Abstract
The oral ingestion of β-alanine, the rate-limiting precursor in carnosine synthesis, has been shown to elevate the muscle carnosine content. Carnosine is thought to act as a physiologically relevant pH buffer during exercise but direct evidence is lacking. Acidosis has been hypothesised to influence oxygen uptake kinetics during high-intensity exercise. The present study aimed to investigate whether oral β-alanine supplementation could reduce acidosis during high-intensity cycling and thereby affect oxygen uptake kinetics. 14 male physical education students participated in this placebo-controlled, double-blind study. Subjects were supplemented orally for 4 weeks with 4.8 g/day placebo or β-alanine. Before and after supplementation, subjects performed a 6-min cycling exercise bout at an intensity of 50% of the difference between ventilatory threshold (VT) and \( \dot{V}{\text{O}}_{2{\text{peak}}} \). Capillary blood samples were taken for determination of pH, lactate, bicarbonate and base excess, and pulmonary oxygen uptake kinetics were determined with a bi-exponential model fitted to the averaged breath-by-breath data of three repetitions. Exercise-induced acidosis was significantly reduced following β-alanine supplementation compared to placebo, without affecting blood lactate and bicarbonate concentrations. The time delay of the fast component (Td1) of the oxygen uptake kinetics was significantly reduced following β-alanine supplementation compared to placebo, although this did not reduce oxygen deficit. The parameters of the slow component did not differ between groups. These results indicate that chronic β-alanine supplementation, which presumably increased muscle carnosine content, can attenuate the fall in blood pH during high-intensity exercise. This may contribute to the ergogenic effect of the supplement found in some exercise modes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
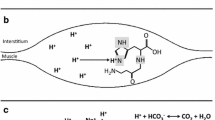
Maintaining acid–base balance is a major challenge during high-intensity exercise, when large amounts of protons are produced and released as a consequence of the anaerobic energy delivery in the active musculature (Hultman and Sahlin 1980). A significant portion of the contraction-induced protons are rapidly transported out of the active myocytes and buffered by the circulating buffers, such as bicarbonate. In addition to the various sarcolemmal ion transporters that can promote this proton efflux (Juel 1998), several intramyocellular pH buffers are available as a first-line defence against exercise-induced acidosis (Parkhouse and McKenzie 1984). These include free inorganic phosphate, creatine phosphate and the histidine residues in both proteins and in histidine-containing dipeptides (HCD). In humans, the HCD are exclusively represented by carnosine (β-alanyl-l-histidine) (Quinn et al. 1992). The relative importance and quantitative contribution to total buffering capacity of these skeletal muscle buffering constituents has been a subject of discussion. Carnosine is likely not the most important buffer in absolute terms, with a relative contribution estimated to be approximately 8–15% in human muscle (Hill et al. 2007; Parkhouse et al. 1985). However, the molecule draws our special attention because of two reasons: (1) its imidazole group has an optimal pKa value of 6.83 (Bate Smith 1938), knowing that intramyocellular pH at rest is 7.0–7.1 and can drop during high-intensity contractions to values as low as 6.3–6.5, and (2) because of the flexibility of its myoplasmic concentration. Recent studies (Harris et al. 2006; Hill et al. 2007; Derave et al. 2007) have shown that oral supplementation with β-alanine, the rate-limiting precursor of the dipeptide synthesis, can elevate muscle carnosine content by 40–80%, depending on the dose (usually between 3.2 and 6.4 g/day) and duration (4–10 weeks). None of the other skeletal muscle buffer pools is nearly as expansible.
