Abstract
The role of α-synuclein (αSyn) in schizophrenia is unknown, whereas in a recent animal model of depression, α- and γ-synuclein have been related to its pathophysiology. Previous biochemical studies in Brodmann area 9 showed significant reduction of αSyn in both chronic schizophrenia and bipolar disorder. Here, prevalence and cerebral distribution of αSyn were examined in 80 autopsy cases of elderly subjects (41 chronic schizophrenia, 12 late live depression/LLD and bipolar disorder/BD, and 27 age-matched controls without neuropsychiatric disorders). Using immunohistochemistry, αSyn-positive lesions (Lewy bodies and neurites) were assessed semiquantitatively. Among 41 chronic schizophrenics, all except one showing low neuritic Braak stages (mean 1.46), three brains (7.3%) revealed only few αSyn-positive inclusions restricted to medullary nuclei. Among 12 LLD and BD patients with mean Braak stage 2.25, αSyn-positive pathology was seen in two cases (16.7%) with clinical LLD, but none in BD. Among 27 controls, showing mean neuritic Braak stage 2.6, seven brains (26%) with higher mean age showed αSyn-positive lesions, either isolated in substantia nigra and nucleus basalis of Meynert (n = 2 each), in medullary nuclei, locus ceruleus and substantia nigra (n = 2), with additional involvement of nucleus basalis (n = 1). This first preliminary study in non-demented psychiatric disorders indicates that αSyn/Lewy pathology in chronic schizophrenia is significantly less frequent than in clinically healthy elderly people (P < 0.01), showing 10–30% of so-called incidental Lewy body disease. Among chronic affective disorders, according to our small cohort, the incidence of Lewy-pathology in LLD appears to be comparable to a healthy elderly population, whereas its occurence in BD is to be elucidated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
α-Synuclein (αSyn) or Lewy pathology is found in 10–30% of clinically healthy people older than 60 years [3, 14, 15, 21, 24]. This finding, termed incidental Lewy body disease (ILBD), has been suggested to represent preclinical Parkinson’s disease (PD), since the distribution of Lewy bodies (LB) in vulnerable brainstem nuclei is similar to that observed in sporadic PD [7, 34], and is associated with moderate reduction of nigral neurons [12, 34]. Recent studies of ILBD have shown decreased striatal dopaminergic immunoreactivity for both tyrosine hydroxylase (TH; the principal enzyme for dopamine synthesis [3]) (33%) and vesicular monoamine transporter 2 (VMAT2/42%), inversely correlated to the substantia nigra (SN) neuronal loss [9, 10]. Both ILBD and PD have a decreased SN concentration of l-ferritin versus controls, which suggests that neurodegeneration is related to a higher availability of free iron, released from the ferritin shell [25]. While in contrast to previous findings, recent studies have found no increased frequency and severity of Alzheimer-related pathology in aged patients with schizophrenia [16, 20, 28, 32], Alzheimer pathology has been suggested to be a prominent morphologic condition in late life major depression that is frequently associated with cognitive impairment [38], suggesting interactions between major depression and AD-lesions [33].
LBs in the amygdala and in cortical areas were found to increase the risk for major depression in Alzheimer’s disease (AD) [26]. On the other hand, biochemical studies in dorsolateral frontal cortex tissue (Brodmann area 9) found a significant reduction of αSyn in both chronic schizophrenia and bipolar disease (BPD) compared to age-matched normal controls, while presynaptic protein 25 (SNAP-25) and synaptophysin were increased [35]. Recent studies in an animal model of depression suggest a role of α- and γ-synuclein in the pathophysiology of this disorder [19].
To the best of our knowledge, up to now, αSyn or LB-related pathology has not been investigated in both chronic schizophrenia and chronic late-life depression (LLD) or bipolar disorders (BD). Therefore, the prevalence of αSyn/LB pathologies was studied in an autopsy cohort of elderly subjects with chronic schizophrenia and LLD or BD.
Materials and methods
The study included a total of 80 elderly individuals from the files of the Institute of Clinical Neurobiology, and the Department of Pathology of OWS Hospital, Vienna, Austria, a large psychiatric hospital with chronic care units.
All cases were retrospectively assessed from hospital charts. The diagnosis of chronic schizophrenia, LLD and BD was assessed according to DSM IV [2] and ICD.10 [39].
