Abstract
Purpose
Sprint interval training (SIT) stimulates rapid metabolic adaptations within skeletal muscle but the nature of neuromuscular adaptions is unknown. Omega-3 polyunsaturated fatty acids (N-3 PUFA) are suggested to enhance neuromuscular adaptations to exercise.
Methods
We measured the neuromuscular adaptations to SIT (Study-1) and conducted a placebo-controlled randomized double blinded study to determine the effect of N-3 PUFA supplementation on neuromuscular adaptations to SIT (Study-2). In Study-1, seven active men (24.4 ± 2.6 years, VO2 peak 43.8 ± 8.7 ml kg min−1) completed 2-weeks of SIT with pre- and post-training 10 km cycling time trials (TT). In Study-2, 30 active men (24.5 ± 4.2 years, VO2 peak 41.0 ± 5.1 ml kg min−1) were randomly assigned to receive N-3 PUFA (2330 mg day−1) (n = 14) or olive oil (n = 16) during 2-weeks of SIT with pre- and post-training TTs. Four week post-training, a SIT session and TT were also performed. Change in neuromuscular function was assessed from resting twitches, quadriceps maximal voluntary contraction (MVC) force, and potentiated twitch force (Q tw).
Results
Study-1 showed that SIT did not elicit significant neuromuscular adaptations. Study-2 showed that N-3 PUFA supplementation had no significant effect on neuromuscular adaptations. Training caused lower MVC force [mean ± SD; N-3 PUFA −9 ± 11%, placebo −9 ± 13% (p < 0.05 time)] and Q tw peripheral fatigue [N-3 PUFA −10 ± 19%, placebo −14 ± 13% (p < 0.05 time)]. TT time was lower after training in all groups [Study-1 −10%, Study-2 N-3 PUFA −8%, placebo −12% (p < 0.05 time)].
Conclusion
Two weeks of SIT improved TT performance in the absence of measurable neuromuscular adaptations. N-3 PUFA supplementation had no significant effect on SIT training adaptations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sprint interval training (SIT) consists of repeated periods of brief maximal exercise interspersed with longer rest periods. Metabolic adaptations within skeletal muscle have been suggested as the primary contributor to increased sprint peak power, fatigue resistance, and exercise capacity (Burgomaster et al. 2005) and cycling performance (Burgomaster et al. 2006). Reported metabolic adaptations include increased muscle glycogen storage, greater muscle mitochondrial number and enzyme concentration, and increased membrane glucose transporters and monocarboxylate transporter concentrations (Burgomaster et al. 2007, 2005; Little et al. 2011).
It is well established that SIT can cause metabolic adaptations; however, it is unknown if neuromuscular adaptations contribute to previously reported improvements in exercise capacity (Burgomaster et al. 2005) and performance (Burgomaster et al. 2006). Previous work has shown that the neuromuscular system is directly involved in the initial adaptations to training (Rich and Cafarelli 2000; Sale 1988). When muscles are challenged with a novel stimulus, the neuromuscular system rapidly adapts, leading to improvements in muscle coordination, activation and ultimately force-generating capacity (Sale 1988). Furthermore, the neuromuscular system has been identified as a primary contributor to increased muscle strength in new exercisers when adopting a new training technique (Griffin and Cafarelli 2003; Rich and Cafarelli 2000).
It is important to investigate the extent of neuromuscular adaptations to SIT to understand the physiological effects of this style of training. Furthermore, investigation of nutritional ergogenic aids to enhance adaptations to SIT has been limited thus far. Cochran et al. (2015a, b) investigated the effect of beta-alanine and altered carbohydrate availability on metabolic adaptations to SIT; however, there as yet to be any investigation of ergogenic aids that might support potential neuromuscular adaptations to SIT. Omega-3 polyunsaturated fatty acid (N-3 PUFA) supplementation has been identified as a potential ergogenic aid for neuromuscular adaptations to exercise (Lewis et al. 2015; Rodacki et al. 2012). N-3 PUFA supplementation increases membrane fluidity and therefore might increase the rate of action potential transmission to the neuromuscular junction and throughout the muscle membrane. Furthermore, there is evidence that N-3 PUFA supplementation can increase acetylcholine concentration and acetylcholinesterase activity at the neuromuscular junction (Patten et al. 2002).
Seal oil was chosen as the experimental N-3 PUFA supplement for this study because mammalian triacylglycerol molecules have N-3 PUFA fats primarily in the sn-1 and sN-3 positions, as opposed to the sn-2 position of fish oil N-3 PUFA (Brockerhoff et al. 1968; Wanasundara and Shahidi 1997). Fats in the sn-3 position are preferentially cleaved by sublingual lipases, and the sn-1 fat is cleaved in the small intestine, while the sn-2 fatty acid is left for later esterification (Bracco 1994; Paltauf et al. 1974). These structural differences enable chylomicrons and chylomicron remnants containing mammalian N-3 PUFA to have a higher rate of clearance from the blood compared to fish oil intake in animal studies (Christensen et al. 1995; Yoshida et al. 1996). These differences in structure and digestibility are thought to increase bioavailability and rate of uptake into membranes; however, this has yet to be examined in humans.
To elucidate the neuromuscular adaptations to SIT and the potential ergogenic effect of N-3 PUFAs on neuromuscular adaptations to SIT, two experiments were performed. Experiment one investigated the neuromuscular adaptations to SIT and experiment two investigated effect of N-3 PUFA supplementation on neuromuscular adaptations to SIT. The purpose of Study 1 was to describe the neuromuscular adaptations to 2-weeks of SIT. We hypothesized that neuromuscular function as measured by quadriceps maximal voluntary contraction (MVC) force would improve following SIT and fatigue would be lower on the last training session compared to the first.
The purpose of Study 2 was to determine the effect of N-3 PUFA supplementation combined with SIT on neuromuscular adaptations compared to placebo. We hypothesized that N-3 PUFA supplementation would enhance neuromuscular adaptations, such that the increase in MVC force would be greater in the N-3 PUFA group compared to placebo.