Carnosine is a pleiotropic molecule, with pH buffering capacity in muscle as only one of several possible other physiological functions (Begum et al. 2005). Carnosine can act as metal chelator, an anti-oxidant and an antiglycation agent. In skeletal muscle, where the majority of the body’s carnosine is found, carnosine can act as a Ca++ sensitizer for the sarcomeres (Dutka and Lamb 2004; Lamont and Miller 1992) and by this mechanism possibly protect against fatigue (Rubtsov 2001). Additionally, exercise-induced oxidative stress is involved in contractile muscle fatigue (Powers and Jackson 2008) and can supposedly be antagonised by the antioxidative effects of carnosine (Kohen et al. 1988). Several lines of evidence suggest that carnosine is ergogenic. β-Alanine supplementation resulted in performance enhancement in some exercise modes, such as a single high-intensity exercise bout (Hill et al. 2007; Stout et al. 2007), in sprint exercise at the end of an endurance cycling race (Van Thienen et al. 2009) and in repeated maximal contraction bouts (Derave et al. 2007). In vitro work on isolated frog muscles has shown that addition of carnosine to the incubation medium can potently antagonise contractile fatigue (Severin et al. 1953; Boldyrev and Petukhov 1978). It remains to be established by which mechanism these ergogenic effects are established. An improved pH buffer capacity certainly is a candidate, because alternative ways to improve the buffer capacity (albeit in blood instead of muscle) by acute oral bicarbonate ingestion have shown to improve performance in exercise modes of similar duration and intensity, i.e. single or repeated maximal exercise bouts of 30 s to 7 min of duration (Linderman and Gosselink 1994). A first aim of the current study was to explore the potential of carnosine to attenuate exercise-induced acidosis in high-intensity exercise of fixed intensity and duration.
The low blood pH resulting from anaerobic work is thought to affect the oxygen uptake (\( \dot{V}{\text{O}}_{2} \)) kinetics of high-intensity exercise. In the transition from rest to exercise at an intensity above the ventilatory threshold (VT) three phases can be distinguished: (1) a short cardio-dynamic phase (15–25 s), (2) a fast component (2–3 min) characterised by an exponential rise in \( \dot{V}{\text{O}}_{2} \) and (3) a slow component where \( \dot{V}{\text{O}}_{2} \) shows a slow but gradual increase towards a steady-state or peak oxygen uptake (\( \dot{V}{\text{O}}_{2{\text{peak}}} \)). Several studies have shown that pre-exercise alkalosis, either elicited by hyperventilation (Hayashi et al. 1999; Ward et al. 1983) or by bicarbonate ingestion (Kolkhorst et al. 2004), slows the fast component of \( \dot{V}{\text{O}}_{2} \), probably by inducing a leftward shift of the oxygen–haemoglobin dissociation curve and reducing the O2 delivery to the working muscles. However, others (Berger et al. 2006) did not observe an effect of pre-exercise metabolic alkalosis on \( \dot{V}{\text{O}}_{2} \) kinetics, and even others (Zoladz et al. 2005) found a speeding of the fast component.
With regard to the nature of the slow component of \( \dot{V}{\text{O}}_{2} \) kinetics, i.e. why \( \dot{V}{\text{O}}_{2} \)/W values are higher above than below the VT, several physiological mechanisms have been investigated, such as elevation in body and/or muscle temperature, cardiac and ventilatory muscle work, auxiliary muscle work, recruitment of fast-twitch fibres and metabolic factors (reviewed in Gaesser and Poole 1996; Zoladz and Korzeniewski 2001). A possible candidate for the latter is the acidosis, because a slow component only occurs at exercise intensities above VT, i.e. where lactate accumulation occurs. In order to investigate this more causally, several interventions with enhancement or attenuation of exercise-induced acidosis have been performed. Zoladz et al. (1998) observed that the magnitude of the slow component is increased following acute pre-exercise acidification induced by ingestion of ammonium chloride. The effects of pre-exercise alkalinisation by oral sodium bicarbonate ingestion are more equivocal. Some authors found a significant reduction of the slow component (Kolkhorst et al. 2004; Berger et al. 2006), whereas several others observed no effect of bicarbonate (Heck et al. 1998; Santalla et al. 2003; Zoladz et al. 1997).
The purpose of the present study was to explore whether 4 weeks of β-alanine supplementation can attenuate exercise-induced acidosis during a fixed 6-min exercise bout at an intensity calculated as 50% of the difference between VT and \( \dot{V}{\text{O}}_{2{\text{peak}}} \). In order to investigate the effect of the possibly suppressed acidosis on the fast and slow component of \( \dot{V}{\text{O}}_{2} \) kinetics, the exercise bout was repeated three times in each condition on separate days, allowing optimal bi-exponential modelling of breath-by-breath data.
