Abstract
We performed two experiments to describe the magnitude of delayed-onset muscle soreness (DOMS) associated with blood flow restriction (BFR) exercise and to determine the contribution of the concentric (CON) versus eccentric (ECC) actions of BFR exercise on DOMS. In experiment 1, nine subjects performed three sets of unilateral knee extension BFR exercise at 35% of maximal voluntary contraction (MVC) to failure with a thigh cuff inflated 30% above brachial systolic pressure. Subjects repeated the protocol with the contralateral limb without flow restriction. Resting soreness (0–10 scale) and algometry (pain–pressure threshold; PPT) were assessed before and 24, 48 and 96 h post-exercise. Additionally, MVC and vastus lateralis cross-sectional area (CSA) were measured as indices of exercise-induced muscle damage. At 24-h post-exercise, BFR exercise resulted in more soreness than exercise without BFR (2.8 ± 0.3 vs 1.7 ± 0.5) and greater reductions in PPT (15.2 ± 1.7 vs. 20 ± 2.3 N) and MVC (14.1 ± 2.5% decrease vs. 1.5 ± 4.5% decrease) (p ≤ 0.05). In experiment 2, 15 different subjects performed three sets of unilateral BFR exercise at 35% MVC with one limb performing only the CON action and the contralateral performing the ECC action. The aforementioned indices of DOMS were assessed before exercise and 24, 48 and 96 h post-exercise. At 24 h post-exercise, CON BFR exercise resulted in more resting soreness than ECC BFR exercise (3.0 ± 0.5 vs. 1.6 ± 0.4), and a greater decrease in MVC (9.8 ± 2.7% decrease vs. 3.4 ± 2.5% decrease) (p ≤ 0.05). These data suggest that knee extension BFR exercise induces mild DOMS and that BFR exercise elicits muscle damage under atypical conditions with low-tension concentric contractions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has previously been reported that resistance exercise performed at loads as low as 20–30% of maximal strength with a moderate degree of blood flow restriction (BFR), induced via a compression cuff on the upper thigh, serves as a potent stimulus for muscle protein synthesis and growth hormone release (Fujita et al. 2007; Pierce et al. 2006). Additionally, BFR exercise training performed chronically has been shown to increase skeletal muscle mass and strength (Abe et al. 2006; Clark et al. 2008). As such, BFR exercise has gained much attention for compensatory muscle adaptation with low mechanical stress that would be clinically valuable in many conditions where more traditional high-load resistance exercise paradigms are contraindicated or not feasible (i.e., conditions with compromised musculotendinous integrity) (Manini and Clark 2009).
We have anecdotally observed that BFR exercise, which incorporates both concentric (CON; shortening) and eccentric (ECC; lengthening) muscle actions, results in delayed-onset muscle soreness (DOMS) (Clark et al. 2008), and it has been suggested that the DOMS associated with this type of training may limit its potential use (Wernbom et al. 2006). At present, there has been very little systematic investigation documenting the DOMS response following BFR exercise. Accordingly, the first aim of the present work was to quantify the amount and timeline of BFR exercise-induced DOMS and to compare these responses to the same exercise protocol performed under normal circulatory conditions (no blood flow restriction).
The mechanism(s) underlying DOMS is (are) generally attributed to the physical breakdown of muscle fibers induced via high force muscle contractions involving a heavy component of ECC muscle actions (Abraham 1977; Armstrong 1990; Black et al. 2008; Newham et al. 1983). While there are numerous differences between CON and ECC muscle actions, two of the most prominent differences involve their respective metabolic cost and neural activation strategy. For example, the metabolic efficiency of CON actions is proportional to the mechanical power generated, but the cost of ECC actions is reduced compared to CON actions (Menard et al. 1991). Additionally, CON actions are well documented as requiring more muscle activation than ECC actions, as evidenced by an increased amplitude of the electromyogram signal (Enoka et al. 2003). The ECC action of exercise results in more force per recruited fiber, resulting in greater mechanical muscle damage and increased DOMS (Clarkson and Hubal 2002; Lieber and Friden 2002; Newham et al. 1987). CON exercise, on the other hand, does not normally result in a significant amount of DOMS (Nosaka and Newton 2002). This assertion was recently supported by increasing specific force using electrical stimulation, which caused more muscle soreness and swelling than a paradigm with the same force level distributed over a larger muscle area (Black and McCully 2008). The observation that low-load BFR exercise that does not employ an excessive ECC action per se causes DOMS is contradictory to that stated in literature. Therefore, the second aim of the present work was to quantify the amount and timeline of muscle soreness and its associated parameters of reduced muscle strength and muscle swelling following BFR exercise performed with CON only actions when compared to ECC only actions.
Methods
General overview of experimental design
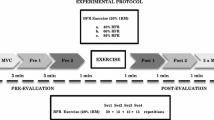
We conducted two experiments. In experiment 1, subjects performed three sets of knee extension resistance exercise with 90-s rest between each set. During experiment 1, the contractions were performed with both the CON and ECC action. The exercise load was 35% of MVC. One of the legs was randomly assigned to perform the exercise task under conditions of BFR. Here, an air compression cuff, located on the proximal thigh was inflated to 30% above the subject’s systolic blood pressure and remained elevated for the duration of the exercise protocol (during exercise sets and the recovery periods). During each set, subjects performed as many contractions with a CON and ECC action as possible to task failure. At the completion of the third set, the subjects were provided a 10-min rest period and then repeated the same protocol on the contralateral limb, except that the compressive cuff was not applied and the subjects performed the same number of repetitions in each set as they did with their limb that performed the BFR exercise. Prior to performing the exercise protocol, and 24, 48 and 96 h after this exercise bout, subjects reported to the laboratory facilities and the following outcome variables were measured: (1) soreness at rest (based on a 0–10 rating scale), (2) pain–pressure threshold (PPT), MVC (to determine functional deficits), and vastus lateralis muscle cross-sectional area (CSA; to assess muscle swelling). In experiment 2, subjects performed an identical protocol as described above, except that they performed BFR exercise with each leg independently (both legs were occluded). One limb performed the CON only action to failure, followed by the other limb performing the same number of repetitions with an ECC action.
Subjects
Nine subjects participated in experiment 1 (7 men, 2 women; 25 ± 5 years, age range: 20–34 years) and 15 different subjects participated in experiment 2 (8 men, 7 women; 23 ± 6 years, range of 18–33 years). Sample sizes were determined by a power analysis designed to detect differences in MVC loss 24-h following the exercise bouts (power = 0.80, two-sided alpha = 0.05). The assumptions on which these calculations were performed were based on pilot data from our laboratory. We chose to power the study for our MVC data as we had the most preliminary data on the reliability of this measurement (Clark et al. 2007). All subjects signed an informed consent approved by the Ohio University Institutional Review Board. Subjects were excluded if they were taking any medications, had performed resistance exercise training in the last 6 months, participated in other types of regular exercise (self-reported participation >60 min per week) or smoked in the previous 6 months. Additionally, participants were excluded if their thighs exceeded the diameter of the cuff (80 cm), if they had hypertension (resting systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg), or had any orthopedic or co-morbid conditions that could limit their ability to perform knee extension exercises. Subjects were required to refrain from strenuous exercise 24 h before the initial exercise session and throughout the course of the study. They were also prohibited from taking any analgesic medication during the study to ensure the accuracy of their perceived muscle soreness.
Resistance exercise protocol for experiment 1
Subjects participating in experiment 1 performed isotonic knee extension with each leg at a load equal to 35% of MVC of the respective leg (described in detail below). During the knee extension exercise task, subjects were seated in a dynamometer (MedX, Ocala, FL, USA), which allowed for strict control of hip and knee joint angles. The backrest was adjusted to sit the subject at a flexed hip position at 80° from the anatomical position and a seat belt was secured to prevent any movement of the hip joint. One leg was randomly chosen to perform knee extension exercise with BFR at the proximal thigh (6 × 83 cm SC5 tourniquet cuff inflated via an E20 Rapid Cuff Inflator, DE Hokanson, Inc., Bellevue, WA) set at 30% above systolic blood pressure (SBP). SBP was determined under resting conditions while the subject sat positioned in the knee extension dynamometer, but before any exercise was performed. Subjects performed a total of three sets, and during each set they exercised to volitional failure [defined as the inability to complete the full range of motion (a 90° range of motion ranging between 108 to 18° of full knee extension)]. During exercise, the non-exercising leg was extended and rested on a pad located under the plantar flexors. Subjects performed a 2-s concentric (shortening) contraction and a 2-s eccentric (lengthening) contraction. The subjects were given a 90-s rest period between each set. The pressure cuff was inflated 10 s before the start of exercise and remained inflated until completion of the protocol. On the contralateral limb, subjects performed the same isotonic knee extension exercise at 35% MVC without BFR. During exercise, they performed the same number of repetitions in each set as the blood flow-restricted limb. The majority of published studies on BFR exercise have used exercise intensities between 20 and 50% of maximal strength and cuff pressures between 30 and 60% above SBP (~160–200 mmHg for normotensive individuals). Accordingly, we chose our intensity (35%) and cuff pressure (30% above SBP) to allow for consistency with and greater generalizability to the current BFR exercise literature. This cuff pressure has been reported to reduce superficial femoral artery blood flow at rest ~30% (Takano et al. 2005), and BFR exercise at intensities ranging between 20 and 40% of maximal strength have been demonstrated to result in muscle fatigue comparable to that of high-intensity exercise (Cook et al. 2007).
Resistance exercise protocol for experiment 2
Subjects participating in experiment 2 performed isotonic knee extension with BFR in a similar method to that described for experiment 1. However, in experiment 2, the subjects performed CON actions only at 35% MVC, while the other leg performed ECC actions only at the same exercise load. During these protocols, the subjects performed their respective action in 2 s, and the investigators manually performed the opposite muscle action through the use of a lever arm attached to the weight stack in 2 s. During this experiment, each subject performed three sets to volitional failure with the CON action limb, followed by performing the same number of repetitions with the ECC action limb.