Methods
Ethics statement
The protocol and consent procedures were approved by the University of Toronto Research Ethics Board. All participants were informed of study procedure and gave written informed consent.
Participants
Males ≥18 years from the University of Toronto area, that were recreationally active (<5 h week−1) but not following a structured exercise program were recruited for these studies. All prospective participants were screened for eligibility by measuring BMI (within 18.5–29.9) and assessing cardiac function using 12-lead electrocardiography (CardioPerfect Workstation, Welych-Allyn, Skaneateles Falls, New York, USA) that was reviewed by a physician. The PAR-Q exercise readiness questionnaire was used for additional physical activity readiness screening (http://www.csep.ca/english/view.asp?x=698).
Participants in Study 2 were further screened for HIV and hepatitis C to prevent any potential adverse events with the study supplement and exercise training. A dietary screen was used to ensure that these participants were not consuming any form of N-3 PUFA supplement or consuming fish ≥3 times per week for 4-weeks prior to beginning the study.
Seven healthy males were recruited for Study 1. This was exploratory in nature and used a sample size similar to training groups in previous SIT studies (Burgomaster et al. 2005, 2006, 2007). Thirty healthy males were recruited for Study 2 separately from Study 1. This sample size was determined from the post-training difference in maximal voluntary contraction force between groups receiving placebo or fish oil N-3 PUFA during resistance training (Rodacki et al. 2012). Using a difference in means of 25 N (SD 15), α = 0.05 and β = 0.8, 14 participants per group are required to detect a significant difference between the placebo and N-3 PUFA group.
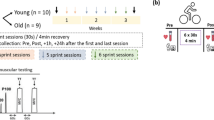
The descriptive data for the participants is shown in Table 1. Figure 1 shows an overview of the progression of each study. Study 1 used a one-way, repeated measures, within-participant design. Study 2 was conducted as a parallel design, placebo-controlled, randomized control trial.
Descriptive characteristics
At the first visit, participants height, weight, and body fat percentage (Omron Fat Loss Monitor, model HBF-306CAN, Omron Healthcare, Bannockburn, Illinois, USA) were measured.
Participants’ VO2max was determined using breath-by-breath gas-analysis (MetaMax 3B, CORTEX, Leipzig, Germany) during an incremental cycling test on an ergometer (Monark 839E, Vansboro, Sweden) as previously described (Lewis et al. 2015). Work rate was set at 50, 100, and 150 W for 2 min each before increasing by 25 W min−1 thereafter. VO2peak was determined as the highest value achieved over a 20-s period. Watt max (W max) was determined from the stage at which participants’ cadence dropped below 50 rpm. Maximum heart rate was the highest recorded during the test (Polar Electro, Finland).
10 km time trial performance test
Participants cycled on an electronically braked ergometer (Monark 839E, Vansboro, Sweden) with no feedback other than distance completed displayed on a computer monitor. Cycling work rate was set at 60% of W max as determined from the VO2max test with the ergometer in hyperbolic mode. This fixed work rate allowed participants to vary their cadence to adjust cycling speed. TT cadence and heart rate were averaged over the test.
Sprint interval training protocol
Training started at least 3 days after visit (V) V2. Six SIT sessions were completed over 2-weeks as previously described (Burgomaster et al. 2005). Participants increased the number of sprints from four to seven and completed four on the final day of training (Fig. 1). Training occurred on alternating days (i.e., Monday, Wednesday, and Friday) with 2 days rest between V5 and V6. Each visit started with a 5-min self-paced warm-up on a stationary cycle ergometer. Participants were then fitted to a mechanically braked cycle ergometer (Monark 894E, Vansboro, Sweden) and repeated 30-s maximal effort sprints against 0.075 kg kg−1 body weight with 4-min of recovery between bouts. Participants were instructed to begin sprinting ~5-s before each interval to achieve a high cadence before the load was automatically applied by the manufacturers’ software (Anaerobic Test Software version 3.2.1.0, Monark). Participants were strongly encouraged by the research team to maintain maximal effort throughout the 30-s intervals. Light pedaling was encouraged between sprints to promote recovery. Peak, mean and minimum power and percent power drop (fatigue index) were recorded for each interval.
Neuromuscular testing
Neuromuscular function was assessed before and 3-min after SIT on V3, V8, and V10. Testing involved a train of 5 maximal stimulations of the femoral nerve to measure twitch characteristics, followed by three MVCs described below. Participants were seated in a custom-built isometric dynamometer with their hip and knee angle fixed at 90° throughout. Force was measured using a load cell (SM-500-I9; Durham Instruments, Pickering, Ontario, Canada) adjusted to each participant. The load cell was connected in a direct line to the right ankle at the level of the malleoli. Change in neuromuscular variables from pre- to post-exercise was considered as neuromuscular fatigue. Data were collected using PowerLab 8/35 (ADInstruments, Colorado Springs, Colorado, USA). The EMG signals were amplified with a Dual BioAmp amplifier (ADInstruments, Colorado Springs, Colorado, USA; bandwidth frequency 10–500 Hz input impedance 200 MΩ, common mode rejection ratio = 85 dB, gain = ±1%). The placement of the EMG and stimulating electrodes were outlined in indelible ink to ensure similar placement after training. All data were transmitted to a laptop and analyzed using a custom analysis program (Matlab 6.0; Mathworks Inc.).
Measurement of global fatigue
Quadriceps peak force was measured during three 5-s maximal voluntary contractions (MVC), each separated by 60-s rest. The highest force achieved was reported as MVC peak force. Change in MVC force was considered as global neuromuscular fatigue.
