Abstract
Carnosine (Carn) occurs in high concentrations in skeletal muscle is a potent physico-chemical buffer of H+ over the physiological range. Recent research has demonstrated that 6.4 g.day−1 of β-alanine (β-ala) can significantly increase skeletal muscle Carn concentrations (M-[Carn]) whilst the resultant change in buffering capacity has been shown to be paralleled by significant improvements in anaerobic and aerobic measures of exercise performance. Muscle carnosine increase has also been linked to increased work done during resistance training. Prior research has suggested that strength training may also increase M-[Carn] although this is disputed by other studies. The aim of this investigation is to assess the effect of 10 weeks resistance training on M-[Carn], and, secondly, to investigate if increased M-[Carn] brought about through β-ala supplementation had a positive effect on training responses. Twenty-six Vietnamese sports science students completed the study. The subjects completed a 10-week resistance-training program whilst consuming 6.4 g.day−1 of β-ala (β-ALG) or a matched dose of a placebo (PLG). Subjects were assessed prior to and after training for whole body strength, isokinetic force production, muscular endurance, body composition. β-Alanine supplemented subjects increased M-[Carn] by 12.81 ± 7.97 mmol.kg−1 dry muscle whilst there was no change in PLG subjects. There was no significant effect of β-ala supplementation on any of the exercise parameters measured, mass or % body fat. In conclusion, 10 weeks of resistance training alone did not change M-[Carn].
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carnosine (β-alanyl-l-histidine) (Carn) occurs in high concentration in skeletal muscle (∼20 mmol.kg−1 dry muscle in humans), with the highest concentrations in type II fibers (Harris et al. 1998; Hill et al. 2007). Carnosine is a di-peptide synthesised in muscle and CNS tissue from histidine and the non-proteogenic amino acid, β-alanine (β-ala). It has been reported to have a number of biochemical functions in vertebrate species including a role as an antioxidant (Boldyrev et al. 1988) and in Ca2+ sensitisation (Batrukova and Rubtsov 1997; Dukta and Lamb 2004). Dukta and Lamb (2004) found that in mechanically skinned rat skeletal muscle fibres the presence of Carn caused an increase in the sensitivity of the contractile fibres to Ca2+. However, with a pKa of 6.83 (arising from the imidozole ring of the histidine residue) (Martin and Edcall 1960), and with such high concentrations occurring in skeletal muscle, Carn is quantitatively important as a physico-buffer of H+ over the physiological range (pH 7.1–6.2) (Harris et al. 1990). Thus Carn may play a significant role in attenuating the decline in intracellular pH during intense exercise and consequently any increase in [Carn] in skeletal muscle could potentially improve performance in exercise where pH decline is a major contributor to fatigue.
Work by Harris et al. (2006) and Hill et al. (2007) has recently demonstrated that 4–10 weeks of supplementation with 6.4 g.day−1 of β-ala can increase M-[Carn] by ∼60–80%. The changes in M-[Carn] resulted in improved performance in a range of exercise modes. Anaerobic exercise capacity during cycle ergometry was been shown by Hill et al. (2007) to increase after β-ala supplementation, and an increased time to exhaustion (TTE) during isometric exercise has been reported by (Ponte et al. 2007). In this last study subjects sustained an isometric contraction at 45% of their maximum voluntary contraction force, until fatigue. Forty-five percent MVC corresponds to the work level resulting in the greatest increase in [lactate] + [pyruvate] in muscle (Ahlborg et al. 1972), and thus presumably also the greatest decline in intramuscular pH. Following supplementation, endurance time at 45% MVC was increased by approximately 9 s from a mean exercise time of 74.6 ± s. Changes in other parameters more usually associated with aerobic exercise capacity, but which might be affected by the release of H+ into plasma lowering pH, have also been shown (Stout et al. 2007). In this study an increase in ventilatory threshold to a higher work intensity following supplementation was observed. These studies indicate that increasing M-[Carn] can aid performance in specific laboratory based protocols where a decline in pH is expected. In contrast only limited work has been done on the effect of increased M-[Carn] on field-based measures of performance.
Resistance training is an important training method for a wide variety of athletes and individuals. Repetitive isometric and dynamic resistance training has been reported to lower pH to ∼6.8 (Schott et al. 1995; Edge et al. 2006). Due to the anaerobic nature of resistance training and reported changes in [H+], increased β-ala ingestion (as a means to increase the muscle carnosine content and buffering capacity) may be an effective aid for resistance training athletes.