Outcome variables
Resting muscle soreness
Subjects were asked to quantify their general level of quadriceps muscle soreness on a verbal analog 0–10 scale at the beginning of each testing session. The value of 10 was anchored as “so sore you can’t move”. The value of 0 was anchored as “no pain or soreness” (Nieman et al. 2006). During this assessment, subjects lay prone and were asked to base their rating on their general perceived soreness associated with the activities they had performed prior to coming to the laboratory (e.g., climbing stairs, walking, etc.).
Pain–pressure threshold soreness
A pressure algometer (Wagner FDN, Greenwich, CT, USA) was used to apply a gradually increasing force to the vastus lateralis (VL) at the midpoint between the fibular head and the iliac crest at rest while each patient was supine. This measurement location was several centimeters distal to the location where the compression cuff was applied. During the PPT test, we used an algometer with a 1-cm2 hard rubber tip, and we took great care to directly apply pressure in the perpendicular plane and at a gradually increasing constant rate (~4 N/s). The subject was instructed to identify to the investigator the moment the pressure generated “slightly uncomfortable pain” and the force at this point was recorded (Andersen et al. 2006). Two trials were obtained and averaged to represent the PPT. The coefficient of variation (CV) for the measurement error in our laboratory of assessing PPT on two separate days is 2.5 ± 1.5%.
Maximal voluntary contraction
Subjects in each group performed unilateral MVCs with the knee positioned 45° from full extension and the hip positioned at 110° in a knee extension dynamometer (MedX, Ocala, FL, USA). Exerted forces were measured by a force transducer (model U1T, HBM Inc., Marlborough, MA, USA; sensitivity of 0.002 mV/N), amplified and recorded at 1,000 Hz using a 16-bit data acquisition card (MP150, BioPac Systems Inc., Santa Barbara, CA, USA). During all strength testing, strong verbal encouragement was provided by the investigators, and subjects were instructed to increase their force over the first second, and then exert a maximum effort for 2–3 s. Three maximal knee extension contractions were performed with a 1–2-min rest period between efforts. If subjects continually recorded more force with increasing trials, or if the trials were not within 5% of each other, additional trials were performed until a plateau was reached. The trial consisting of the highest value was considered the MVC force. This protocol was repeated on each subsequent visit to monitor muscle strength. The CV for the measurement error in our laboratory for assessing MVC on two separate days is 3.0 ± 0.7%.
Vastus lateralis cross-sectional area (CSA)
B-mode ultrasonography (MyLab25, BiosoundEasote, Inc., Indianapolis, IN) using a 7.5-MHz linear-array probe (LA522E, BiosoundEasote, Inc.) was used to obtain a cross-section axial-plane image of the VL muscle. Prior to imaging, subjects were supine for 15 min to minimize the potential effect of fluid shifts on the area measurements. A panoramic software function was utilized to acquire 2D images by reconstructing consecutive frames (VPAN, BiosoundEasote, Inc.). Prior to scanning, the depth gain was adjusted to optimize the VL field of view. A single image was obtained from each leg halfway between the iliac crest and the lateral epicondyle of the femur with the subject supine and the leg straight and relaxed. The probe was oriented in the axial plane with the transducer being perpendicular to the VL muscle. The scan began at the medial border of the VL and progressed to the lateral border along a pre-marked section on the leg that was surrounded by a foam trough attached to the leg and filled with ~12 mm of conductive gel (REF4238, Chattanooga Group, Hixson, TN), which allowed minimal pressure to be applied and avoided compression of the muscle. The mechanical scan was performed at a velocity of ~2.5 cm/s at a frame sampling rate of 7 Hz. Images were saved for subsequent analysis and imported into digitizing software (MyLabDesk, BiosoundEasote, Inc.) to calculate the VL CSA by manually defining the leading edge of its fascial borders. The CV for the measurement error in our laboratory of manually defining the VL of the same image on different days in ten subjects is 0.8 ± 0.3%, while the error in quantifying the VL CSA from different scan images (error from tracing plus the mechanical sweep) is 2.3 ± 0.5%. Ultrasound-derived measurement of VL CSA was previously validated against magnetic resonance imaging (MRI) (Reeves et al. 2004). Additionally, to evaluate our panoramic reconstruction, we compared VL CSA measurements obtained with the ultrasound to those obtained with magnetic resonance imaging as previously described (Clark et al. 2007) and observed that the respective values were in agreement (percent difference of 0.7%, which easily falls within the range of the measurement error associated with the imaging modalities).
Statistical analysis
Repeated-measures analysis of variance procedures were performed to determine changes between exercise mode over time for the two experiments. The within-subjects factors were exercise protocol (two factors) and time (four factors). Separate analyses were performed for the two experiments (experiment 1: BFR exercise vs. exercise without restriction; experiment 2: CON BFR exercise vs. ECC BFR exercise). Sidak post hoc tests were used to test significant main effects and/or interactions. For all analyses, a preset alpha level of significance equal to 0.05 was required for statistical significance. Eta-squared effect sizes are also reported to aid in the interpretation of the magnitude of effects. The SPSS statistical package (version 14.0, Chicago, IL) was used for data analysis. Data are presented as mean ± SE.
