://doi.org/10.14814/phy2.13636
Contessa P, De Luca CJ, Kline JC (2016) The compensatory interaction between motor unit firing behavior and muscle force during fatigue. J Neurophysiol 116(4):1579–1585. https://doi.org/10.1152/jn.00347.2016
Damas F, Libardi CA, Ugrinowitsch C, Vechin FC, Lixandrao ME, Snijders T, Nederveen JP, Bacurau AV, Brum P, Tricoli V, Roschel H, Parise G, Phillips SM (2018) Early- and later-phases satellite cell responses and myonuclear content with resistance training in young men. PLoS ONE 13(1):e0191039. https://doi.org/10.1371/journal.pone.0191039
De Luca CJ, Erim Z (1994) Common drive of motor units in regulation of muscle force. Trends Neurosci 17(7):299–305
De Luca CJ, Hostage EC (2010) Relationship between firing rate and recruitment threshold of motoneurons in voluntary isometric contractions. J Neurophysiol 104(2):1034–1046. https://doi.org/10.1152/jn.01018.2009
De Luca CJ, Adam A, Wotiz R, Gilmore LD, Nawab SH (2006) Decomposition of surface EMG signals. J Neurophysiol 96(3):1646–1657. https://doi.org/10.1152/jn.00009.2006
Del Vecchio A, Casolo A, Negro F, Scorcelletti M, Bazzucchi I, Enoka R, Felici F, Farina D (2019) The increase in muscle force after 4 weeks of strength training is mediated by adaptations in motor unit recruitment and rate coding. J Physiol. https://doi.org/10.1113/JP277250
Dimmick HL, Miller JD, Sterczala AJ, Trevino MA, Herda TJ (2018) Vastus lateralis muscle tissue composition and motor unit properties in chronically endurance-trained vs. sedentary women. Eur J Appl Physiol. https://doi.orrg/10.1007/s00421-018-3909-9
Eddinger TJ, Moss RL (1987) Mechanical properties of skinned single fibers of identified types from rat diaphragm. Am J Physiol 253(2 Pt 1):C210–218
Enoka RM, Duchateau J (2015) Inappropriate interpretation of surface EMG signals and muscle fiber characteristics impedes understanding of the control of neuromuscular function. J Appl Physiol 119(12):1516–1518. https://doi.org/10.1152/japplphysiol.00280.2015
Erskine RM, Fletcher G, Folland JP (2014) The contribution of muscle hypertrophy to strength changes following resistance training. Eur J Appl Physiol 114(6):1239–1249. https://doi.org/10.1007/s00421-014-2855-4
Farina D, Holobar A, Merletti R, Enoka RM (2010) Decoding the neural drive to muscles from the surface electromyogram. Clin Neurophysiol 121(10):1616–1623. https://doi.org/10.1016/j.clinph.2009.10.040
Farina D, Merletti R, Enoka RM (2014) The extraction of neural strategies from the surface EMG: an update. J Appl Physiol 117(11):1215–1230. https://doi.org/10.1152/japplphysiol.00162.2014
Gabriel DA, Kamen G, Frost G (2006) Neural adaptations to resistive exercise: mechanisms and recommendations for training practices. Sports Med 36(2):133–149
Hakansson CH (1956) Conduction velocity and amplitude of the action potential as related to circumference in the isolated fibre of frog muscle. Acta Physiol Scand 37(1):14–34. https://doi.org/10.1111/j.1748-1716.1956.tb01338.x
Helms ER, Cronin J, Storey A, Zourdos MC (2016) Application of the repetitions in reserve-based rating of perceived exertion scale for resistance training. Strength Cond J 38(4):42–49. https://doi.org/10.1519/SSC.0000000000000218
Henneman E, Olson CB (1965) Relations between structure and function in the design of skeletal muscles. J Neurophysiol 28:581–598
Herda TJ, Trevino MA, Sterczala AJ, Miller JD, Wray ME, Dimmick HL, Gallagher PM, Fry AC (2019) Muscular strength and power are correlated with motor unit action potential amplitudes, but not myosin heavy chain isoforms in sedentary males and females. J Biomech 86:251–255. https://doi.org/10.1016/j.jbiomech.2019.01.050
Hu X, Rymer WZ, Suresh NL (2013a) Assessment of validity of a high-yield surface electromyogram decomposition. J Neuroeng Rehabil 10:99. https://doi.org/10.1186/1743-0003-10-99
Hu X, Rymer WZ, Suresh NL (2013b) Motor unit pool organization examined via spike-triggered averaging of the surface electromyogram. J Neurophysiol 110(5):1205–1220. https://doi.org/10.1152/jn.00301.2012
Hu X, Rymer WZ, Suresh NL (2013c) Reliability of spike triggered averaging of the surface electromyogram for motor unit action potential estimation. Muscle Nerve 48(4):557–570. https://doi.org/10.1002/mus.23819
Jenkins NDM, Miramonti AA, Hill EC, Smith CM, Cochrane-Snyman KC, Housh TJ, Cramer JT (2017) Greater neural adaptations following high- vs low-load resistance training. Front Physiol 8:331. https://doi.org/10.3389/fphys.2017.00331
Kamen G, Knight CA (2004) Training-related adaptations in motor unit discharge rate in young and older adults. J Gerontol A Biol Sci Med Sci 59(12):1334–1338
Knight CA, Kamen G (2001) Adaptations in muscular activation of the knee extensor muscles with strength training in young and older adults. J Electromyogr Kinesiol 11(6):405–412
Martinez-Valdes E, Laine CM, Falla D, Mayer F, Farina D (2016) High-density surface electromyography provides reliable estimates of motor unit behavior. Clin Neurophysiol 127(6):2534–2541. https://doi.org/10.1016/j.clinph.2015.10.065
McManus L, Hu X, Rymer WZ, Lowery MM, Suresh NL (2015) Changes in motor unit behavior following isometric fatigue of the first dorsal interosseous muscle. J Neurophysiol 113(9):3186–3196. https://doi.org/10.1152/jn.00146.2015
Miller JD, Sterczala AJ, Trevino MA, Wray ME, Dimmick HL, Herda TJ (2019) Motor unit action potential amplitudes and firing rates during repetitive muscle actions of the first dorsal interosseous in children and adults. Eur J Appl Physiol. https://doi.org/10.1007/s00421-019-04090-0
Moritani T, deVries HA (1979) Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med 58(3):115–130
Nawab SH, Chang SS, De Luca CJ (2010) High-yield decomposition of surface EMG signals. Clin Neurophysiol 121(10):1602–1615. https://doi.org/10.1016/j.clinph.2009.11.092
Olson CB, Carpenter DO, Henneman E (1968) Orderly recruitment of muscle action potentials. Arch Neurol 19(6):591–597
Patten C, Kamen G, Rowland DM (2001) Adaptations in maximal motor unit discharge rate to strength training in young and older adults. Muscle Nerve 24(4):542–550
Person RS, Kudina LP (1972) Discharge frequency and discharge pattern of human motor units during voluntary contraction of muscle. Electroencephalogr Clin Neurophysiol 32(5):471–483
Pope ZK, Hester GM, Benik FM, DeFreitas JM (2016) Action potential amplitude as a noninvasive indicator of motor unit-specific hypertrophy. J Neurophysiol 115(5):2608–2614. https://doi.org/10.1152/jn.00039.2016
Pucci AR, Griffin L, Cafarelli E (2006) Maximal motor unit firing rates during isometric resistance training in men. Exp Physiol 91(1):171–178. https://doi.org/10.1113/expphysiol.2005.032094
Rich C, Cafarelli E (2000) Submaximal motor unit firing rates after 8 wk of isometric resistance training. Med Sci Sports Exerc 32(1):190–196
Snijders T, Smeets JS, van Kranenburg J, Kies AK, van Loon LJ, Verdijk LB (2016) Changes in myonuclear domain size do not precede muscle hypertrophy during prolonged resistance-type exercise training. Acta Physiol (Oxf) 216(2):231–239. https://doi.org/10.1111/apha.12609
