Abstract
Carnosine is an abundant dipeptide in human skeletal muscle with proton buffering capacity. There is controversy as to whether training can increase muscle carnosine and thereby provide a mechanism for increased buffering capacity. This study investigated the effects of 5 weeks sprint training combined with a vegetarian or mixed diet on muscle carnosine, carnosine synthase mRNA expression and muscle buffering capacity. Twenty omnivorous subjects participated in a 5 week sprint training intervention (2–3 times per week). They were randomized into a vegetarian and mixed diet group. Measurements (before and after the intervention period) included carnosine content in soleus, gastrocnemius lateralis and tibialis anterior by proton magnetic resonance spectroscopy (1H-MRS), true-cut biopsy of the gastrocnemius lateralis to determine in vitro non-bicarbonate muscle buffering capacity, carnosine content (HPLC method) and carnosine synthase (CARNS) mRNA expression and 6 × 6 s repeated sprint ability (RSA) test. There was a significant diet × training interaction in soleus carnosine content, which was non-significantly increased (+11%) with mixed diet and non-significantly decreased (−9%) with vegetarian diet. Carnosine content in other muscles and gastrocnemius buffer capacity were not influenced by training. CARNS mRNA expression was independent of training, but decreased significantly in the vegetarian group. The performance during the RSA test improved by training, without difference between groups. We found a positive correlation (r = 0.517; p = 0.002) between an invasive and non-invasive method for muscle carnosine quantification. In conclusion, this study shows that 5 weeks sprint training has no effect on the muscle carnosine content and carnosine synthase mRNA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Muscle buffering capacity is an important mechanism related to high-intensity exercise performance. A positive correlation exists between muscle buffering capacity and high-intensity exercises performance (Bishop et al. 2004; Edge et al. 2006b; Nevill et al. 1989). Cross-sectional studies have shown that athletes who are regularly involved in anaerobic high-intensity sprint-type activities have a higher muscle buffering capacity than endurance athletes or untrained subjects (Sahlin and Henriksson 1984; Parkhouse et al. 1985; Edge et al. 2006b). The in vivo muscle buffering capacity is a dynamic buffering system that includes not only the in vitro muscle buffer capacity, but also the bicarbonate/CO2 buffer system, and the transsarcolemmal ion fluxes (Juel 1998). Despite the limited number of studies, it is generally accepted that training can lead to an increased in vivo muscle buffer capacity (Sharp et al. 1986; Troup et al. 1986; Juel 1998). This increase could possibly be explained by an increased lactate/H+ co-transport; due to an increased expression of monocarboxylate transporters and Na+/H+ exchange proteins (Bishop et al. 2008; Juel et al. 2004; Iaia et al. 2008; Juel 1998). The in vitro muscle buffering capacity is the physicochemical buffer capacity, including muscle proteins, inorganic phosphate and histidine-containing dipeptides (HCD), of which carnosine, with a pKa of 6.83, is the main buffer in humans (Parkhouse and McKenzie 1984; Hirakoba 1999; Hultman and Sahlin 1980). For in vitro muscle buffering capacity, there is less consistency about what kind of training can increase the muscle buffering capacity. Some researchers find an increased in vitro muscle buffering capacity following a chronic training program in humans (Bell and Wenger 1988; Edge et al. 2006a; Gibala et al. 2006; Weston et al. 1997) others find no effect (Edge et al. 2006c; Harmer et al. 2000; Mannion et al. 1994; Nevill et al. 1989; Pilegaard et al. 1999) and Bishop et al. (2008, 2009) indicate even a decrease of buffering capacity due to some types of training. There is still no consensus on what specific training modality leads to an increased in vitro muscle buffering capacity. To find an answer to this question, it is necessary to know which component(s) of the in vitro muscle buffering capacity are sensitive to training stimuli. The metabolite carnosine has been suggested as a candidate (Juel 1998; Parkhouse and McKenzie 1984).
Carnosine (beta-alanyl-l-histidine) is a cytoplasmic dipeptide present in relatively high concentrations in the human skeletal muscle (Harris et al. 1990; Derave et al. 2010). It is synthesized from the precursors l-histidine and beta-alanine by carnosine synthase. Parkhouse et al. (1985) showed a positive correlation (r = 0.69) between muscle carnosine content and the in vitro muscle buffering capacity. The increased muscle buffering capacity of sprinters, compared with marathon runners or untrained subjects, is associated with their higher skeletal muscle carnosine concentrations. According to Harris et al. (1990), carnosine could contribute to ~7% of the total in vitro muscle buffering capacity, in humans, although this is probably an underestimate.
It has been suggested that training effects on in vitro muscle buffer capacity are related to an increase in carnosine (Juel 1998; Parkhouse and McKenzie 1984). However, there is still no consensus about the impact of training intervention on the muscle carnosine content. One study from Suzuki et al. (2004) found a doubling in muscle carnosine due to 8 weeks sprint training when untrained subjects performed two times a week 1 or 2 Wingates (30 s all-out sprints) during 8 weeks. Most other studies, however, did not find an effect of a chronic training program on the muscle carnosine content (Kendrick et al. 2008; Kendrick et al. 2009; Mannion et al. 1994). In the study of Kendrick et al. 2009, for example, 14 Vietnamese students undertook a 4 week isokinetic one-leg training program. The muscle carnosine content of the trained leg did not increase significantly (+2.10 ± 4.79 mmol/kg dw). The inconsistency between the currently available studies may possibly be due to the type of training and the selected training protocol.
