Abstract
Purpose
Blood-flow restricted resistance exercise training (BFRE) is suggested to be effective in rehabilitation training, but more knowledge is required about its potential muscle damaging effects. Therefore, we investigated muscle-damaging effects of BFRE performed to failure and possible protective effects of previous bouts of BFRE or maximal eccentric exercise (ECC).
Methods
Seventeen healthy young men were allocated into two groups completing two exercise bouts separated by 14 days. One group performed BFRE in both exercise bouts (BB). The other group performed ECC in the first and BFRE in the second bout. BFRE was performed to failure. Indicators of muscle damage were evaluated before and after exercise.
Results
The first bout in the BB group led to decrements in maximum isometric torque, and increases in muscle soreness, muscle water retention, and serum muscle protein concentrations after exercise. These changes were comparable in magnitude and time course to what was observed after first bout ECC. An attenuated response was observed in the repeated exercise bout in both groups.
Conclusion
We conclude that unaccustomed single-bout BFRE performed to failure induces significant muscle damage. Additionally, both ECC and BFRE can precondition against muscle damage induced by a subsequent bout of BFRE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Low-load resistance training coupled with partially restricted blood flow has been proposed as an efficient, yet gentle training alternative to heavy resistance training, to counteract muscle wasting from aging or disease and may be of special benefit for rehabilitating patients/athletes that are unable to tolerate the high mechanical load on the joints inherent of traditional heavy resistance exercise (Takarada et al. 2000). Accordingly, this training method, often referred to as low-load blood flow restricted exercise (BFRE), has been reported to induce hypertrophy and increased muscle strength to a similar degree as traditional heavy resistance exercise, despite training loads as low as 20–30 % of 1RM (Madarame et al. 2008; Nielsen et al. 2012). Furthermore, BFRE has been reported to be beneficial for elderly individuals, cardiac rehabilitation patients (Madarame et al. 2013) and individuals recovering from ACL reconstruction (Ohta et al. 2003).
However, the use of BFRE in patient populations requires that unwanted side effects are minimal. In this regard, it is of concern whether BFRE, similarly to eccentric exercise, can result in exercise-induced muscle damage (EIMD). In support of a muscle damaging effect of BFRE, some studies have observed muscle force loss and delayed onset muscle soreness (DOMS) during the first few days following a single bout BFRE conducted to concentric failure (Umbel et al. 2009; Wernbom et al. 2012). On the contrary, other studies have not observed prolonged decrements in maximum voluntary contraction (MVC) and/or increases in muscle soreness following BFRE (Loenneke et al. 2013; Thiebaud et al. 2013, 2014). Despite the conflicting reports it seems as if BFRE can inflict muscle damage under certain conditions.
EIMD has traditionally been associated with exercise involving eccentric actions (Newham et al. 1983; Vissing et al. 2008) and is characterized by prolonged loss of muscle strength, development of muscle soreness, increases in serum intramuscular enzymes and water retention in the days following damaging exercise (Takahashi et al. 1994; Vissing et al. 2008). Also, it is well established that when performing subsequent bouts of similar exercise, the EIMD is attenuated, a phenomenon referred to as the “repeated bout effect” (Nosaka and Clarkson 1995).
It is not known whether the muscle damage response, and the underlying mechanisms, following BFRE are similar or different from those observed after eccentric exercise. However, the quite similar time-course of post-exercise force loss and muscle soreness among subjects exposed BFRE as well as eccentric exercise, suggest that it may be. Furthermore, no study has yet investigated if a single bout of BFRE or ECC may precondition against muscle damage elicited with repeated bouts of BFRE.
Eccentric exercise and BFRE might differ in neuromuscular recruitment pattern i.e. eccentric contractions are suggested to involve low motor unit activation (Komi et al. 1987) combined with possible preferential recruitment of high-threshold type II fibres (Nardone et al. 1989), whereas BFRE is suggested to recruit type I fibres (at least at the beginning of exercise before fatigue onset) (Wernbom et al. 2009; Farup et al. 2015). The different recruitment patterns might cause differences in fibre-type specific damage. Accordingly, other studies have observed indications of a higher stress and damage imposed on type I fibres from BFRE (Wernbom et al. 2012; Cumming et al. 2014). This is contrary to what is commonly observed after eccentric exercise where the inflicted fibre damage primarily is observed in type II fibres (Friden et al. 1983).
In addition to differences in recruitment pattern, BFRE and eccentric exercise might differ in their underlying damage mechanisms. The initial muscle damage inflicted by eccentric contractions is suggested related to mechanical factors (Armstrong et al. 1991). However, since BFRE does not involve a high mechanical load, but rather encompasses a high degree of metabolic stress (Suga et al. 2010), BFRE-induced muscle damage would presumably be related to other factors e.g. metabolite build-up (Takarada et al. 2000; Suga et al. 2010) and/or disturbances in ion-homeostasis due to muscle activation under ischemic conditions (Fredsted et al. 2005), which might lead to activation of intracellular proteolytic systems (Fredsted et al. 2007). If EIMD is induced by BFRE, one would expect it to precondition against EIMD with repeated BFRE. This would be of interest to elucidate, to resolve the applicability of BFRE for patient populations and/or if prior accustomisation should be implemented in a BFRE training strategy. Furthermore, initial knowledge of whether eccentric exercise and BFRE are similar or different in terms of underlying mechanisms and/or neuromuscular recruitment pattern, can be obtained by investigating if EIMD produced by eccentric exercise can precondition against EIMD when repeated exercise is conducted as BFRE.
Therefore, one aim of the present study was to conduct a comparative investigation of the muscle-damaging effect of a single bout of BFRE to failure versus a bout of maximal eccentric exercise. Another aim was to investigate the capability of BFRE versus eccentric exercise to precondition against EIMD induced by subsequent BFRE.
We hypothesized; (1) that a first bout of BFRE as well as eccentric exercise would inflict muscle damage; (2) that repeated BFRE would produce a repeated bout effect, and; (3) that high-intensity eccentric exercise would not precondition against EIMD inflicted by a subsequent bout of BFRE.
Methods
Subjects
Eighteen healthy young men were included in the study. One subject dropped out due to personal reasons. All subjects were informed of the purpose and risks of the study and provided written informed consent in accordance with the standards of the Declaration of Helsinki. The study was approved by the Ethical Committee for Region Midtjylland (ref. no. M-201430314). Exclusion criteria were; (1) a history of musculoskeletal injuries in the lower extremities; (2) participation in resistance training for the lower extremities during the last 6 months prior to inclusion; (3) use of medications that could potentially influence the results; (4) metal-containing implants. Six applicants were excluded as a result of these criteria. All subjects were recreationally active in activities such as swimming, running, cycling and team sports but none of the subjects were performing resistance training. The subjects were instructed to refrain from strenuous activities the last 72 h before the two exercise sessions and to avoid use of any pain-relieving medicaments throughout the study.