Hoffman et al. (2006) reported that subjects supplemented with β-ala and creatine (Cr) increased fat free mass and had a threefold increase in strength after 10 weeks of resistance training compared to a placebo group. There was also a Cr only group which displayed the same change in strength but did not show any body compositional changes. It was suggested that a significantly increased mean training volume per week (reps × sets × load × number of sessions) in the β-ala + Cr group caused the increase in mass, resulting in greater changes in strength. Unfortunately a β-ala alone group was not included and therefore it cannot be concluded if β-ala alone can improve the efficiency of resistance training. However, it was only in the β-ala + Cr group that a reduction in subcutaneous body fat and increased muscle mass occurred concurrent with increased mean training volume. Hoffman et al. is currently the only study that has tested the potential of increased M-[Carn] to improve resistance exercise performance.
In addition to supplying additional dietary β-ala there is some evidence that chronic prolonged training may also increase Carn synthesis. Tallon et al. (2005) found M-[Carn] of 43 mmol.kg−1 dry muscle in experienced bodybuilders, whilst Kim et al. (2006) reported concentrations of 34 mmol.kg−1 dry muscle in elite Korean speed skaters. Both postulated that regular exposure to extreme skeletal muscle acidosis brought about an up-regulation in Carn synthesis to increase M-[Carn] 1.5 to twofold. However, in the case of Tallon et al. the possible use of anabolic–androgenic steroids and a very high meat content of the diet (hence high β-alanine content) in the subject group may have influenced M-[Carn]. Further as no pre-training data could be taken it is not known whether those individuals who display inherently high M-[Carn] are naturally advantageous to partake in high intensity exercise and therefore self select for the above sports. It does, however, highlight the possible advantage of raised M-[Carn] when engaging in resistance training.
The effect of relatively acute bouts of training (months vs. years) to regulate M-[Carn] is less clear. Suzuki et al. (2004) doubled the M-[Carn] of six Japanese students after 8 weeks of sprint cycle training. The training used a 30 s Wingate sprint cycling protocol and over the 8 week period 28 bouts were completed. The mean post value of M-[Carn] was 47.4 mmol.kg−1 dry muscle, an increase of 25.1 mmol.kg−1 dry muscle. This seems a radical change in Carn synthesis from just 14 min of exercise. In contrast Mannion et al. (1994) showed no change in M-[Carn] after 16 weeks of intensive isokinetic training, and Harris et al. (2007) also saw no changes after a combined sprint/resistance training program.
In light of the inconclusive data on the effect of training on M-[Carn], as well as the possibility of raised M-[Carn] improving the efficiency of resistance training, this study has two aims. First, to investigate the effect of 10 weeks of resistance training on M-[Carn] and secondly to determine if β-ala supplementation alone improves the outcomes of a training program designed to increase strength and muscle mass.
Method
Ethical approval was given by the relevant committees at the University of Chichester, Chichester, UK and the University of Physical Education and Sports Science II, Ho Chi Minh City, Vietnam.
Subjects
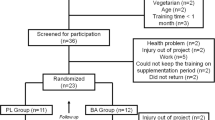
Twenty-six male Vietnamese physical education students completed the study. Subjects were fit and healthy having completed health history questionnaires, and were not currently involved in any programme of resistance training. The subjects were divided into two groups––a β-alanine supplemented group (β-ALG) and a Placebo group (PLG)––using a matched pairs design based on body mass and whole body strength. Subject characteristics are shown in Table 1.
Study design
The study was divided into four phases; familiarisation, pretesting, training and post testing. During the first phase, subjects were familiarised with the various laboratory and resistance training techniques to be used. All exercise data, body composition data and muscle biopsies were taken pre- and post-training. During the training phase subjects were exposed to both treatments (i.e. resistance training and supplementation).