In the current study, a similar training protocol as in the study of Suzuki et al. (2004) will be used. Therefore, an increase in muscle carnosine can be expected following this training program. Since beta-alanine is the rate limiting precursor of carnosine synthesis (Bakardjiev and Bauer 1994; Dunnett and Harris 1999; Hill et al. 2007) and since it is a natural constituent of meat, fish and poultry (Abe 2000; Boldyrev 2007), this training effect will probably occur only if sufficient substrate for carnosine synthesis is present. Except from the minimal traces of beta-alanine recently found in vegetable oils (Sanchez-Hernandez et al. 2011), beta-alanine containing dipeptides are virtually absent in plants and therefore, chronic vegetarians have lower muscle carnosine content than omnivorous subjects (Harris et al. 2007; Everaert et al. 2010). It can thus be expected that a combination of the above-mentioned training program with a vegetarian diet will probably not lead to an increase in muscle carnosine. Secondly we expected that, if carnosine is an important buffer and increases dramatically, the in vitro muscle buffer capacity and therefore, possibly also the performance during repeated sprint exercise would be more pronounced when training with a mixed diet, compared to a vegetarian diet. To our knowledge, the interaction of diet and sprint training on muscle buffer capacity is unexplored. Third, we aim to investigate whether training induces an upregulation of the carnosine synthase (CARNS) transcript, as this is a likely mechanism by which a training-induced increase in muscle carnosine would take place. Finally the current study will also make a direct comparison between the invasive and non-invasive techniques for measuring muscle carnosine.
Materials and methods
Subjects
Twenty healthy non-vegetarian students (11 males and 9 females) volunteered to participate in this study. They gave their informed consent and the study was approved by the local Ethics Committee (Ghent University Hospital, Belgium). All subjects were physically active (weekly 2–3 h of sport), but were not involved in sport competition or organized training. After the baseline measurements, the subjects were allocated to a vegetarian (VD) and mixed diet (MD) group, matched for gender, age and muscle carnosine concentration at group level. As a result of an injury, there was a drop out of one man in the VD group. The MD group consisted of five females and five males and the VD group was composed of four females and five males. The subjects’ age, weight and height were 21.5 ± 1.7 years, 69.9 ± 10.3 kg and 1.72 ± 0.07 m for MD and 20.8 ± 1.4 years, 60.4 ± 7.3 kg and 1.74 ± 0.11 m for VD group, respectively (all parameters were not significantly different, except weight; p = 0.02).
Study design
Diet
Both groups were asked to follow a specific diet for 5 weeks. All subjects of the MD group followed a beta-alanine-rich diet, which included the daily intake of meat or fish. The ovo-lacto-vegetarians on the other hand did not eat meat and fish, but eggs and dairy products were allowed. In addition, all subjects completed a food diary during three, randomly selected, days. The diaries were analyzed with the Becel nutrition software (Becel institute, BINS V3.0). To prevent a creatine deficiency in the VD group, subjects from both groups, were supplemented with 1 g/day of creatine monohydrate (100% Creapure TM 1000 mg; VerdePharma, Belgium). The beta-alanine consumption by meat and fish consumption was calculated as described before (Baguet et al. 2009).
Training protocol
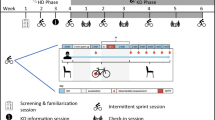
Both groups completed the same sprint training program for 5 weeks. The training frequency was two times a week (minimum 1 day rest in between) during the first 2 weeks and three times a week (Monday, Wednesday and Friday) from week 3. The training program consisted of alternating cycling and running efforts. During the cycling training sessions, subjects performed 1 or 2 (with 20 min rest in between) 30 s all-out cycling sprints (Wingate test) on a mechanically braked cycle ergometer (Monark ergomedic 894Ea, Peak bike). Resistance was set to 7 and 8% of the bodyweight for females and males, respectively. During the running sessions, the students performed 1 or 2 (with a passive rest period of 20 min in between) maximal runs of 200 m.
Measurements
Proton MRS (1H-MRS)
Before and after 5 weeks sprint training, the carnosine content was measured in three skeletal muscles (soleus, gastrocnemius lateralis and tibialis anterior) of the lower leg by proton magnetic resonance spectroscopy (1H-MRS), as previously described (Baguet et al. 2010a). The subjects lay in supine position on their back and the lower leg was fixed in a holder with the angle of the ankle at 20° plantar flexion. All the MRS measurements were performed on a three Tesla whole body MRI scanner (Siemens Trio, Erlangen) equipped with a knee-coil. Single voxel point-resolved spectroscopy (PRESS) sequence with the following parameters was used: repetition time (TR) = 2,000 ms, echo time (TE) = 30 ms, number of excitations = 128, 1,024 data points, spectral bandwidth of 1,200 Hz, and a total acquisition time of 4.24 min. The average voxel size of the soleus, gastrocnemius lateralis and tibialis anterior was 40 × 12 × 28 mm, 40 × 10 × 28 mm and 40 × 13 × 24 mm, respectively. Following shimming procedures, the linewidth of the water signal was on average 26.9, 27.5 and 30.6 Hz for soleus, gastrocnemius lateralis and tibialis anterior, respectively. A 500-ml spherical container filled with an aqueous solution of 20 mM carnosine (Sigma-Aldrich) was used as an external reference for absolute quantification. The following equation was used to determine the carnosine concentration in vivo, based on the C2-H resonance at 8 ppm:
[C m] = [C r] × (S m × V r × C T1r × C T2r × T m)/(S r × V m × C T1m × C T2m × T r)
Where [C m] is the carnosine concentration in vivo; [C r] is the concentration of the external reference phantom (20 mM); S m and S r are the estimated signal peak areas of the muscle and reference phantom, respectively, obtained by curve fitting performed in the frequency domain and were also corrected for differences in coil loading between phantom and the muscle; V m and V r are the volumes of the voxels in vivo and in the phantom, respectively; C T1m, C T2m, C T1r and C T2r are correction factors for the T1 and T2 relaxation times in vivo and in the phantom, respectively; T m and T r are the temperatures in vivo and in the phantom, respectively. The formulae used to calculate the correction factors are:
For the determination of T1 and T2 relaxation times, the carnosine signal was measured with a PRESS sequence by varying TR and TE, respectively, and the resulting peak areas were fitted to mono-exponential functions. The T1 and T2 relaxation times of in vitro carnosine were determined and were found to be 2616 ± 20 ms and 250 ± 29 ms, respectively. For the determination of T1 and T2 relaxation times in vivo five healthy subjects (2 males and 3 females; age: 21 to 26 year) were scanned for the soleus, five (3 males and 2 females; age: 21 to 25 year) for the gastrocnemius lateralis and five for the tibialis anterior muscle (5 females; 22 to 26 year). T1 was measured using a TE of 30 ms and 8 TRs varying from 1,500 ms to 6,000 ms, while T2 was measured using a TR of 4,000 ms and 6 TEs varying from 30 ms to 200 ms. For each measurement 128 averages were acquired. The T1 relaxation times were found to be 1,488 ± 377 ms, 1,771 ± 225 ms and 1,692 ± 432 ms in soleus, gastrocnemius lateralis, and tibialis anterior, respectively, which are similar values as mentioned in Ramadan et al. (2010), a study performed at 7 T. The T2 relaxation times were found to be 152 ± 28 ms, 106 ± 50 ms and 64 ± 32 ms in soleus, gastrocnemius lateralis and tibialis anterior, respectively, which is slightly higher for soleus and gastrocnemius, than in Ramadan et al. (2010), which is expected, since T2 decreases with increasing field strength.
Muscle biopsy
Before and after 5 weeks sprint training muscle biopsies were taken from the right gastrocnemius lateralis at rest, with 14 Gauge true-cut biopsy needle (Bard Magnum Biopsy gun; Bard, Inc., New Jersey, USA). Under ultrasonographic guidance (Ultrasonography Pro Sound SSD-5000, ALOKA Co., Ltd., Tokyo, Japan. with probe UST-5545, frequency 5–13 MHz), two muscle samples (~15 mg) were taken following local anesthesia (lidocaine 1%, Linisol®). The samples were then immediately frozen in liquid nitrogen and stored at −80°C until subsequent analysis.
In vitro muscle buffering capacity; titration method (β m in vitro)
The first muscle sample was freeze-dried and 2.78 ± 0.43 mg was homogenized in a solution containing sodium fluoride (NaF 10 mM) at a dilution of 10 mg dw/ml homogenizing solution. The pH was measured with a microelectrode (MI-415-4 cm pH combination electrode, Microelectrodes inc., USA) connected to a pH meter (WTW series, inoLab 730, VWR Belgium). Sodium hydroxide (NaOH) was added to the homogenates to adjust the initial pH to 7.2. Thereafter, the homogenate was titrated to a pH of 6.1 by serial addition of 4 μl hydrochloric acid (HCl 10 mM). The number of moles H+ (per kg dw) required to change the pH from 7.1 to 6.5 was calculated and normalized to the whole pH unit. The titration was performed twice and the average value of the two measurements was used.
High performance liquid chromatography (HPLC) analysis
An aliquot of 100 μl of the homogenate, to calculate β m in vitro, was deproteinized by adding sulfosalicylic acid (35%, 9:1) and centrifuged at 14,000 g for 5 min. The supernatants (100 μl) were then dried under vacuum (40°C). Dried residues were resolved with 40 μl of coupling reagent: methanol-triethylamine-H2O-phenylisothiocyanate (7:1:1:1) and allowed to react for 20 min at room temperature. The samples were dried again and resolved in 100 μl of sodium acetate buffer (10 mM, pH 6.4). The same method was applied to standard solutions of carnosine (Sigma-Aldrich) with distilled water. The derivatized samples (20 μl) were applied to a Waters HPLC system with an Atlantis HILIC Silica column (4.6 × 150 mm, 5 μm) and UV detector (wavelength 254 nm). The column was equilibrated with buffer A [10 mM sodium acetate adjusted to pH 6.4 with 6% acetic acid] and buffer B [60% acetonitrile—40% buffer A].
Repeated sprint ability test (6 × 6 s RSA)
Before and after the training period, all subjects performed a 6 × 6 s repeated sprint ability test (RSA) on an electromagnetically braked cycle ergometer (Lode, Excalibur sport; Groningen, The Netherlands). The RSA test was preceded by a standardized warm-up including 5 min cycling at 80 W, followed by a passive rest period of 90 s and one practice sprint of 6 s. After warming-up, the subjects rested 5 min before the start of the RSA. The RSA test consisted of six maximal 6 s-sprints with 24 s slow pedaling in between. About 5 s before the start of each sprint the subjects were asked to accelerate until the display showed ~80 RPM. They were allowed to perform in standing position and strong verbal encouragement was provided. The cycle ergometer was used in the linear mode and the linear factor was set at 7 and 8% of the body weight for females and males, respectively.
Lactate measurements
Capillary blood samples (7 μl) were taken from a hyperaemic ear lobe in order to determine the lactate concentration after warm-up, after 6 × 6 s RSA and after 5 min active recovery (Analox instruments Ltd., London, UK).
RNA isolation, cDNA synthesis and real-time-PCR
From the second biopsy, total RNA was isolated using the TriPure Isolation Reagent (Roche) followed by a purification with the RNeasy Mini Kit (Qiagen). An on-column DNase treatment was performed using the RNase‐Free DNase Set (Qiagen). RNA was quantified using a Nonodrop 2000C spectrophotometer (Thermo Scientific) and RNA purity was assessed using the A260/A280 ratio. Using a blend of oligo(dT) and random primers, 150 ng RNA was reversed transcripted with the iScript cDNA Synthesis kit (Biorad), according to the manufacturer’s instructions. RT‐PCR was carried out on a Lightcycler 480 system (Roche) using an 8 μl reaction mix containing 3 μl template cDNA (1/10 dilution), 300 nM forward and reverse primers and 4 μl SYBR Green PCR Master Mix (Applied Biosystems, Belgium). The cycling conditions comprised a polymerase activation at 95°C for 10 min, followed by 45 cycles at 95°C for 15 s, 60°C for 30 s and 72°C for 30 s. Primer sequences of CARNS (gene ID: 57571 forward: GGCGTCAGCAAGAAGTTCGT; reverse: CCGGTGCTCTGTCATGTCAA) were designed using Primer Express software 3.0 (Applied Biosystems). Primer sequences of the reference gene EEF1A1 (gene ID: 1915, forward: CTGGCAAGGTCACCAAGTCT; reverse: CCGTTCTTCCACCACTGAT) were selected from the RTprimerDB site (http://medgen.ugent.be/rtprimerdb/). Sequence specificity was confirmed using NCBI Blast analysis (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Since geNorm ranked EEF1A1 as the best reference gene, this was used to normalize the expression of CARNS and primers. To control the specificity of amplification, data melting curves were inspected and PCR efficiency was calculated ( 99.9 and 102% for CARNS and EEF1A1, respectively). Relative gene expression levels were calculated via the delta delta‐Ct method (Derveaux et al. 2010).
Statistics
All correlations were evaluated by Pearson’s correlations. A 2 × 2 general linear model repeated-measures ANOVA was used to evaluate the muscle carnosine content, the relative CARNS mRNA expression and in vitro muscle buffering capacity, with “diet group” (VD vs. MD) as between-subjects factor and “time” (pre vs. post) as a within-subjects factor. In case of a significant interaction effect, a post hoc analysis was performed. The performance improvement during the 6 × 6 s RSA test was evaluated with a 2 × 6 (diet × sprint number) general linear model repeated-measures ANOVA. An independent sample t test was used for the nutritional analysis (VD vs. MD) and a paired sample t test was used to analyze lactate values (SPSS statistical software, SPSS 16.0, Chicago, IL). Values are presented as means ± SD, and significance was assumed at p ≤ 0.05. The normalized mRNA expressions were log transformed for further analysis.
Results
Muscle carnosine content
Before (pre) and after (post) the training period the muscle carnosine content was measured in three lower leg muscles by 1H-MRS. The baseline carnosine concentrations of the soleus (p = 0.712), gastrocnemius lateralis (p = 0.944) and tibialis anterior (p = 0.969) were not significantly different between the MD and VD group. Males had a significant higher muscle carnosine content compared to females (soleus: p < 0.001, gastrocnemius: p = 0.01, tibialis: p < 0.001). 5 weeks sprint training resulted in a significant interaction effect (p = 0.020) in the soleus (Fig. 1). The post hoc analysis showed that the carnosine content of the soleus was non-significantly increased (+11%) in the MD group and non-significantly decreased (−9%) with VD. The effects of training and diet on the carnosine content of the gastrocnemius lateralis muscle were measured by both 1H-MRS and by HPLC. As seen in Table 1, both methods show that the mean carnosine content of the gastrocnemius lateralis remained stable after 5 weeks sprint training, in both groups (interaction effect: 1H-MRS p = 0.879 and HPLC p = 0.182). Table 1 indicates that the muscle carnosine content of the tibialis anterior did not change (p = 0.063) following the intervention.
Muscle buffering capacity (β m)
Five weeks sprint training did not result in a change (p = 0.514) in in vitro muscle buffering capacity in any group. As can be observed in Fig. 2, the muscle buffering capacity was 93.3 ± 21.6 mmol H+/kg dw.pH and 106.2 ± 11.9 mmol H+/kg dw.pH before and 97.1 ± 26.5 mmol H+/kg dw.pH and 102.7 ± 19.6 mmol H+/kg dw.pH after the training period, for the VD and MD group, respectively. Figure 3 shows a rather modest, yet significant (p = 0.047) positive (r = 0.343) correlation between in vitro muscle buffering capacity and carnosine concentration of the gastrocnemius lateralis (measured by HPLC).