Results
Experiment 1: blood flow-restricted exercise versus exercise without restriction
Performance characteristics
The mean MVC for the BF exercise leg was 751.7 ± 55.1 N and 707.8 ± 54.5 N for the exercise without restriction leg (p > 0.05). The two respective exercise modes were equally matched on an individual subject basis for the number of repetitions performed, with the average number of repetitions to failure being: 45.2 ± 8.6 for the first set, 29.6 ± 3.5 for the second, and 27.6 ± 5.5 for the third. The average amount of time under ischemia for the BFR exercise limb was 9.8 ± 3.1 min.
Resting muscle soreness
We observed an exercise mode by time interaction for resting muscle soreness (p = 0.04; eta-squared = 0.51; Fig. 1a). Follow-up analysis revealed that mean resting muscle soreness 24 h after exercise increased from 0.1 ± 0.1 to 2.8 ± 0.3 (p < 0.01) and was 2.4 ± 0.6 48-h after exercise in BFR exercise (p = 0.02) with a subsequent return to baseline after 96 h (0.7 ± 0.4; p = 0.45). Exercise without restriction increased from 0.1 ± 0.1 to 1.7 ± 0.5 24-h after exercise (p = 0.05) and returned to baseline 48-h (1.4 ± 0.6; p = 0.20) and 96 h after exercise (0.3 ± 0.3; p = 0.92). Additionally, 24 h after exercise, BFR exercise demonstrated more soreness than exercise without restriction (p = 0.04), but no differences in soreness between the two exercise modes existed at other time points (p > 0.05).
The time course of changes in resting muscle soreness (0–10 scale, a), pain–pressure threshold (b), vastus lateralis cross-sectional area (c), and maximal voluntary contraction (MVC, d) for low-load blood flow-restricted (BFR) exercise compared to low-load exercise without restriction. Significant exercise mode by time interactions were followed up with Sidak post hoc tests, and asterisks based on these analyses are shown as follows: *different from baseline value; †between task differences at the corresponding time point
Pain–pressure threshold soreness
We observed an exercise mode by time interaction for PPT (p < 0.01; eta-squared = 0.44; Fig. 1b). Follow-up analysis revealed that BFR exercise did not alter the PPT at any time point compared to baseline (20.8 ± 2.4 N) [24-h post-exercise, (15.2 ± 1.7 N), p = 0.14; 48-h post-exercise, (17.1 ± 2.2 N), p = 0.54; and 96-h post-exercise, (18.9 ± 2.8 N), p = 0.89]. Exercise without restriction did not alter the PPT at any time point compared to baseline (21.1 ± 2.0 N) [24-h post-exercise, (20 ± 2.3 N), p = 0.99; 48-h post-exercise, (21.6 ± 2.3 N), p = 0.99; 96-h post-exercise, (20.6 ± 3.0 N), p = 0.99]. Differences between the two exercise modes were observed at 24- and 48-h post-exercise, with BFR exercise demonstrating a lower PPT than exercise without restriction (p < 0.01), but no differences were observed between the two exercise modes at baseline or 96-h post-exercise (p > 0.05).
Cross-sectional area
We did not observe an exercise mode by time interaction for VL CSA (p = 0.08; eta-squared = 0.24; Fig. 1c) (BFR exercise: baseline, 26.2 ± 2.3 cm2; 24-h post-exercise, 27.1 ± 2.3 cm2; 48-h post-exercise, 27.3 ± 2.3 cm2; 96-h post-exercise, 26.6 ± 2.3 cm2; exercise without restriction: baseline, 27.5 ± 2.8 cm2; 24-h post-exercise, 27.2 ± 2.7 cm2; 48-h post-exercise, 27.5 ± 2.7 cm2; 96-h post-exercise, 27.4 ± 2.8 cm2). We did observe a time main effect (p = 0.03) indicating that when collapsed across exercise modes, there was a significant increase in VL CSA 24- and 48-h post-exercise (p = 0.02).
Maximum voluntary contraction
We observed an exercise mode by time interaction for MVC (p = 0.01; eta-squared = 0.38; Fig. 1d). Follow-up analysis revealed that BFR exercise did not alter the MVC at any time point compared to baseline (751.8 ± 55.1 N) [24-h post-exercise, (652.7 ± 66.8 N), p = 0.11; 48-h post-exercise, (700.6 ± 78.6 N), p = 0.84; and 96-h post-exercise, (682.0 ± 70.7 N), p = 0.35]. Exercise without restriction did not alter the MVC at any time point compared to baseline (707.9 ± 54.5 N) [24-h post-exercise, (701.2 ± 68.3 N), p = 0.99; 48-h post-exercise, (699.8 ± 64.1 N), p = 0.99; 96-h post-exercise, (698.8 ± 70.8 N), p = 0.99]. Differences between the two exercise modes were observed at 24-h post-exercise, with the BFR exercise demonstrating a greater reduction in MVC than exercise without restriction (p = 0.04, but no differences were observed between the two exercise modes at baseline, 48- or 96-h post-exercise (p > 0.05).