Exercise testing
Subjects were tested both before and after training using three laboratory or gym based protocols. Whole body strength (WBS) was assessed by performance of three gym lifts; box squat (BS––lower body), bench press (BP––upper body) and deadlift (DL––upper + lower body). Subjects performed progressively heavier loads up to one repetition maximum (1RM) with a minimum of 2 min rest between attempts. The BS was performed as per a free squat but with subjects squatting onto a box set at a height resulting in a sub-parallel squat (i.e. with the hip-ending below the level of the knee-joint). The tests were conducted in the order of BS, BP and DL. The cumulative total, of the three 1RM’s, was defined as WBS. Isokinetic force production (IFP) was assessed using an isokinetic dynometer (Kin Com, Chattecx Co., Chattancoga, TN, USA). The subjects performed 90° isokinetic extensions of the knee from a starting angle (shank relative femur) of 90°, at an angular velocity of 180°.s−1. Isokinetic force production of the quadriceps muscle group was determined as the largest magnitude force (isokinetic force) recorded during three consecutive extensions. The flexion portion of each lift was passive. Finally an upper arm curl test (CT) was used to determine fatigue resistance in the flexor muscles of the upper arm. After a suitable warm-up, subjects performed one high repetition set within a target range of 20–40 reps, of single armed curls until momentary concentric fatigue occurred. An appropriate load enabling 20–40 reps was determined during the familiarisation phase.
Subjects were required to arrive at the laboratory or gym in a well rested state having done no intensive or unaccustomed exercise in the previous 24 h. Subjects were discouraged from drinking alcohol in the preceding 24 h or drinking excessive amounts of coffee or other caffeine containing beverages. Whole body strength and IFP were assessed on separate days at least 48 h apart. The CT was performed on the same day and prior to the IFP test. All tests were carried out by the same investigator (IK).
Body composition
Mass and calliper assessments of skin-folds were taken pre- and post-training to measure body compositional changes over the training period. A four site method was used for the calliper method; bicep brachii, triceps brachii sub-scapular and supra-iliac crest (Durin and Wormsley 1974). The mean of the three measurements taken at each site was used to calculate body density from which the Siri equation was used to estimate percentage of body fat (BF% = (([4.95/density]−4.50) × 100)).
Supplementation protocol
β-ALG (n = 13) received 800 mg × 8/day × 4 weeks β-ala (Carnosyn™, NAI, San Marcos, CA, USA) supplied as 800 mg in gelatine capsules, whilst PLG (n = 13) were given 800 mg maltodextrin again in capsule form and of identical appearance. The study was conducted double blind.
Training
Both groups trained 4 days/week for a 10 week period. The aim of the training was to elicit an increase in both whole body strength and muscle mass. Two sessions per week were upper body dominant and two were lower body dominant (Table 2). All sessions were supervised by the same qualified instructor (IK), and comprised multiple exercises performed over multiple sets, each set executed close to, or to concentric fatigue. Training was conducted in a progressive overload manner, striving to increase load over time within the given repetition/set scheme for each lift.
Muscle biopsies
Percutaneous muscle biopsies were taken from the vastus lateralis at week 0 and week 11. Biopsies were taken after local anaesthesia of the skin using a 6 mm muscle biopsy needle (Stille AB, Solna, Sweden) with suction (Bergström 1962). The samples were frozen and stored at 85°C until freeze dried when a portion of muscle from each biopsy was dissected free of obvious fat, connective tissue and blood and then powdered (Harris et al. 1974).
One to 3 mg of powdered muscle were extracted using 1 ml of water and vortexed for 3 min at ∼4°C. Ten microlitres of extract was then diluted with 1 ml of water, filtered through a 0.4 μm centrifugal-filter (Pall, Portsmouth, UK) prior to being derivatised with ortho-phthaldialdehyde/mecaptoproprionic acid reagent for 3 min (Dunnett and Harris 1997). The extract was then analysed for M-[Carn] by HPLC using fluorescence detection (Dunnett and Harris 1997). Water extracts of freeze-dried muscle showed no degradation of Carn, or the appearance of β-ala from the degradation of Carn, over 1 h of storage at ∼4°C.
Statistical analysis
Values are presented as means ± SD. Changes before and after training were compared within and between treatment groups by means of t tests for both paired and unpaired observations. Significance was set at p ≤ 0.05. To establish the normality of the distribution of the data, in each case, the results were analysed for skewness and kurtosis and a Kolmagaror–Smirev test was performed.