At autopsy, most of the brains were sagittally sectioned; one half was deep-frozen for biochemistry and the other half was fixed in 4% buffered aqueous solution of formaldehyde. Multiple blocks of neocortex (frontal, temporal, occipital), hippocampal formation including amygdala and parahippocampal area, basal ganglia, midbrain, pons and medulla oblongata were embedded in paraffin. In addition to routine stains, immunohistochemistry for phosphorylated tau-protein (antibody AT-8, Innogenetics, Heiden, Germany), β-amyloid (Aβ) (monoclonal antibody 4G8, Signet Labs, Dedham, MA, USA), and αSyn (monoclonal and polyclonal rabbit antibodies, Chemicon, Hofheim, Germany, and Signet Labs, Dedham, MA, USA) were performed. Sections were deparaffinized and incubated with primary antibodies overnight at 4°C, then visualized by the avidin–biotin–peroxidase complex method using diaminobenzidine (DAB) as chromogen (for methods see [22]). Coexistent AD pathology was rated according to the criteria of the Consortium to Establish a Registry on Alzheimer’s disease/CERAD [27], and the Braak staging of neuritic AD-changes [6]. The LB pathology was assessed semiquantitatively and staged according to Braak et al. [7].
Three autopsy groups were compared in this study: (1) 41 elderly persons (21 males, 20 females, aged 50–95, mean 67.9 ± 7.6 years) with chronic schizophrenia or schizophrenic residua according to DSM-IV or ICD.10 (F 20.5); (2) 12 elderly persons (three males, nine females) with LLD (F34) or BD (F31) (six patients each; age 63–89, mean 73.7 ± 6.5 years), and (3) 27 age-matched controls (10 males, 17 females, aged 68–84, mean 72.8 ± 5.7 years) without clinical history of neurological or psychiatric disorders or cognitive impairment.
Statistical methods
Differences between the control, schizophrenia and LLD/BD groups were assessed by Kruskal–Wallis one-way analysis and χ2 test. The significance level was set at P < 0.05.
Results
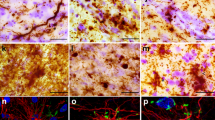
(1) The group of chronic schizophrenics, most of them showing duration of illness up to 55 years, had died from pneumonia, pulmonary embolism or myocardial infarction (ten each), bolus death, uremia or shock (2–4). Cognitive impairment was recorded in ten patients (24.4%). CERAD scores were 0–A, only one demented female aged 67 years scored CERAD C and neuritic Braak stage V, whereas the others had Braak stage IV (n = 6), II (n = 2), I or II (n = 8 each) and 0 (n = 16), with a mean of 1.46. Brain weight was 1,020–1,800 (mean 1,196 ± 50) g. Mild uni- or bilateral abnormal pre-α cell clusters or other subtle cytoarchitectonic abnormalities in the entorhinal cortex [5, 11, 18] were seen in 19/41 cases (46.3%). Negative αSyn histochemistry was found in 38 cases (92.7%), whereas positive deposits were observed in three brains (7.3%) aged 68–80 (mean 72) years: few Lewy neurites in dorsal vagal nucleus (dmX) (male aged 68 years), singular pre-LBs in nucleus ambiguus (female aged 68 years), and a few LBs and Lewy neurites in both dmX and reticular formation (non-demented male aged 86 years) (Fig. 1a, b). None of the brains showed any αSyn or LB lesions in pons, midbrain or other cerebral areas; no apparent loss of nigral neurons was observed.
(2) The group of LLD and BD (six cases each) also had shown chronic illness for many years or decades. Cognitive impairment or dementia (MMSE 14-18/30) was reported in four patients (33%). Causes of death were pulmonary embolism, myocardial infarction and pneumonia. Brain weight was 1,114–1,335 (mean 1,210 ± 86) g, CERAD scores were 0–A; Braak stages were V in a demented female aged 71 years; II and IV (three each), II (n = 1), 0 and I (2 each) (mean 2.25). Mild neuronal abnormality in the transentorhinal cortex of one side was observed in a demented female aged 87 years. αSyn immunohistochemistry was negative in ten brains (83.3%), including all BD cases, whereas αSyn-positive deposits were seen in two cases (16.7%), both clinically diagnosed LLD: Lewy neurites in dmX and reticular formation in a demented female aged 84 years with LLD, and moderate numbers of LBs and Lewy neurites in medullary nuclei, locus ceruleus (LC) and a few in SN associated with very mild nigral cell loss in a demented female aged 87 years with LLD, but without clinical parkinsonian signs or symptoms. Demented patients with αSyn-positive deposits were significantly older than those without (mean 85.0 vs. 71.3 years, P < 0.01).