Repeated sprint ability test (6 × 6 s RSA)
The typical 6 × 6 s RSA profile showed that the mean power output per sprint decreased from sprint 1 to sprint 6 independent of group (at baseline: total mean decrease of ~170 W and following training: total mean decrease of ~100 W). As seen in Fig. 4a, b, 5 weeks sprint training caused an increase in the power output per sprint (p < 0.05) for both MD and VD group. The mean power output for all sprints together (p < 0.001; pre: 765 ± 196 W and post: 852 ± 228 W) increased following 5 weeks sprint training independent of the diet group (p = 0.707). Figure 4c shows a significant main effect of sprint number (p = 0.010) for the training-induced increase in power output per sprint. The subjects improved more in sprint 6 in comparison with sprint 1 (Δpost–pre: S1 = +48.58 ± 93.92 W; S6 = +114.68 ± 51.00 W), without differences between groups (p = 0.392). A moderate to strong positive correlation was observed between baseline muscle carnosine content of the gastrocnemius lateralis (1H-MRS) and both mean (p = 0.004; r = 0.63) and maximal (p = 0.005; r = 0.62) power output of the RSA test (pre). There was no significant correlation between performance on the RSA test (mean power output: p = 0.707; max: p = 0.262) and in vitro muscle buffering capacity. The lactate concentration before and after the training period was measured after warm-up, after the 6 × 6 s RSA test and after 5 min active recovery. For both groups, the lactate concentration following the 6 × 6 s RSA test was ~1.2 mmol/l higher after the training period (pre: 7.6 ± 1.4 mmol/l and post: 8.8 ± 1.5 mmol/l; p = 0.021).
Mean power output for the vegetarian (a) and mixed diet group (b) during the 6 × 6 s repeated sprint ability test (RSA) before (pre) and after (post) 5 weeks sprint training. Sprints 1–6 (S1–S6) are shown on the x-axis. Data are mean ± SD of 9 subjects in the vegetarian and 10 subjects in the mixed diet group. Different from pre (*p < 0.05). Different from sprint 1 (S1) ($ p < 0.05). c Increase in sprint power after 5 weeks sprint training for the vegetarian (VD) and mixed diet (MD) group
Diet registration
The analysis of the food diaries can be found in Table 2. Both groups consumed the same amount of carbohydrates, fat and alcohol and there were no differences between MD and VD group regarding total energy intake. There was only a tendency that the VD group consumed on average less (~17%) proteins than MD group (p = 0.078). Due to their meat and fish consumption, the MD group consumed on average 0.36 ± 0.23 g beta-alanine a day (range: 0.067–0.812 g/day), while the VD group consumed hardly any beta-alanine.
Measurement of muscle carnosine content: comparison of 1H-MRS and HPLC
The muscle carnosine concentration of the gastrocnemius lateralis muscle was measured by an invasive (HPLC technique using a true-cut biopsy) and a non-invasive method (1H-MRS). As shown in Fig. 5, there was a significant (p = 0.002) positive correlation (r = 0.517) between the two measurement methods. The dry weight muscle carnosine content (measured by HPLC) was back-calculated into a wet weight value by multiplying the dry weight value by the percentage weight remained after freeze-drying. The wet weight carnosine concentration values of both methods (HPLC and 1H-MRS) were comparable (p = 0.56).
CARNS mRNA expression
The baseline CARNS mRNA expression was not different (p = 0.628) between males (0.41 ± 0.36) and females (0.48 ± 0.33). The interaction effect (training × diet) was not significant (p = 0.076). Figure 6 shows that the CARNS mRNA expression of the MD group non-significantly increased (p = 0.325) from 0.42 ± 0.31 to 0.60 ± 0.39 and significantly decreased (p = 0.023) in the VD group from 0.57 ± 0.43 to 0.34 ± 0.28.
Discussion
This is the first study that investigated the effect of sprint training in combination with a vegetarian diet on the muscle carnosine content and muscle buffering capacity. Beta-alanine, which is hardly present in vegetarian food, is the rate limiting precursor of carnosine synthesis. The hypothesis that muscle carnosine would increase after 5 weeks sprint training, provided beta-alanine was present in the diet, cannot be confirmed. The carnosine content did not significantly increase in soleus, gastrocnemius and tibialis anterior. This further isolates the findings by Suzuki et al. (2004) who showed a massive increase (+113%) in carnosine in the vastus lateralis muscle, with a similar training program as in our study, whereas almost all other studies found no effect irrespective of various training programs (Kendrick et al. 2008; Kendrick et al. 2009; Mannion et al. 1994). The current study confirms that there is no effect of a short training period on the muscle carnosine content. It could be argued that a 5-week training program is too short, given the slow loading and unloading profile of muscle carnosine (Baguet et al. 2009). However, even at mRNA level of carnosine synthase, which could be expected to be a more early sign of increased carnosine synthesis, there is no training effect visible.