Measurement of central neuromuscular fatigue
Quadriceps voluntary activation (VA) was used to measure central neuromuscular fatigue. A high-frequency doublet (100 Hz; 10 ms inter-stimulus interval) was given at 2.5-s into the MVC and at 2-s after the contraction with the muscle in a potentiated state as described by (Millet et al. 2011). Stimulation intensity was set at 120% of optimal intensity. The ratio of the amplitude of the superimposed twitch during the MVC over the potentiated twitch (Q tw) was used to calculate voluntary activation as follows:
Stimulations of the femoral nerve were delivered from a high-voltage (400 V) constant current stimulator (Biopac, BSLSTMA, Santa Barbara, California, USA), controlled by a custom-designed program (LabChart 7, ADInstruments, Colorado Springs, Colorado, USA). A square wave, 1-ms stimulation was delivered from a cathode (10 mm diameter) (Kendall 100, Covidien, Saint-Laurent, Quebec, Canada) placed over the femoral nerve at the fAemoral triangle beneath the inguinal ligament. The anode (5 × 10 cm, DJO, Vista, California, USA) was placed on the lower portion of the gluteal fold opposite to the cathode. Determination of optimal stimulation intensity was achieved by increasing stimulation intensity until a further 5 mA increase did not alter peak quadriceps twitch force and vastus lateralis M-wave amplitude and was reassessed at each visit.
Skin on the vastus lateralis (VL), vastus medialis (VM), and the patella were shaved and cleaned with alcohol wipes to ensure low signal impedance (Z < 5 kΩ). EMG signals were recorded using Ag–AgCl electrodes (Kendall 100, Covidien, Saint-Laurant, Quebec, Canada; inter-electrode distance 25 mm) placed lengthwise over the mid-belly of the muscle with the reference electrode over the patella (Hermens et al. 2000). Muscle activation was determined by calculating the EMG root mean square (RMS) averaged over 0.2-s periods from when muscle force began to plateau until immediately before the superimposed stimulation (Martin et al. 2010). Mean EMG RMS MVCs was normalized by mean M AMP (RMS/M AMP) as a measure of descending neural drive.
Measurement of peripheral neuromuscular fatigue
The femoral nerve was stimulated with a train of five stimulations, each separated by 5-s rest at 100% of optimal intensity. Twitch peak force (tPF), rate of force development (tRFD), time to peak (tTP), and time to half relaxation (tHRT) and total twitch area under the curve (tAUC) were measured and averaged. Corresponding M-wave data from the lateralis (VL) were measured for amplitude (M AMP).
The rate of force development (RFD) from 20 and 80% of peak force was calculated for each MVC and averaged. The electromechanical delay (EMD) was calculated from the time difference from the increase in quadriceps EMG activity and force >10 standard deviations from resting baseline.
Changes in Q tw is a sensitive method for detecting neuromuscular fatigue from cycling (Amann and Dempsey 2008) and provides a method to quantify peripheral fatigue compared to the previously described critical threshold of peripheral neuromuscular fatigue (Amann et al. 2006; Thomas et al. 2015). Five seconds after Q tw, a low frequency doublet (10 Hz; 100 ms inter-stimulus interval) was given. The ratio of this stimulation to Q tw was used to measure low-force frequency fatigue (LFF) as previously described (Verges et al. 2009).
Figure 1 shows an overview of the protocol used to assess global fatigue as well as central and peripheral neuromuscular fatigue.
Training and dietary controls
All participants were asked to refrain from any strenuous activity for 24 h prior to testing and were instructed not to participate in any exercise beyond activities of daily living during the 2-weeks of training. In Study 2, participants were allowed to return to their pre-study exercise habits during the 4-week washout period.
Participants were asked to complete a 3-day food record culminating on V2. During the study, participants were encouraged to eat similar foods and not alter their diet with the exception of the provided supplement in Study 2. Prior to the second and third TT (Study 2), participants were given a copy of their food record for the day prior to the TT and asked to follow the food choices and quantities as closely as possible.
Study 2 experimental and placebo supplements
Participants in Study 2 were randomized to either the experimental N-3 PUFA group or placebo. The supplement was given to the participant in a sealed opaque envelope at the end of V3. Participants consumed their supplement for the duration of training (12-days). Participants consumed 5 mL of seal oil containing 1115 mg N-3 PUFA (375 mg EPA, 230 mg DPA, 510 mg DHA and with 5000 IU retinyl palmitate and vitamin D3) (Auum Inc., Timmons, Canada) (NPN 80021190) or 5 mL olive oil (Bertolli, Mississauga, Canada) matched with 5000 IU retinyl palmitate and vitamin D (Auum Inc., Timmons, Canada). Participants were instructed to take 1–2.5 mL servings orally twice daily, and to let the oil remain in the mouth for 1-min before swallowing to allow for potential sublingual absorption (Paltauf et al. 1974).
Randomization and concealment
Participants were assigned a sequential study ID based on their enrollment. Treatment assignment (N-3 PUFA or placebo) was determined from a computer-generated random number sequence using variable block sizes to enhance concealment. Supplement bottles were then sealed in opaque envelopes and labeled with participant ID by a researcher not involved in the study. Participants received their supplement at the end of the first SIT session. Study researchers were unblinded after neuromuscular and plasma N-3 PUFA analysis was completed.
Blood sampling
A resting 8 mL blood sample from the antecubital vein was collected into K3-EDTA Vacutainer tubes (BD Vacutainer, Mississauga, Canada) at the beginning of Visit 3, 8, and 10. Samples were centrifuged (3000 rpm for 15 min at 4 °C). Plasma samples were collected and stored at 80 C for later analysis of plasma N-3 PUFA concentration.
At visit 3, 8, and 10, a finger prick blood lactate sample was taken prior to training and 3-min after sprint 4 (Lactate Scout, Cardiff, UK).
Lipid extraction and gas chromatography-flame ionization detection
All lipid extraction techniques have been previously described by Chen et al. (2011). To extract total lipids, Folch, Lees, and Sloane Stanley’s method was applied by use of chloroform:methanol:0.88% KCl (2:1:0.75 by vol.) with a known quantity of heptadecanoic acid (17:0) as internal standard. Following extraction, total lipid extracts (TLE) were heated for 1h at 100 °C with 14% boron trifluoridemethanol in methanol to transesterify lipids to fatty acid methyl esters (FAME; ester-linked fatty acids). Gas chromatography-flame ionization detection (GC-FID) was used to quantify FAME.
A Varian-430 gas chromatograph (Varian, Lake Forest, California, USA) with an Agilent capillary column (DB-23ms; 30 m · 0.25 mm i.d. · 0.25 μm film thickness) and an FID was used to analyze FAME injected in splitless mode. Injector and detector ports were set at 250 °C, and helium carrier gas was set at a constant flow rate of 0.7 mL/min. A specific temperature program was used during FAME elution; it was set at 50 °C for 2 min, slowly increasing by 20 °C/min, and held at 170 °C for 1 min, then at 3 °C/min and held at 212 °C for 5 min to complete the run at 28 min. Retention times of authentic FAME standards (Nu-Chek Prep, Inc., Elysian, MN, USA) were used to identify peaks. Internal standard (17:0) peaks were compared to FAME peaks to calculate fatty acid concentrations, with final values expressed percent concentration in plasma.
Statistics
All data are presented as means ± SD (SPSS v22; IBM Corp., Armonk, NY, USA). Mauchley’s test of sphericity was used to assess the variance of data and where violations occurred, corrections were made using the Greenhouse–Geisser adjustment to prevent inflation of F ratios.
Study 1 Wingate and TT data were analyzed using a paired samples t test. Neuromuscular data were analyzed using a one-way repeated measures analysis of variance (ANOVA) with Bonferroni’s pairwise comparison to determine differences between times.
Two analyses were performed in Study 2 to examine effect of N-3 PUFA supplementation on adaptations to training (V2/3 vs. V8/9) and the subsequent effect of N-3 PUFA washout and detraining compared to baseline (V2/3 vs. V10/11). This analysis plan was selected as V10 and V11 data were exploratory and to account for the number of participants lost to follow-up after V9 (Fig. 2). Wingate, plasma fatty acid composition, TT, and within visit neuromuscular percent change data were analyzed using a group (2) by time (2) repeated measures ANOVA. Neuromuscular data were analyzed with a group (2) by time (4) repeated measures ANOVA. Where there was a main effect for time, Bonferroni’s pairwise comparison to determine differences between times. Study 2 descriptive data were analyzed using an independent samples t test. Significance was set at p ≤ 0.05.
Study 1 results
Adaptations to SIT
In response to training, participant’s body weight decreased from 82.1 ± 12.4 kg to 81.0 ± 13.0 kg (p = 0.007) with no change in body fat [13.4 ± 7.8 vs. 12.9 ± 7.8% (p = 0.2)].
After 2-weeks of SIT, first sprint peak power increased 21% from 710 ± 210 W to 860 ± 230 W (p = 0.05) and peak power in the fourth sprint tended to be higher as well, 670 ± 160 W compared to 790 ± 180 W (p = 0.08). Average power in the first sprint was unchanged at 645 ± 161 W compared to 655 ± 140 W (p = 0.4), whereas average power in the fourth sprint increased 12.5% from 480 ± 100 W to 540 ± 110 W (p = 0.03). There was no change in the percent power drop on the first or fourth sprint.
Global fatigue
MVC force was not significantly different between pre-V3 and pre-V8 (Table 2).
Central neuromuscular fatigue
Quadriceps VA was not different within or between V3 and V8. No differences were observed within or between V3 and V8 for EMG RMS/ M AMP (p = 0.1) measured during MVCs.
Peripheral neuromuscular fatigue
tPT was not different between pre-V3 and pre-V8, but was significantly lower after each training session (p < 0.001). tRFD was lower after V3 training (p = 0.02) but not after V8 (p = 0.1). tAUC was not different between pre-V3 and pre-V8 (p = 0.7), but was significantly lower after each training session (p = 0.001). There were no differences within or between V3 and V8 for tTP (p = 0.6) or tHRT (p = 0.6) (data not shown). Q tw was not different between pre-V3 and pre-V8 (p = 0.8), but was significantly lower after each training session (p < 0.02). There was a significant increase in LFF after V3 and V8 training sessions (p < 0.05). There were no differences between or within V3 and V8 for MVC RFD (p = 0.1) or EMD (p = 0.8). No differences were found within or between V3 and V8 for M-wave M AMP (p = 0.2).
10 km TT performance
TT time was improved by 10% after training, decreasing from 1263 ± 244 s to 1137 ± 248 s (p < 0.001). Mean RPM tended to increase in response training from 95 ± 10 to 100 ± 13 (p = 0.1), while heart rate was not different between tests (p = 0.8).
Study 2 results
Of the 30 participants that started the study, two did not complete the training protocol and one participant’s data was excluded for inconsistent effort (n = 27) (Fig. 2). Seven participants were lost to follow-up for visit 10 (n = 20).
Adaptations to SIT
Neither SIT nor placebo and N-3 PUFA supplementation had any significant effect on body mass for either group; however, body composition showed a main effect for time with lower percent body fat (p = 0.05).
After 2-weeks of SIT, there were no interaction effects, main effects for group or time in first sprint peak power (p = 0.2), average power (p = 0.3), or minimum power (p = 0.4) (Fig. 3). First sprint percent power drop (fatigue index) showed a main effect for time from V3 to V8 and decreased from 59 ± 12 to 53 ± 11% (p = 0.04). Fourth sprint average power showed a main effect for time from V3 to V8 and increased from 366 ± 71 to 390 ± 89 W (p = 0.02).
After 4-weeks of washout, there were no interaction effects, main effects for group or time in first sprint peak power (p = 0.4), average power (p = 0.4), minimum power (p = 0.2), percent power drop (p = 0.8).