Study design
The subjects were randomly assigned to either a group that conducted two bouts of BFRE (BB) or a group that conducted eccentric exercise (ECC) in a first bout and BFRE in a second bout (EB). The two exercise bouts (BB1/BB2 and EB1/EB2) were separated by 14 days and were performed as unilateral knee extension exercises with a randomly chosen leg (dominant vs. non-dominant). The BFRE consisted of five sets of knee extensions to volitional failure with 30 % of 1RM whereas the eccentric exercise consisted of 150 maximal eccentric contractions. A schematic overview of the study design is presented in Fig. 1. At least 7 days prior to the first exercise session, the subjects underwent a unilateral 1RM knee extension test and the subject’s anthropometrics were recorded (Table 1). MRI scans were conducted 4 days before and 3 days after each exercise session. Tests of muscle function and muscle soreness as well as collection of blood samples were conducted immediately before each exercise session and at 1 h, 1, 2, 4, and 7 days post-exercise in the following order; blood sampling, evaluation of muscle soreness and test of muscle function (Fig. 1).
Schematic presentation of the study timeline. Bout 1 consisted of BFRE (BB1) or ECC (EB1) and bout 2 consisted of BFRE (BB2 and EB2). The two exercise sessions were separated by 14 days. RM repetition maximum, MR magnetic resonance, performance and damage measurements included test of muscle function, muscle soreness and blood sampling
Maximal unilateral knee extensor strength
To establish the training load for the BFRE protocol, the unilateral concentric 1RM strength of the subjects was tested in a knee extension machine (Technogym Selection Line Leg Extension, Technogym SpA, Cesena, Italy). The subjects did a standardized warm-up consisting of a 5 min low-intensity exercise (~100 W) on a stationary ergometer bike (Monark Ergomedic 818E, Monark, Varberg, Sweden). For the 1RM test, subjects performed two warm up sets of five repetitions with increasing loads. After the warm up sets, the first 1RM trial was performed with a load that the investigators were confident the subject could lift. For each successful trial the load was increased by 5 kg until nearing 1RM, where loads were increased by 1–2.5 kg until failure. Trials were accepted if the subject reached full knee extension and 1RM was generally determined within 5–6 trials. Previous studies have shown that submaximal eccentric contractions can confer a protective effect against EIMD inflicted by high-intensity eccentric exercise performed within the following weeks (Chen et al. 2012). Thus, to avoid preconditioning of the subjects, both warm up sets and 1RM trials were performed with the subject lifting the load utilizing only concentric contractions (knee extension phase). An investigator lowered the weight to liberate the subject from performing the eccentric part of the movement. All settings of the knee extension machine were noted for each subject and saved for later use.
Muscle function testing
Subsequent to a standardized warm-up of 5 min low intensity exercise (~100 W) on a stationary ergometer bike (Monark Ergomedic 818E, Monark, Varberg, Sweden), the subjects were seated on an isokinetic dynamometer (Humac Norm, CSMI, Stoughton, Massachusetts, USA) with 90° hip flexion and the axis of rotation of the subjects knee aligned with the axis of the lever arm. The test leg was attached to the dynamometer arm approximately 3 cm proximal to the medial malleolus and the other leg was placed behind a stabilization bar. Restraining straps were put on the torso and hip to minimize accessory movements. MVC was measured at 70° (0° equals full extension). The subjects were instructed to avoid any countermovement and to contract as “fast and forcefully as possible” until torque began to decline (approximately 4 s). Standard verbal encouragement and visual feedback was provided during each contraction. Subjects were given four trials, however, if a subject continued to improve, additional trials were provided. All attempts were interspaced with 1 min recovery time.
After the MVC measurements, the torque elicited through electrical stimulation was measured at two different stimulation frequencies to assess low frequency fatigue (a measure of excitation–contraction coupling function). Following shaving and cleaning of the skin with alcohol swabs, two electrodes (ValuTrode Neurostimulation Electrodes CF5090, 2″ × 3.5″, Axelgaard Manufacturing Co., Ltd, Denmark) were placed across the main muscle belly. Electrode positions were marked to ensure the same positioning in each trial. At the first test session the subjects were familiarized with the stimulation protocol and an electrical current level acceptable to each individual was determined (150–220 mA) (these current levels produced torque >15 Nm). The stimulation protocol consisted of two trains of pulses, separated by a minimum of 20 s, with a duration of 1 s at stimulation frequencies of 20 and 100 Hz, respectively. The subjects were instructed to relax as much as possible during the stimulations.
Torque recordings from all trials from the MVC measurements and stimulation protocol were sampled at 1500 Hz and the offline analyses were performed in custom-made software (Labview 2011, National Instruments Corporation, Austin, Texas, USA). During offline analysis, all trials were visually inspected, and trials exhibiting countermovement were eliminated from further analysis. Torque data was corrected for limb weight and MVC was defined as the peak torque measurement from the best trial. Torque produced from the 20 and 100 Hz stimulation frequencies was defined as the average torque during the last 200 ms of the stimulation train where a torque plateau was observed.
Blood sampling
Blood samples were collected from an antecubital vein immediately before and 1 h, 1, 2, 4 and 7 days after each exercise session. After centrifugation at 1900g for 10 min at 5 °C, plasma aliquots were divided into Eppendorf tubes and stored at −80 °C. Samples were analyzed for creatine kinase (CK) (upper detection limit 20,000 IU/L) and myoglobin (Mb) (lower detection limit 52 µg/L) by use of a commercial kit applied in a multi-analyzer system (Cobas c 311/501, Rotkreuz, Switzerland) at the Department of Clinical Biochemistry at Aarhus University Hospital.
Muscle soreness
Muscle soreness was evaluated using a 100 mm visual analog scale (VAS) where 0 represented “no soreness” and 100 represented “extreme soreness”. Both the exercise and control leg were evaluated immediately before each exercise session and at 1 h, 1, 2, 4 and 7 days post-exercise. The subjects were asked to sit and rise from a chair using the leg that was evaluated and place a mark on the 100 mm line according to their perceived soreness in the knee extensors during the movement. To provide a reference, the subjects were allowed to see their previous ratings of soreness within the current bout. However, subjects were not allowed to see their soreness ratings from the first exercise bout when rating soreness after the second exercise bout.
Magnetic resonance imaging protocol
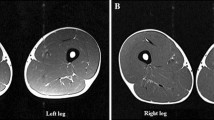
The T2 relaxation time and anatomical cross-sectional area (CSA) of m. quadriceps of both legs were obtained using images acquired with a 3 T magnetic resonance imaging (MRI) scanner (Siemens Skyra, Erlangen, Germany). The subjects were placed in a supine position with the feet entering the scanner first, the legs fully extended and a distance between the feet of approximately 10 cm. An initial frontal and sagittal localizer scan was applied as an introductory scout scan used to correctly place the location of the first proximally positioned slice level (top of caput femoris) in the following T2-mapping scan. The T2 scan was a multi-echo spin echo scan. It consisted of 40 slices separated by 14 mm and was acquired as two separate stacks. Slice thickness was 4 mm, field-of-view was 420 × 250 mm2 and acquisition matrix was 320 × 190 giving a pixel resolution of 1.3 × 1.3 mm2. Eight echoes from 20 to 160 ms were acquired, the repetition time was 3.35 s and total scan time for both stacks was 11:36 min.