Experiment 2: concentric versus eccentric actions
Performance characteristics
The mean MVC for the CON BFR exercise leg was 643.0 ± 48.2 N and 606 ± 43.8 N for the ECC BFR exercise leg (p > 0.05). The two respective exercise modes were equally matched on an individual subject basis for the number of repetitions performed, with the average number of repetitions to failure being: 62.5 ± 8.7 for the first set, 39.2 ± 7.2 for the second, and 33.5 ± 5.3 for the third. The average amount of time under ischemia for each leg was 12.2 ± 1.4 min.
Resting muscle soreness
We observed a muscle action by time interaction for resting muscle soreness (p < 0.01; eta-squared = 0.34; Fig. 2a). Follow-up analysis revealed that mean resting muscle soreness 24 h after CON BFR exercise increased from 0.1 ± 0.1 to 3.0 ± 0.5 (p < 0.01), remained elevated above baseline after 48 h (1.9 ± 0.6; p = 0.03) and returned to baseline after 96 h (0.5 ± 0.2; p = 0.16). ECC BFR exercise also resulted in increased resting muscle soreness when compared to baseline (0.1 ± 0.1) (24-h post-exercise: 1.6 ± 0.4, p < 0.01), remained elevated above baseline after 48 h (0.9 + 0.3, p = 0.05) and returned to baseline after 96 h (0.4 ± 0.1, p = 0.22). Differences between the two muscle actions were observed at 24- and 48-h post-exercise, with the CON BFR exercise mode demonstrating more soreness than ECC BFR exercise (p < 0.01 and p = 0.04, respectively), but no differences were observed between the two muscle actions at baseline or 96-h post-exercise (p > 0.05).
The time course of changes in resting muscle soreness (0–10 scale, a), pain–pressure threshold (b), vastus lateralis cross-sectional area (c), and maximal voluntary contraction (MVC, d) for blood flow-restricted (BFR) exercise with a concentric action only compared to BFR exercise with an eccentric action only. Significant exercise mode (contraction type) by time interactions were followed up with Sidak post hoc tests, and asterisks based on these analyses are shown as follows: *different from baseline value; †between task differences at the corresponding time point
Pain–pressure threshold soreness
We did not observe a muscle action by time interaction for PPT (p = 0.47; eta-squared = 0.06; Fig. 2a) (CON BFR exercise: baseline, 24.0 ± 2.2 N; 24-h post-exercise, 20.6 ± 2.2 N; 48-h post-exercise, 21.9 ± 2.3 N; 96-h post-exercise, 24.5 ± 2.4 N; ECC BFR exercise: baseline, 25.0 ± 2.7 N; 24-h post-exercise, 22.6 ± 2.5 N; 48-h post-exercise, 23.9 ± 2.7 N; 96-h post-exercise, 25.0 ± 2.6 N). We did observe a time main effect (p < 0.01) indicating that when collapsed across muscle actions, there was a significant increase in PPT 24-h post-exercise (p = 0.05).
Cross-sectional area
We did not observe a muscle action by time interaction for VL CSA (p = 0.31; eta-squared = 0.10; Fig. 2c) (CON BFR exercise: baseline, 23.8 ± 1.7 cm2; 24-h post-exercise, 24.7 ± 2.0 cm2; 48-h post-exercise, 24.5 ± 1.8 cm2; 96-h post-exercise, 24.2 ± 1.8 cm2; ECC BFR exercise: baseline, 23.4 ± 1.3 cm2; 24-h post-exercise, 23.8 ± 1.4 cm2; 48-h post-exercise, 25.3.7 ± 1.5 cm2; 96-h post-exercise, 23.3 ± 1.5 cm2). We did observe a time main effect (p < 0.01) indicating that when collapsed across muscle actions, there was a significant increase in VL CSA 24-h post-exercise (p = 0.05).
Maximum voluntary contraction
We observed an exercise mode by time interaction for MVC (p = 0.04; eta-squared = 0.16; Fig. 2d). Follow-up analysis revealed that MVC was reduced in comparison to baseline (751.8 ± 55.1 N) 24 h after CON BFR exercise (652.7 ± 66.8 N, p = 0.04), but that this difference had returned to baseline 48- and 96-h after exercise [48-h post-exercise, (700.6 ± 78.6 N), p = 0.84; and 96-h post-exercise, (682.0 ± 70.7 N), p = 0.35]. ECC BFR exercise did not alter the MVC at any time point compared to baseline (606.1 ± 43.8 N) [24-h post-exercise, (578.9 ± 39.3 N), p = 0.48; 48-h post-exercise, (591.7 ± 39.5 N), p = 0.95; 96-h post-exercise, (613.6 ± 47.7 N), p = 0.99]. Differences between the two exercise modes were observed at 24- and 48-h post-exercise, with the CON BFR exercise demonstrating a greater reduction in MVC than ECC BFR exercise (p = 0.01 and 0.04, respectively), but no differences were observed between the two exercise modes at baseline or 96-h post-exercise (p > 0.05).