(3) In the group of aged controls (cause of death mainly myocardial infarction, less frequently pneumonia, shock, cardiac decompensation), neuropathology showed nothing beyond age-related changes, with brain weight 1,100–1,300 (mean 1,198 ± 65) g. AD pathology scored CERAD 0–B and Braak stages 0–4 (mean 2.6 ± 0.5). Twenty brains (74%) showed negative αSyn immunohistochemistry, seven brains of clinically unremarkable persons without clinical parkinsonism (age at death 69–95, mean 84.0 ± 7.6 years) revealed αSyn positive deposits in dmX, reticular formation, LC and/or SN, with very little nigral cell loss in a non-demented male aged 93 years (neuritic Braak stage III) (Fig. 2a), and in medullary nuclei plus LC or nucleus basalis of Meynert (NBM) or amygdala (one case each—non-demented female aged 83 and male aged 86 years, respectively) (Fig. 2b), one brain showed αSyn-positive deposits in SN and limbic cortex, and isolated involvement of SN and NBM was also observed in one case each. Thus, 26% of these elderly controls were morphologically equivalent to ILBD. They were significantly older than those without αSyn-positive deposits (mean 84.0 vs. 73.6 years; P < 0.01).
In none of the cases of the present cohort, αSyn-positive deposits were observed in cingulate cortex, CA 2/3 subregion of hippocampus or isocortex.
Discussion
While the pathophysiology of schizophrenic disorders has been related to increased/disordered dopamine activity in prefrontal and striatal networks [8, 17, 36], the role of αSyn that interacts with dopamine production/metabolism [13] and has important implications for neurodegeneration in Parkinson’s disease (PD), other neurodegenerations [4, 23, 30, 37], and aging brain [1], schizophrenia and affective disorders is unknown. As yet, there is no clear evidence as to the contributions of pre- and post-synaptic constituents of the dopaminergic synapse to the pathophysiology of schizophrenia and depression [29]. LBs, mainly containing pathologically misfolded αSyn and synucleins may be related to increased risk of major depression in AD [26] and in animal models of depression [19], but a single biochemical study showed significant reduction of αSyn in cortex of humans with chronic schizophrenia and BD [35]. To the best of our knowledge, no post-mortem studies of αSyn in patients with chronic schizophrenia, LLD and BD have been performed.
The present study in 80 autopsy cases of elderly subjects—41 chronic schizophrenics, 12 LLD or BD and 27 age-matched controls without neuropsychiatric disorders, using immunohistochemistry and current diagnostic criteria for schizophrenia, depression, AD, Lewy-pathology and neuritic Alzheimer staging, gave the following results:
All three cohorts were similar in age, had comparable brain weight and neuritic Braak stages (P not significant). In the control group, 26% of the patients, being significantly older than the two other cohorts (P < 0.05), showed αSyn-positive deposits mainly in lower brainstem with occasional extension to SN and NBM, some of them conforming to the definition of ILBD. The incidence of ILBD in this cohort was comparable to previous ones, ranging between 10 and 30% [3, 14, 15, 21, 24]. By contrast, in the group of chronic schizophrenics only 7.3% revealed mild αSyn-positive deposits exclusively in medullary nuclei without involvement of other brain areas (P < 0.01). In the small cohort of LLD/BD, αSyn-positive deposits seen in 16.7% were also confined to medullary nuclei with mild involvement of SN in a single brain, all clinically presenting with LLD. None of the cases of the three cohorts showed αSyn-positive deposits in basal forebrain, hippocampus or isocortex.
The cohort of LLD/BD showed a frequency of αSyn pathology slightly less than aged controls, but was too small for statistical evaluation, while the group of chronic schizophrenics revealed significantly less frequent and less severe αSyn/Lewy pathology than aged controls (P < 0.01), which is in line with a single biochemical study showing significant reduction of αSyn in frontal cortex in chronic schizophrenics (and BD).
The mean age of ILBD cases in healthy controls was significantly higher than in both αSyn negative controls and the two other groups (P < 0.05). The age of ILBD in healthy elderly controls has been shown to be higher than in αSyn-negative cases [21], suggesting an age-dependence of ILBD frequency [3]. Although in the present cohort, the AD Braak stages were comparatively low in all three groups, it cannot be excluded that a slightly higher AD Braak stage in controls versus schizophrenics (2.6 vs. 1.46; P < 0.05) may suggest some relation between AD lesions and αSyn pathology. Additive or synergistic actions between both pathologies have been suggested with mutual increase of the propensity of tau and αSyn aggregation [31].
The molecular basis and clinical relevance of statistically decreased αSyn pathology in schizophrenic brain versus aged controls is unknown and needs further elucidation as will be necessary for its incidence and relevance in chronic affective disorders.
References
Al-Wandi A, Ninkina N, Millership S, