Abstract
Conflicting findings have been reported regarding muscle damage with low-intensity resistance exercise with blood flow restriction (BFR) by pressure cuffs. This study investigated muscle function and muscle fibre morphology after a single bout of low-intensity resistance exercise with and without BFR. Twelve physically active subjects performed unilateral knee extensions at 30% of their one repetition maximum (1RM), with partial BFR on one leg and the other leg without occlusion. With the BFR leg, five sets were performed to concentric torque failure, and the free-flow leg repeated the exact same number of repetitions and sets. Biopsies were obtained from vastus lateralis before and 1, 24 and 48 h after exercise. Maximum isometric torque (MVC) and resting tension were measured before and after exercise and at 4, 24, 48, 72, 96 and 168 h post-exercise. The results demonstrated significant decrements in MVC (lasting ≥48 h) and delayed onset muscle soreness in both legs, and increased resting tension for the occluded leg both acutely and at 24 h post-exercise. The percentage of muscle fibres showing elevated intracellular staining of the plasma protein tetranectin, a marker for sarcolemmal permeability, was significantly increased from 9% before exercise to 27–38% at 1, 24 and 48 h post-exercise for the BFR leg. The changes in the free-flow leg were significant only at 24 h (19%). We conclude that an acute bout of low-load resistance exercise with BFR resulted in changes suggesting muscle damage, which may have implications both for safety aspects and for the training stimulus with BFR exercise.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last decade, the method of low-load strength training with blood-flow restriction (BFR, also often referred as “vascular occlusion” or “vascular restriction”) has attracted a lot of attention. Several studies have demonstrated that BFR by pressure cuffs in combination with low-intensity training (20–50% of 1RM) induces muscle growth at rates comparable to those seen with conventional strength training (Takarada et al. 2000b, 2002, 2004; Kubo et al. 2006). Notably, this mode of training has been shown to produce strength gains and hypertrophy not only in untrained individuals ranging from young to elderly (Takarada et al. 2000b, 2004; Kubo et al. 2006; Karabulut et al. 2010), but also in well-trained athletes (Takarada et al. 2002), as well as in patients recovering after anterior cruciate ligament surgery (Ohta et al. 2003). Taken together, these findings suggest that low-intensity strength training with cuff occlusion has a broad application potential (Wernbom et al. 2008).
In some studies, regimes with a remarkably high training frequency of two BFR training sessions per day for up to 6 days per week (i.e., a total of 12 training sessions per week) have been tried successfully over short periods (1–2 weeks), resulting in significant increases in muscle cross-sectional area (CSA) and strength despite the very short training periods (Abe et al. 2005; Fujita et al. 2008). Indirect measures of muscle damage such as creatine kinase (CK) and myoglobin did not increase appreciably in these and other studies (e.g., Takarada et al. 2000a), thus supporting that a fast recovery is possible.
In contrast, Wernbom et al. (2006, 2009) observed moderate to severe delayed onset muscle soreness (DOMS) in the quadriceps after acute bouts of low-intensity knee extensions with cuff occlusion, which were performed with multiple sets to concentric torque failure. Similarly, Umbel et al. (2009) observed mild DOMS after an acute bout of BFR training involving the knee extensors, as well as significant decrements (~10–15%) in maximum voluntary isometric torque and increases in vastus lateralis muscle CSA at 24–48 h post-exercise. Moreover, a recent case report revealed very high serum levels of CK (>12,000 U/L) and extreme muscle soreness in an individual 48–96 h after an acute bout of low-load BFR training (Iversen and Røstad 2010). CK values in excess of 10,000 U/L are considered diagnostic for rhabdomyolysis (Clarkson et al. 2006). Collectively, these observations indicate a potential for muscle damage with the first bout of BFR strength training even at low loads (20–30% of 1RM).
Prolonged anoxia causes calcium (Ca2+) influx and loss of cell membrane integrity both in myotube culture (Ørtenblad et al. 2003) and in muscle preparations (Fredsted et al. 2005). Interestingly, Fredsted et al. (2005, 2007) noted that these changes occurred much faster when the anoxia was combined with electrical stimulation. Furthermore, hypoxic exercise (12.7% O2) resulted in increased uptake of Evans Blue Dye (EBD) in rat skeletal muscle, suggesting compromised membrane integrity (Xu et al. 2008). EBD is known to bind to albumin (Straub et al. 1997; Hamer et al. 2002), hence any increase in intracellular EBD staining presumably reflects increased albumin uptake by the muscle. Albumin has been used as a marker for skeletal muscle damage caused by eccentric exercise (McNeil and Khakee 1992). Thus, not only high mechanical stress but also hypoxia, especially in combination with exercise, can result in damage to the muscle cell membrane.
A few studies on the acute effects of training with cuff occlusion have been published (e.g., Cook et al. 2007; Yasuda et al. 2008, 2009; Umbel et al. 2009); but except for the study of Umbel et al. (2009), muscle function was studied for a limited time period, from pre-exercise to the first few minutes following the bout. Furthermore, no morphological data at the muscle fibre level following an acute BFR exercise bout has been reported in the literature to date. If a first-time bout can induce muscle damage, this would potentially have implications for the prescription and safety of BFR resistance exercise. This concern may be especially relevant to disused muscle. For example, unloading is known to markedly increase the vulnerability of human skeletal muscle to relatively light eccentric exercise (Ploutz-Snyder et al. 1996). Given that BFR resistance exercise may be used not only in training of healthy individuals but also in rehabilitation settings, it is important to establish whether or not low-load BFR resistance exercise can induce symptoms of muscle damage.
Therefore, the aim of the present investigation was to study neuromuscular fatigue and recovery as well as fibre morphology following an acute bout of low-load resistance exercise with and without BFR. Specifically, we hypothesised that resistance exercise with BFR would result in signs of muscle damage and that these would be greater than those induced by exercise at the same volume and load without BFR.
Materials and methods
Subjects
Eight male subjects (mean ± SD, 26 ± 3 year, height 180 ± 6 cm, body mass 80 ± 10 kg) and four female subjects (24 ± 2 year, height 171 ± 6 cm, body mass 66 ± 8 kg) were recruited from the student population at the Norwegian School of Sport Sciences. All subjects were exercising on a regular basis (e.g., running, cycling, cross-country skiing, alpine skiing, skating, trekking) and had previous experience with resistance training, but some were not performing heavy strength training at the time of the study while others were very well trained in this regard. The participants were instructed not to perform any strenuous activities involving their quadriceps during the last 72 h before the main test. They were informed of the experimental risks and signed an informed consent document prior to the investigation. The study complied with the standards set by the Declaration of Helsinki and was approved by the Regional Ethics Committee of Southern Norway.