Discussion
The purpose of this work was to characterize the degree of DOMS associated with low-load blood flow-restricted resistance exercise in comparison to low-load exercise without restriction, and to determine the relative contribution of the concentric versus eccentric action of BFR exercise on mediating the DOMS response. The novel finding of our first experiment was that, on average, BFR exercise resulted in subjects reporting mean resting muscle soreness of 2.8 out of 10 at rest in the day following the exercise bout, along with increased sensitivity to pressure, and a 14% loss of muscle strength at 24-h post-exercise; whereas exercise without restriction minimally affected any of these variables. Our follow-up experiment revealed that BFR exercise employing a CON only muscle action results in greater resting muscle soreness and exercise-induced weakness than BFR exercise employing an ECC only muscle action, suggesting that the CON BFR exercise results in somewhat more DOMS than ECC BFR exercise. This observation is particularly interesting, as it is counter to the traditionally touted mechanisms of DOMS, which include high mechanical stress and strain on the muscle that are particularly pronounced during ECC actions. The clinical and scientific implications of this work are discussed below.
Previous work has shown that low-load BFR exercise increases both muscle strength and size in a quantitatively similar fashion to high-load exercise (Clark et al. 2008; Manini and Clark 2009; Takarada et al. 2000). To our knowledge, only one other study has reported the effect of BFR exercise on DOMS, and they reported that four sets of low-load BFR knee extension exercise resulted in subjects reporting DOMS at rest on the magnitude of 5.5 out of a 10-point scale (Wernbom et al. 2006). Interestingly though, the aforementioned study, which utilized trained individuals, also reported that low-load exercise (20–30% MVC) without BFR also resulted in substantial DOMS (7.0 out of 10), which is in contrast to the results on low-load exercise from the present study as well as that reported by others using 30% of MVC to muscular failure (Tiidus and Ianuzzo 1983).The results from the present study suggest that low-load BFR exercise produces comparable soreness to previous experiments involving high-load eccentric exercise (Tiidus and Ianuzzo 1983). However, not all of our indices were significantly altered post-exercise and some demonstrated discrepancies. Specifically, the PPT measurement did not differ between the CON and ECC BFR exercise protocols, although the perceived soreness on a visual analog scale was greater with CON BFR than ECC BFR. It is likely that the indexes that did not show significance have more between-subject variability, lower reliability and/or sensitivity to DOMS. As such, it is possible that we were underpowered to detect differences in all of the indexes of DOMS.
While the exact mechanisms explaining exercise-induced DOMS have not been completely identified, the initial injury is generally ascribed to mechanical disruption of fibers and subsequent damage associated with inflammatory processes, and to changes in excitation–contraction coupling within the muscle (Clarkson and Hubal 2002). It is well documented that ECC muscle actions, especially the ones involving high levels of force, result in both muscle damage and soreness (Clarkson and Hubal 2002; Lieber and Friden 2002). As such, our finding of low-load BFR exercise with a CON action causing more DOMS than the ECC protocol suggests that the mechanism(s) of DOMS associated with BFR exercise may be different from those associated with high-force ECC actions. While the present study did not assess the mechanisms of DOMS, a brief discussion of the potential regulators is warranted.
The typical explanation for ECC contractions causing more DOMS than CON or isometric contractions is related to mechanical stress and insult (Black and McCully 2008; Clarkson and Hubal 2002; Lieber and Friden 2002). This is attributed to ECC actions requiring less fiber recruitment than CON actions at the same absolute load (Enoka et al. 2003), thus resulting in greater load per individual fiber (increased stress). Recent evidence suggests that this mechanical stress results in cytoskeletal damage and membrane disruption that are mediated primarily by increases in Ca2+ influx into muscle cells and subsequent activation of calpain (a proteolytic enzyme) (Zhang et al. 2008). Based on the CON action in the present study resulting in greater DOMS, it seems unlikely that the mechanical stress mediated the DOMS response. However, ischemia–reperfusion, another paradigm resulting in muscle damage, has also been linked to a similar phenomenon as Ca2+ influx into cells in a variety of organs (Deshpande et al. 1987; Faust et al. 1988; Hossmann et al. 1983). Skeletal muscles are generally thought to tolerate up to 2.5 h of global ischemia with minimal risk of injury (Pang et al. 1995), and models of ischemia–reperfusion injury in skeletal muscle are in the order of 4–6 h (Harris et al. 1986; Idstrom et al. 1990). Thus, at first glance the 10–15 min of ischemia experienced associated with BFR exercise appears too short to induce ischemia–reperfusion injury. However, typical studies on ischemia–reperfusion injury are performed in non-contracting skeletal muscle, which has a very low rate of ATP utilization, and when electrical stimulation is used to increase the rate of energy depletion, the time required to induce ischemia–reperfusion is dramatically expedited (e.g., 5–20 min of ischemia) (Welsh and Lindinger 1993). Accordingly, it seems probable that the BFR exercise model results in ischemia–reperfusion injury and may cause DOMS. Additionally, ischemia–reperfusion injury is associated with a dramatic increase in reactive oxygen species that are thought to be associated with the induction of muscle damage (Murphy and Steenbergen 2008). Reactive oxygen species have also been suggested to be associated with exercise-induced DOMS (Close et al. 2004). With respect to BFR exercise, the coupling of hypoxia with high levels of NADH and FADH2 from glucose substrate metabolism, as would be expected in the present study, serves as a potent stimulus for reactive oxygen species production (Clanton 2007). Accordingly, it is possible that the combination of the BFR coupled with exercise in the present model resulted in an interaction of these proposed mechanisms contributing to DOMS.
Other possible explanations of the development of DOMS following BFR exercise could be related to compression injury from the application of the cuff or the enhanced metabolic acidosis (Abe et al. 2006; Fujita et al. 2007). It seems unlikely that the former explanation plays a role as we measured the PPT and muscle CSA several centimeters distal to where the compression cuff was applied; however, further study of placing a cuff proximal to the exercising muscles would be required to eliminate this possibility. With respect to the latter, lactic acid accumulation was a popular theory for the etiology of DOMS historically (Francis 1983), but in more recent years, its mechanistic role in DOMS has been diminished (Miles and Clarkson 1994).
There are several limitations and delimitations of the present work. For example, we took a reductionist approach and controlled for the number of contractions performed in each experiment. Because we were interested in determining the absolute effect of BFR, we chose to standardize the number of repetitions between the limbs (e.g., the control limb did the same number of repetitions as the BFR exercise limb). It is difficult to know, but our findings could have been different if we had standardized the protocols on a relative basis and had both legs exercise until task failure. Another potential limitation of the present work was that we did not standardize our muscle soreness outcome to a given task (e.g., stair climbing). It is possible that a more standardized stimulus could have affected the absolute magnitude of perceived soreness. Additionally, as we wished to standardize the exercise protocols based on absolute mechanical work, in both experiments we had to have the subjects perform one given protocol first. As such, we could not randomize the order of the exercise protocols, and it is possible that an order effect could have affected our outcome measures.
In summary, this study characterized the effect of BFR exercise on DOMS. We observed that, on average, BFR exercise resulted in subjects reporting a mean soreness of 2.8 out of 10 at rest in the day following the exercise bout, along with increased sensitivity to pressure, and a loss of muscle strength, whereas low-load exercise minimally affected these variables. Additionally, we evaluated the role of the CON and ECC muscle actions in contributing to the BFR exercise-induced DOMS. We found that BFR exercise employing a CON only muscle action results in a greater amount of resting muscle soreness and loss of muscle strength than ECC BFR exercise. This observation is particularly interesting as it is counter to the traditionally touted mechanisms of exercise-induced DOMS, which is associated with high mechanical stress on the muscle that is particularly pronounced during ECC actions (Black et al. 2008; Black and McCully 2008; Clarkson and Hubal 2002; Lieber and Friden 2002; Newham et al. 1987).
References
Abe T, Kearns CF, Sato Y (2006) Muscle size and strength are increased following walk training with restricted venous blood flow from the leg muscle, Kaatsu-walk training. J Appl Physiol 100:1460–1466
Abraham WM (1977) Factors in delayed muscle soreness. Med Sci Sports 9:11–20
Andersen H, Arendt-Nielsen L, Danneskiold-Samsoe B, Graven-Nielsen T (2006) Pressure pain sensitivity and hardness along human normal and sensitized muscle. Somatosens Mot Res 23:97–109
Armstrong RB (1990) Initial events in exercise-induced muscular injury. Med Sci Sports Exerc 22:429–435
Black CD, McCully KK (2008) Force per active area and muscle injury during electrically stimulated contractions. Med Sci Sports Exerc 40:1596–1604
Black CD, Elder CP, Gorgey A, Dudley GA (2008) High specific torque is related to lengthening contraction-induced skeletal muscle injury. J Appl Physiol 104:639–647
Clanton TL (2007) Hypoxia-induced reactive oxygen species formation in skeletal muscle. J Appl Physiol 102:2379–2388
Clark BC, Cook SB, Ploutz-Snyder LL (2007) Reliability of techniques to assess human neuromuscular function in vivo. J Electromyogr Kinesiol 17:90–101
Clark BC, Manini TM, Guiler M, Williams PS, Hoffman RL, Kushnick MR (2008) Relative safety and efficacy of blood flow-restricted resistance exercise. The Physiologist 51:4.2
Clarkson PM, Hubal MJ (2002) Exercise-induced muscle damage in humans. Am J Phys Med Rehabil 81:S52–S69
Close GL, Ashton T, Cable T, Doran D, MacLaren DP (2004) Eccentric exercise, isokinetic muscle torque and delayed-onset muscle soreness: the role of reactive oxygen species. Eur J Appl Physiol 91:615–621
Cook SB, Clark BC, Ploutz-Snyder LL (2007) Effects of exercise load and blood-flow restriction on skeletal muscle function. Med Sci Sports Exerc 39:1708–1713