The femur length was calculated as the difference between the position of the first proximal slice level located at the top of caput femoris and the position of the slice level corresponding to the knee joint line (both given by the software program OsiriX, version 4.1 32-bit). Following this, T2 relaxation times and whole-muscle CSAs were obtained from the acquired images at 1/3, 1/2 and 2/3 of the femur length representing a proximal, mid and distal level. The mean of both the CSA and T2 obtained at the three slice positions was used for further analysis.
The CSA was manually outlined three times at each slice level by the same investigator using OsiriX. The intra-individual correlation of the three measurements was r 2 = 0.99 and an inter-individual correlation of r 2 = 0.99 was calculated.
The scanner calculated T2 maps by fitting an exponential relaxation function to the 8 acquired echo times. At the same three slices as used for the CSA calculations a region-of-interest (ROI), corresponding to the whole m. quadriceps, was outlined with special care to avoid inclusion of subcutaneous fat, fascia, blood vessels and bone structures. This ROI was chosen instead of individual muscle portions since it has previously been shown that the location of increased T2 can vary among subjects (Nosaka and Clarkson 1996). T2 from each ROI outlined were recorded using OsiriX. Three ROIs (representing the whole m. quadriceps) were outlined at each slice level (inter and intra-individual correlation in T2 analysis was r 2 = 0.99).
Blood flow restriction exercise protocol
The BFRE protocol consisted of five sets of unilateral knee extensions at 30 % of 1RM in a knee extension machine (Technogym Selection Line Leg Extension, Technogym SpA, Cesena, Italy) with partial BFR. A 135 mm wide curved tourniquet cuff (VBM, Medizintechnik, GmbH, Germany) was wrapped around the proximal part of the thigh and inflated to a pressure of 100 mmHg by a digital tourniquet system with automatic regulation of pressure (Digital Tourniquet 9000, VBM, Medizintechnik, GmbH, Germany). This pressure cuff was inflated immediately before the first set and remained inflated throughout the exercise session. All sets were performed to volitional failure. Recovery time between sets was 45 s and subjects remained seated in the leg extension machine during recovery. The cadence was 15 repetitions per min (2 s each for the concentric and eccentric part of the duty cycle), which was controlled with a metronome set to 60 bpm. No rest was permitted between the repetitions. Range of motion was aimed to be ~90°–0° (0° equals full extension), and the subjects were instructed to extend their knee as much as possible for each repetition, however, failure was defined as when the subject were not able to extend the knee to <10° (corresponding to the range of motion during the eccentric exercise protocol). Subjects were verbally encouraged to complete as many repetitions as possible in each set. The number of completed repetitions in each set and the total BFR time was noted.
Eccentric exercise protocol
Only the EB group did eccentric exercise in the first exercise session. The eccentric exercise protocol consisted of 15 sets of 10 maximal isokinetic eccentric contractions for the knee extensors in an isokinetic dynamometer (Humac Norm, CSMI, Stoughton, Massachusetts, USA). The range of motion was 10°–90° (0° equals full extension) and contraction speed was set to 30°/s. Recovery time between sets and repetitions were 60 and 3 s, respectively. Subjects received verbal encouragement and visual feedback throughout the exercise to ensure maximal effort. All dynamometer settings were identical to the settings during muscle function testing.
Statistics
The sample size was determined by completing a power analysis where serum CK was chosen as the primary outcome. Serum CK was chosen because of the very high inter-individual variation. Thus, expectedly, we would be able to detect significant changes in other outcomes with less variation. Power analysis (power = 0.8, α = 0.05) was based on Larsen et al. (2007) and expected serum CK levels of 1400 ± 1000 IU/L following BFRE. The analysis showed that seven subjects in each group where required. We included nine subjects in each group to account for potential dropouts.
Data were checked for normality of distribution by Shapiro–Wilk test and QQ plots. Data from blood samples (CK and Mb) were not normally distributed and were log-transformed before doing the statistical analysis. The log-transformed data were back-transformed before data presentation. Differences in anthropometric measures and strength measures at baseline, and differences in training data (number of repetitions and occlusion time) within and between groups were assessed by paired and unpaired t tests, respectively.
To test whether BFRE and eccentric exercise induced indications of muscle damage, differences between the pre-value and later time points for indicators of muscle damage (MVC, 20:100 Hz ratio, muscle soreness and serum activity of CK and Mb) were identified by a one-way repeated measures ANOVA with Student–Newman–Keuls post hoc test. Paired t tests were used to evaluate exercise-induced changes in muscle CSA and T2.
The accumulated changes for the first 4 days following exercise in indicators of muscle damage (MVC, 20:100 Hz ratio, muscle soreness, serum activity of CK and Mb) were evaluated as area under curve (AUC), and were calculated by integrating the change in each indicator with respect to time (h) from pre to 4 days post-exercise. We chose a period comprising the first 4 days following exercise rather than 7 days because no data was collected at 5 and 6 days post-exercise.
To investigate whether there were group differences in the magnitude of muscle damage within each bout, differences in AUC, peak muscle soreness, ΔCSA and ΔT2 relaxation time between groups within bout 1 and 2, respectively, were evaluated by unpaired t tests (BB1 vs. EB1 and BB2 vs. EB2).
To investigate whether there was an attenuated response in indicators of muscle damage after the second bout within the BB group, differences in AUC, peak muscle soreness, ΔCSA and ΔT2 relaxation time between bout 1 and 2 were evaluated by paired t tests.
To investigate whether eccentric exercise protects against muscle damage inflicted by BFRE, unpaired t tests were used to identify differences in AUC, peak muscle soreness, ΔCSA and ΔT2 relaxation time between the first bout of BFRE in each group (BB1 vs. EB2).
To test for correlations between ΔCSA and ΔT2 relaxation time, linear regression analysis was performed.
Alpha level was set to p ≤ 0.05 and data are presented as mean ± SE unless otherwise stated in figure texts. Statistical analysis was made in Sigmaplot version 11.0 (Systat Software, San Jose, CA, USA) and graphical presentations were made in GraphPad version 6.0 (GraphPad Software, La Jolla, CA, USA).
Results
As shown in Table 1, no differences between groups were observed in anthropometric measures or baseline measures of muscle strength. In addition, no differences were observed between groups within each bout in AUC, peak muscle soreness, ΔCSA and ΔT2 relaxation time (BB1 vs. EB1 and BB2 vs. EB2).
Maximal voluntary contraction
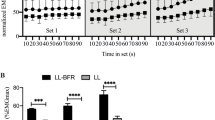
As shown in Fig. 2 MVC was reduced by 24 ± 3 and 16 ± 4 % at 1 h following the first bout of exercise in the EB and BB group, respectively. Following this, MVC gradually recovered during post-exercise recovery but was still significantly reduced at 4 days post-exercise by 9 ± 5 and 8 ± 3 %, in the EB and BB group, respectively. At 7 days post-exercise, MVC had returned to baseline in both groups.
Time course of exercise-induced changes in a MVC (normalized to the pre-value), b 20:100 Hz ratio and c muscle soreness at 1 h, 1, 2, 4 and 7 days after each exercise bout. ^ p < 0.05; ^^ p < 0.01; ^^^ p < 0.001; significantly different from pre-values within BB. *p < 0.05; **p < 0.01; ***p < 0.001; significantly different from pre-values within EB. Symbols indicate mean and error bars indicate ±SE. Accumulated changes in d MVC, e 20:100 Hz ratio and f muscle soreness from pre to 4 days post-exercise at each exercise bout. # p < 0.05; ## p < 0.01; significant difference between bouts
Following the second bout, MVC was reduced by 11 ± 3 and 9 ± 3 % at 1 h post-exercise in the EB and BB group, respectively. By 1 day, MVC had returned to baseline in the EB group but was still significantly reduced by 8 ± 2 % in the BB group. At 2 days post-exercise, MVC had returned to baseline in the BB group (Fig. 2).
As shown in Fig. 2, AUCMVC was ~68 % lower after the second exercise bout compared to the first within the BB group. Furthermore, when comparing the first bout of BFRE in each group (BB1 vs. EB2), AUCMVC was ~71 % lower in the EB group (Fig. 2).
20:100 Hz ratio
Following the first exercise bout in the EB group, the 20:100 Hz ratio decreased by ~52 % at 1 h post-exercise and gradually recovered during the following days but was still reduced by ~14 % at 7 days (Fig. 2). In the BB group, the 20:100 Hz ratio was reduced by ~30 and ~15 % at 1 h and 1 day following the first exercise bout, respectively. No differences were observed at 2 or 4 days but a ~14 % lower ratio was observed at 7 days.
A paired t test revealed that the pre-value was lower before bout 2 compared to bout 1 (0.71 vs. 0.58) in the BB group. At 1 h after the second bout both groups had a reduced 20:100 Hz ratio but no differences were observed at other time points.
Within the BB group, AUC20:100 Hz was lower after the second bout compared to the first. No difference between the first bouts of BFRE in each group (BB1 vs. EB2) was observed (Fig. 2).
Muscle soreness
Muscle soreness was elevated at 1 h–4 days after the first exercise bout in both groups, peaking at 2 days (EB ~4.4 cm and BB ~5.0 cm), and returned to baseline at 7 days. After the second bout, the BB group experienced muscle soreness at 1 h, 1 and 2 days post-exercise (1.5–2.4 cm) (Fig. 2).
AUCsoreness was ~57 % lower after the second bout compared to the first in the BB group (Fig. 2). No significant differences in AUCsoreness were observed between the first bouts of BFRE in each group (BB1 vs. EB2; p = 0.10) but when examining the highest soreness ratings irrespective of time points, a lower peak soreness was observed after the first bout of BFRE in the EB group compared to the first bout in the BB group (BB1 5.0 cm vs. EB2 2.9 cm; p < 0.05). No changes in soreness were observed in the control leg in either group (data not shown).
Serum activity of muscle enzymes
Before the first exercise bout serum CK was 215 ± 47 and 137 ± 26 IU/L in the EB and BB group, respectively. No significant changes in CK were observed at 1 h in either group but at 1 day serum CK had increased in the EB group (1060 ± 226 IU/L, p < 0.01) while no significant changes were observed within the BB group. At 2 days post-exercise serum CK was increased in both groups (EB; 744 ± 146, BB; 1837 ± 1308 IU/L, p < 0.05) and at 4 days serum CK peaked with a 13 and 36-fold increase in the EB (2936 ± 1904 IU/L, p < 0.01) and BB (4954 ± 2773 IU/L, p < 0.001) group, respectively. At 7 days post-exercise serum CK had returned to baseline in the BB group but was still increased in the EB group (1230 ± 547 IU/L, p < 0.05). A large inter-subject variation in CK changes was observed and two subjects in the BB group had peak CK values >15,000 IU/L.
Serum Mb increased after the first bout in both groups. Before exercise serum Mb was 52 ± 1 (lower detection limit 52 μg/L) in the EB group. Following the first exercise bout a bimodal response was observed in the EB group with peaks at 1 h (345 ± 96 μg/L, p < 0.001) and 4 days post-exercise (307 ± 158 μg/L, p < 0.05). No significant changes were observed at other time-points. Following the first exercise bout in the BB group, no significant changes were observed in serum Mb at 1 h and 1 day post-exercise but at 2 and 4 days serum Mb was increased from a pre-value of 52 ± 0 to 948 ± 575 μg/L (p < 0.001) and 601 ± 395 μg/L (p < 0.01), respectively. At 7 days post-exercise serum Mb had returned to baseline. No changes in serum CK or Mb were observed in either group after the second exercise bout.
Within the BB group, the AUCCK was ~99 % lower after the second bout of exercise compared to the first. With regards to Mb, a ~99 % lower AUCMb was observed after BFRE in the EB group (EB2) compared to the first bout of BFRE in the BB group (BB1) (Fig. 3).
Magnetic resonance imaging data
CSA Following the first exercise bout, mean CSA did not increase significantly (p = 0.078) in the BB group, whereas CSA increased by ~2 % (p < 0.05) in the EB group. No changes were observed in either group after the second bout. However, when comparing the changes in CSA following each bout in the BB group, only a tendency (p = 0.084) towards a lower CSA change was observed, and no difference between bouts was observed within the EB group. Furthermore, no differences were observed when comparing the changes in CSA following the first bout of BFRE in each group (BB1 vs. EB2) (Table 2).
T2 Following the first exercise bout, mean T2 increased by ~16 and ~6 % in the BB and EB group, respectively. No significant changes were observed in either group after the second bout. When comparing the changes in T2 following each bout in the BB group, T2 change was reduced after the second bout compared to the first. When comparing ΔT2 following the first bout of BFRE in each group (BB1 vs. EB2), ΔT2 was lower after EB2 compared to BB1 (Table 2). No changes were observed in non-exercised legs (data not shown).
Correlations
A positive correlation between ΔCSA and ΔT2 relaxation time following bout 1 was observed in the BB group (r 2 = 0.87, p < 0.001). No correlation was observed between ΔCSA and ΔT2 relaxation time following bout 1 in the EB group or following bout 2 in either group.
