Abstract
Background
Prolonged surgical operative time is associated with postoperative adverse outcomes following total knee arthroplasty (TKA). Increasing operating room efficiency necessitates the accurate prediction of surgical operative time for each patient. One potential way to increase the accuracy of predictions is to use advanced predictive analytics, such as machine learning. The aim of this study is to use machine learning to develop an accurate predictive model for surgical operative time for patients undergoing primary total knee arthroplasty.
Methods
A retrospective chart review of electronic medical records was conducted to identify patients who underwent primary total knee arthroplasty at a tertiary referral center. Three machine learning algorithms were developed to predict surgical operative time and were assessed by discrimination, calibration and decision curve analysis. Specifically, we used: (1) Artificial Neural Networks (ANNs), (2) Random Forest (RF), and (3) K-Nearest Neighbor (KNN).
Results
We analyzed the surgical operative time for 10,021 consecutive patients who underwent primary total knee arthroplasty. The neural network model achieved the best performance across discrimination (AUC = 0.82), calibration and decision curve analysis for predicting surgical operative time. Based on this algorithm, younger age (< 45 years), tranexamic acid non-usage, and a high BMI (> 40 kg/m2) were the strongest predictors associated with surgical operative time.
Conclusions
This study shows excellent performance of machine learning models for predicting surgical operative time in primary total knee arthroplasty. The accurate estimation of surgical duration is important in enhancing OR efficiency and identifying patients at risk for prolonged surgical operative time.
Level of evidence
Level III, case control retrospective analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the continued growth in utilization of total knee arthroplasty (TKA), healthcare workers and administrations have begun to scrutinize operating room (OR) efficiency in total knee arthroplasty [1,2,3]. Allocation of OR time and appropriate scheduling of case length are parameters closely associated with OR efficiency [4], which is vital for delivering efficient and cost-effective care to arthroplasty patients. The accurate prediction of surgical operative time in primary TKA, which tends to be a more predictable and reproducible surgical procedure, may be an ideal starting point when analyzing and attempting to improve OR efficiency.
Historically, the preoperative estimation of individual surgical operative time has been done based on surgeon’s estimates, or by an electronic medical record (EMR) based system which averaged previous case durations. However, prior studies demonstrated that the accuracy of surgeon’s estimates as well as EMR scheduling system lack accurate predictions of surgical operative time due to variations in preoperative data [5, 6]. Determinants for surgical operative time are based upon multiple perioperative factors, which may be beyond the abilities of previous estimation models. These limitations may be circumvented by estimating surgical operative time using machine learning (ML) algorithms. Recently, a number of studies have demonstrated the feasibility of using ML models to improve OR planning and efficiency through the accurate prediction of case duration in non-arthroplasty patients [7]. Therefore, this study aimed to develop and validate machine learning models to predict surgical operative time for patients undergoing primary total knee arthroplasty.
Materials and methods
Patient cohort
With Institutional Review Board (IRB) approval, we identified 10,089 consecutive primary TKA procedures that were performed at a single tertiary institution. Patients with missing perioperative data were excluded as were patients with simultaneous bilateral TKA surgery and less than 2 years of follow-up. A total of 10,021 primary TKA patients remained for the development and validation of machine learning algorithms. Surgical operative time was defined as the time from first incision to completion of wound closure. This duration was selected due to its medical and economic importance in concordance with prior literature [8]. Surgical operative time did not include anesthesia time nor the OR room turn over time between cases.
Variables
Electronic medical records were used to manually review patient and procedural variables associated with prolonged surgical operative time [9, 10]. Collected patient data included: (1) age, (2) gender, (3) body mass index (BMI), (4) ethnicity, (5) American Society of Anesthesiologist Physical Status score (ASA score), (6) medical comorbidities and (7) Charlson comorbidity index (CCI; Table 1). Procedural variables included for analysis involved: (1) indication for primary TKA (post-traumatic vs primary osteoarthritis), (2) anesthesia type, (3) tranexamic acid usage, (4) component fixation method (cemented vs non-cemented), (5) tourniquet use as well as (6) tourniquet time and pressure, (7) implant type and (8) prior knee surgeries.