Results
Whole body strength
There was a significant increase in WBS (Fig. 1) from pre to post training in both β-ALG and PLG (261.92 ± 34.43–312.50 ± 35.41 kg and 265.00 ± 29.88–311.35 ( 40.40 kg, respectively). However, there was no significant difference in the change in WBS between β-ALG (+19.67 ± 5.53%) and PLG (+17.46 ± 6.37%), the absolute changes being 50.58 ± 11.55 kg (p < 0.001) vs. 46.35 ± 19.30 kg (p < 0.001), respectively.
Isokinetic force production
There was a significant increase in IFP (Fig. 1) pre to post training in both β-ALG and PLG (569.77 ± 91.71–637.46 × 101.93°N and 537.69 ± 88.27–599.38 ± 76.89°N, respectively). However, there was no significant difference in the change in IFP between β-ALG (+12.11 ± 8.70%) and PLG (+12.55 ± 12.09%), the absolute change being 67.69 ± 47.66°N (p < 0.001) vs. 61.69 ± 56.63°N (p < 0.01), respectively.
Upper arm curl test
There was a significant increase in CT (Fig. 1) performance pre to post training in both β-ALG and PLG (25.15 ± 13.32–32.77 ± 14.63 repetitions, and 25.00 ± 12.10–31.69 ± 13.84 repetitions, respectively). However, there was no significant difference in the change in CT between β-ALG (+35.65 ± 23.60%) and PLG (+30.59 ± 24.24%), the absolute change being 7.62 ± 4.65 repetitions (p < 0.001) vs. 6.69 ± 5.42 repetitions (p < 0.001), respectively.
Body composition
Mass (Fig. 1) was significantly increased posttraining by 2.06 ± 1.13 kg in β-ALG (+3.44 ± 1.93%) (p < 0.001) and by 2.27 ± 1.42 kg in PLG (+4.00 ± 2.69%) (p < 0.001). There was no significant difference in the change in mass between the two groups. There was no change in percentage of body fat in either group posttraining.
Muscle biochemistry
M-[Carn] was significantly increased in β-ALG after the supplementation/training period (23.96 ± 5.94–36.77 ± 8.26 mmol.kg−1 dry muscle, respectively; mean change +12.81 ± 7.97 mmol.kg−1 dry muscle, p < 0.001). There were no significant changes in the PLG (29.17 ± 9.82–27.29 ± 9.52 mmol.kg−1 dry muscle, mean change 1.87 ( 3.36 mmol.kg−1 dry muscle, p > 0.05) (Fig. 2). However, there are two subjects in the PLG that displayed unusually high pre-M-[Carn] (43.94 and 48.3 l mmol.kg−1 dry muscle), despite reanalysis. These values would represent the upper range for the post-M-[Carn] results in the β-ALG. Removing these outliers (indicated by an asterisk in Fig. 2) the mean values for PLG become; 27.08 ± 6.37–25.27 ± 6.13 mmol.kg−1 dry muscle with a mean change 1.81 ± 2.92 mmol.kg−1 dry muscle. However, there is still no statistically significant change pre–post in the PLG. The difference in the mean change in M-[Carn] between β-ALG and PLG was highly significant (p < 0.001).
Discussion
The aims of this project were to study the effect of 10-weeks resistance training on M-[Carn] and measures of whole body strength.
As previously reported, ingestion of 6.4 g.day−1 of the amino acid β-ala resulted in significantly elevated M-[Carn]. The present study again confirms the efficacy of this supplementary protocol in increasing M-[Carn] with a mean increase of 12.81 mmol.kg−1 dry muscle (a mean change of 59%) similar to an increase of ∼15 mmol.kg−1 dry muscle or 60–80% reported by Harris et al. (2006) and Hill et al. (2007). Taking all the three studies together, in which supplementation periods varied from 4 to 10 weeks and the total amount of β-ala given ranged from 145.6 g (over 4 weeks) to 448 g (over 10 weeks), the combined data suggests an upper limit to the increase in M-[Carn] with a threshold for the absolute concentration of ∼40–45 mmol.kg−1 dry muscle. Paradoxically this was the concentration observed by Tallon et al. (2005) in chronically trained UK body builders, by Kim et al. (2006) in Korean speed skaters, and by Suzuki et al. (2004) in subjects trained for 4 weeks.