Sterczala AJ, Herda TJ, Miller JD, Ciccone AB, Trevino MA (2017) Age-related differences in the motor unit action potential size in relation to recruitment threshold. Clin Physiol Funct Imaging. https://doi.org/10.1111/cpf.12453
Sterczala AJ, Miller JD, Trevino MA, Dimmick HL, Herda TJ (2018) Differences in the motor unit firing rates and amplitudes in relation to recruitment thresholds during submaximal contractions of the first dorsal interosseous between chronically resistance trained and physically active men. Appl Physiol Nutr Metab. https://doi.org/10.1139/apnm-2017-0646
Stock MS, Thompson BJ (2014) Effects of barbell deadlift training on submaximal motor unit firing rates for the vastus lateralis and rectus femoris. PLoS ONE 9(12):e115567. https://doi.org/10.1371/journal.pone.0115567
Thompson CK, Negro F, Johnson MD, Holmes MR, McPherson LM, Powers RK, Farina D, Heckman CJ (2018) Robust and accurate decoding of motoneuron behaviour and prediction of the resulting force output. J Physiol 596(14):2643–2659. https://doi.org/10.1113/JP276153
Trevino MA, Herda TJ, Fry AC, Gallagher PM, Vardiman JP, Mosier EM, Miller JD (2016) The influence of myosin heavy chain isoform content on mechanical behavior of the vastus lateralis in vivo. J Electromyogr Kinesiol 28:143–151. https://doi.org/10.1016/j.jelekin.2016.04.005
Trevino MA, Sterczala AJ, Miller JD, Wray ME, Dimmick HL, Ciccone AB, Weir JP, Gallagher PM, Fry AC, Herda TJ (2018) Sex-related differences in muscle size explained by amplitudes of higher-threshold motor unit action potentials and muscle fibre typing. Acta Physiol (Oxf):e13151. https://doi.org/10.1111/apha.13151
Van Cutsem M, Duchateau J, Hainaut K (1998) Changes in single motor unit behaviour contribute to the increase in contraction speed after dynamic training in humans. J Physiol 513(Pt 1):295–305
Vila-Cha C, Falla D, Farina D (2010) Motor unit behavior during submaximal contractions following six weeks of either endurance or strength training. J Appl Physiol 109(5):1455–1466. https://doi.org/10.1152/japplphysiol.01213.2009
Walker S, Hulmi JJ, Wernbom M, Nyman K, Kraemer WJ, Ahtiainen JP, Hakkinen K (2013) Variable resistance training promotes greater fatigue resistance but not hypertrophy versus constant resistance training. Eur J Appl Physiol 113(9):2233–2244. https://doi.org/10.1007/s00421-013-2653-4
Watanabe K, Holobar A, Kouzaki M, Ogawa M, Akima H, Moritani T (2016) Age-related changes in motor unit firing pattern of vastus lateralis muscle during low-moderate contraction. Age (Dordr) 38(3):48. https://doi.org/10.1007/s11357-016-9915-0
Author information
Authors and Affiliations
Contributions
AJS and TJH conceived and designed research. AJS, JDM, HLD, MEW and MAT acquired and analyzed data. AJS and TJH drafted the manuscript. AJS, JDM, HLD, MEW, MAT and TJH reviewed and revised the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Additional information
Communicated by William J. Kraemer .
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sterczala, A.J., Miller, J.D., Dimmick, H.L. et al. Eight weeks of resistance training increases strength, muscle cross-sectional area and motor unit size, but does not alter firing rates in the vastus lateralis. Eur J Appl Physiol 120, 281–294 (2020). https://doi.org/10.1007/s00421-019-04273-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-019-04273-9