Methods
Subjects
Fourteen male physical education students volunteered to participate in this study. All subjects were physically active, but not involved in sports competition or organised training. The subjects’ age, weight, height and maximal oxygen uptake were 21.9 ± 1.5 years, 74.9 ± 8.3 kg, 1.80 ± 0.05 m and 55.5 ± 3.6 mL/(kg min) for placebo and 21.1 ± 0.7 years, 71.8 ± 8.8 kg, 1.78 ± 0.07 m and 57.1 ± 4.7 mL/(kg min) for β-alanine group, respectively (NS). Subjects reported that they did not take any other oral supplement during the study nor had taken nutritional supplements in the 3 months prior to the study. Subjects were asked to abstain from exercise 24 h before each test and to maintain their normal physical activity during the study. During this study they did not participate in regular or organised training. The subjects gave their informed consent and the study was approved by the local Ethics Committee (Ghent University Hospital, Belgium).
Experimental protocol
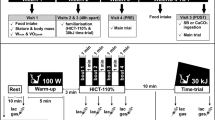
The subjects of this placebo-controlled, double-blind study, were randomised, based on their (\( \dot{V}{\text{O}}_{2{\text{peak}}} \)) and blood pH at the end of a 6-min cycling exercise at a power output equivalent to 50% of the difference between VT and \( \dot{V}{\text{O}}_{2{\text{peak}}} \) (50% Δ) into a control and experimental group. They were supplemented for 4 weeks with either placebo (maltodextrine) or β-alanine (Carnosyn™, National Alternatives International, San Marcos, USA). Supplements were provided in capsules of 400 mg and were administered each day as six divided doses, with at least 2 h in between ingestions. Daily doses consisted of 2.4 g/day during the first 2 days, 3.6 g/day during the subsequent 2 days, and from then on 4.8 g/day until the end of the supplementation. In a recent study on a similar study population, we have shown that this supplementation schedule leads to significant elevations in the carnosine content of both slow-twitch and fast-twitch muscle types (Baguet et al. 2009). Each subject performed a maximal ramp exercise test on an electromagnetically braked cycle ergometer (Lode, Excalibur sport; Groningen, The Netherlands) to determine \( \dot{V}{\text{O}}_{2{\text{peak}}} \) and ventilatory threshold (VT). Pedalling frequency was kept between 75 and 80 rpm. After a warm-up of 3 min at 50 W, the work load was increased by 30 W/min to the point the subjects failed to continue to pedal at 75 rpm. The gas exchange threshold (GET) was determined as the point at which there was the beginning of a systematic increase in \( {{\dot{V}{\text{E}}} \mathord{\left/ {\vphantom {{\dot{V}{\text{E}}} {\dot{V}{\text{O}}_{2} }}} \right. \kern-\nulldelimiterspace} {\dot{V}{\text{O}}_{2} }} \), but not in \( {{\dot{V}{\text{E}}} \mathord{\left/ {\vphantom {{\dot{V}{\text{E}}} {\dot{V}{\text{CO}}_{2} }}} \right. \kern-\nulldelimiterspace} {\dot{V}{\text{CO}}_{2} }} \), by two independent experienced researchers. For all subjects, work rates equivalent to 50% Δ were calculated.
There were two test periods (Pre and Post) with 4 weeks of supplementation with β-alanine or placebo in between. Each test period lasted 1 week and included on Monday, Wednesday and Friday a 6-min cycle exercise bout at a power output equivalent to 50% Δ. The 50% Δ test was preceded by 3 min of baseline pedalling at 10 W and was followed by 4 min of unloaded pedalling. The exercise test sessions were conducted at the same time of the day for each subject to account for any possible circadian rhythm effects.
Measurements
Before and during the exercise bouts, \( \dot{V}{\text{O}}_{2} \) was measured continuously on a breath-by-breath basis by means of a computerised O2–CO2 analyser-flowmeter combination (Jaeger Oxycon Pro, Germany). Prior to each exercise test, the gas analysers (an O2-analyser with functioning based on the differential-paramagnetic principle and an infrared CO2-analyser) were calibrated and the volume calibration (‘triple V’ transducer) was conducted. Capillary blood samples (150 μL) were taken from a hyperaemic ear lobe in order to determine blood gas analysis (GEM, Premier™ 3000, Instrumentation Laboratory) at rest (following warm-up), after 3 min cycling at 50% Δ, after 6 min cycling at 50% Δ and after 4 min recovery. The blood samples were taken in two of the three tests per condition (Wednesday and Friday) and the data of the two samples were averaged. In the blood samples pH and lactate were measured and bicarbonate and base excess were calculated (base excess = (1 − 0.014[Hb]) × ([HCO3 −] − 24 + (1.43[Hb] + 7.7) (pH − 7.4))). In subjects where blood lactate exceeded 15 mmol/L during exercise, Lactate Pro strips (Arkray Inc, Kyoto, Japan) were used for blood lactate determination.