Experimental design
To examine the acute effects of dynamic low-intensity strength training with BFR on muscle function in the quadriceps, a within-subjects study design was used in which one leg (dominant or non-dominant in a randomised fashion) was tested with cuff occlusion and the other leg was tested without occlusion. To match the volume of work, and because BFR reduces the dynamic endurance at low loads (Wernbom et al. 2006, 2009), the occluded leg was always exercised before the free-flow leg.
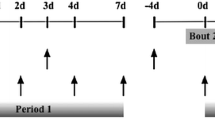
All subjects participated in a familiarisation session 1 week (range 4–11 days) before the main experiment. In the main trial, the subjects were tested for maximal voluntary isometric strength immediately before the exercise bout, at 1 and 2 min post-exercise, and at 4, 24, 48, 72, 96 and 168 h post-exercise (Fig. 1). Muscle contractility was also tested with neuromuscular electrical stimulation (NMES) of the vastus medialis at the same test occasions following isometric testing, except at 168 h post-exercise testing, where no NMES was performed.
Muscle function testing and familiarisation protocols
A week before the main exercise test, the subjects were tested for their unilateral 1RM strength for each leg in a variable resistance knee extension machine (Leg Extension model 66478, Gym2000, Geithus, Norway). The subjects warmed up 5 min on a stationary bicycle at a light load (~75 watts) and then moved to the knee extension apparatus. The 1RM was determined according to the procedures of Staron et al. (1990). Tagesson and Kvist (2007) demonstrated high inter and intra-rater intraclass correlation (ICC) values for the unilateral knee extension 1RM test (ICC = 0.90–0.96), supporting that this test is clinically reliable.
After 1RM for each leg had been established, the subjects practiced maximum voluntary isometric contractions (MVC) in the same knee-extension machine, with the lever arm of the machine fixed at an angle corresponding to 90° of knee flexion (full knee extension is here defined as 0°). Isometric force was measured by a strain gauge (HBM U2AC2, Darmstadt, Germany) connected to the lever arm of the knee extension machine, and the signal was amplified and recorded at 1,000 Hz using a 16-bit data acquisition card (MP150, BioPac Systems Inc., CA). The back rest was set at a hip joint angle of ~80° of flexion (fully extended hip here defined as 0°), and belts were secured across the subjects hips and shoulders to reduce any movements of the hips, and to minimise assistance from other muscle groups. The subjects were instructed to apply force as rapid and hard as possible and to maintain maximal effort for at least 4 s.
After the MVC practice, the subjects were familiarised with NMES. Two self-adhesive electrodes (5 × 10 cm, Polartrode, Medi-Stim, Oslo, Norway) were placed longitudinally on the vastus medialis and the positions of the electrodes were marked on the skin with indelible ink to ensure the same positioning in each trial. The stimulation protocol comprised two trains of stimuli with a duration of 200 ms each and with a frequency of 20 Hz, and two trains of stimuli of 200 ms duration and 50 Hz frequency. Each square-wave pulse lasted 0.5 ms, and the voltage was fixed at 120 V. One minute separated the last isometric MVC attempt and the NMES testing, and 5–10 s separated each NMES-induced contraction.
After resting for 5 min after the NMES testing, a load of 30% of 1RM was chosen and the subject was then familiarised with the partial vascular occlusion and the cadence of repetitions to be used during the main test. For each leg, one set of 15 coupled concentric-eccentric knee extensions was performed with cuff occlusion, followed by a second set of five repetitions after 45 s of rest, during which the partial occlusion was maintained.
Main exercise and testing protocols
At a subsequent session after the 1RM testing, with ~7 days between the sessions, the subjects reported to the laboratory for the main test. The subjects warmed up 5 min on a stationary bicycle at a light load (~75 watts) and then moved to the knee extension apparatus. After warming up with three submaximal isometric contractions with gradually increasing effort for each contraction, the subjects performed two MVCs, with 30 s of rest between them. The highest value of the two attempts was used as baseline MVC. After the MVC, the skin above the vastus medialis muscle was washed with isopropanol and the self-adhesive electrodes were applied for the NMES, which proceeded as described above. From the forces generated at 20 and 50 Hz, the ratio between 20 and 50 Hz (20/50 Hz ratio) was calculated and used as an index of low-frequency fatigue. While the ankle was strapped to the lever arm in conjunction with the NMES testing, passive force against the lever arm was also measured as an indirect index of passive muscle tension.
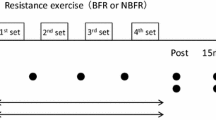
After 5 min of rest, the subjects were tested in the unilateral knee extension exercise at 30% of 1RM, during partial blood flow restriction (see further below). After inflation of the pressure cuff, the subjects performed as many repetitions as possible for a total of five sets for the occluded leg. The subjects remained seated in the machine during the rest periods, and the rest period between each set was 45 s for both the non-occluded and the occluded leg. The range of motion was between 100° and 20° of knee flexion (0° = full extension) and the cadence was 20 repetitions per minute (1.5 s each for the concentric and the eccentric muscle actions) which was controlled with a metronome set at 40 bpm. No rest was permitted between the repetitions, and the subjects were instructed to stop the eccentric phase just before the weight stack touched down to avoid relaxation of the quadriceps. The number of complete repetitions performed in each set was noted.
When the five sets for the occluded leg had been completed, and the muscle function tests (1 and 2 min post-exercise MVC, as well as the 20 and 50 Hz NMES at ~3 min post-exercise) had been performed, the pressure cuff was deflated (total time under partial occlusion: ~9 min). After 10 min rest, the subjects performed exactly the same number of sets and repetitions and muscle function testing for the other leg as with the occluded leg, but without the pressure cuff.
Blood flow restriction
A curved tourniquet cuff of 135 mm in width with a 100 mm wide pneumatic bag inside of it was connected to a surgical tourniquet system (Zimmer A.T.S. 2000, Zimmer Patient Care, Dover, OH) with automatic regulation of the pressure. The cuff was wrapped around the proximal part of the thigh and inflated to a pressure of 90–100 mmHg just before exercise with BFR, and the pressure was maintained throughout the session until both the exercise and the muscle function tests had been completed, after which the cuff was deflated.