Ten weeks training without supplementation, however, did not result in any change in M-[Carn], the mean contents in PLG being 29.17 ± 9.92 mmol.kg−1 dry muscle before and 27.29 ± 9.52 mmol.kg−1 dry muscle after training. The balance of evidence prior to this study was that short term intensive training did not alter Carn synthesis (Mannion et al. 1994; Kim et al. 2006 in favour versus Suzuki et al. 2004 against). This data, therefore, agrees with Mannion et al. and Kim et al. who showed no effect of 10–16 weeks of intensive training on M-[Carn]. As this study represents the third training modality (isokinetic training, sprint + resistance training and resistance training) that has been studied and shown to have no effect on M-[Carn] it seems probable that the only way to raise M-[Carn] in the short term is to increase β-ala intake either through meat ingestion or by supplementation. There are potentially three limitations to M-[Carn] synthesis: the in vivo synthesis of β-ala in the liver from uracil degradation (Fritzon 1957), the transport of β-ala via the blood and its uptake into muscle, and, finally the activity of carnosine synthase (CS) in muscle. The absence of any training effect in this study, in contrast to the effect of β-ala supplementation, suggests that even if transport and CS activity were up-regulated synthesis remains limited by the availability of β-ala synthesis which itself does not appear to have been affected by training. Certainly this would seem to be the case over 10–16 weeks of training. The results of the present study do not exclude the possibility of an interaction between training and β-ala supplementation on M-[Carn] synthesis although as noted the increase in this in this study was very similar to that shown in previous work.
However, as shown by Tallon et al. (2005) and Kim et al. (2006) increased M-[Carn], concentrations, ca. 40–45 mmol.kg−1 dry muscle, are possible with chronic training suggesting that training may elevate β-ala synthesis in the long-term, or alternatively increase the efficiency by which dietary supplied β-ala is assimilated to carnosine in muscle. We are at a loss, however, to explain the dramatic increase in M-[Carn] with 4 weeks training (without β-ala supplementation) reported by Suzuki et al. (2004) other than to suggest that training caused the subjects to ingest greater amounts of meat or fish containing β-ala, such as tuna (which is rich in carnosine and anserine as well as being a popular item in the Japanese diet).
Hoffman et al. (2006) reported improved strength and body composition (mean reduction in percentage of body fat whilst retaining the same mean mass) after 10 weeks of resistance training in a group consuming β-ala + Cr as compared to a placebo group. The control group of subjects had a mean increase in bench press and squat strength of ∼5 kg compared to the β-ala + Cr group of ∼12 and 25 kg (bench press and squat, respectively), a twofold to fourfold increase in performance due to the supplementation regime. The rational for this large ergogenic effect was the ability of increased M-[Carn] to prolong each bout of resistance exercise (more repetitions per set) and in turn a greater mean volume of training per week. This increase in work resulted in an increase in mechanical stress and in turn a greater adaptive process occurred. In contrast, there were no significant differences in posttraining performance between groups in the present study. Neither the Δ for WBS or IFP were significantly different between β-ALG and PLG. The training protocol resulted in mean increases in the squat of 19.04 and 20.38 kg, the bench press 9.23 and 8.27 kg, and, the dead lift 22.31 and 17.69 kg (β-ALG and PLG, respectively). This demonstrates the greater effectiveness of the current training program to increase strength. In comparing training programs it is clear that in Hoffman et al. the training was generally of a higher volume than used in this study (i.e. greater no of repetitions × sets). However, there was a greater focus on lower repetition strength work (for the core exercises) in the current training programme. This may well account for the different results. The higher repetitions per set and greater volume may have meant a greater extent of muscle acidosis during the training sessions in the study of Hoffman et al. This could account for the greater ergogenic effect of increased M-[Carn] (assumed to be similar to the present study although no biopsies were taken), as compared to the greater strength gain but lack of ergogenic effect in the present study. Further, the duration of the rest periods between exercises would also have had a profound effect on muscle pH, longer periods allowing for greater removal and buffering of H+. During the current study the rest periods could not be standardised due to the varied numbers of subjects per training session. Rest periods ranged from 2 to 5 min. If rest periods during the Hoffman et al. training were much tighter controlled and shorter again this might have increased the chance of detecting an effect of elevated M-[Carn] on performance. Finally, the difference in the training status of the subject groups between studies may have affected the outcome. In this study, despite being trained athletes with good strength to body weight ratios, the subjects had limited experience in resistance training methods. This was in comparison with the resistance trained subjects in Hoffman et al. (2006). One would naturally expect greater increases in strength in subjects which had no previous experience of resistance training.