Global fatigue
Quadriceps MVC force showed a main effect for time from V3 vs. V8 and V3 vs. V10 (p < 0.001) (Table 3). Pairwise comparisons showed MVC force was also lower at pre-V8 compared to pre-V3 (p = 0.006) and that post-SIT MVC force was lower at after all SIT session (p < 0.001). MVC force percent change from V3 to V8 showed an interaction effect as the placebo group decreased 11% and the N-3 PUFA group was unchanged (p = 0.04). A main effect for time showed V10 percent change was lower than V3 (p = 0.03). Figure 4 shows representative force trainings from V3 and V8.
Central neuromuscular fatigue
VA showed a main effect for time from V3 vs. V8 and V3 vs. V10 (p < 0.02) (Table 3). Pairwise comparisons showed that pre-V8 tended to be lower than pre-V3 (p = 0.055). VA percent change showed no difference between V3 and V8 or V3 and V10 (p > 0.05). There were no interaction, time or group effects for EMG RMS/M AMP between V3 and V10 (p = 0.08) or V3 and V11 (p = 0.6) (Table 3).
Peripheral neuromuscular fatigue
Peripheral neuromuscular data is shown in Table 3. tPF showed a main effect for time from V3 vs. V8 and V3 vs. V10 (p < 0.001). Pairwise comparisons showed that post-SIT tPF was lower after all SIT session (p < 0.01). tPF percent change showed a main effect for time and was lower at V8 and V10 compared to V3 (p < 0.02).
tRFD showed a main effect for time from V3 vs. V8 and V3 vs. V10 (p < 0.001). Pairwise comparisons showed that post-SIT tRFD was lower at after all SIT session (p < 0.01). Pairwise comparison showed that post V10 was higher than post V3 (p = 0.03). tRFD percent change showed no difference between V3 and V8 but V10 was lower compared to V3 (p < 0.02).
tAUC showed a main effect for time from V3 vs. V8 and V3 vs. V10 (p < 0.03). Pairwise comparisons showed that post-SIT tAUC was lower at after all SIT session (p < 0.01). tAUC percent change showed no difference between V3 and V8 or V3 and V10 (p > 0.05). tTP and tHRT were not significantly different at V8 or V10 compared to V3.
MVC RFD showed a main effect for time from V3 vs. V8 and V3 vs. V10 (p < 0.01). Pairwise comparisons showed that post-SIT Q tw was lower at after all SIT V3 and V8 only (p < 0.01). RFD percent change at V8 or V10 was not significantly different from V3.
Q tw showed a main effect for time from V3 vs. V8 and V3 vs. V10 (p < 0.001). Pairwise comparisons showed that pre-V8 was lower than pre-V3 and post-SIT Q tw was lower after all SIT session (p < 0.01). Q tw percent change showed a main effect for time and was lower at V8 and V10 compared to V3 (p < 0.02).
LFF showed a main effect for time from V3 vs. V8 and V3 vs. V10 (p < 0.001). Pairwise comparisons showed that post-SIT LFF was higher after all SIT session (p < 0.03). LFF percent change at V8 and V10 was not significantly different from V3.
There were no interaction, time or group effects for M AMP between V3 and V10 (p = 0.2) or V3 and V11 (p = 0.7) (Table 3).
Blood lactate
Blood lactate showed no difference between groups but a main effect for time, increasing at V3 from 1.9 ± 0.8 to 15.1 ± 2.7 mmol L−1 and at V8 from 1.9 ± 0.5 to 14.5 ± 3.3 mmol L−1 (p < 0.001). At V10, lactate increased from 2.0 ± 0.4 to 13.7 ± 5.5 mmol L−1 (p < 0.001).
Plasma N-3 PUFA
Plasma N-3 PUFAs are shown in Table 4. Between V3 and V8, there was an interaction effect for eicosapentaenoic acid (EPA) showed an interaction effect with the N-3 PUFA group increasing 40% and placebo group decreasing 5% at visit 8 (p = 0.02). Docosapentaenoic acid (DPA) did not show an interaction effect (p = 0.6) but a main effect for time was present with the N-3 PUFA and placebo groups increasing (p = 0.04). Docosahexaenoic acid (DHA) tended to be higher with the N-3 PUFA group increasing 15% compared to 6% placebo group (p = 0.06); however, a main effect of time (p = 0.02). There was a main effect of time for N-6 PUFA arachidonic acid (ARA) that decreased during supplementation (p = 0.003). The N-6 PUFA:N-3 PUFA showed an interaction effect with a 20% decrease in the N-3 PUFA group compared to a 5% decrease in the placebo group. At V10, a main effect for time was still present for DPA and ARA (p < 0.05).
10 km TT performance
There was a main effect for time as TT time decreased from V2 to V9 by −8 and −12% for the N-3 PUFA and placebo groups, respectively (p < 0.001) (Fig. 5). Comparison of V2 and V11 showed a main effect for time with a decrease of −10% for both groups (p = 0.001).
Cycling cadence showed a main effect for time as mean RPM increased from V2 to V9 (p = 0.02) (Fig. 5). At V11, RPM tended to be higher than V2 (p = 0.06). Mean heart rate was not different between groups or time at V9 (p = 0.4) or V11 (p = 0.2).
Discussion
These studies are the first to report changes in neuromuscular function following acute Wingate-based SIT and investigate the role of neuromuscular adaptations in overall adaptations to SIT. Findings from Study 1 and 2 demonstrate that acute SIT causes neuromuscular fatigue primarily from peripheral factors. Two weeks of SIT does not appear to promote measurable neuromuscular adaptations, and in fact this type of training appears to cause residual neuromuscular fatigue. However, we did observe cycling-specific training adaptations measured from Wingate variables and improved 10 km TT performance in both studies that might have been the result of previously described metabolic adaptations (Burgomaster et al. 2007, 2005; Little et al. 2011).