Analysis
The breath-by-breath \( \dot{V}{\text{O}}_{2} \) data from each test were initially examined to exclude errant breaths caused by coughing, swallowing, sighing, etc., and those values exceeding local mean by more than 4 standard deviations were deleted. The breath-by-breath \( \dot{V}{\text{O}}_{2} \) data from each test were subsequently linearly interpolated to give 1 s-values. For each subject and each condition, the three identical repetitions were time-aligned to the start of exercise, superimposed, and ensemble averaged to reduce the breath-to-breath noise and enhance the underlying physiological response characteristics. The baseline \( \dot{V}{\text{O}}_{2} \) was defined as the average \( \dot{V}{\text{O}}_{2} \) measured during baseline pedalling between 150 and 30 s before the start of the 50% Δ bout. The initial cardiodynamic component was ignored by eliminating the first 20 s of data after the onset of exercise. Subsequently, each averaged response was described using a bi-exponential model with the following equation:
This model includes amplitudes (A), time constants (τ) and delay times (Td) for the \( \dot{V}{\text{O}}_{2} \) fast (subscript 1) and the \( \dot{V}{\text{O}}_{2} \) slow (subscript 2) component which were determined using a non linear least-square algorithm. Because the asymptotic value A2 may represent a higher value than that actually reached at the end of the exercise, the value of the \( \dot{V}{\text{O}}_{2} \) slow exponential term at the end of exercise was defined as A2′.
The O2 deficit was computed by integrating the difference between the \( \dot{V}{\text{O}}_{2} \) requirement for the exercise (assumed to be represented by the average \( \dot{V}{\text{O}}_{2} \) during the last 30 s of exercise) and the actual measured \( \dot{V}{\text{O}}_{2} \) from t = 0 to t = 360.
Statistics
A 2 × 2 repeated measures analysis of variance (RM ANOVA) was used to evaluate pH, lactate, bicarbonate, base excess, \( \dot{V}{\text{O}}_{2} \) kinetic parameters (A, τ and Td), \( \dot{V}{\text{O}}_{2} \), ventilation (\( \dot{V}{\text{E}} \)) and CO2 output (\( \dot{V}{\text{CO}}_{2} \)) with ‘group’ (placebo vs. β-alanine) as between-subjects factor and ‘time’ (Pre and Post) as a within-subjects factor (SPSS statistical software, SPSS Inc, Chicago, USA). Values are presented as mean ± SD and significance was assumed at p < 0.05.
Results
Blood gas analysis
Table 1 shows an overview of the blood gas analysis parameters. Blood pH at rest was approximately 7.41–7.42 and was not affected by supplementation. The cycling exercise at an intensity of 50% Δ elicited a marked acidosis towards values around 7.20 at the sixth minute. There was no significant interaction effect of the absolute pH values at 6 min of exercise (Ex6), but Fig. 1 shows that the pH difference between baseline and Ex6 (i.e. the exercise-induced acidosis) is significantly different between β-alanine and placebo group over the time (interaction effect; p = 0.031). As a result of 4 weeks’ supplementation the ΔpH from baseline to the end of high-intensity cycling decreased with 0.015 units in the β-alanine group and increased with 0.012 pH units in the placebo group. Blood lactate increased to values of ~13 mmol/L at the end of exercise and slightly declined at 4 min into recovery. Bicarbonate and base excess markedly decreased during high-intensity exercise. None of these parameters (lactate, bicarbonate and base excess) showed a significant group effect or interaction.
Pulmonary gas exchange
Figure 2 shows a typical graph of the \( \dot{V}{\text{O}}_{2} \) before (Pre) and after (Post) β-alanine and placebo supplementation. As shown in Table 2, the \( \dot{V}{\text{O}}_{2} \) profile contains a clear slow component (A2′) of ~500 mL, as can be expected for exercise intensities above the VT. For both groups there were no significant differences (p > 0.05) in \( \dot{V}{\text{O}}_{2} \) throughout exercise prior to or after supplementation. As shown in Table 3 the \( \dot{V}{\text{E}} \) was ~22 L/min after warm up and increased fivefold throughout the 6-min intensive cycling, with no differences between both groups. For both groups the \( \dot{V}{\text{CO}}_{2} \) was ~750 mL/min after warm-up and increased to ~4,100 mL/min after a 6-min cycling exercise at 50% Δ before and after supplementation. Also for \( \dot{V}{\text{CO}}_{2} \) there were no significant differences between β-alanine and placebo (Table 3).