Since the carnosine synthase gene was just recently cloned (Drozak et al. 2010), this is—to our knowledge—the first study, which investigated the CARNS mRNA expression in human skeletal muscle. The enzymatic regulation of muscle carnosine probably depends mainly on CARNS, but it is still unclear how its activity and expression is regulated. The current study shows that the gender difference, which is clearly present for muscle carnosine concentration (Mannion et al. 1992), seemed to be absent in CARNS expression. Furthermore, based on the results in Fig. 6, it can be suggested that beta-alanine availability is probably an important factor in CARNS expression, as this significantly declined in the VD group. This suggestion is further supported by the significant interaction effect (diet × training) in the soleus muscle carnosine content. This interaction effect showed a non-significant increase of ~11% in the MD group and in the VD group a non-significant decrease of ~9%, which could have partially resulted from differential CARNS expression.
Carnosine’s quantitative contribution to the in vitro buffer capacity of human muscle is estimated to be 7–8% (Harris et al. 1990; Hill et al. 2007). Indeed, in the current study a positive correlation was found between muscle carnosine content and in vitro muscle buffering capacity. A substantial increase in muscle carnosine content, such as the 80% increase in vastus lateralis carnosine concentration elicited by 10 weeks of oral beta-alanine supplementation, has been calculated to increase carnosine’s contribution from 8.2 to 13.5% and elevate the total in vitro buffer capacity by 6% (Hill et al. 2007). Likewise, oral beta-alanine supplementation reduces the magnitude of exercise-induced acidosis in healthy volunteers (Baguet et al. 2010b). If training would induce a 113% increase in muscle carnosine content, as suggested by Suzuki et al. (2004), this would mean a sizeable 9% increase in muscle buffer capacity. Since the current study did not find an effect of training on the muscle carnosine content, we did not expect that the in vitro buffer capacity would be affected. Indeed, our results indicate no change in in vitro buffer capacity in the gastrocnemius lateralis with training and diet intervention.
Sprint training improved the performance on a RSA test in both groups. The observation that subjects improved more in the final sprints as compared to the first sprint and that higher lactate concentrations were reached post-training could suggest that the in vitro muscle buffering capacity is increased. However, this was not the case (in the gastrocnemius lateralis). Other possible explanations of the performance improvement are an increase in aerobic capacity (McMahon and Wenger 1998) or a neural effect (Ross et al. 2001). The performance enhancement could also be due to an increase in lactate/H+ transport capacity (Juel and Halestrap 1999; Pilegaard et al. 1999; Mohr et al. 2007; Thomas et al. 2007) or to bicarbonate buffering, since in vitro buffer capacity does not include this components (Hultman and Sahlin 1980).
The finding that the performance improvement was not different between both diet groups, suggests that acute vegetarianism has no acute adverse effects on adaptations to sprint exercise. However, it should be noted that in the current study, all subjects received 1 g creatine a day, similar to dietary intake of creatine by meat consumption (Venderley and Campbell 2006), to prevent a creatine deficiency in VD group and to isolate the effect of beta-alanine deprivation. Ovo-lacto vegetarians, who do not take creatine supplements, can possibly develop a creatine deficiency (Lukaszuk et al. 2002; Burke et al. 2003) and therefore, possibly experience a decrease in performance. In addition, it should also be mentioned that the intervention only lasted 5 weeks and that the results of the current study can, therefore, not be generalized for a longer intervention period.
The current study also aimed to validate the quantification of muscle carnosine by 1H-MRS, using the HPLC analysis of muscle biopsy tissue as the criterion measure. This is the first study, which compared the invasive and non-invasive methods to measure muscle carnosine, within the same study. The current study found a significant positive correlation (r = 0.517) between muscle carnosine measured by HPLC and 1H-MRS and also the absolute (wet weight) concentrations seem to match well.
Conclusion
In conclusion, 5 weeks sprint training does not induce an increase in muscle carnosine content nor in CARNS mRNA content, whereas deprivation of dietary beta-alanine (vegetarianism) seems to slightly affect these factors negatively. Although this could in the long run signify a new disadvantageous aspect of vegetarian diet on muscle adaptations to sprint training, the current vegetarian diet intervention during 5 weeks of training did not negatively affect performance or muscle buffer capacity.
References
Abe H (2000) Role of histidine-related compounds as intracellular proton buffering constituents in vertebrate muscle. Biochemistry (Mosc) 65:757–765
Baguet A, Reyngoudt H, Pottier A, Everaert I, Callens S, Achten E, Derave W (2009) Carnosine loading and washout in human skeletal muscles. J Appl Physiol 106:837–842
Baguet A, Bourgois J, Vanhee L, Achten E, Derave W (2010a) Important role of muscle carnosine in rowing performance. J Appl Physiol 109:1096–1101
Baguet A, Koppo K, Pottier A, Derave W (2010b) Beta-Alanine supplementation reduces acidosis but not oxygen uptake response during high-intensity cycling exercise. Eur J Appl Physiol 108:495–503
Bakardjiev A, Bauer K (1994) Transport of beta-alanine and biosynthesis of carnosine by skeletal muscle cells in primary culture. Eur J Biochem 225:617–623
Bell GJ, Wenger HA (1988) The effect of one-legged sprint training on intramuscular pH and nonbicarbonate buffering capacity. Eur J Appl Physiol Occup Physiol 58:158–164
Bishop D, Edge J, Goodman C (2004) Muscle buffer capacity and aerobic fitness are associated with repeated-sprint ability in women. Eur J Appl Physiol 92:540–547
Bishop DJ, Edge J, Thomas C, Mercier J (2008) Effects of high-intensity training on muscle lactate transporters and post-exercise recovery of muscle lactate and hydrogen ions in women. Am J Physiol Regul Integr Comp Physiol 295(6):R1991–R1998
Bishop D, Edge J, Mendez-Villanueva A, Thomas C, Schneiker K (2009) High-intensity exercise decreases muscle buffer capacity via a decrease in protein buffering in human skeletal muscle. Pflugers Arch 458:929–936
Boldyrev A (2007) Carnosine and oxidative stress in cells and tissues. Nova Science Publishers, New York
Burke DG, Chilibeck PD, Parise G, Candow DG, Mahoney D, Tarnopolsky M (2003) Effect of creatine and weight training on muscle creatine and performance in vegetarians. Med Sci Sports Exerc 35:1946–1955
Derave W, Everaert I, Beeckman S, Baguet A (2010) Muscle carnosine metabolism and beta-alanine supplementation in relation to exercise and training. Sports Med 40:247–263
Derveaux S, Vandesompele J, Hellemans J (2010) How to do successful gene expression analysis using real-time PCR. Methods 50:227–230
Drozak J, Veiga-da-Cunha M, Vertommen D, Stroobant V, Van SE (2010) Molecular identification of carnosine synthase as ATP-grasp domain-containing protein 1 (ATPGD1). J Biol Chem 285:9346–9356
Dunnett M, Harris RC (1999) Influence of oral beta-alanine and l-histidine supplementation on the carnosine content of the gluteus medius. Equine Vet J Suppl 30:499–504
Edge J, Bishop D, Goodman C (2006a) The effects of training intensity on muscle buffer capacity in females. Eur J Appl Physiol 96:97–105
Edge J, Bishop D, Hill-Haas S, Dawson B, Goodman C (2006b) Comparison of muscle buffer capacity and repeated-sprint ability of untrained, endurance-trained and team-sport athletes. Eur J Appl Physiol 96:225–234
Edge J, Hill-Haas S, Goodman C, Bishop D (2006c) Effects of resistance training on H+ regulation, buffer capacity, and repeated sprints. Med Sci Sports Exerc 38:2004–2011
Everaert I, Mooyaart A, Baguet A, Zutinic A, Baelde H, Achten E, Taes Y, De Heer E, Derave W. 2010. Vegetarianism, female gender and increasing age, but not CNDP1 genotype, are associated with reduced muscle carnosine levels in humans. Amino Acids
Gibala MJ, Little JP, van EM, Wilkin GP, Burgomaster KA, Safdar A, Raha S, Tarnopolsky MA et al (2006) Short-term sprint interval versus traditional endurance training: similar initial adaptations in human skeletal muscle and exercise performance. J Physiol 575:901–911
Harmer AR, McKenna MJ, Sutton JR, Snow RJ, Ruell PA, Booth J, Thompson MW, Mackay NA, Stathis CG, Crameri RM, Carey MF, Eager DM (2000) Skeletal muscle metabolic and ionic adaptations during intense exercise following sprint training in humans. J Appl Physiol 89:1793–1803
Harris RC, Marlin DJ, Dunnett M, Snow DH, Hultman E (1990) Muscle buffering capacity and dipeptide content in the thoroughbred horse, greyhound dog and man. Comp Biochem Physiol A Comp Physiol 97:249–251
Harris RC, Jones G, Hill C, Kendrick IP, Boobis L, Kim CK, Kim HJ, Dang VH, Edge J, Wise JA (2007) The carnosine content in V Lateralis of vegetarians and omnivores (abstract). FASEB J 6:A944
Hill CA, Harris RC, Kim HJ, Harris BD, Sale C, Boobis LH, Kim CK, Wise JA (2007) Influence of beta-alanine supplementation on skeletal muscle carnosine concentrations and high-intensity cycling capacity. Amino Acids 32:225–233
Hirakoba K (1999) Buffering capacity in human skeletal muscle: a brief review. Bull Faculty Compt Sci Syst Eng Kyushu Inst Technol (Hum Sci) 12:1–21
Hultman E, Sahlin K (1980) Acid–base balance during exercise. Exerc Sport Sci Rev 8:41–128
Iaia FM, Thomassen M, Kolding H, Gunnarsson T, Wendell J, Rostgaard T, Nordsborg N, Krustrup P, Nybo L, Hellsten Y, Bangsbo J (2008) Reduced volume but increased training intensity elevates muscle Na+–K+ pump alpha1-subunit and NHE1 expression as well as short-term work capacity in humans. Am J Physiol Regul Integr Comp Physiol 294:R966–R974
Juel C (1998) Muscle pH regulation: role of training. Acta Physiol Scand 162:359–366