Model development
We developed three supervised machine learning algorithms in concordance with prior literature [11,12,13]: (1) artificial neural networks (ANN), (2) random forests (RF), and (3) k-nearest neighbors (KNN). The TKA dataset as shown in Table 1 was randomly divided into 2 datasets using an 80:20 split ratio [14, 15]: a dataset for the training of machine learning models (8,016 TKAs) and a dataset to test machine learning model performance (2,005 TKAs). A recursive feature elimination technique (popular technique to select feature most relevant in predicting the target variable) was utilized to determine patient and surgical factors for final modeling [16, 17]. A fivefold cross-validation was repeated 5 times to develop and assess all candidate models in the training set. We applied a grid-search algorithm to determine each algorithm’s hyperparameters during the training phase [18,19,20]: (1) ANN: number of hidden layer nodes; (2) RF: number of trees and boosting parameter; (3) KNN: mixing parameter α (Ridge regularization α = 0; Lasso regularization α = 0) and number of nearest neighbors.
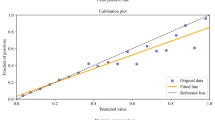
Model accuracy was defined using the area under the receiver operating curve (AUC) [21]. Machine learning models no better than chance have an AUC of 0.5, with perfect candidate models demonstrating an AUC of 1 [22]. Machine learning model calibration was performed using a calibration plot. The Brier score was used to assess overall model performance [23]. The Brier score, defined as mean squared difference between predicted probabilities and observed frequencies of events in a given population, is 0 for perfect candidate models [24].
Statistical analysis
All data analysis was performed using Matlab (MathWorks Inc., Natick, MA, USA), Anaconda (Anaconda Inc., Austin, TX, USA) and Python (Python Software Foundation, Wilmington, DE, USA)[25].
Results
A total of 10,021 patients underwent primary total knee joint arthroplasty. The mean age of the patient cohort is 74.2 ± 22.7 years and the mean body mass index is 32.3 ± 6.4 kg/m2. The average follow-up time was 2.8 ± 1.1 years. Patient demographics and surgical variables for TKA patients are summarized in Table 1. The mean surgical operative time was 98.9 ± 32.6 min. The machine learning models demonstrated an average 11 min (SD: 3.4 min) improvement in absolute difference between predicted surgical operative time and actual surgical operative time, when compared to the conventionally used electronic medical record system.
Model parameters were optimized using a coarse-grained grid-search algorithm with repeated random sub-sampling validation. The optimal ANN had two hidden layers with 18 neurons each. The optimal RF consisted of 110 trees, with the number of predictors for each node set to default. The optimal KNN learning rate was 0.3 with a sub-sampling coefficient of 0.80 and a 24 nearest neighbors.
In the training dataset, all machine learning models demonstrated excellent model discrimination. The AUC for the candidate models ranged from 0.77 for k-nearest neighbor to 0.83 for neural networks (Table 2). The calibration intercept ranged from – 0.19 to 0.22, with the best intercept for neural networks (intercept of 0.05; Table 2). The calibration slope varied between 0.92 and 1.18 across the three candidate models (Table 2). The lowest Brier score error was achieved by neural networks (Brier score of 0.053). In the testing set of TKA patients, the AUC for the three candidate models ranged from 0.78 to 0.82 (Table 3). The highest AUC was achieved by neural networks (AUC = 0.82; Table 3). The Brier score errors in the testing set varied between 0.053 and 0.055, with the lowest Brier score error for neural networks (Brier score of 0.053, Table 3).
Decision curve analysis demonstrated that the three machine learning models all achieved higher net benefits for TKA patients, when compared to the default strategies of changing management for all patients or no patient. The variables significantly associated with surgical operative time were younger age (< 45 years), female gender, ASA score, Charlson Comorbidity Index (CCI), high BMI (> 40 kg/m2), indication for TKA (post-traumatic), tranexamic acid non-usage, and operating surgeon (Fig. 1). The strongest predictors for surgical operative time were younger age (< 45 years), tranexamic acid non-usage, and high BMI (> 40 kg/m2; Fig. 2).