\( \dot{V}{\text{O}}_{2} \) kinetic parameters
In the fast component of the \( \dot{V}{\text{O}}_{2} \) kinetics, a significant interaction (p = 0.007) in the time delay (Td1) was observed, which resulted from both a decrease over time (−2.2 s post vs. pre) in the β-alanine group and an increase (+3.9 s) in the placebo group. The time constant (tau1), however, tended to display an opposite pattern (p = 0.088 for the interaction effect), i.e. a slowing in the β-alanine group (+1.2 s) and a speeding (−4.8 s) in the placebo group. Therefore, the oxygen deficit, which is influenced by both the Td1 and tau1, was not affected by either intervention (p = 0.937). The amplitude of the slow component (A2′) was not affected by β-alanine supplementation. For the time constant (tau2) and time delay (Td2) of the slow component, there was a tendency for an interaction effect (p = 0.082 and p = 0.068, respectively) (Table 2).
Discussion
The primary goal of the present study was to investigate the role of muscle carnosine in the acid–base balance during high-intensity exercise. According to the current working hypothesis, an increased intramyocellular content of carnosine would attenuate the drop in intracellular pH during high-intensity contractions. The smaller transsarcolemmal concentration gradient of [H+] decreases the acid efflux from the active muscle cells and results in less pronounced circulating acidosis. Evidence for this hypothesis is now presented in the current results. A 6-min exercise bout at a fixed intensity above the VT (50% of the difference between VT and \( \dot{V}{\text{O}}_{2{\text{peak}}} \)) in healthy male subjects resulted in a decline in capillary blood pH from ~7.4 to ~7.2, yet this acidosis, when expressed as the difference between baseline and end-exercise, but not in absolute values, was less pronounced after subjects were supplemented with β-alanine for 4 weeks compared to placebo. Post-supplementation, the exercise-induced acidosis was 19% smaller in the β-alanine group compared to the placebo group (Fig. 1). Although we did not measure the carnosine concentration, our two previous studies demonstrated that all subjects had increased muscle carnosine content in 4–5 weeks of β-alanine supplementation (4–6 g/day) (Baguet et al. 2009; Derave et al. 2007). This suggests that the difference between groups is related to the presumable elevation in muscle carnosine content (Harris et al. 2006; Baguet et al. 2009). This supports the earlier suggestion of Hill et al. (2007) that the physicochemical buffer property of the dipeptide probably in part underlies the ergogenic potential of β-alanine supplementation. However, it does not exclude the additional contribution of other factors.
Traditionally, the importance of carnosine as a physicochemical buffer in human skeletal muscle has been largely ignored, because various calculations and measurements have designated its relative contribution to only 8–15% of total buffer capacity (Hill et al. 2007; Parkhouse et al. 1985; Hultman and Sahlin 1980). Indeed, in various other vertebrates the HCD contribute more in both absolute and relative terms (Abe 2000). In the middle gluteal muscle of the thoroughbred horse, for example, the carnosine concentration is 6.7-fold higher than in human vastus lateralis, increasing its relative contribution to total buffer capacity to 30.6% (Harris et al. 1990; Sewell et al. 1992). The significant reduction in exercise-induced acidosis, observed in the present study following β-alanine supplementation (and a presumable increase in muscle carnosine content of 40–50%), illustrates that the importance of carnosine as a pH buffer should not be dismissed. The cause for the discrepancy between its calculated small importance and its observed physiological larger importance remains to be established. Part of the explanation may be found in the fact that most changes in contracting muscle occur in the narrow range of pH around the value of 6.8, where carnosine exerts its maximal effect, leading to an underestimation of carnosine’s relative contribution.