Discussion
The main findings of the present study were; firstly, that both blood flow restricted exercise performed to volitional failure and maximal eccentric exercise inflict muscle damage as indicated by the response in several indicators of muscle damage (i.e. MVC, serum activity of intramuscular enzymes, muscle soreness, muscle swelling and T2 relaxation time). Secondly, the muscle damage induced by BFRE was comparable in magnitude to that inflicted by maximal eccentric exercise, and thirdly, preceding bouts of both BFRE and maximal eccentric exercise confers a protective effect against muscle damage induced by a repeated exercise bout conducted as BFRE, as reflected by the attenuated response in several of the indicators of muscle damage.
Effects of a first-bout BFRE on muscle damage indicators
Prolonged force loss is considered one of the most valid and reliable indirect measures of muscle damage (Warren et al. 1999). In the present study, MVC was decreased at 1–96 h after the first bout of BFRE in the BB group (BB1) indicating EIMD. The force loss observed at 1 and 24 h post-exercise was accompanied by a decrease in the 20:100 Hz ratio suggesting that the decreased MVC was, in part, due to E–C coupling failure (Ingalls et al. 1998). In line with these findings, using a very similar exercise protocol as the present study, Wernbom et al. (2012) found force decrements lasting 48 h after BFRE, which was only accompanied by a significantly decreased 20:50 Hz ratio at the time point immediately after exercise, where cuff pressure was still applied (Wernbom et al. 2012). Also, Umbel et al. (2009) observed a force loss lasting 24 h after concentric BFRE. The magnitude of changes in MVC observed in the present study are quantatively comparable to the changes observed by Wernbom et al. (2012) and Umbel et al. (2009), albeit with minor differences in the time course of MVC recovery.
In contrast, other studies have not observed prolonged decrements in force following BFRE (Thiebaud et al. 2013, 2014; Loenneke et al. 2013). Loenneke et al. (2013) and Thiebaud et al. (2013, 2014) used an exercise protocol consisting of four sets with a pre-set repetition scheme (30-15-15-15), whereas subjects worked to volitional failure in all sets in our study as well as in the other two studies exhibiting prolonged BFRE-induced loss of force (Umbel et al. (2009), Wernbom et al. (2012)). In addition, Loenneke et al. (2013) used subjects who were accustomed to resistance training and the subjects completed a familiarization session a week prior to the main experiment (Loenneke et al. 2013). Finally, since the exercise equipment used varied, torque curves were likely different between studies. Together, differences in exercise protocols (failure vs. non-failure), training status of the subjects (non-resistance trained vs. resistance trained) and use of familiarization procedures may explain why prolonged force loss following BFRE is seen in some studies (Umbel et al. 2009; Wernbom et al. 2012), and not in others (Thiebaud et al. 2013, 2014; Loenneke et al. 2013).
The time-course and degree of muscle soreness we observed after the first bout of BFRE is similar to what was observed by Wernbom et al. (2012). In addition, several other studies have reported muscle soreness after BFRE, but with varying magnitude (Umbel et al. 2009; Wernbom et al. 2009; Wilson et al. 2013; Thiebaud et al. 2013). This variation is conceivably because different methods are used to evaluate muscle soreness ratings (e.g. soreness when palpating (Thiebaud et al. 2013), general soreness (Wernbom et al. 2012) and retrospective ratings (Umbel et al. 2009)).
We assessed plasma levels of CK and Mb as indicators of possible damage to the muscle membrane. Until now, only one study has investigated the serum CK response to acute BFRE (Takarada et al. 2000). In Takaradas’ study, blood samples were collected during the first 24 h following BFRE and no changes in CK were observed. In agreement, no significant changes were observed within the first 24 h following the first bout of BFRE in the BB group in the present study. However, large increases were observed at 2 and 4 days post-exercise. The observed time-course of the increase in CK and Mb is similar to what has previously been observed with eccentric exercise (Vissing et al. 2008).
To our knowledge, the present study is the first to use MRI to assess prolonged muscle swelling (CSA) and muscle water retention (T2 relaxation time) after BFRE. Following the first exercise bout in the BB group, we found elevated T2 at 3 days post-exercise, whereas only a tendency (p = 0.078) towards an increased CSA at the same time-point was observed. Elevated T2 at 3 days post-exercise has previously been shown after damaging eccentric exercise (Nosaka and Clarkson 1996; Foley et al. 1999) where the prolonged increase in signal intensity is thought to indicate increased tissue water content possibly due to injury to connective tissue, increased capillary permeability or degradation of proteins in the muscle cell (Takahashi et al. 1994).
A single previous study has observed muscle swelling in the arm musculature 48 h after a first-bout of BFRE (Farup et al. 2015), whereas, most previous studies have merely observed acute muscle swelling following BFRE (Umbel et al. 2009; Loenneke et al. 2013; Thiebaud et al. 2013; Wilson et al. 2013). Prolonged muscle swelling might indicate EIMD (Nosaka and Clarkson 1996).
We only examined CSA at 3 days post-exercise and found no significant changes in BB group. However, when using only one post-exercise time-point it is possible that we did not find significantly increased CSAs because we missed the peak of CSA change. Umbel et al. (2009) reported significant increases in muscle CSA at 24 h post-exercise when data from both BFR concentric and BFR eccentric legs were combined. From their figures, it appears that muscle swelling after BFR exercise peaked at 24 h and then declined. An association between increases in CSA and T2, following eccentric exercise, has previously been proposed (Takahashi et al. 1994) and in the present study, a positive correlation (r 2 = 0.87) was observed 3 days after BFRE between CSA and T2.
Collectively, our results suggest that a first bout of BFRE completed to failure induces muscle damage for up to 4 days as indicated by the response in the multiple indicators of muscle damage investigated, thus supporting hypothesis (1).
Effects of maximal eccentric exercise on muscle damage indicators
Contrary to the low-load, low-volume BFRE performed by the BB group, the first exercise bout in the EB group consisted of high-intensity, high-volume eccentric exercise, which has consistently been shown to induce muscle damage (Stupka et al. 2001; Raastad et al. 2010). We observed force loss, accompanied by a decreased 20:100 Hz ratio, that gradually returned to baseline during the following days, as well as increased muscle soreness and increased serum CK and Mb. Acknowledging the differences in the magnitude of these changes, for single-bout eccentric exercise, we found a similar time-course and response in the indicators of EIMD as previously shown in studies on eccentric exercise (Vissing et al. 2008; Raastad et al. 2010). Furthermore, also similar to a previous finding regarding muscle swelling and T2 in m. quadriceps femoris following eccentric exercise (Takahashi et al. 1994), we observed significant muscle swelling and increased T2.
When employing un-paired t tests to compare the accumulated changes in each indicator of muscle damage during the first 4 days following the first bout in each group (BB1 vs. EB1), represented by the AUC, no differences were observed. Accordingly, the response in most indicators of EIMD to BFRE was comparable in magnitude to what was observed following ECC, despite the low load and volume utilized in BFRE, suggesting that the two types of exercise used in this study, in most aspects, produce a comparable extent of muscle damage. It should be noted, however, that the BFRE and ECC bouts were not matched for work, intensity or repetition number. Therefore the similarity in damage responses seen here does not necessarily indicate that these exercise modalities are equally damaging when expressed relative to muscle work performed.
Repeated bout effect BFRE/BFRE
In agreement with our original hypothesis, a repeated bout effect was observed within the BB group, as shown by the attenuated response in several of the measured indicators of muscle damage following the second bout.
One striking difference in the response to the second bout compared to the first in the BB group was in serum activity of CK, in which a 36-fold increase in serum CK was observed following the first bout and no changes were observed after the second bout. This is similar to what has previously been observed with repeated muscle-damaging eccentric exercise (Newham et al. 1987; Vissing et al. 2008). Furthermore, MVC initially decreased to a similar extent after the second bout of BFRE compared to the first, but recovered faster and the accumulated changes, reflected by AUCMVC, were lower. This result resembles previous observations after repeated eccentric exercise (Newham et al. 1987; Vissing et al. 2008). Furthermore, repeated eccentric exercise has been shown to induce a reduced decrease in the 20:100 Hz ratio after the second bout (Newham et al. 1987), however, in our study the 20:100 Hz ratio was somewhat comparable between the two bouts in the BB group, except for a significantly lower pre-value at bout 2 compared to bout 1. We speculate that the lower pre-value, rather than a repeated bout effect, gives rise to the lower AUC after bout 2. With regards to CSA and T2, no increases were observed after the second bout in the BB group, and a significant difference between bouts was observed for T2 change. This is in line with a study by Farup et al. (2015), who reported long-lasting muscle swelling (3–48 h) after a first bout of BFRE, which was diminished when the BFRE bout was performed after 5 weeks of BFRE training (Farup et al. 2015). Furthermore, these findings are similar to studies on repeated eccentric exercise that have reported reduced increases in T2 and CSA after the second bout of exercise (Foley et al. 1999; Nosaka et al. 2001; Chen et al. 2012).
Like in previous studies on repeated eccentric exercise (Newham et al. 1987; Vissing et al. 2008), subjects still experienced muscle soreness after the second bout of BFRE in the BB group, but both the AUCsoreness and peak muscle soreness were lower after the second bout compared to the first.
All in all, the repeated bout effect of two bouts of BFRE is substantial, and comparable to what has previously been observed with repeated eccentric exercise (Newham et al. 1987; Foley et al. 1999; Nosaka et al. 2001; Vissing et al. 2008), thus supporting hypothesis (2).
Repeated bout effect eccentric exercise/BFRE
Contrary to our original hypothesis (3), maximal eccentric exercise had a protective effect on the subsequent bout of BFRE in the EB group, which was very similar to the one observed with two bouts of BFRE.
Clearly, eccentric exercise and BFRE are very different in terms of exercise intensity and volume and no efforts were made to match total work between the two types of exercise in our study. Accordingly, the lower damage response observed in the second bout compared to the first within the EB group might emerge simply because maximal eccentric exercise induces more, or a different type of, muscle damage compared to BFRE. Therefore, in order to investigate whether eccentric exercise protects against muscle damage inflicted by BFRE, we compared the response in indicators of muscle damage when BFRE was performed without preceding exercise and when it was performed subsequent to a bout of maximal eccentric exercise (first bout in BB group, BB1 vs. second bout in EB group, EB2). This analysis showed that the AUC of MVC and serum Mb, peak muscle soreness, and ΔT2 were all lower after the first bout of BFRE in the EB group (EB2) compared to the first bout of BFRE in the BB group (BB1), suggesting a protective effect of maximal eccentric exercise against damage elicited by BFRE. The attenuated response after the second bout in the EB group very much resembles what we observed after repeated BFRE and what has previously been observed after repeated eccentric exercise (Newham et al. 1987; Foley et al. 1999; Nosaka et al. 2001; Vissing et al. 2008).
Collectively, our results show that both maximal eccentric exercise and BFRE induces a comparable repeated bout effect, suggesting that the adaptive response triggered by both types of exercise is, at least in part, similar, which might be due to similarities in the mechanisms causing the muscle damage and/or which muscle fibres that are predominantly damaged.
It has been hypothesized that eccentric contractions cause muscle damage due to a high stress on a small number of active fibres (Moritani et al. 1987) resulting in a high mechanical strain (Lieber and Friden 1993) and damage to primarily type II fibres (Friden et al. 1983). As previously discussed, working to volitional failure might be decisive for inducing muscle damage with BFRE and it has been suggested that during BFRE, type II fibres are gradually recruited as type I fibres fatigue (Wernbom et al. 2009; Farup et al. 2015), implying that recruitment of type II fibres might be essential for causing muscle damage with BFRE. Thus, with fatigue and consequent low force output in some fibres due to the combined effects of ischemia and exercise, the remaining force-producing fibres may be subjected to greater mechanical stresses and strains than the nominally low load (30 % of 1RM) would suggest.
Hence, if muscle damage observed after BFRE is due to the mechanical strain put upon newly recruited type II fibres during the last repetitions, this could explain the protective effect of high-intensity eccentric contractions, which also recruits these fibers. However, the few BFRE studies that have examined fibre-type specific muscle damage have implied higher stress (and damage) in type I fibres with BFRE protocols identical to ours (Wernbom et al. 2012; Cumming et al. 2014). Alternatively, if muscle damage inflicted by BFRE is due to mechanical and/or metabolic stresses on type I fibres, the protective effect of preceding eccentric exercise might be mediated by mechanisms that make the muscle less susceptible to EIMD across all fibre-types, such as increased whole muscle stiffness, changes in the muscles length-tension relationship, increased or strengthened connective tissue and/or removal/replacement of weak fibres (McHugh 2003). Accordingly, isolated maximal eccentric exercise has previously been shown to protect against force loss, muscle soreness and serum CK induced by downhill running despite the differences in exercise modality (Eston et al. 1996).
Because the load used during BFRE is very low, a purely mechanical explanation for the induced muscle damage seems unlikely. However, other shared mechanisms in the pathways leading to EIMD might explain the repeated bout effects seen here. Such mechanisms could possibly include activation of proteolytic systems e.g. autophagy-lysosomal, calcium-dependent calpains and the ubiquitin protease system (Pasiakos and Carbone 2014). Accordingly, it has previously been theorized that EIMD is mediated by increased Ca2+ influx into muscle cells (Zhang et al. 2008) that may trigger Ca2+-activated proteases (Belcastro et al. 1998; Zhang et al. 2008). BFRE might increase intracellular Ca2+ due to muscle activation during ischemia (Fredsted et al. 2005), which lowers the energy status of the cell and impedes clearing of Ca2+ entering the cell (Fredsted et al. 2007).