An example of a local, individual patient-level explanation for the model predictions by neural networks is shown in Fig. 3. For a 43 year old non-obese (BMI = 28 kg/m2) male TKA patient with ASA score 3 and Charlson comorbidity index of 3.36, who was operated using tranexamic acid, the predicted probability of an operative time greater than 85 min is 17.6%. Younger age (< 45 years), higher Charlson comorbidity index, ASA score of 3 and post-traumatic TKA indication increased the probability of a longer operative time, whereas tranexamic acid usage, low BMI (< 40 m/kg2) and male gender decreased the probability of a longer operative time.
Discussion
The efficient use of operating room (OR) time is significantly associated with health care spending [26]. With a high cost of use estimated at $36 per minute, under- and overestimation due to inaccurate prediction of surgical operative time can cause inefficiency in OR utilization and staffing. Thus, the accurate estimation of surgical duration is critical to enhancing OR efficiency and identifying patients at risk for prolonged surgical operative time. Regarding the estimation of surgical operative time, there have been accuracy improvement efforts [27]. Traditionally, one common approach was based on surgeon’s personal experience. However, according to a previous study by Laskin et al., surgeons overestimate surgical operative time up to 32% of the time, and underestimate it 42%_of the time [28]. Alternatively, electronic medical record (EMR)-based approaches have been used to calculate surgical operative time based on previous data for the same procedure and/or surgeon. Despite modestly higher accuracies (12%) compared to surgeon’s estimates [29], prior studies have demonstrated the limitations of this approach in terms of the inability to consider multiple significant influential factors [30, 31]. Additionally, these prior works demonstrated poor performance for EMR-based predictions of surgical operative time for patients with non-standard medical history, where the EMR system may struggle to provide comparative data [30, 31]. Furthermore, a lack of complete and reliable information in EMR systems provides a significant risk for a poor estimation of surgical operative time [32].
Due to the diverse multifactorial nature of OR time predictions, including patient characteristics and surgical environments [33], recent studies have investigated the possibility of utilizing machine learning (ML) techniques to improve the accuracy of these estimations. Previously, a pilot study including 990 operative cases over a variety of non-orthopedic specialties by Tuwatananurak et al. presented that ML models showed a 7 min improvement in absolute difference between predicted case duration and actual case duration, when compared to conventional electronic medical record systems [7]. Similarly, a recent large retrospective study by Bartek et al. demonstrated a high predictive capability for ML models to predict case-time duration across a broad spectrum of non-orthopedic departments in a tertiary medical center [34]. Despite these prior studies utilizing non-orthopedic patient populations, only a small sized retrospective study by Wu et al. described an improved accuracy for ML-based predictions, when compared to surgeon's own predictions in revision THA [35]. In this present study, we report an 11 min improvement in absolute difference between predicted surgical operative time and actual surgical operative time, when compared to the conventionally used electronic medical record system, which highlights the strong potential of machine learning models for the prediction of surgical operative time. Additionally, the clinical utility of the machine learning models is supported by an opportunity to reduce healthcare costs, with an estimated $36 per minute for each minute of surgical operative time. As prior modeling studies did not develop computational tools which significantly improve the absolute difference between predicted surgical operative time and actual surgical operative time [36], the presented machine learning models have potential to assist in clinical practice.
All machine learning candidate ML models showed excellent performance on discrimination, calibration and decision curve analysis for surgical operative time. ML algorithms have the strength to become more accurate and predictive as additional data are given because these algorithms have the ability to learn and improve from repetitive experiences with nonlinear complicated data [37]. Thus, the excellent predictive abilities of these ML models may be a direct reflection of the multifactorial etiology of surgical operative time in patients. In a recent modeling study including all subspecialties by Bartek et al., the ML-based model performed better in predicting case duration than a linear regression model. Based on the result of this present study, the ANN model provided superior predictions (AUC = 0.82), when compared to two other ML algorithms; random forest (RF) and k-nearest neighbor (KNN). Regarding the superiority of ANN, a series of previous studies has proved the accuracy of ANN models for making complex medical decisions [36]. Although direct comparison is limited, the predictive value of this ANN model was comparable to the values of recent ANN-based predictive models applied to various aspects of primary TKA including length of stay, charges, and disposition [38].