Using the same type of cuff, we previously demonstrated that this level of pressure results in decreased muscle endurance at ~30% of 1RM (Wernbom et al. 2009), thus confirming that the degree of occlusion was sufficient to affect muscle performance. In addition, we determined in separate experiments with Doppler ultrasound that the pressure at which the femoral artery blood flow ceased (measured in the seated position) was in the range of 150–205 mmHg for the subjects. Generally, the male subjects required higher pressures (170–205 mmHg) than the female subjects (150–170 mmHg) for complete occlusion. Based on this information and our experiences regarding the tolerability of different pressures during exercise, it was decided on a cuff pressure of 100 mmHg for the male subjects and 90 mmHg for the female subjects in the exercise part of the study.
Notably, these training pressures are considerably lower than those that have typically been reported in many of the studies on BFR exercise, where a narrow cuff (33 mm in width) has often been used. With a 33 mm cuff and pressures of 160–180 mmHg, Takano et al. (2005) reported a ~70% reduction of the femoral blood flow at rest in the seated position. However, it is important to note that wider cuffs are generally more effective in occluding the blood flow than more narrow ones (Crenshaw et al. 1988; Graham et al. 1993). For example, Crenshaw et al. (1988) determined that with a 45 mm thigh cuff, the pressure needed for complete occlusion of blood flow in the lower limb (measured with the subjects in supine position) was on average ~280 mmHg, and in some cases more than 400 mmHg; while with a 120 mm cuff, the pressure needed for full occlusion was ~150 mmHg. Thus, we decided on a relatively wide cuff and a low pressure during training to minimise compressive and shear forces on soft tissues (Crenshaw et al. 1988; Graham et al. 1993).
Muscle soreness ratings
Muscle soreness was self-rated by the subjects before the exercise bout and at 4, 24, 48, 72 and 96 h post-exercise on a 100 mm visual analog scale (VAS), where 0 represented “no soreness” and 10 represented “extreme soreness” (Wernbom et al. 2006). Subjects were asked to place a mark on the line on the paper according to the general level of perceived soreness at the time of assessment. A blank scale was used each time to avoid bias from preceding ratings.
Muscle biopsy sampling
Biopsies were obtained from m. vastus lateralis in the free-flow leg before exercise and in both legs 1, 24 and 48 h after exercise (monitored for each leg). Hence, the number of biopsies was limited to three for the BFR leg and four in the free-flow leg. The rationale for this design was to minimise any impact of the procedure itself on the muscle samples (particularly those from the BFR leg), as multiple biopsies can inflict muscle damage and negatively influence recovery around the sampled area (Constantin-Teodosiu et al. 1996). During the biopsy, subjects laid supine, and the procedure was performed under local anesthesia (Xylocain® adrenaline, 10 mg ml−1 + 5 μg ml−1; AstraZeneca, Södertälje, Sweden).
The first muscle sample from each leg was taken from the vastus lateralis approximately midway between the origin and the insertion, from a location which was distal to the part that was compressed by the tourniquet during training (occluded leg). In the free-flow leg, the first incision was taken from the corresponding location and this incision was also used for the second biopsy (1 h post-exercise) but the needle was angled in a different direction so that the sample was taken about 5 cm from the first. The second (24 h) and third incisions (48 h) were placed approximately 3 and 6 cm proximally to the first incision. Care was taken to avoid affected tissue from earlier biopsies.
Biopsies were taken from 10 out of the 12 participants, i.e., 2 subjects completed the exercise protocol without any biopsies. Due to some technical difficulties with the biopsy sampling, complete sets of biopsies (including both limbs and all time points) were achieved in eight of the subjects, therefore N = 8 for tetranectin stainings.
Light microscopic immunofluorescence on cross sections
The muscle samples were rinsed in physiological saline and visible fat and connective tissue were removed. From one part of the biopsy, 8 μm-thick cross-sections were cut and later stained with antibodies against dystrophin’s COOH-terminus (anti-rabbit; 1:2,000; Dys 2, Novocastra Laboratories Ltd, Newcastle, UK) and tetranectin (anti-mouse; 1:300; Hyb-130-11, AntibodyShop, Gentofte, Denmark). Tetranectin is a plasma protein which like albumin and EBD is a good marker for muscle fibre damage resulting from eccentric exercise (Kalhovde et al. unpublished data). The primary antibodies were diluted in blocking solution [1% BSA, and 0.05% Tween-20 in TBS (TBST)] and the sections were incubated for 2 h at room temperature or overnight at 4°C. The sections were then incubated for 45–60 min with appropriate secondary antibodies (Alexa Fluor 488 and 594, Invitrogen, Carlsbad, CA, USA) and mounted with coverslips (Fluoromount-G, Chemi-Teknik AS, Oslo, Norway, or ProLong Gold Antifade Reagent with DAPI, Invitrogen-Molecular Probes, Eugene, OR). The muscle sections were washed in TBST between each step.
Images were captured using a digital camera (Olympus DP72, Olympus-Europa GmbH, Hamburg, Germany) connected to a light microscope (Olympus BX61) with appropriate filters. Pictures were taken with 4×, 10×, 20×, and 40× magnification objectives, where 4× was used for overview images and 10× was used for evaluations of the number of positive fibres. Intact fibres on each sample were counted, but the outermost layer of muscle fibres in the samples was excluded to avoid areas damaged by sectioning, as were other areas and fibres with visible artifacts (folds, tears, etc.). The mean number of counted fibres for each time point per individual was 238 (range 43–882). The program Axiovision (Zeiss, Oberkochen, Germany) was used to analyse the images and the degree of staining for tetranectin was determined with the measure mean green density (MGD). The stainings for the COOH-terminus were inspected to help identification of the borders of the muscle fibres.
Evaluation of tetranectin staining
McNeil and Khakee (1992) and Clarke et al. (1993) used threshold values for determining the number of albumin-positive fibres in the sections. A somewhat similar threshold-based model was adopted for the purposes of the present investigation. The contours of each counted fibre were outlined just beneath the sarcolemma, and the MGD value of the cytoplasm was measured with the Axiovision program. Pilot studies indicated that subjectively determined positive fibres had an intracellular staining (as indicated by MGD) at least 50% stronger than presumably negative fibres. This corresponded to about three standard deviations above the mean of negative fibres in a baseline sample. Therefore, this threshold was chosen for MGD.