Neuromuscular adaptations to SIT
Findings from Study 1 and 2 indicate that 2-weeks of SIT, with or without N-3 PUFA supplementation, does not stimulate neuromuscular adaptations as measured by quadriceps MVC force, contrary to our hypotheses. A single session of SIT was a potent stimulator of neuromuscular fatigue, eliciting a −14 to 29% change in MVC force, which is similar to the −18% change reported by Fernandez et al. (Fernandez-del-Olmo et al. 2011) after two Wingates. Surprisingly, there was no evidence of central neuromuscular fatigue in the present studies; however, peripheral neuromuscular fatigue was pervasive as evident from lower resting twitch characteristics including tPF (−41 to 56%) and Q tw (−36 to 49%). Here, we report Q tw peripheral fatigue beyond the previously suggested critical threshold of ~35% after constant cycling exercise (Amann and Dempsey 2008; Amann et al. 2006, 2009). In our neuromuscular testing protocol, we did not have any MVCs without superimposed stimulation. Reports have shown that study participants might not provide maximal force in anticipation of the superimposed stimulus (Button and Behm 2008). Some participants did show an acute decrease in force prior to stimulation; however, this was after peak force was achieved. To further address this limitation, we selected the highest force reading of the three MVCs per testing session.
It is possible that the intermittent nature of SIT training, compared to constant cycling, allowed the metabolic stress within the muscles to partially resolve between each bout, thus enabling participants to continue training at maximal effort while allowing fatigue to progressively increase after each sprint. We measured a significant increase in blood lactate in response to training; however, it is likely that metabolic stress from multiple factors, including the accumulation of H + and inorganic phosphates, altered calcium balance, and glycogen depletion, were involved in the manifestation such high peripheral neuromuscular fatigue. Interestingly, recreationally active participants in the study by Place et al. (Place et al. 2015) showed higher MVC force loss and LFF compared to the present study. This could have been the result of higher baseline fitness, as these participants had a mean VO2max 10 mL kg min−1 higher than the present study. This higher level of fitness could have resulted in higher fatigue from more cycling work completed.
Despite the intense nature of the SIT protocol and acute neuromuscular fatigue measured during the first training session, neuromuscular adaptations to SIT at V8 were not apparent in either study. Study 2 participants showed residual training-induced neuromuscular fatigue in pre-V8 neuromuscular testing. Residual peripheral neuromuscular fatigue was also present as measured from Q tw. Given this residual fatigue, it is surprising that there was no low-force frequency fatigue from training. Neuromuscular measurement after V8 training showed similar global and peripheral neuromuscular fatigue as measured after V3 training. Percent change in Q tw was significantly less than that measured at V3; however, we do not interpret this as a training adaptation given the pre-training fatigue.
The exploratory endpoint of V10 showed that participants’ neuromuscular function returned to pre-training baseline. Training-induced global and peripheral fatigue was attenuated in both groups as demonstrated by MVC and Q tw suggesting SIT-induced neuromuscular adaptations might have been realized during the post-training period. Previously observed increases in glucose transporter 4 and cytochrome C oxidase subunit-4 during 6-weeks of SIT are maintained up to 6 weeks post-training (Burgomaster et al. 2007); however, the observed increase in monocarboxylate transporter (MCT) that regulate muscle lactate and H + are only maintained 1-week post-training (Burgomaster et al. 2007). Therefore, the attenuated neuromuscular fatigue observed at V10 might be the result of both neural and metabolic adaptations to SIT.
N-3 PUFA supplementation does not alter neuromuscular adaptations to SIT
Plasma N-3 PUFA EPA concentrations increased, while DHA tended to be higher in response to 12-days of supplementation and subsequently returned to baseline over the 4-week washout period (Table 4). Previous reports have suggested that N-3 PUFA supplementation can enhance neuromuscular adaptations to exercise training in well-trained males (Lewis et al. 2015) and previously sedentary elderly females (Rodacki et al. 2012). From these studies and others, N-3 PUFAs have been suggested to enhance adaptations to exercise through a variety of mechanisms including increased acetylcholine concentration and acetylcholinesterase activity at the neuromuscular junction (Patten et al. 2002); altered membrane dynamics from increased N-3 PUFA incorporation that could increase nerve conduction velocity (Stiefel et al. 1999); or improved peripheral blood flow (Schiano et al. 2008). In the present study, N-3 PUFA supplementation had no effect on adaptations to SIT or detraining. This might have been the result of the supplementation duration used in the present study. Previous findings have suggested that 3-weeks of N-3 PUFA supplementation is the minimum duration to promote any neuromuscular ergogenic effects (Lewis et al. 2015) as 14-days of supplementation is necessary for incorporation into muscle membranes (McGlory et al. 2014). Alternatively, the intensity of training could have overwhelmed the potential influence of N-3 PUFA as was observed in recent evaluation of beta-alanine on adaptations to SIT (Cochran et al. 2015b). As such, future SIT investigations could evaluate a higher N-3 PUFA supplementation dose during a longer period of training.
SIT adaptations are cycling specific
SIT training data indicates participants in Study 1 improved cycling force generating a capacity (peak power), whereas Study 2 participants showed improvements in muscle endurance (percent power drop). Previous investigation of SIT has demonstrated an improvement in cycling performance more consistent with Study 1 as a result of increased muscle glycogen storage and metabolic capacity (Burgomaster et al. 2005); however, these metabolic adaptations along with increased muscle MCT concentration could also enhance cycling performance as observed in Study 2.