Based on the ANN algorithm, the strongest predictors for surgical operative time were younger age (< 45 years), high BMI (> 40 kg/m2), and tranexamic acid non-usage. Due to diverse potential variables [39], independently evaluating the effect of individual variables on surgical operative time has posed significant challenges. A retrospective database study by Sodhi et al. found that younger age and obesity were predictors of longer surgical operative times in TKA patients (p < 0.001) [40]. A retrospective study by Liabaud et al. reported that surgical operative time for TKA patients increased by 0.933 min when the BMI increased by 1 kg/m2 [41]. Additionally, in a large database study, Wang et al. presented that TKA patients with higher BMI required significantly longer surgical operative time [42]. In terms of tranexamic acid, a retrospective cohort study by Mufarrih et al. found that for unilateral TKA patients, tranexamic acid usage led to a significant reduction in total surgical operative time (p < 0.001) [43]. Similarly, a study by Stoicea et al. reported that tranexamic acid usage was associated with a significant reduction in surgical operative time, using a study cohort involving 564 primary and revision TKA patients [44]. Equally, in a prospective study with 43 primary TKA patients, Guerreiro et al. showed the beneficial effect of tranexamic acid usage during TKA surgery with regards to surgical operative time as well as blood loss [45].
In comparison to prior retrospective studies, the present ML study demonstrated an increasingly significant impact of high body mass index on surgical operative time following primary TKA. Both, Sodhi et al. as well as Liabaud et al. demonstrated a moderately strong effect of obesity on surgical operative time, with other patient factors being of greater significance [41, 42]. This discrepancy may be due to the use of ML algorithms in the present study, with ML models being shown to provide more accurate data analysis compared to conventional statistical approaches [46], in the setting of large and complex datasets with noisy or incomplete information.
With the current focus on quality improvement initiatives, the ability of ML for predicting patients expected to require a longer surgical operative time can enhance patient care, as well as improve healthcare resource utilization and overall efficiency. The US National Healthcare Safety Network (NHSN) index predicts surgical risks for infections based on 3 factors, surgical operative time being one of them [47]. Periprosthetic joint infection (PJI), one of the most devastating complications following TJA, is strongly associated with prolonged surgical operative time [33]. Increased surgical operative times are also an independent risk factor for a multitude of other postoperative complications following TJA [33, 48]. Thus, more accurate prediction of case duration based on ML models might enable anticipated response in efforts to mitigate complications and undesirable sequelae for at risk TKA patients. In addition, a prior study by Sodhi et al. demonstrated that increased surgical operative times had the greatest effect on length of stay in primary TKA patients [40]. Therefore, coupled with the higher risk of complications, prolonged surgical operative time has a significant effect on the utilization of healthcare resources associated with hospital stays. The increased resource utilization posed by lengthy surgical operative time is also correlated to inaccurate operating room (OR) scheduling. A more accurate prediction of surgical operative time with these ML models may aid in optimizing surgical case scheduling for TKA patients, as they allow the incorporation and interpretation of multiple diverse patient and procedural factors with the potential for optimizing perioperative planning between patient, surgeon, and hospital.
There are potential limitations in the present study. First, our ML models were developed based on data from a single tertiary institution. The data may not be generalizable in other practice settings. Specifically, patients demonstrated an average BMI greater 30 kg/m2 indicating an overweight population as per definition of the Center for Disease Control. Nonetheless, similar BMI ranges were reported in similar studies on this topic [12, 21, 49]. Second, this study has inherent limitations of retrospective design such as bias and an inability to control for confounding factors. Third, the accuracy values did not exceed 90%, which merits future refined ML-based prediction models. However, despite the above limitations, in the context of homogeneous sampling and characterization of surgical operative time, this current single institutional study has clinical feasibility as patients undergo the same healthcare protocols.
In conclusion, this study shows excellent performance of machine learning models for predicting surgical operative time in primary total knee arthroplasties. The accurate estimation of surgical duration is important in enhancing OR efficiency and identifying patients at risk for prolonged operative time. These models have the potential to enhance utilization and efficiency of OR and may assist in allocation of healthcare resources associated with TKA.
References
Halawi MJ, Molloy R, Barsoum WK (2016) Maximizing efficiency in the operating room for total joint artroplasty. Am J Orthop (Belle Mead NJ) 45:E233–E235
Attarian DE, Wahl JE, Wellman SS, Bolognesi MP (2013) Developing a high-efficiency operating room for total joint arthroplasty in an academic setting. Clin Orthop Relat Res 471:1832–1836. https://doi.org/10.1007/s11999-012-2718-4
Oganesyan R, Klemt C, Esposito J et al (2021) Knee arthroscopy prior to revision TKA Is associated with increased re-revision for stiffness. J Knee Surg. https://doi.org/10.1055/s-0040-1722662
Dexter F, Epstein RH (2005) Operating room efficiency and scheduling. Curr Opin Anesthesiol 18:195
Master N, Zhou Z, Miller D et al (2017) Improving predictions of pediatric surgical durations with supervised learning. Int J Data Sci Anal 4:35–52. https://doi.org/10.1007/s41060-017-0055-0
Larsson A (2013) The accuracy of surgery time estimations. Prod Plan Control 24:891–902. https://doi.org/10.1080/09537287.2012.666897
Tuwatananurak JP, Zadeh S, Xu X et al (2019) Machine learning can improve estimation of surgical case duration: a pilot study. J Med Syst. https://doi.org/10.1007/s10916-019-1160-5
Khanuja HS, Solano MA, Sterling RS et al (2019) Surgeon mean operative times in total knee arthroplasty in a variety of settings in a health system. J Arthroplasty 34:2569–2572. https://doi.org/10.1016/j.arth.2019.06.029
Wang Q, Goswami K, Shohat N et al (2019) Longer operative time results in a higher rate of subsequent periprosthetic joint infection in patients undergoing primary joint arthroplasty. J Arthroplasty 34:947–953. https://doi.org/10.1016/j.arth.2019.01.027
Bredow J, Boese CK, Flörkemeier T et al (2018) Factors affecting operative time in primary total hip arthroplasty: a retrospective single hospital cohort study of 7674 cases. Technol Health Care 26:857–866. https://doi.org/10.3233/THC-171015
Borjali A, Chen AF, Muratoglu OK et al (2020) Detecting total hip replacement prosthesis design on plain radiographs using deep convolutional neural network. J Orthop Res Off Publ Orthop Res Soc 38:1465–1471. https://doi.org/10.1002/jor.24617
Kunze KN, Karhade AV, Sadauskas AJ et al (2020) Development of machine learning algorithms to predict clinically meaningful improvement for the patient-reported health state after total hip arthroplasty. J Arthroplasty 35:2119–2123. https://doi.org/10.1016/j.arth.2020.03.019
Klemt C, Harvey MJ, Robinson MG et al (2022) Machine learning algorithms predict extended postoperative opioid use in primary total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-021-06812-4
Karhade AV, Schwab JH, Bedair HS (2019) Development of machine learning algorithms for prediction of sustained postoperative opioid prescriptions after total hip arthroplasty. J Arthroplasty 34:2272-2277.e1. https://doi.org/10.1016/j.arth.2019.06.013
Yeo I, Klemt C, Robinson MG et al (2022) The use of artificial neural networks for the prediction of surgical site infection following TKA. J Knee Surg. https://doi.org/10.1055/s-0041-1741396
Darst BF, Malecki KC, Engelman CD (2018) Using recursive feature elimination in random forest to account for correlated variables in high dimensional data. BMC Genet 19:1–6. https://doi.org/10.1186/s12863-018-0633-8
Karhade AV, Ogink PT, Thio QCBS et al (2019) Machine learning for prediction of sustained opioid prescription after anterior cervical discectomy and fusion. Spine J 19:976–983. https://doi.org/10.1016/j.spinee.2019.01.009
Mallow GM, Siyaji ZK, Galbusera F et al (2021) Intelligence-based spine care model: a new era of research and clinical decision-making. Glob Spine J 11:135–145. https://doi.org/10.1177/2192568220973984
Ben-Ari A, Chansky H, Rozet I (2017) Preoperative opioid use is associated with early revision after total knee arthroplasty: a study of male patients treated in the veterans affairs system. J Bone Joint Surg Am 99:1–9. https://doi.org/10.2106/JBJS.16.00167