A further difference in results from the two studies is in body compositional changes. Hoffman et al. reported a decrease in percentage of body fat (1.21%) whilst maintaining mass in their β-ala + Cr group. This subject group managed to simultaneously decrease body fat whilst increasing muscle mass. This would result in an increase in fat free mass, proportional to the mass of the lost body fat. However, our results showed no change in percentage of body fat but an increase in overall mass in both groups with a mean increase of 2.06 kg in β-ALG and 2.27 kg in the PLG. Again, the only possible mechanism for this discrepancy is the already mentioned differences in training protocols resulting in a greater metabolic demand for energy provision during the training in the study by Hoffman et al. with an increase in subcutaneous fat loss.
The discussion of training volume aside the fact that Hoffman et al. did not use a β-ALG, independent of Cr, means that it is hard to compare the results of the two studies. It is also important to note that the Cr alone group had similar strength increases to the β-ala + Cr group, but without the same increase in volume of work, and reduction in percentage of body fat.
It was notable that the change in CT performance was not significantly different between β-ALG and PLG (7.62 vs. 6.69, respectively). Of any of the testing protocols performed in this study, the upper arm muscular endurance test would be the one most likely to be affected by an increase in H+ buffering capacity. It is possible that the time under tension was not long enough to produce a significant accumulation of H+. It could also be that the training effect on performance was greater than that resulting from changes in buffering and therefore masked any ergogenic effect from β-ala supplementation.
In common with many training studies values for n where small, whilst SD relatively high. The combination of which inevitably results in a low power of statistical analysis. In this study, values of power ranged from 0.09 to 0.15, giving rise to potential type two errors. It is therefore possible that there were small changes in performance through β-ala supplementation that went undetected by the statistical analysis. However, there was no observable trend in results which could suggest a possible type two error. The mean % change in performance variables (Fig. 1) does not indicate a consistently greater change in performance for either treatment group across the four dependent variables (changes in; whole body strength, isokinetic force production, curl test performance and mass).
The main findings of this study are that short term intensive training alone did not influence M-[Carn]. The results presented here are in agreement with the work of Mannion et al. (1994) and Kim et al. (2006). Therefore in the short term at least the only method to manipulate M-[Carn] is through appropriate supplementation with β-ala or increased ingestion of foods (meat and fish) which are significant sources of this. It also appeared that training + supplementation did not result in any additive effect beyond supplementation alone. However, further research using an appropriate design is needed to clarify whether or not this is indeed the case and also in elucidating the effect of prolonged intensive training.
Secondly enhancing M-[Carn] did not result in an improved training effect (force production and muscle hypertrophy) through whole body resistance training. This is opposed to the only work prior to this using a resistance training model, and suggests that the structure of any resistance training program and its goals will heavily influence potential ergogenic effects of β-ala. More research is needed to appropriately define the exact effect of raised M-[Carn] on different modes of resistance training.