Participants in both studies decreased 10 km TT time (−8 to 12%) after 2-weeks of SIT, which is consistent with previous findings (Burgomaster et al. 2006). After 4-weeks of detraining, TT time was still improved (−10%) compared to V2, which is consistent with SIT-associated maintenance of glucose transporter 4 and cytochrome C oxidase subunit-4 during detraining (Burgomaster et al. 2007). Improved TT performance was related to higher cycling cadence, which provides some evidence of cycling-specific neuromuscular adaptations. However, the metabolic cost of higher cadence cycling is fueled by carbohydrate-based metabolism (Beelen and Sargeant 1993), thus, suggesting that cycling-specific neuromuscular and metabolic adaptations might have occurred in concert to improve TT performance.
Conclusions
Findings from these studies show that a single SIT session causes peripheral neuromuscular fatigue. Furthermore, 2-weeks of SIT with or without N-3 PUFA supplementation does not enhance neuromuscular function measured by change quadriceps MVC force, contrary to our hypothesis. The residual neuromuscular fatigue observed from training in Study 2 suggests neuromuscular adaptations may not have been realized without a post-training recovery period. N-3 PUFA supplementation increased plasma concentration of EPA, DPA, and DHA, but did not significantly enhance or impair adaptations to training from similar improvements in 10 km TT performance between studies and groups. It appears that the stress and neuromuscular fatigue from training were more pervasive than the previously reported ergogenic effect of N-3 PUFA supplementation during prolong training (Rodacki et al. 2012). As such, 2-weeks of SIT training improved 10 km cycling TT performance; however, previously reported metabolic adaptations to SIT appear to have been the primary contributor to these cycling adaptations with neuromuscular adaptations potentially playing a secondary role.
Abbreviations
- DHA:
-
Docosahexaenoic acid
- DPA:
-
Docosapentaenoic acid
- EMD:
-
Electromechanical delay
- EMG:
-
Electromyography
- EPA:
-
Eicosapentaenoic acid
- HR:
-
Heart rate
- LFF:
-
Low-force frequency fatigue
- M AMP :
-
M-wave amplitude
- N-3:
-
Omega-3
- MCT:
-
Monocarboxylate transporter
- MVC:
-
Maximal voluntary contraction
- PUFA:
-
Polyunsaturated fatty acid
- Q tw :
-
Potentiated twitch force
- RFD:
-
Rate of force development
- SIT:
-
Sprint interval training
- tAUC:
-
Twitch area under the curve
- tHRT:
-
Time to half relaxation
- tPF:
-
Twitch peak force
- tRFD:
-
Twitch rate of force development
- tTP:
-
Twitch time to peak force
- TT:
-
Time trial
- VA:
-
Voluntary activation
- VL:
-
Vastus lateralis
- VM:
-
Vastus medialis
- VO2 :
-
Volume of oxygen consumed
- W max :
-
Watt max
References
Amann M, Dempsey J (2008) Locomotor muscle fatigue modifies central motor drive in healthy humans and imposes a limitation to exercise performance. J Physiol 586:161–173
Amann M, Eldridge M, Lovering R, Stickland M, Pegelow D, Dempsey J (2006) Arterial oxygenation influences central motor output and exercise performance via effects on peripheral locomotor muscle fatigue in humans. J Physiol 575:937–952
Amann M, Proctor L, Sebranek J, Pegelow D, Dempsey J (2009) Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol 587:271–283
Beelen A, Sargeant A (1993) Effect of prior exercise at different pedalling frequencies on maximal power in humans. Eur J Appl Physiol 66:102–107
Bracco U (1994) Effect of triglyceride structure on fat absorption. Am J Clin Nutr 60:1002S–1009S
Brockerhoff H, Hoyle R, Hwang P, Litchfield C (1968) Positional distribution of fatty acids in depot triglycerides of aquatic animals. Lipids 3:24–29
Burgomaster KA, Hughes SC, Heigenhauser GL, Bradwell SN, Gibala MJ (2005) Six sessions of sprint interval training increases muscle oxidative potential and cycle endurance capacity in humans. J Appl Physiol 98:1985–1990
Burgomaster KA, Heigenhauser G, Gibala MJ (2006) Effect of short-term sprint interval training on human skeletal muscle carbohydrate metabolism during exercise and time-trial performance. J Appl Physiol 100:2041–2047
Burgomaster KA, Cermak NM, Philips SM, Benton C, Bonen A, Gibala MJ (2007) Divergent response of metabolite transport proteins in human skeletal muscle after sprint interval training and detraining. Am J Physiol Regul Integr Comp Physiol 292:R1970–R1976
Button D, Behm D (2008) The effect of stimulus anticipation on the interpolated twitch technique. J Sport Sci Med 7:520–524
Chen C, Liu Z, Bazinet R (2011) Rapid de-esterification and loss of eicosapentaenoic acid from rat brain phospholipids: an intracerebroventricular study. J Neurochem 116:363–373
Christensen M, Mortimer B, Hoy C, Redgrave T (1995) Clearance of chylomicrons following fish oil and seal oil feeding. Nutr Res 15:359–368
Cochran A, Myslik F, MacInnis M, Percival M, Bishop D, Tarnopolsky M, Gibala MJ (2015a) Manipulating carbohydrate availability between twice-daily sessions of high-intensity interval training over two weeks improves time-trial performance. Int J Sport Nutr Exerc Metab 25(5):463–470
Cochran A, Percival M, Thompson S, Gillen J, MacInnis M, Potter M, Tarnopolsky M, Gibala MJ (2015b) Beta-alanine supplementation does not augment the skeletal muscle adaptive response to six weeks of sprint interval training. Int J Sport Nutr Exerc Metab 25(6):541–549
Fernandez-del-Olmo M, Rodriguez FA, Iglesias M, Marina M, Benitez A, Vallejo L, Accero R (2011) Isometric knee extensor fatigue following a Wingate test: peripheral and central mechanisms. Scand J Med Sci Sports 23(1):57–65