Four presumably negative fibres from each section, two peripheral and two centrally located fibres, were chosen based on having the lowest MGD values of the peripheral and central fibres, respectively. Central fibres were within the middle third of the length and the middle third of the width of the picture, and peripheral fibres were consequently located outside these limits. The mean MGD of these four fibres was calculated and fibres with a staining ≥50% stronger than this mean were counted as tetranectin-positive. Values for positive fibres are given as percentage (%) of the total number of counted fibres.
Statistical analyses
To identify statistically significant changes in muscle function variables and tetranectin from baseline, one-way repeated-measures ANOVA with Dunnett’s post-hoc test was performed for both the BFR and the free-flow leg. A two-way ANOVA (with Bonferroni’s post hoc test) was used to assess differences between the legs. Unless otherwise indicated, data are presented as mean ± SE. The level of significance for all statistical analyses was p < 0.05.
Results
Exercise protocols and muscle function testing
The number of repetitions performed before concentric torque failure gradually decreased from 28 ± 5 in the first set, to 10 ± 2 in the second set and to 6 ± 1 in the fifth set (mean ± SD). MVC was reduced more in the occluded leg than in the non-occluded leg when measured 1 and 2 min after exercise (−62 ± 15% vs. −22 ± 4%, and −61 ± 14% vs. −21 ± 4%, p < 0.01, mean ± SD) when partial BFR was still maintained, but not at later time points (Fig. 2). At 4 h post-exercise, MVC was reduced by 15 ± 4% in the occluded leg and 12 ± 3% in the non-occluded leg. At 72 h post-exercise, the MVC was no longer significantly different from baseline, although a trend was noted in the BFR leg (10 ± 4% reduced MVC, p < 0.10).
The 20/50 Hz ratio decreased in the occluded leg at 3 min post-exercise, but was no longer significantly lowered at 4 h post. In the non-occluded leg, no significant changes were noted at any time point. Only at 3 min post-exercise the difference between the conditions was significant (p < 0.01).
The passive tension increased for the occluded leg (8.5 ± 2.3%, p < 0.01) and also for the free-flow leg (5.3 ± 2.3%, p < 0.05) at 3 min post-exercise (Fig. 3). Thereafter, passive tension declined gradually in both legs with the last significant time point for the occluded leg at 24 h post. At no time point the difference between the conditions was significant.
Muscle soreness was increased at 24–72 h after exercise in both legs (p < 0.01 for all three time points in both legs; Fig. 4), and was not significantly different between the conditions, but when a t test was used to compare the highest values irrespective of the time points, peak DOMS was greater for the BFR leg (p < 0.05).
Tetranectin immunofluorescence on cross-sections
The percentage of muscle fibres showing elevated tetranectin staining increased from 9% before exercise to 31% at 1 h and 38% at 24 h (p < 0.01) and 27% at 48 h post-exercise (p < 0.05) in the occluded leg (Fig. 5). For the non-occluded leg, tetranectin-positive fibres were significantly increased only at 24 h post-exercise (18%, p < 0.05). At 24 h, the percentage of muscle fibres with elevated tetranectin staining was significantly greater in the occluded leg (p < 0.05). Control markings with a secondary antibody only showed very faint staining and no changes with exercise. Figure 6 shows examples of the tetranectin stainings.
a–d. Examples of tetranectin stainings from the same subject, before and 1 and 48 h after BFR-exercise, and control staining with secondary antibody only. Note the increased number of diffusely stained fibres, as well as some granular fibres, particularly at 48 h post-exercise in this example (a pre, b 1 h, c 48 h, d secondary antibody only)
To gain insight into whether one or both of the major fibre types (type I and II) showed increased tetranectin, a subsample of muscle sections was stained with SC-71, which marks type IIa myosin and also to a lesser extent type IIx (Smerdu and Soukup 2008). They were then compared with neighbouring sections stained with tetranectin. These comparisons indicated that the majority of the tetranectin-positive muscle fibres were type I fibres (Fig. 7).
a–d. Examples of tetranectin stainings versus fibre types from two subjects, 1 h postexercise. In both these cases, two sections were stained with SC-71 (type II fibres) to identify the tetranectin-positive fibres, but only one section is shown here. Note that type I fibres show a generally greater response than type II fibres in these examples (a and c tetranectin staining, b and d SC-71 staining)
Discussion
There were three main findings of the present study: firstly, there were markedly larger acute reductions (at 1 and 2 min post-exercise) in MVC in the occluded leg compared to the free-flow leg. Secondly, part of the decline in MVC was longer-lasting (at least 48 h), especially in the occluded leg. Thirdly, an increased occurence of fibres with elevated tetranectin was observed after exercise in both legs, especially in the occluded leg.
Acute force decrements
A large part of the acute fatigue after exercise in the BFR leg was probably of a metabolic origin, as the isometric force improved vastly between the release of the pressure cuff and the 4 h post-exercise MVC measurements. Low-load coupled concentric-eccentric knee extensions to exhaustion with complete cuff occlusion have been demonstrated to lead to depletion of phosphocreatine (PCr) levels in muscle fibres from the vastus lateralis (Ingemann-Hansen et al. 1981). Similarly, Krustrup et al. (2009) reported that when submaximal concentric knee extensor exercise was combined with complete restriction of blood flow, both type I and type II fibres were depleted of PCr. With PCr breakdown, there is an increase in inorganic phosphate (Pi). Because Pi is an important factor in muscle fatigue (Allen et al. 2008), accumulation of Pi is likely contributing to the decline in MVC as well as the slow recovery observed while the cuff was still inflated.
When the blood flow is totally occluded, almost no recovery of PCr levels and force production capability takes place (Harris et al. 1976; Colliander et al. 1988; Meyer et al. 2008). Meyer et al. (2008) further reported that partial occlusion (~120 mmHg, cuff width not stated) in combination with exercise resulted in a slower recovery of PCr compared to free-flow conditions. Similarly, the partial occlusion in the current experiment impacted on the recovery, as demonstrated by the lack of increase in MVC between 1 and 2 min post-exercise. In contrast, as little as 1 min of free circulation results in a marked recovery of MVC after BFR exercise (Yasuda et al. 2008, 2009).
Although reduced pH alone has little effect on force and velocity (Allen et al. 2008), data from Karatzaferi et al. (2008) suggests that low pH has additive effects to that of Pi on the velocity of shortening and the isometric force of single muscle fibres at close to physiological temperatures. As a bout of low-load occlusion training results in large increases in intramuscular lactate (Ingemann-Hansen et al. 1981) and lowered pH in plasma (Fujita et al. 2007), we suggest that accumulation of phosphate ions and hydrogen ions are at least in part responsible for the acute fatigue in occlusion training. However, we cannot rule out other causes, such as inhibitory effects of low pH on the central nervous system (Kent-Braun 1999), and effects of cuff occlusion on the excitability of nerve and/or muscle cells.
Prolonged force decrements and increased resting tension
Regarding the longer-lasting part of the force decrements, we believe that this reflects some degree of muscle damage, and the prolonged increases in tetranectin staining and resting tension lend support to this view. However, the decrease in MVC in the present study is smaller than what has been reported after acute bouts of severe high-force eccentric exercise (e.g., Hellsten et al. 1997; Brown et al. 1997; Paschalis et al. 2005; Raastad et al. 2010).
The BFR exercise protocol was pre-dominantly concentric, and submaximal eccentric muscle actions are known to induce less damage and decline in muscle function than maximal eccentric muscle actions (Paschalis et al. 2005). Further explanations include (1) the comparatively low volume of BFR exercise, (2) that the duration of ischemia may not have been long enough to induce major damage, (3) that the subjects were physically active and in some cases strength trained, and (4) that they were familiarised with BFR exercise before the main experiment.
Nevertheless, at least one “high-responder” could be identified in the present study based on MVC decrements of 46–49% at 48–72 h post-exercise. We have previously observed a few high-responders to an acute bout of BFR resistance exercise with multiple sets to failure, as judged from their reports of severe DOMS and long-lasting (1–2 weeks) decreases in muscle function, and in one case also suspected myoglobinuria (Wernbom, unpublished observations).
Elevations in tetranectin staining
The molecular weight of tetranectin in humans was reported to be approximately 68 kDa (Clemmensen et al. 1986), making it slightly heavier than albumin (67 kDa). New data from our laboratory (Kalhovde et al. unpublished data) suggests that severe eccentric exercise causes markedly increased intracellular tetranectin staining. Interestingly, strong granular-like staining appeared at the same locations as myofibrillar disruptions, suggesting that this pattern may be a good indication of myofibrillar damage and subsequent remodelling.
In the current study, fibres with a strong granular-like staining were also observed in some samples, suggesting that low-load BFR resistance training can induce myofibrillar disruptions. Nevertheless, in other samples, such staining patterns were almost absent despite visibly elevated intracellular tetranectin fluorescence in the muscle fibres. Thus, the dominant pattern of tetranectin staining was a general and diffuse increase in the cytoplasm.
The cytoplasmic presence of tetranectin suggests an increased sarcolemmal permeability. We base this on the following arguments: first, tetranectin does not normally accumulate in any appreciable amounts in adult human muscle tissue (Albrechtsen and Wever unpublished, cited in Xu et al. 2001). Second, not only the early but also the later increases in immunostaining seem too rapid to be accounted for by any elevations in de novo synthesis. Wewer et al (1998) found no staining in injured myofibres from mice until at 3 days post-injury. Third, in several of the tetranectin-positive fibres, accumulation was visible not only in the sarcoplasma but even more so at and just beneath the sarcolemma (Figs. 6, 8). Finally, in situ hybridization suggests that tetranectin is not produced intracellularly in human skeletal muscle after exercise-induced damage (Kalhovde et al. unpublished data).
a–c. Examples of tetranectin and dystrophin stainings at ×40, from the same subject as in Fig. 7 a, b 1 h after BFR-exercise. Note the tetranectin staining at the membrane in some of the positive fibres, resulting in yellow/orange colours in the merged picture. Note also that some of the type II fibres (refer to Fig. 7 a, b) have weak tetranectin staining at the membrane and very fine granular staining in the cytoplasm (a dystrophin, b tetranectin, c merged)
The increase in staining intensity in tetranectin-positive fibres was typically in the range of 50–80%, suggesting mild damage; but some fibres exceeded 100% (and even 200–500% for strongly granular fibres). Similarly, variations in the staining of albumin, EBD and Procion Orange are evident in the literature; e.g., in the papers of McNeil and Khakee (1992), Petrof et al. (1993), Zhu et al. (2000), Xu et al. (2008), Ojima et al. (2010). The elevated tetranectin staining at the later time points is consistent with observations on EBD, which can be detected in muscle fibres for several days after exercise-induced damage (Xu et al. 2008).
Possible causes of muscle damage with BFR resistance exercise
We postulate that the combination of exercise and ischemic conditions in the working muscle may be a major cause of damage with BFR resistance exercise. Importantly, muscle activation during anoxia shortens the time required for increased Ca2+ influx to take place (Fredsted et al. 2005). A possible mediator of sarcolemma damage is phospholipase A2 (PLA2) (Duncan and Jackson 1987). Isoforms of PLA2 in skeletal muscle are activated by several stimuli, including ischemia, exercise, stretch and cell swelling (Ørtenblad et al. 2003; Burkholder 2009). Activation of PLA2 results in the production of arachidonic acid (AA) and metabolites downstream of AA such as prostaglandins and leukotrienes, and in lysophopholipids such as lysophosphatidylcholine (LPC) (Ørtenblad et al. 2003; Burkholder 2009). Both the AA and the LPC arms of the PLA2 pathway may cause increased production of reactive oxygen species and taurine loss from myofibres (Ørtenblad et al. 2003).
Interestingly, Umbel et al. (2009) reported DOMS, muscle swelling and decreased muscle torque after a bout of pure concentric knee extensions with BFR (3 sets to failure at 35% of MVC). This suggests that ischemia, like anoxia, increases the susceptibility of the working muscle so that normally not damaging exercise may become damaging. Another factor may be the eccentric muscle actions that are inherent in most BFR dynamic strength training regimes (see Wernbom et al. 2009, for further discussion on this point).