References
Ahlborg B, Bergrström J, Ekelund LG, Guarineri G, Harris RC, Hultman E, Nordesjö LO (1972) Muscle metabolism during isometric exercise performed at constant force. J Appl Physiol 33:224–228
Batrukova MA, Rubtsov AM (1997) Histidine-containing dipeptides as endogenous regulators of the activity of sarcomplasmic Ca-release channels. Biochim Biophys Acta 21:142–150
Bergström J (1962) Muscle electrolytes in man. Determination by neutron activation analysis on needle biopsy specimens. A study on normal subjects, kidney patients and patients with chronic diarrhoea. Scand J Clin Lab Invest 14:100–110
Boldyrev AA, Dupin AM, Pindel EV, Severin SE (1988) Antioxidative properties of histidine-containing diepeptides from skeletal muscles of vertebrates. Comp Biochem Physiol 89:245–250
Dukta TL, Lamb GD (2004) Effects of carnosine on excitation–contraction coupling in mechanically-skinned rat skeletal muscle. J Muscle Res Cell Motil 25:203–213
Dunnett M, Harris RC (1997) High-performance liquid chromatographic determination of imidazole dipeptides, histidine, 1-methylhistidine and 3-methylhistadine in equine and camel muscle and individual muscle fibres. J Chromatogr B 688:47–55
Durin JV, Wormsley J (1974) Body fat assessment from total body density and its estimation form skinfold thickness: measurements on 481 men and women from ages 17–72. Br J Nutr 32:77–97
Edge J, Hill-Haas S, Goodman C, Bishop D (2006) Effects of resistance training on H+ regulation, buffer capacity, and repeated sprints. Med Sci Sports Exerc 38:2004–2011
Fritzon P (1957) The catabolism of C14-labeled uracil, dihydro-uracil and β-ureidopropioninc acid in rat liver slices. J Biol Chem 226:223–228
Harris RC, Hultman E, Nordejö LO (1974) Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculos quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest 33:109–120
Harris RC, Marlin DJ, Dunnett M, Snow DH, Hultman E (1990) Muscle buffering capacity and dipeptide content in the thoroughbred horse, greyhound dog and man. Comp Biochem Physiol 97A:249-251
Harris RC, Dunnett M, Greenhaff PL (1998) Carnosine and taurine contents in individual fibres of human vastus alteralis muscle. J Sports Sci 16:639–643
Harris RC, Tallon MJ, Dunnett M, Boobis LH, Coakley J, Kim HJ, Fallowfield JL, Chester CA, Sale C, Wise JA (2006) The absorption of orally supplied β-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids 30:279–289
Harris RC, Kim HJ, Kim CK, Lee YW, Sale C, Harris DB, Wise JA (2007) The effect of a supplement containing β-alanine on muscle carnosine synthesis and exercise capacity, during 12 weeks of combined endurance and weight training. J Sport Sci 25:336–337
Hill CC, Harris RC, Kim HJ, Harris BD, Sale C, Boobis LH, Kim CK, Wise JA (2007) Influence of β-alanine supplementation on skeletal muscle carnosine concentrations and high intensity cycling capacity. Amino Acids 32:225–233
Hoffman J, Ratamess N, Kang J, Mangine G, Faigenbaum A, Stout J (2006) Effects of creatine and β-alanine supplementation on performance and endocrine responses in strength/power athletes. Int J Sport Nutr Exerc Metab 16:430–446
Kim JH, Kim CJ, Changkeun K, Harris RC, Harris DB, Sale C, Wise JA (2006) Effect on muscle fibre morphology and carnosine content after 12 days training of korean speed skaters. Med Sci Sports Exerc 37:S-192
Mannion AF, Jakeman PM, Willan PL (1994) Effects of isokinetic training of the knee extensors on high-intensity exercise performance and skeletal muscle buffering. Eur J Appl Physiol Occup Physiol 68:356–361
Martin R, Edcall JT (1960) The association of divalent cations with acylacted histidine derivatives. J Am Chem Soc 82:1107
Ponte J, Harris RC, Hill CA, Sale C, Jones GA, Kim HJ, Wise JA (2007) Effect of 14–28 days of β-alanine supplementation on isometric endurance of the knee extensors. J Sport Sci 25:334
Schott J, McCully K, Rutherford OM (1995) The role of metabolites in strength training. II. Short versus long isometric contractions. Eur J Appl Physiol Occup Physiol 71:337–341
Stout JR, Cramer JT, Zoeller RF, Torok D, Costa P, Hoffman JR, Harris RC, O’Kroy J (2007) Effects of β-alanine supplementation on the onset of neuromuscular fatigue and ventilatory thresholds in women. Amino Acids 32:381–386
Suzuki Y, Ito O, Takahashi H, Takahashi K (2004) Effect of carnosine and anserine supplementation on skeletal muscle carnosine concentration in humans. Intl J Sport Health Sci 2:105–110
Tallon MJ, Harris RC, Boobis LH, Fallowfield JL, Wise JA (2005) The carnsoine content of vastus lateralis is elevated in resistance-trained bodybuilders. J Strength Cond Res 19:725–729
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kendrick, I.P., Harris, R.C., Kim, H.J. et al. The effects of 10 weeks of resistance training combined with β-alanine supplementation on whole body strength, force production, muscular endurance and body composition. Amino Acids 34, 547–554 (2008). https://doi.org/10.1007/s00726-007-0008-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-007-0008-3