In the present study the circulating bicarbonate and lactate concentrations were not different between conditions, which allows for the following interpretations. Given the identical lactate levels, the lower degree of acidosis evident in the β-alanine supplemented group, is not caused by a lower anaerobic component of total energy delivery. Hence, smaller acidosis genuinely represents better buffering capacity, and not smaller acid production. The identical bicarbonate levels suggest that the enhanced intracellular buffering capacity (by β-alanine-induced carnosine loading) is not compensated by sparing of extracellular buffering capacity (mainly bicarbonate). Carnosine has been implicated as an activator of carbonic anhydrase activity (Temperini et al. 2005), but since in the current study neither the circulating bicarbonate level and pCO2, nor the pulmonary CO2 output differed between groups, it seems unlikely that β-alanine supplementation attenuated acidosis through carbonic anhydrase activity. Thus, the buffering actions of circulating bicarbonate and intracellular carnosine are additive in order to better protect the ‘milieu interieur’ against the homeostatic perturbation of (extreme) acidosis. This is somewhat different from the effects observed by Suzuki et al. (2006) during repeated sprint exercise after subjects were acutely supplemented with a 1.5 g carnosine/anserine or placebo mixture. In that study, the enhanced circulating buffering capacity by dipeptide supplementation was compensated by a decreased utilisation (sparing) of bicarbonate (Suzuki et al. 2006). Therefore, acute dipeptide supplementation enhances the relative contribution of non-bicarbonate buffering with only little effect on total blood buffer capacity. The emerging conclusion of the latter and the current study is that carnosine and bicarbonate can work as additive in physicochemical buffering, provided they are located in different compartments, i.e. carnosine intracellularly and bicarbonate in the circulation.
An additional goal of the current study was to explore the role of acidosis in the \( \dot{V}{\text{O}}_{2} \) kinetics during high-intensity exercise. The Td1 of the fast component was significantly shorter following β-alanine compared to placebo supplementation, which suggests faster kinetics. The physiological significance of this finding, however, is probably limited because the effect was not sufficient to alter the calculated oxygen deficit. The latter is probably due to the fact that the time constant (tau1) changed, albeit not significantly (p = 0.088), in the direction of slower kinetics (larger tau) following β-alanine supplementation. Taken together, the effect of reduced exercise-induced acidosis following β-alanine supplementation on the fast component of the \( \dot{V}{\text{O}}_{2} \) kinetics is very limited. This is in agreement with the recent study by Berger et al. (2006) that reported no alteration in the fast component following induced metabolic alkalosis. The fact that other studies did find a significantly faster (shorter tau1, Zoladz et al. 2005) or slower (longer tau1, Kolkhorst et al. 2004) kinetics following metabolic alkalosis, may be caused by the shortcoming that kinetic modelling was based on only one repetition per condition, rather than several like transitions in the current study and that of Berger et al. (2006).
As outlined in the introduction, some but not all studies that have experimentally altered pre-exercise blood pH (alkalinisation or acidification) support a role for proton accumulation in the slow component of \( \dot{V}{\text{O}}_{2} \). In the study by Berger et al. (2006), the appearance of the slow component (TD) is significantly delayed and the absolute \( \dot{V}{\text{O}}_{2} \) above baseline at the end of exercise was significantly reduced following sodium bicarbonate ingestion. In the current study, the absolute end-exercise \( \dot{V}{\text{O}}_{2} \) and A2′ were not different between conditions. Therefore, the present study does not support a role for acidosis in the physiological basis of the slow component. At first sight, our results appear to be in contradiction with the study from Berger et al. (2006) and others (Kolkhorst et al. 2004; Forbes et al. 2005). However, the magnitude of experimental alteration of pH is less pronounced in the current study compared with the latter studies. Furthermore, the experimental intervention is essentially different in nature. In the bicarbonate supplementation studies, the resting pH is substantially elevated beyond values that lie within the physiological variation range. In the present study, however, the chronic β-alanine supplementation has no direct effect on resting blood pH, but the enhanced buffer capacity suppresses the acidosis that results from high-intensity work. Thus, although the absolute pH cannot be excluded as a contributing factor, the magnitude of the decline in pH during exercise is not a factor involved in the slow component of \( \dot{V}{\text{O}}_{2} \) kinetics.
Conclusions
It can be concluded from the current data that chronic β-alanine supplementation can reduce acidosis during high-intensity exercise. This indicates that carnosine may act as a physiologically meaningful physicochemical buffer in human skeletal muscle and may provide at least a part of the explanation for the ergogenic effect of the β-alanine supplement found in some exercise modes. Additionally, these data do not support an important role for acidosis in the oxygen deficit or in the origin of the slow component of \( \dot{V}{\text{O}}_{2} \) kinetics during high-intensity exercise.