Deshpande JK, Siesjo BK, Wieloch T (1987) Calcium accumulation and neuronal damage in the rat hippocampus following cerebral ischemia. J Cereb Blood Flow Metab 7:89–95
Enoka RM, Christou EA, Hunter SK, Kornatz KW, Semmler JG, Taylor AM, Tracy BL (2003) Mechanisms that contribute to differences in motor performance between young and old adults. J Electromyogr Kinesiol 13:1–12
Faust KB, Chiantella V, Vinten-Johansen J, Meredith JH (1988) Oxygen-derived free radical scavengers and skeletal muscle ischemic/reperfusion injury. Am Surg 54:709–719
Francis K (1983) Delayed muscle soreness: a review. J Orthop Sports Phys Ther 5:10–13
Fujita S, Abe T, Drummond MJ, Cadenas JG, Dreyer HC, Sato Y, Volpi E, Rasmussen BB (2007) Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J Appl Physiol 103:903–910
Harris K, Walker PM, Mickle DA, Harding R, Gatley R, Wilson GJ, Kuzon B, McKee N, Romaschin AD (1986) Metabolic response of skeletal muscle to ischemia. Am J Physiol 250:H213–H220
Hossmann KA, Paschen W, Csiba L (1983) Relationship between calcium accumulation and recovery of cat brain after prolonged cerebral ischemia. J Cereb Blood Flow Metab 3:346–353
Idstrom JP, Soussi B, Elander A, Bylund-Fellenius AC (1990) Purine metabolism after in vivo ischemia and reperfusion in rat skeletal muscle. Am J Physiol 258:H1668–H1673
Lieber RL, Friden J (2002) Mechanisms of muscle injury gleaned from animal models. Am J Phys Med Rehabil 81:S70–S79
Manini TM, Clark BC (2009) Blood flow-restricted exercise and skeletal muscle health. Exerc Sport Sci Rev 37:78–85
Menard MR, Penn AM, Lee JW, Dusik LA, Hall LD (1991) Relative metabolic efficiency of concentric and eccentric exercise determined by 31P magnetic resonance spectroscopy. Arch Phys Med Rehabil 72:976–983
Miles MP, Clarkson PM (1994) Exercise-induced muscle pain, soreness, and cramps. J Sports Med Phys Fitness 34:203–216
Murphy E, Steenbergen C (2008) Mechanisms underlying acute protection from cardiac ischemia–reperfusion injury. Physiol Rev 88:581–609
Newham DJ, Mills KR, Quigley BM, Edwards RH (1983) Pain and fatigue after concentric and eccentric muscle contractions. Clin Sci (Lond) 64:55–62
Newham DJ, Jones DA, Clarkson PM (1987) Repeated high-force eccentric exercise: effects on muscle pain and damage. J Appl Physiol 63:1381–1386
Nieman DC, Henson DA, Dumke CL, Oley K, McAnulty SR, Davis JM, Murphy EA, Utter AC, Lind RH, McAnulty LS, Morrow JD (2006) Ibuprofen use, endotoxemia, inflammation, and plasma cytokines during ultramarathon competition. Brain Behav Immun 20:578–584
Nosaka K, Newton M (2002) Concentric or eccentric training effect on eccentric exercise-induced muscle damage. Med Sci Sports Exerc 34:63–69
Pang CY, Yang RZ, Zhong A, Xu N, Boyd B, Forrest CR (1995) Acute ischaemic preconditioning protects against skeletal muscle infarction in the pig. Cardiovasc Res 29:782–788
Pierce JR, Clark BC, Ploutz-Snyder LL, Kanaley JA (2006) Growth hormone and muscle function responses to skeletal muscle ischemia. J Appl Physiol 101:1588–1595
Reeves ND, Maganaris CN, Narici MV (2004) Ultrasonographic assessment of human skeletal muscle size. Eur J Appl Physiol 91:116–118
Takano H, Morita T, Iida H, Asada K, Kato M, Uno K, Hirose K, Matsumoto A, Takenaka K, Hirata Y, Eto F, Nagai R, Sato Y, Nakajima T (2005) Hemodynamic and hormonal responses to a short-term low-intensity resistance exercise with the reduction of muscle blood flow. Eur J Appl Physiol 95:65–73
Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, Ishii N (2000) Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol 88:2097–2106
Tiidus PM, Ianuzzo CD (1983) Effects of intensity and duration of muscular exercise on delayed soreness and serum enzyme activities. Med Sci Sports Exerc 15:461–465
Welsh DG, Lindinger MI (1993) Energy metabolism and adenine nucleotide degradation in twitch-stimulated rat hindlimb during ischemia–reperfusion. Am J Physiol 264:E655–E661
Wernbom M, Augustsson J, Thomee R (2006) Effects of vascular occlusion on muscular endurance in dynamic knee extension exercise at different submaximal loads. J Strength Cond Res 20:372–377
Zhang BT, Yeung SS, Allen DG, Qin L, Yeung EW (2008) Role of the calcium–calpain pathway in cytoskeletal damage after eccentric contractions. J Appl Physiol 105:352–357
Acknowledgments
This study was supported in part by grants from the Ohio University Research Committee (to BC Clark) and the Ohio University College of Osteopathic Medicine (research fellowship to JD Umbel).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Umbel, J.D., Hoffman, R.L., Dearth, D.J. et al. Delayed-onset muscle soreness induced by low-load blood flow-restricted exercise. Eur J Appl Physiol 107, 687–695 (2009). https://doi.org/10.1007/s00421-009-1175-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-009-1175-6