Consequently, despite possible differences in how Ca2+ enters the cell, muscle damage inflicted by eccentric exercise and BFRE might both be mediated by increased Ca2+, and the repeated bout effect could thus be due to an adaptation in proteolytic systems, such as calcium-dependent proteases, after the second bout. As evidence against such a contention, previous studies have failed to show a repeated bout effect on for instance calpain/calpastatin expression despite repeated bout effect on other parameters (Stupka et al. 2001; Vissing et al. 2008), although this does not rule out the possibility that the muscle becomes less sensitive to activity in proteolytic systems. Collectively, our results suggest an overlap of the mechanisms causing EIMD in BFRE and eccentric exercise and in the subsequent adaptations that make the muscle more resilient to the second bout of exercise.
Limitations
In the present study, we used a very homogenous subject population of active, healthy young males (19–25 years). Consequently, the results may not be generalizable to other populations e.g. women, the elderly, and various patient groups.
Two limitations of the present study are that we did not investigate the recruitment pattern of BFRE and eccentric exercise or measure fibre-type specific damage through analysis on muscle biopsies. Thus, we were unable to determine whether the previously discussed possible differences in recruitment patterns between BFRE and eccentric exercise result in fibre-type specific damage.
Another limitation is that we only conducted MRI scans 3 days post-exercise and thus we might have missed potential effects of the exercise. Furthermore, multiple MRI scans would have enabled us to evaluate the time-course of changes in CSA and T2.
Finally, all subjects were instructed to refrain from strenuous exercise during the study period but no efforts were made to control the level of physical activity during the study period. Consequently, some subjects might have ignored this instruction, e.g. two subjects from the EB group reported to the main experiment with muscle soreness (VAS 2.3 and 2.1 cm) in m. quadriceps because of prior exercise.
Conclusion
In conclusion, based on the responses observed in several of the indirect indicators of muscle damage used in this study, a first-time bout of BFRE induces muscle damage in non-resistance trained subjects performing multiple working sets to volitional failure. Furthermore, a protective effect of previous damaging exercise is seen when BFRE is performed subsequent to either BFRE or maximal eccentric exercise. This finding suggests that similar damage mechanisms and/or subsequent adaptations may be operating in both BFRE and eccentric exercise.
Perspectives
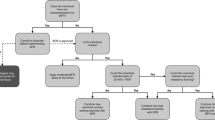
We recommend that BFRE is carefully introduced to untrained people and patient populations as it has the potential to cause muscle damage accompanied by muscle soreness, which could increase the risk of discontinuing the training due motivational reasons. Furthermore, the very high training frequency reported in some studies (Nielsen et al. 2012) might not be preferable initially if the BFRE is performed to failure because of the decrements in muscle strength and the occurrence of muscle soreness. In order to avoid muscle damage, an accustomization period could be applied when commencing BFRE to avoid the negative effects of muscle damage or one could consider not working to volitional failure, as this is not necessary for inducing muscular hypertrophy with BFRE (Madarame et al. 2008).
Abbreviations
- ANOVA:
-
Analysis of variance
- AUC:
-
Area under curve
- BFRE:
-
Blood flow restriction exercise
- CK:
-
Creatine kinase
- CSA:
-
Cross-sectional area
- DOMS:
-
Delayed onset muscle soreness
- ECC:
-
Eccentric exercise
- EIMD:
-
Exercise-induced muscle damage
- Mb:
-
Myoglobin
- MRI:
-
Magnetic resonance imaging
- MVC:
-
Maximum voluntary contraction
- ROI:
-
Region of interest
References
Armstrong RB, Warren GL, Warren JA (1991) Mechanisms of exercise-induced muscle fibre injury. Sports Med 3:184–207
Belcastro AN, Shewchuk LD, Raj DA (1998) Exercise-induced muscle injury: a calpain hypothesis. Mol Cell Biochem 1–2:135–145
Chen HL, Nosaka K, Chen TC (2012) Muscle damage protection by low-intensity eccentric contractions remains for 2 weeks but not 3 weeks. Eur J Appl Physiol 2:555–565. doi:10.1007/s00421-011-1999-8
Cumming KT, Paulsen G, Wernbom M, Ugelstad I, Raastad T (2014) Acute response and subcellular movement of HSP27, alphaB-crystallin and HSP70 in human skeletal muscle after blood-flow-restricted low-load resistance exercise. Acta Physiol (Oxf) 4:634–646. doi:10.1111/apha.12305
Eston RG, Finney S, Baker S, Baltzopoulos V (1996) Muscle tenderness and peak torque changes after downhill running following a prior bout of isokinetic eccentric exercise. J Sports Sci 4:291–299. doi:10.1080/02640419608727714
Farup J, de Paoli F, Bjerg K, Riis S, Ringgard S, Vissing K (2015) Blood flow restricted and traditional resistance training performed to fatigue produce equal muscle hypertrophy. Scand J Med Sci Sports. doi:10.1111/sms.12396
Foley JM, Jayaraman RC, Prior BM, Pivarnik JM, Meyer RA (1999) MR measurements of muscle damage and adaptation after eccentric exercise. J Appl Physiol (1985) 6:2311–2318
Fredsted A, Mikkelsen UR, Gissel H, Clausen T (2005) Anoxia induces Ca2+ influx and loss of cell membrane integrity in rat extensor digitorum longus muscle. Exp Physiol 5:703–714. doi:10.1113/expphysiol.2005.030247
Fredsted A, Gissel H, Madsen K, Clausen T (2007) Causes of excitation-induced muscle cell damage in isometric contractions: mechanical stress or calcium overload? Am J Physiol Regul Integr Comp Physiol 6:R2249–R2258. doi:10.1152/ajpregu.00415.2006
Friden J, Sjostrom M, Ekblom B (1983) Myofibrillar damage following intense eccentric exercise in man. Int J Sports Med 3:170–176
Ingalls CP, Warren GL, Williams JH, Ward CW, Armstrong RB (1998) E–C coupling failure in mouse EDL muscle after in vivo eccentric contractions. J Appl Physiol (1985) 1:58–67
Komi PV, Kaneko M, Aura O (1987) EMG activity of the leg extensor muscles with special reference to mechanical efficiency in concentric and eccentric exercise. Int J Sports Med 8:22–29
Larsen RG, Ringgaard S, Overgaard K (2007) Localization and quantification of muscle damage by magnetic resonance imaging following step exercise in young women. Scand J Med Sci Sports 17(1):76–83