References
Abe H (2000) Role of histidine-related compounds as intracellular proton buffering constituents in vertebrate muscle. Biochemistry (Mosc) 65:757–765
Baguet A, Reyngoudt H, Pottier A, Everaert I, Callens S, Achten E, Derave W (2009) Carnosine loading and washout in human skeletal muscles. J Appl Physiol 106:837–842
Bate Smith EC (1938) The buffering of muscle in rigor; protein, phosphate and carnosine. J Physiol (Lond) 92:336–343
Begum G, Cunliffe A, Leveritt M (2005) Physiological role of carnosine in contracting muscle. Int J Sport Nutr Exerc Metab 15:493–514
Berger NJ, McNaughton LR, Keatley S, Wilkerson DP, Jones AM (2006) Sodium bicarbonate ingestion alters the slow but not the fast phase of VO2 kinetics. Med Sci Sports Exerc 38:1909–1917
Boldyrev AA, Petukhov VB (1978) Localization of carnosine effect on the fatigued muscle preparation. Gen Pharmacol 9:17–20
Derave W, Ozdemir MS, Harris RC, Pottier A, Reyngoudt H, Koppo K, Wise JA, Achten E (2007) Beta-alanine supplementation augments muscle carnosine content and attenuates fatigue during repeated isokinetic contraction bouts in trained sprinters. J Appl Physiol 103:1736–1743
Dutka TL, Lamb GD (2004) Effect of carnosine on excitation–contraction coupling in mechanically-skinned rat skeletal muscle. J Muscle Res Cell Motil 25:203–213
Forbes SC, Raymer GH, Kowalchuk JM, Marsh GD (2005) NaHCO3-induced alkalosis reduces the phosphocreatine slow component during heavy-intensity forearm exercise. J Appl Physiol 99:1668–1675
Gaesser GA, Poole DC (1996) The slow component of oxygen uptake kinetics in humans. Exerc Sport Sci Rev 24:35–71
Harris RC, Marlin DJ, Dunnett M, Snow DH, Hultman E (1990) Muscle buffering capacity and dipeptide content in the thoroughbred horse, greyhound dog and man. Comp Biochem Physiol A Physiol 97:249–251
Harris RC, Tallon MJ, Dunnett M, Boobis L, Coakley J, Kim HJ, Fallowfield JL, Hill CA, Sale C, Wise JA (2006) The absorption of orally supplied beta-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids 30:279–289
Hayashi N, Ishihara M, Tanaka A, Yoshida T (1999) Impeding O(2) unloading in muscle delays oxygen uptake response to exercise onset in humans. Am J Physiol 277:R1274–R1281
Heck KL, Potteiger JA, Nau KL, Schroeder JM (1998) Sodium bicarbonate ingestion does not attenuate the VO2 slow component during constant-load exercise. Int J Sport Nutr 8:60–69
Hill CA, Harris RC, Kim HJ, Harris BD, Sale C, Boobis LH, Kim CK, Wise JA (2007) Influence of beta-alanine supplementation on skeletal muscle carnosine concentrations and high intensity cycling capacity. Amino Acids 32:225–233
Hultman E, Sahlin K (1980) Acid–base balance during exercise. Exerc Sport Sci Rev 8:41–128
Juel C (1998) Muscle pH regulation: role of training. Acta Physiol Scand 162:359–366
Kohen R, Yamamoto Y, Cundy KC, Ames BN (1988) Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. Proc Natl Acad Sci USA 85:3175–3179
Kolkhorst FW, Rezende RS, Levy SS, Buono MJ (2004) Effects of sodium bicarbonate on VO2 kinetics during heavy exercise. Med Sci Sports Exerc 36:1895–1899
Lamont C, Miller DJ (1992) Calcium sensitizing action of carnosine and other endogenous imidazoles in chemically skinned striated muscle. J Physiol 454:421–434
Linderman JK, Gosselink KL (1994) The effects of sodium bicarbonate ingestion on exercise performance. Sports Med 18:75–80
Parkhouse WS, McKenzie DC (1984) Possible contribution of skeletal muscle buffers to enhanced anaerobic performance: a brief review. Med Sci Sports Exerc 16:328–338
Parkhouse WS, McKenzie DC, Hochachka PW, Ovalle WK (1985) Buffering capacity of deproteinized human vastus lateralis muscle. J Appl Physiol 58:14–17
Powers SK, Jackson MJ (2008) Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88:1243–1276