Juel C, Halestrap AP (1999) Lactate transport in skeletal muscle—role and regulation of the monocarboxylate transporter. J Physiol 517(Pt 3):633–642
Juel C, Klarskov C, Nielsen JJ, Krustrup P, Mohr M, Bangsbo J (2004) Effect of high-intensity intermittent training on lactate and H + release from human skeletal muscle. Am J Physiol Endocrinol Metab 286:E245–E251
Kendrick IP, Harris RC, Kim HJ, Kim CK, Dang VH, Lam TQ, Bui TT, Smith M, Wise JA (2008) The effects of 10 weeks of resistance training combined with beta-alanine supplementation on whole body strength, force production, muscular endurance and body composition. Amino Acids 34(4):547–554
Kendrick IP, Kim HJ, Harris RC, Kim CK, Dang VH, Lam TQ, Bui TT, Wise JA (2009) The effect of 4 weeks beta-alanine supplementation and isokinetic training on carnosine concentrations in type I and II human skeletal muscle fibres. Eur J Appl Physiol 106:131–138
Lukaszuk JM, Robertson RJ, Arch JE, Moore GE, Yaw KM, Kelley DE, Rubin JT, Moyna NM (2002) Effect of creatine supplementation and a lacto-ovo-vegetarian diet on muscle creatine concentration. Int J Sport Nutr Exerc Metab 12:336–348
Mannion AF, Jakeman PM, Dunnett M, Harris RC, Willan PL (1992) Carnosine and anserine concentrations in the quadriceps femoris muscle of healthy humans. Eur J Appl Physiol Occup Physiol 64:47–50
Mannion AF, Jakeman PM, Willan PL (1994) Effects of isokinetic training of the knee extensors on high-intensity exercise performance and skeletal muscle buffering. Eur J Appl Physiol Occup Physiol 68:356–361
McMahon S, Wenger HA (1998) The relationship between aerobic fitness and both power output and subsequent recovery during maximal intermittent exercise. J Sci Med Sport 1:219–227
Mohr M, Krustrup P, Nielsen JJ, Nybo L, Rasmussen MK, Juel C, Bangsbo J (2007) Effect of two different intense training regimens on skeletal muscle ion transport proteins and fatigue development. Am J Physiol Regul Integr Comp Physiol 292:R1594–R1602
Nevill ME, Boobis LH, Brooks S, Williams C (1989) Effect of training on muscle metabolism during treadmill sprinting. J Appl Physiol 67:2376–2382
Parkhouse WS, McKenzie DC (1984) Possible contribution of skeletal muscle buffers to enhanced anaerobic performance: a brief review. Med Sci Sports Exerc 16:328–338
Parkhouse WS, McKenzie DC, Hochachka PW, Ovalle WK (1985) Buffering capacity of deproteinized human vastus lateralis muscle. J Appl Physiol 58:14–17
Pilegaard H, Domino K, Noland T, Juel C, Hellsten Y, Halestrap AP, Bangsbo J (1999) Effect of high-intensity exercise training on lactate/H + transport capacity in human skeletal muscle. Am J Physiol 276:E255–E261
Ramadan S, Ratai EM, Wald LL, Mountford CE (2010) In vivo 1D and 2D correlation MR spectroscopy of the soleus muscle at 7T. J Magn Reson 204:91–98
Ross A, Leveritt M, Riek S (2001) Neural influences on sprint running: training adaptations and acute responses. Sports Med 31:409–425
Sahlin K, Henriksson J (1984) Buffer capacity and lactate accumulation in skeletal muscle of trained and untrained men. Acta Physiol Scand 122:331–339
Sanchez-Hernandez L, Marina ML, Crego AL (2011) A capillary electrophoresis-tandem mass spectrometry methodology for the determination of non-protein amino acids in vegetable oils as novel markers for the detection of adulterations in olive oils. J Chromatogr (in Press)
Sharp R, Costill D, Fink W, King D (1986) Effects of eight weeks of bicycle ergometer sprint training on human muscle buffering capacity. Int J Sports Med 7:13–17
Suzuki Y, Ito O, Takahashi H, Takamatsu K (2004) The effect of sprint training on skeletal muscle carnosine in humans. Int J Sport Health Sci 2:105–110
Thomas C, Bishop D, Moore-Morris T, Mercier J (2007) Effects of high-intensity training on MCT1, MCT4, and NBC expressions in rat skeletal muscles: influence of chronic metabolic alkalosis. Am J Physiol Endocrinol Metab 293:E916–E922
Troup JP, Metzger JM, Fitts RH (1986) Effect of high-intensity exercise training on functional capacity of limb skeletal muscle. J Appl Physiol 60:1743–1751
Venderley AM, Campbell WW (2006) Vegetarian diets : nutritional considerations for athletes. Sports Med 36:293–305
Weston AR, Myburgh KH, Lindsay FH, Dennis SC, Noakes TD, Hawley JA (1997) Skeletal muscle buffering capacity and endurance performance after high-intensity interval training by well-trained cyclists. Eur J Appl Physiol Occup Physiol 75:7–13
Acknowledgments
This study was financially supported by grants from the Research Foundation—Flanders (FWO 1.5.149.08 and G.0046.09) Audrey Baguet is a recipient of a PhD-scholarship from the Research Foundation—Flanders. The practical contribution of Jonathan Dehenau, Job Franssen and Bavo Verhasselt is greatly acknowledged.
The experiments of this manuscript comply with the current laws of Belgium.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jacques R. Poortmans.
Rights and permissions
About this article
Cite this article
Baguet, A., Everaert, I., De Naeyer, H. et al. Effects of sprint training combined with vegetarian or mixed diet on muscle carnosine content and buffering capacity. Eur J Appl Physiol 111, 2571–2580 (2011). https://doi.org/10.1007/s00421-011-1877-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-011-1877-4