Lieber RL, Friden J (1993) Muscle damage is not a function of muscle force but active muscle strain. J Appl Physiol (1985) 2:520–526
Loenneke JP, Thiebaud RS, Fahs CA, Rossow LM, Abe T, Bemben MG (2013) Blood flow restriction does not result in prolonged decrements in torque. Eur J Appl Physiol 4:923–931. doi:10.1007/s00421-012-2502-x
Madarame H, Neya M, Ochi E, Nakazato K, Sato Y, Ishii N (2008) Cross-transfer effects of resistance training with blood flow restriction. Med Sci Sports Exerc 2:258–263. doi:10.1249/mss.0b013e31815c6d7e
Madarame H, Kurano M, Fukumura K, Fukuda T, Nakajima T (2013) Haemostatic and inflammatory responses to blood flow-restricted exercise in patients with ischaemic heart disease: a pilot study. Clin Physiol Funct Imaging 1:11–17. doi:10.1111/j.1475-097X.2012.01158.x
McHugh MP (2003) Recent advances in the understanding of the repeated bout effect: the protective effect against muscle damage from a single bout of eccentric exercise. Scand J Med Sci Sports 2:88–97
Moritani T, Muramatsu S, Muro M (1987) Activity of motor units during concentric and eccentric contractions. Am J Phys Med 6:338–350
Nardone A, Romano C, Schieppati M (1989) Selective recruitment of high-threshold human motor units during voluntary isotonic lengthening of active muscles. J Physiol 409:451–471
Newham DJ, McPhail G, Mills KR, Edwards RH (1983) Ultrastructural changes after concentric and eccentric contractions of human muscle. J Neurol Sci 1:109–122
Newham DJ, Jones DA, Clarkson PM (1987) Repeated high-force eccentric exercise: effects on muscle pain and damage. J Appl Physiol 4:1381–1386
Nielsen JL, Aagaard P, Bech RD, Nygaard T, Hvid LG, Wernbom M, Suetta C, Frandsen U (2012) Proliferation of myogenic stem cells in human skeletal muscle in response to low-load resistance training with blood flow restriction. J Physiol Pt 17:4351–4361. doi:10.1113/jphysiol.2012.237008
Nosaka K, Clarkson PM (1995) Muscle damage following repeated bouts of high force eccentric exercise. Med Sci Sports Exerc 9:1263–1269
Nosaka K, Clarkson PM (1996) Changes in indicators of inflammation after eccentric exercise of the elbow flexors. Med Sci Sports Exerc 8:953–961
Nosaka K, Sakamoto K, Newton M, Sacco P (2001) How long does the protective effect on eccentric exercise-induced muscle damage last? Med Sci Sports Exerc 9:1490–1495
Ohta H, Kurosawa H, Ikeda H, Iwase Y, Satou N, Nakamura S (2003) Low-load resistance muscular training with moderate restriction of blood flow after anterior cruciate ligament reconstruction. Acta Orthop Scand 1:62–68. doi:10.1080/00016470310013680
Pasiakos SM, Carbone JW (2014) Assessment of skeletal muscle proteolysis and the regulatory response to nutrition and exercise. IUBMB Life 7:478–484. doi:10.1002/iub.1291
Raastad T, Owe SG, Paulsen G, Enns D, Overgaard K, Crameri R, Kiil S, Belcastro A, Bergersen L, Hallen J (2010) Changes in calpain activity, muscle structure, and function after eccentric exercise. Med Sci Sports Exerc 1:86–95. doi:10.1249/MSS.0b013e3181ac7afa
Stupka N, Tarnopolsky MA, Yardley NJ, Phillips SM (2001) Cellular adaptation to repeated eccentric exercise-induced muscle damage. J Appl Physiol (1985) 4:1669–1678
Suga T, Okita K, Morita N, Yokota T, Hirabayashi K, Horiuchi M, Takada S, Omokawa M, Kinugawa S, Tsutsui H (2010) Dose effect on intramuscular metabolic stress during low-intensity resistance exercise with blood flow restriction. J Appl Physiol (1985) 6:1563–1567. doi:10.1152/japplphysiol.00504.2009
Takahashi H, Kuno S, Miyamoto T, Yoshioka H, Inaki M, Akima H, Katsuta S, Anno I, Itai Y (1994) Changes in magnetic resonance images in human skeletal muscle after eccentric exercise. Eur J Appl Physiol Occup Physiol 5:408–413
Takarada Y, Nakamura Y, Aruga S, Onda T, Miyazaki S, Ishii N (2000) Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. J Appl Physiol (1985) 1:61–65
Thiebaud RS, Yasuda T, Loenneke JP, Abe T (2013) Effects of low-intensity concentric and eccentric exercise combined with blood flow restriction on indices of exercise-induced muscle damage. Interv Med Appl Sci 2:53–59. doi:10.1556/IMAS.5.2013.2.1
Thiebaud RS, Loenneke JP, Fahs CA, Kim D, Ye X, Abe T, Nosaka K, Bemben MG (2014) Muscle damage after low-intensity eccentric contractions with blood flow restriction. Acta Physiol Hung 2:150–157. doi:10.1556/APhysiol.101.2014.2.3
Umbel JD, Hoffman RL, Dearth DJ, Chleboun GS, Manini TM, Clark BC (2009) Delayed-onset muscle soreness induced by low-load blood flow-restricted exercise. Eur J Appl Physiol 6:687–695. doi:10.1007/s00421-009-1175-6
Vissing K, Overgaard K, Nedergaard A, Fredsted A, Schjerling P (2008) Effects of concentric and repeated eccentric exercise on muscle damage and calpain–calpastatin gene expression in human skeletal muscle. Eur J Appl Physiol 3:323–332. doi:10.1007/s00421-008-0709-7
Warren GL, Lowe DA, Armstrong RB (1999) Measurement tools used in the study of eccentric contraction-induced injury. Sports Med 1:43–59
Wernbom M, Jarrebring R, Andreasson MA, Augustsson J (2009) Acute effects of blood flow restriction on muscle activity and endurance during fatiguing dynamic knee extensions at low load. J Strength Cond Res 8:2389–2395. doi:10.1519/JSC.0b013e3181bc1c2a
Wernbom M, Paulsen G, Nilsen TS, Hisdal J, Raastad T (2012) Contractile function and sarcolemmal permeability after acute low-load resistance exercise with blood flow restriction. Eur J Appl Physiol 6:2051–2063. doi:10.1007/s00421-011-2172-0
Wilson JM, Lowery RP, Joy JM, Loenneke JP, Naimo MA (2013) Practical blood flow restriction training increases acute determinants of hypertrophy without increasing indices of muscle damage. J Strength Cond Res 11:3068–3075. doi:10.1519/JSC.0b013e31828a1ffa
Zhang BT, Yeung SS, Allen DG, Qin L, Yeung EW (2008) Role of the calcium–calpain pathway in cytoskeletal damage after eccentric contractions. J Appl Physiol (1985) 1:352–357. doi:10.1152/japplphysiol.90320.2008
Acknowledgments
The study was funded by Helga and Peter Kornings Foundation and by the Danish Council for Independent Research (FSS). We acknowledge the expert technical assistance provided by Janni Mosgaard Jensen and Gitte Kaiser Hartvigsen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by William J. Kraemer.
Rights and permissions
About this article
Cite this article
Sieljacks, P., Matzon, A., Wernbom, M. et al. Muscle damage and repeated bout effect following blood flow restricted exercise. Eur J Appl Physiol 116, 513–525 (2016). https://doi.org/10.1007/s00421-015-3304-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-015-3304-8