Klemt C, Tirumala V, Barghi A et al (2022) Artificial intelligence algorithms accurately predict prolonged length of stay following revision total knee arthroplasty. Knee Surg Sport Traumatol Arthrosc. https://doi.org/10.1007/s00167-022-06894-8
Cohen-Levy WB, Klemt C, Tirumala V et al (2022) Artificial neural networks for the prediction of transfusion rates in primary total hip arthroplasty. Arch Orthop Trauma Surg. https://doi.org/10.1007/s00402-022-04391-8
Klemt C, Tirumala V, Smith EJ et al (2020) Development of a preoperative risk calculator for re-infection following revision surgery for periprosthetic joint infection. J Arthroplasty. https://doi.org/10.1016/j.arth.2020.08.004
Ferro CAT (2007) Comparing probabilistic forecasting systems with the Brier score. Weather Forecast 22:1076–1088. https://doi.org/10.1175/WAF1034.1
Mendez JH, Mehrani A, Randolph P, Stagg S (2019) Throughput and resolution with a next-generation direct electron detector. IUCrJ 6:1007–1013. https://doi.org/10.1107/S2052252519012661
Klemt C, Drago J, Tirumala V, Kwon Y-M (2021) Asymmetrical tibial polyethylene geometry-cruciate retaining total knee arthroplasty does not fully restore in-vivo articular contact kinematics during strenuous activities. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-020-06384-9
Gabriel RA, Wu A, Huang CC et al (2016) National incidences and predictors of inefficiencies in perioperative care. J Clin Anesth 31:238–246. https://doi.org/10.1016/j.jclinane.2016.01.007
Timoney N, Procter L, Liau J et al (2016) The effects of surgeons and anesthesiologists on operating room efficiency. Interdiscip Neurosurg 5:38–42. https://doi.org/10.1016/j.inat.2016.06.001
Laskin DM, Abubaker AO, Strauss RA (2013) Accuracy of predicting the duration of a surgical operation. J Oral Maxillofac Surg 71:446–447. https://doi.org/10.1016/j.joms.2012.10.009
Wu A, Huang C-C, Weaver MJ, Urman RD (2016) Use of historical surgical times to predict duration of primary total knee arthroplasty. J Arthroplasty 31:2768–2772. https://doi.org/10.1016/j.arth.2016.05.038
Eijkemans MJC, van Houdenhoven M, Nguyen T et al (2010) Predicting the unpredictable: a new prediction model for operating room times using individual characteristics and the surgeon’s estimate. Anesthesiology 112:41–49. https://doi.org/10.1097/ALN.0b013e3181c294c2
Stepaniak PS, Heij C, Mannaerts GHH et al (2009) Modeling procedure and surgical times for current procedural terminology-anesthesia-surgeon combinations and evaluation in terms of case-duration prediction and operating room efficiency: a multicenter study. Anesth Analg 109:1232–1245. https://doi.org/10.1213/ANE.0b013e3181b5de07
Freeman NLB, McGinigle KL, Leese PJ (2019) Using electronic medical records to identify enhanced recovery after surgery cases. EGEMS (Washington, DC) 7:34
Duchman KR, Pugely AJ, Martin CT et al (2017) Operative time affects short-term complications in total joint arthroplasty. J Arthroplasty 32:1285–1291. https://doi.org/10.1016/j.arth.2016.12.003
Bartek MA, Saxena RC, Solomon S et al (2019) Improving operating room efficiency: machine learning approach to predict case-time duration. J Am Coll Surg 229:346-354.e3. https://doi.org/10.1016/j.jamcollsurg.2019.05.029
Wu A, Weaver MJ, Heng MM, Urman RD (2017) Predictive model of surgical time for revision total hip arthroplasty. J Arthroplasty 32:2214–2218. https://doi.org/10.1016/j.arth.2017.01.056
Sutradhar R, Barbera L (2020) Comparing an artificial neural network to logistic regression for predicting ED visit risk among patients with cancer: a population-based cohort Study. J Pain Symptom Manage 60:1–9. https://doi.org/10.1016/j.jpainsymman.2020.02.010