Griffin S, Cafarelli E (2003) Neural excitability following resistance training studied with transcranial magnetic stimulation. Med Sci Sports Exerc 35:S293
Hermens H, Frericks B, Disselhorst-Klug C, Rau G (2000) Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 10:361–374
Lewis E, Radonic P, Wolever T, Wells G (2015) 21 days of mammalian omega-3 fatty acid supplementation improves aspects of neuromuscular function and performance in male athletes compared to olive oil placebo. J Int Soc Sports Nutr 12:28. doi:10.1186/s12970-015-0089-4
Little JP, Safdar A, Bishop D, Tarnopolsky M, Gibala MJ (2011) An acute bout of high-intensity interval training increases the nuclear abundance of PGC-1α and activates mitochondrial biogenesis in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 300:R1303–R1310
Martin V, Kerherve H, Messonnier LA, Banfi J, Geyssant A, Bonnefoy R, Feasson L, Millet GY (2010) Central and peripheral contributions to neuromuscular fatigue induced by a 24-h treadmill run. J Appl Physiol 108:1224–1233
McGlory C, Galloway SD, Hamilton DL, McClintock C, Breen L, Dick JD, Bell J, Tipton K (2014) Temporal changes in human skeletal muscle and blood lipid composition with fish oil supplementation. Prostaglandins Leukot Essent Fatty Acids 90:199–206
Millet GY, Tomazin K, Verges S, Vincent C, Bonnefoy R, Boisson R, Gergele L, Feasson L, Martin V (2011) Neuromuscular consequences of an extreme mountain ultra-marathon. PLoS One 6:e17059
Paltauf F, Esfandi F, Holasek A (1974) Stereo-specificity of lipases. Enzymatic hydrolysis of enantiomeric aklyl diglycerides by lipoprotein lipase, lingual lipase and pancreatic lipase. FEBS Lett 40:119–123
Patten G, Abeywardena M, Mcmurchie E, Jahangiri A (2002) Dietary fish oil increases acetylcholine and eicosanoid induced contractility of isolated rat ileum. J Nutr 132:2506–2513
Place N, Ivarsson N, Venckunas T, Neyroud D, Brazaitis M, Cheng A et al (2015) Ryanodine receptor fragmentation and sarcoplasmic reticulum Ca2 + leak after one session of high-intensity interval exercise. Proc Natl Acad Sci 112:15492–15497
Rich C, Cafarelli E (2000) Submaximal motor unit firing rates after 8 wk of isometric resistance training. Med Sci Sports Exerc 32:190–196
Rodacki C, Rodacki A, Pereira G, Naliwaiko K, Coelho I, Pequito D, Rernandes L (2012) Fish-oil supplementation enhances the effects of strength training in elderly women. Am J Clin Nutr 95:428–436
Sale D (1988) Neural adaptation to resistance training. Med Sci Sports Exerc 20:S135–S145
Schiano V, Laurenzano E, Brevetti G, De Maio J, Lanero S, Scopacasa F, Chiariello M (2008) Omega-3 polyunsaturated fatty acid in peripheral arterial disease: effect on lipid pattern, disease severity, inflammation profile, and endothelial function. Clin Nutr 27:241–247
Stiefel P, Ruiz-Gutierrez V, Gajon E, Acosta D, Garcia-Donas M, Madrazo J, Villar J, Carneado J (1999) Sodium transport kinetics, cell membrane lipid composition, neural conduction and metabolic control in type 1 diabetic patients. Changes after a low-dose n-3 fatty acid dietary intervention. Ann Nutr Metab 43:113–120
Thomas K, Goodall S, Stone M, Howatson G, St Clair Gibson A, Ansley L (2015) Central and peripheral fatigue in male cyclists after 4-, 20-, and 40-km time trials. Med Sci Sports Exerc 47:537–546
Verges S, Maffiuletti N, Kerherve H, Decorte N, Wuyam B et al (2009) Testing involved a train of 5 stimulations of the femoral nerve to measure twitch characteristics, followed by 3 MVCs described below. J Appl Physiol 106:701–710
Wanasundara U, Shahidi F (1997) Positional distribution of fatty acids in triacylglycerols of seal blubber oil. J Food Lipids 4:51–64
Yoshida H, Kumamaru J, Mawatari M, Ikeda I, Imaizumi K, Tsuji H, Seto A (1996) Lympatic absorption of seal and fish oils and their effecton lipid metabolism and eicosanoid production in rats. Biosci Biotechnol Biochem 60:1293–1298
Acknowledgements
The authors appreciate the help of Cathie Kessler for her technical assistance building the isometric dynamometer and MatLab program for analysis. Thank you to Dr. Bazinet’s laboratory for performing plasma fatty acid analysis and to Ms. Joy Hur assisting with data management. The omega-3 and placebo supplements for this study were donated by Auum Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
EJHL was supported by an Ontario Graduate Scholarship, Banting and Best Diabetes Centre studentship and a Peterborough K. M. Hunter scholarship. Equipment for this study was purchased from an Ontario Ministry of Tourism Culture and Sport, Research Program in Applied Sport Sciences operating grant to EJHL.
Conflict of interest
EJHL has received speaking fees and conference travel support from Auum Inc. TMSW is a part owner, President, and Medical Director of Glycemic Index Laboratories, Toronto, Canada and has authored several popular diet books on the glycemic index for which he has received royalties from Phillipa Sandall Publishing Services and CABI Publishers. He has received consultant fees, honoraria, travel funding, or served on the scientific advisory board for McCain Foods, Temasek Polytechnic, Singapore, Glycemic Index Symbol program, CreaNutrition AG, and the National Sports and Conditioning Association. His wife is an employee and part owner of Glycemic Index Laboratories.
Additional information
Communicated by Nicolas Place.
Rights and permissions
About this article
Cite this article
Lewis, E.J.H., Stucky, F., Radonic, P.W. et al. Neuromuscular adaptations to sprint interval training and the effect of mammalian omega-3 fatty acid supplementation. Eur J Appl Physiol 117, 469–482 (2017). https://doi.org/10.1007/s00421-017-3539-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-017-3539-7