Quinn PJ, Boldyrev AA, Formazuyk VE (1992) Carnosine: its properties, functions and potential therapeutic applications. Mol Aspects Med 13:379–444
Rubtsov AM (2001) Molecular mechanisms of regulation of the activity of sarcoplasmic reticulum Ca-release channels (ryanodine receptors), muscle fatigue, and Severin’s phenomenon. Biochemistry (Mosc) 66:1132–1143
Santalla A, Perez M, Montilla M, Vicente L, Davison R, Earnest C, Lucia A (2003) Sodium bicarbonate ingestion does not alter the slow component of oxygen uptake kinetics in professional cyclists. J Sports Sci 21:39–47
Severin SE, Kirzon MV, Kaftanova TM (1953) Effect of carnosine and anserine on action of isolated frog muscles (article in Russian). Dokl Akad Nauk SSSR 91:691–694
Sewell DA, Harris RC, Marlin DJ, Dunnett M (1992) Estimation of the carnosine content of different fibre types in the middle gluteal muscle of the thoroughbred horse. J Physiol (Lond) 455:447–453
Stout JR, Cramer JT, Zoeller RF, Torok D, Costa P, Hoffman JR, Harris RC (2007) Effects of beta-alanine supplementation on the onset of neuromuscular fatigue and ventilatory threshold in women. Amino Acids 32:381–386
Suzuki Y, Nakao T, Maemura H, Sato M, Kamahara K, Morimatsu F, Takamatsu K (2006) Carnosine and anserine ingestion enhances contribution of nonbicarbonate buffering. Med Sci Sports Exerc 38:334–338
Temperini C, Scozzafava A, Puccetti L, Supuran CT (2005) Carbonic anhydrase activators: X-ray crystal structure of the adduct of human isozyme II with l-histidine as a platform for the design of stronger activators. Bioorg Med Chem Lett 15:5136–5141
Van Thienen R, Van Proeyen K, Vanden Eynde B, Puype J, Lefere T, Hespel P (2009) Beta-alanine improves sprint performance in endurance cycling. Med Sci Sports Exerc 41:898–903
Ward SA, Whipp BJ, Koyal S, Wasserman K (1983) Influence of body CO2 stores on ventilatory dynamics during exercise. J Appl Physiol 55:742–749
Zoladz JA, Korzeniewski B (2001) Physiological background of the change point in VO2 and the slow component of oxygen uptake kinetics. J Physiol Pharmacol 52:167–184
Zoladz JA, Duda K, Majerczak J, Domanski J, Emmerich J (1997) Metabolic alkalosis induced by pre-exercise ingestion of NaHCO3 does not modulate the slow component of VO2 kinetics in humans. J Physiol Pharmacol 48:211–223
Zoladz J, Duda K, Majerczak J, Emmerich J, Domanski J (1998) Pre-exercise acidification induced by ingestion of NH4Cl increases the magnitude of the slow component of VO2 kinetics in humans. J Physiol Pharmacol 49:443–455
Zoladz JA, Szkutnik Z, Duda K, Majerczak J, Korzeniewski B (2005) Preexercise metabolic alkalosis induced via bicarbonate ingestion accelerates VO2 kinetics at the onset of a high-power-output exercise in humans. J Appl Physiol 98:895–904
Acknowledgments
This study was financially supported by grants from the Research Foundation—Flanders (FWO 1.5.149.08 and G.0046.09). Audrey Baguet is a recipient of a PhD-scholarship from the Research Foundation—Flanders (FWO). We thank Dr. John Wise and Natural Alternatives International (San Marcos, CA) for generously providing the β-alanine (CarnoSyn) and placebo supplements. We thank Peter Van Mossevelde and Tim Decleir for their practical contributions and Dr. Jacques Bouckaert for his valuable advice. The experiments of this manuscript comply with the current laws of Belgium.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Susan Ward.
Rights and permissions
About this article
Cite this article
Baguet, A., Koppo, K., Pottier, A. et al. β-Alanine supplementation reduces acidosis but not oxygen uptake response during high-intensity cycling exercise. Eur J Appl Physiol 108, 495–503 (2010). https://doi.org/10.1007/s00421-009-1225-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-009-1225-0