Myers TG, Ramkumar PN, Ricciardi BF et al (2020) Artificial intelligence and orthopaedics. J Bone Jt Surg. https://doi.org/10.2106/jbjs.19.01128
Ramkumar PN, Karnuta JM, Navarro SM et al (2019) Deep learning preoperatively predicts value metrics for primary total knee arthroplasty: development and validation of an artificial neural network model. J Arthroplasty 34:2220-2227.e1. https://doi.org/10.1016/j.arth.2019.05.034
Silber JH, Rosenbaum PR, Zhang X, Even-Shoshan O (2007) Influence of patient and hospital characteristics on anesthesia time in medicare patients undergoing general and orthopedic surgery. Anesthesiology 106:356–364. https://doi.org/10.1097/00000542-200702000-00025
Sodhi N, Anis HK, Gold PA et al (2019) Operative times can predict and are correlated with lengths-of-stay in primary total knee arthroplasty: a nationwide database study. J Arthroplasty 34:1328–1332. https://doi.org/10.1016/j.arth.2019.03.024
Liabaud B, Patrick DAJ, Geller JA (2013) Higher body mass index leads to longer operative time in total knee arthroplasty. J Arthroplasty 28:563–565. https://doi.org/10.1016/j.arth.2012.07.037
Girardi FM, Liu J, Guo Z et al (2019) The impact of obesity on resource utilization among patients undergoing total joint arthroplasty. Int Orthop 43:269–274. https://doi.org/10.1007/s00264-018-4059-8
Mufarrih SH, Malik AT, Qureshi NQ et al (2018) The effect of tranexamic acid in unilateral and bilateral total knee arthroplasty in the South Asian population: A retrospective cohort study. Int J Surg 52:25–29. https://doi.org/10.1016/j.ijsu.2018.02.005
Stoicea N, Moran K, Mahmoud A-R et al (2018) Tranexamic acid use during total hip arthroplasty: A single center retrospective analysis. Medicine (Baltimore) 97:e10720. https://doi.org/10.1097/MD.0000000000010720
Guerreiro JPF, Badaro BS, Balbino JRM et al (2017) Application of tranexamic acid in total knee arthroplasty - prospective randomized trial. Open Orthop J 11:1049–1057. https://doi.org/10.2174/1874325001711011049
Helm JM, Swiergosz AM, Haeberle HS et al (2020) Machine learning and artificial intelligence: definitions, applications, and future directions. Curr Rev Musculoskelet Med 13:69–76. https://doi.org/10.1007/s12178-020-09600-8
Surace P, Sultan AA, George J et al (2019) The association between operative time and short-term complications in total hip arthroplasty: an analysis of 89,802 surgeries. J Arthroplasty 34:426–432. https://doi.org/10.1016/j.arth.2018.11.015
Ravi B, Jenkinson R, O’Heireamhoin S et al (2019) Surgical duration is associated with an increased risk of periprosthetic infection following total knee arthroplasty: a population-based retrospective cohort study. EClinicalMedicine 16:74–80. https://doi.org/10.1016/j.eclinm.2019.09.015
Bonner BE, Castillo TN, Fitz DW et al (2019) Preoperative opioid use negatively affects patient-reported outcomes after primary total hip arthroplasty. J Am Acad Orthop Surg. https://doi.org/10.5435/JAAOS-D-18-00658
Funding
This study did not receive any funding. All authors report no conflict of interest or financial disclosures. There is no funding source.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose. The authors have no conflicts of interest to declare that are relevant to the content of this article. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. The authors have no financial or proprietary interests in any material discussed in this article. The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent.
Informed consent was obtained for the retrospective patient chart review.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yeo, I., Klemt, C., Melnic, C.M. et al. Predicting surgical operative time in primary total knee arthroplasty utilizing machine learning models. Arch Orthop Trauma Surg 143, 3299–3307 (2023). https://doi.org/10.1007/s00402-022-04588-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-022-04588-x