The tetranectin uptake appeared to be most pronounced in type I fibres (Fig. 7). We speculate that being recruited already from the start, the type I fibres may have subjected to longer durations of stress than the type II fibres. The data on MVC, DOMS and tetranectin indicate that some damage occurred also in the free-flow leg, which is consistent with studies on fatiguing resistance exercise at 40–50% of 1RM (Volek et al. 2002; Pullinen et al. 2010). We estimate that during the first two or three sets with the free-flow leg, our subjects had only 3–5 repetitions left before failure, based on their subjective reports and our previous data.
Relevance to previous BFR resistance exercise studies
In this study, all five BFR exercise sets were performed to concentric torque failure, whereas in some of the high-frequency training studies (e.g., Abe et al. 2005; Fujita et al. 2008), it is unclear whether subjects had performed all-out in any set during training. For example, using an identical protocol to that of Fujita et al. (2007, 2008), Fry et al. (2010) noted that at the end of the BFR exercise bout, their subjects reported values of 7–8 on a 0–10 scale of perceived exertion, indicating a high but still submaximal level of effort. This is an important point, as the electromyography (EMG) levels are at their highest in the last few repetitions in each set (Wernbom et al. 2009), which may coincide with the lowest oxygenation and energy levels.
With regard to increased membrane permeability, it should be noted that serum CK is a less sensitive marker than myoglobin (Virtanen et al. 1993), possibly due to its higher molecular weight (88 vs. 18 kDa). Indeed, serum myoglobin, but not CK, increased 5–6 fold at 30–180 min after a strenuous squat protocol (5 sets of 15–20 repetitions at 50% of 1RM) known to cause exercise-induced hypoxia (Volek et al. 2002). However, delayed elevations (48–96 h) of myoglobin and CK were observed after acute bouts of fatiguing resistance exercise at 40–50% of 1RM (Volek et al. 2002; Pullinen et al. 2010).
It is thus possible that previous studies on BFR resistance exercise may have missed both early and delayed elevations of serum markers, and/or that the markers employed have not been sufficiently sensitive. Alternatively, the protocols were not severe enough to induce marked increases in membrane permeability. Future studies should investigate multiple markers of muscle damage over an extended time-course after acute bouts of BFR exercise.
Limitations
Because we used threshold values as a basis for the evaluation of the stainings, we may have missed more subtle changes in membrane permeability, especially in the type II fibres. Furthermore, we obtained samples only from the vastus lateralis, and the biopsies were taken from areas distal to the tourniquet. Thus, we cannot exclude that greater damage may have occurred in the other muscle bellies, and/or in the muscle tissues beneath the pressure cuff.
Perspectives
Temporary elevations in sarcolemmal permeability are not necessarily only associated with damage; mild to moderate changes could also contribute to mechanotransduction processes (Clarke et al. 1993; Grembowicz et al. 1999; Miyake and McNeil, 2003), and thus potentially provide insights into the mechanisms of muscle hypertrophy with BFR exercise. Of note, a BFR resistance exercise protocol with multiple sets of knee extensions to failure at 20% of 1RM, with wide cuffs and pressures similar to those used in the present study, was recently shown to induce muscle fibre hypertrophy and strength gains when performed very frequently for 1–2 weeks (Nielsen et al. 2011; Frandsen et al. 2011).
Nevertheless, the potential for damage to local tissues, as well as the still relatively unexplored systemic responses of BFR resistance exercise (possibly including both beneficial and detrimental effects), need to be addressed further. For example, repeated bouts of high venous pressure may constitute one factor underlying the development of varicosities and valvular insufficiencies in superficial veins (Kölegård and Eiken 2011). The risk of muscle damage caused by compression and ischemia is further underscored by the recent findings of Kacin and Strazar (2011) of attenuation of muscle hypertrophy in the quadriceps and even muscle atrophy of the vastus intermedius at the levels beneath the cuff after short-term low-load resistance exercise with BFR at high occlusion pressures (>230 mmHg).
Conclusion
Based on the prolonged torque decrements and the indications of increased membrane leakiness, there is a potential for muscle damage with a first-time bout of BFR resistance training with multiple sets of all-out efforts. We therefore recommend that this mode of exercise should be introduced carefully, with a low volume and/or effort initially.
References
Abe T, Yasuda T, Midorikawa T, Sato Y, Kearns CF, Inoue K, Koizumi K, Ishii N (2005) Skeletal muscle size and circulating IGF-1 are increased after two weeks of twice daily kaatsu resistance training. Int J Kaatsu Training Res 1:7–14
Allen DG, Lamb GD, Westerblad H (2008) Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88:287–332
Brown SJ, Child RB, Day SH, Donnelly AE (1997) Exercise-induced skeletal muscle damage and adaptation following repeated bouts of eccentric muscle contractions. J Sports Sci 15:215–222
Burkholder TJ (2009) Stretch-induced ERK2 phosphorylation requires PLA2 activity in skeletal myotubes. Biochem Biophys Res Commun 386:60–64
Clarke MS, Khakee R, McNeil PL (1993) Loss of cytoplasmic basic fibroblast growth factor from physiologically wounded myofibers of normal and dystrophic muscle. J Cell Sci 106:121–133
Clarkson PM, Kearns AK, Rouzier P, Rubin R, Thompson PD (2006) Serum creatine kinase levels and renal function measures in exertional muscle damage. Med Sci Sports Exerc 38:623–627
Clemmensen I, Petersen LC, Kluft C (1986) Purification and characterization of a novel, oligomeric, plasminogen kringle 4 binding protein from human plasma: tetranectin. Eur J Biochem 156:327–333
Colliander EB, Dudley GA, Tesch PA (1988) Skeletal muscle fiber type composition and performance during repeated bouts of maximal, concentric contractions. Eur J Appl Physiol Occup Physiol 58:81–86
Constantin-Teodosiu D, Casey A, Short AH, Hultman E, Greenhaff PL (1996) The effect of repeated muscle biopsy sampling on ATP and glycogen resynthesis following exercise in man. Eur J Appl Physiol Occup Physiol 73:186–190
Cook SB, Clark BC, Ploutz-Snyder LL (2007) Effects of exercise load and blood-flow restriction on skeletal muscle function. Med Sci Sports Exerc 39:1708–1713
Crenshaw AG, Hargens AR, Gershuni DH, Rydevik B (1988) Wide tourniquet cuffs more effective at lower inflation pressures. Acta Orthop Scand 59:447–451
Duncan CJ, Jackson MJ (1987) Different mechanisms mediate structural changes and intracellular enzyme efflux following damage to skeletal muscle. J Cell Sci 87:183–188
Frandsen U, Nielsen JL, Jensen L, Nygaard T, Bech RD, Wernbom M, Suetta C, Aagaard P. Short-term BFR resistance training increase skeletal muscle myofiber size without concomitant increase in capillary density. [abstract] Med Sci Sports Exerc 2011; 43 (5 Suppl): p751
Fredsted A, Mikkelsen UR, Gissel H, Clausen T (2005) Anoxia induces Ca2+ influx and loss of cell membrane integrity in rat extensor digitorum longus muscle. Exp Physiol 90:703–714
Fredsted A, Gissel H, Madsen K, Clausen T (2007) Causes of excitation-induced muscle cell damage in isometric contractions: mechanical stress or calcium overload? Am J Physiol Regul Integr Comp Physiol 292:R2249–R2258
Fry CS, Glynn EL, Drummond MJ, Timmerman KL, Fujita S, Abe T, Dhanani S, Volpi E, Rasmussen BB (2010) Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J Appl Physiol 108:1199–1209
Fujita S, Abe T, Drummond MJ, Cadenas JG, Dreyer HC, Sato Y, Volpi E, Rasmussen BB (2007) Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J Appl Physiol 103:903–910
Fujita T, Brechue WF, Kurita K, Sato Y, Abe T (2008) Increased muscle volume and strength following six days of low-intensity resistance training with restricted muscle blood flow. Int J Kaatsu Training Res 4:1–8
Graham B, Breault MJ, McEwen JA, Mcgraw RW (1993) Occlusion of arterial flow in the extremities at subsystolic pressures through the use of wide tourniquet cuffs. Clin Orthop 286:257–261
Grembowicz KP, Sprague D, McNeil PL (1999) Temporary disruption of the plasma membrane is required for c-fos expression in response to mechanical stress. Mol Biol Cell 10:1247–1257
Hamer PW, McGeachie JM, Davies MJ, Grounds MD (2002) Evans Blue Dye as an in vivo marker of myofibre damage: optimising parameters for detecting initial myofibre membrane permeability. J Anat 200:69–79
Harris RC, Edwards RH, Hultman E, Nordesjö LO, Nylind B, Sahlin K (1976) The time course of phosphorylcreatine resynthesis during recovery of the quadriceps muscle in man. Pflügers Arch 367:137–142
Hellsten Y, Frandsen U, Orthenblad N, Sjødin B, Richter EA (1997) Xanthine oxidase in human skeletal muscle following eccentric exercise: a role in inflammation. J Physiol 498:239–248
Ingemann-Hansen T, Halkjaer-Kristensen J, Halskov O (1981) Skeletal muscle phosphagen and lactate concentrations in ischaemic dynamic exercise. Eur J Appl Physiol Occup Physiol 46:261–270
Iversen E, Røstad V (2010) Low-load ischemic exercise-induced rhabdomyolysis. Clin J Sport Med 20:218–219
Kacin A, Strazar K (2011) Frequent low-load ischemic resistance exercise to failure enhances muscle oxygen delivery and endurance capacity. Scand J Med Sci Sports. doi:10.1111/j.1600-0838.2010.01260.x. [Epub ahead of print]
Karabulut M, Abe T, Sato Y, Bemben MG (2010) The effects of low-intensity resistance training with vascular restriction on leg muscle strength in older men. Eur J Appl Physiol 108:147–155
Karatzaferi C, Franks-Skiba K, Cooke R (2008) Inhibition of shortening velocity of skinned skeletal muscle fibers in conditions that mimic fatigue. Am J Physiol Regul Integr Comp Physiol 294:R948–R955
Kent-Braun JA (1999) Central and peripheral contributions to muscle fatigue in humans during sustained maximal effort. Eur J Appl Physiol 80:57–63
Kölegård R, Eiken O (2011) Distensibility in human veins as affected by 5 weeks of repeated elevations of local transmural pressure. Eur J Appl Physiol [Epub ahead of print]
Krustrup P, Söderlund K, Relu MU, Ferguson RA, Bangsbo J (2009) Heterogeneous recruitment of quadriceps muscle portions and fibre types during moderate intensity knee-extensor exercise: effect of thigh occlusion. Scand J Med Sci Sports 19:576–584
Kubo K, Komuro T, Ishiguro N, Sato Y, Ishii N, Kanehisa H, Fukunaga T (2006) Effects of low-load resistance training with vascular occlusion on the mechanical properties of muscle and tendon. J Appl Biomech 22:112–119
McNeil PL, Khakee R (1992) Disruptions of muscle fiber plasma membranes: role in exercise-induced damage. Am J Pathol 140:1097–1109
Meyer RA, Slade JM, Towse TF, Olive JL, Forbes SC (2008) Phosphocreatine resynthesis during recovery after exercise with blood flow occlusion. Med Sci Sports Exerc 40(5 Suppl):S349 [abstract]
Miyake K, McNeil PL (2003) Mechanical injury and repair of cells. Crit Care Med 31(8 Suppl):S496–S501
Nielsen JL, Aagaard P, Bech RD, Nygaard T, Wernbom M, Suetta C, Frandsen U (2011) Rapid increases in myogenic satellite cells expressing Pax-7 with blood flow restricted low-intensity resistance training. Med Sci Sports Exerc 43(5 Suppl):752
Ohta H, Kurosawa H, Ikeda H, Iwase Y, Satou N, Nakamura S (2003) Low-load resistance muscular training with moderate restriction of blood flow after anterior cruciate ligament reconstruction. Acta Orthop Scand 74:62–68
Ojima K, Kawabata Y, Nakao H, Nakao K, Doi N, Kitamura F, Ono Y, Hata S, Suzuki H, Kawahara H, Bogomolovas J, Witt C, Ottenheijm C, Labeit S, Granzier H, Toyama-Sorimachi N, Sorimachi M, Suzuki K, Maeda T, Abe K, Aiba A, Sorimachi H (2010) Dynamic distribution of muscle-specific calpain in mice has a key role in physical-stress adaptation and is impaired in muscular dystrophy. J Clin Invest 120:2672–2683
Ørtenblad N, Young JF, Oksbjerg N, Nielsen JH, Lambert IH (2003) Reactive oxygen species are important mediators of taurine release from skeletal muscle cells. Am J Physiol Cell Physiol 284:C1362–C1373
Paschalis V, Koutedakis Y, Jamurtas AZ, Mougios V, Baltzopoulos V (2005) Equal volumes of high and low intensity of eccentric exercise in relation to muscle damage and performance. J Strength Cond Res 19:184–188
Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL (1993) Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA 90:3710–3714
Ploutz-Snyder LL, Tesch PA, Hather BM, Dudley GA (1996) Vulnerability to dysfunction and muscle injury after unloading. Arch Phys Med Rehabil 77:773–777
Pullinen T, Mero A, Huttunen P, Pakarinen A, Komi PV (2010) Resistance exercise-induced hormonal response under the influence of delayed onset muscle soreness in men and boys. Scand J Med Sci Sports. doi:10.1111/j.1600-0838.2010.01238.x. [Epub ahead of print]
Raastad T, Owe SG, Paulsen G, Enns D, Overgaard K, Crameri R, Kiil S, Belcastro A, Bergersen L, Hallén J (2010) Changes in calpain activity, muscle structure, and function after eccentric exercise. Med Sci Sports Exerc 42:86–95
Smerdu V, Soukup T (2008) Demonstration of myosin heavy chain isoforms in rat and humans: the specificity of seven available monoclonal antibodies used in immunohistochemical and immunoblotting methods. Eur J Histochem 52:179–190
Staron RS, Malicky ES, Leonardi MJ, Falkel JE, Hagerman FC, Dudley GA (1990) Muscle hypertrophy and fast fiber type conversions in heavy resistance-trained women. Eur J Appl Physiol Occup Physiol 60:71–79
Straub V, Rafael JA, Chamberlain JS, Campbell KP (1997) Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. J Cell Biol 139:375–385
Tagesson SK, Kvist J (2007) Intra- and interrater reliability of the establishment of one repetition maximum on squat and seated knee extension. J Strength Cond Res 21:801–807
Takano H, Morita T, Iida H, Asada K, Kato M, Uno K, Hirose K, Matsumoto A, Takenaka K, Hirata Y, Eto F, Nagai R, Sato Y, Nakajima T (2005) Hemodynamic and hormonal responses to a short-term low-intensity resistance exercise with the reduction of muscle blood flow. Eur J Appl Physiol 95:65–73
Takarada Y, Nakamura Y, Aruga S, Onda T, Miyazaki S, Ishii N (2000a) Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. J Appl Physiol 88:61–65
Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, Ishii N (2000b) Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol 88:2097–2106
Takarada Y, Sato Y, Ishii N (2002) Effects of resistance exercise combined with vascular occlusion on muscle function in athletes. Eur J Appl Physiol 86:308–314
Takarada Y, Tsuruta T, Ishii N (2004) Cooperative effects of exercise and occlusive stimuli on muscular function in low-intensity resistance exercise with moderate vascular occlusion. Jpn J Physiol 54:585–592
Umbel JD, Hoffman RL, Dearth DJ, Chleboun GS, Manini TM, Clark BC (2009) Delayed-onset muscle soreness induced by low-load blood flow-restricted exercise. Eur J Appl Physiol 107:687–695
Virtanen P, Viitasalo JT, Vuori J, Väänänen K, Takala TE (1993) Effect of concentric exercise on serum muscle and collagen markers. J Appl Physiol 75:1272–1277
Volek JS, Kraemer WJ, Rubin MR, Gómez AL, Ratamess NA, Gaynor P (2002) L-Carnitine L-tartrate supplementation favorably affects markers of recovery from exercise stress. Am J Physiol Endocrinol Metab 282:E474–E482
Wernbom M, Augustsson J, Thomeé R (2006) Effects of vascular occlusion on muscular endurance in dynamic knee extension exercise at different submaximal loads. J Strength Cond Res 20:372–377
Wernbom M, Augustsson J, Raastad T (2008) Ischemic strength training: a low-load alternative to heavy resistance exercise? Scand J Med Sci Sports 18:401–416
Wernbom M, Järrebring R, Andreasson MA, Augustsson J (2009) Acute effects of blood flow restriction on muscle activity and endurance during fatiguing dynamic knee extensions at low load. J Strength Cond Res 23:2389–2395
Wewer UM, Iba K, Durkin ME, Nielsen FC, Loechel F, Gilpin BJ, Kuang W, Engvall E, Albrechtsen R (1998) Tetranectin is a novel marker for myogenesis during embryonic development, muscle regeneration, and muscle cell differentiation in vitro. Dev Biol 200:247–259
Xu X, Gilpin B, Iba K, Maier A, Engvall E, Albrechtsen R, Wewer UM (2001) Tetranectin in slow intra- and extrafusal chicken muscle fibers. J Muscle Res Cell Motil 22:121–132
Xu YM, Li JP, Fang WB, Wang RY (2008) Effects of hypoxic uphill exercise on changes in sarcolemma integrity and dystrophin content in rat skeletal muscle. J Exerc Sci Fit 6(2):97–105
Yasuda T, Brechue WF, Fujita T, Sato Y, Abe T (2008) Muscle activation during low-intensity muscle contractions with varying levels of external limb compression. J Sports Sci Med 7:467–474
Yasuda T, Brechue WF, Fujita T, Shirakawa J, Sato Y, Abe T (2009) Muscle activation during low-intensity muscle contractions with restricted blood flow. J Sports Sci 27:479–489
Zhu E, Comtois AS, Fang L, Comtois NR, Grassino AE (2000) Influence of tension time on muscle fiber sarcolemmal injury in rat diaphragm. J Appl Physiol 88:135–141
Acknowledgments
The authors thank the dedicated group of subjects for their time and effort. We also thank Dr Satu Koskinen and Dr Eva Runesson for helpful comments and suggestions. Sincere apologies to scientific colleagues whose work was not cited in this paper due to space constraints. This project was in part supported by a grant from the Swedish National Centre for Research in Sports (Grant: CIF 125/05). Preliminary results for muscle function were presented at the 6th International Conference of Strength Training, Colorado Springs, Colorado, October 30–November 2 2008.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by William J. Kraemer.
Rights and permissions
About this article
Cite this article
Wernbom, M., Paulsen, G., Nilsen, T.S. et al. Contractile function and sarcolemmal permeability after acute low-load resistance exercise with blood flow restriction. Eur J Appl Physiol 112, 2051–2063 (2012). https://doi.org/10.1007/s00421-011-2172-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-011-2172-0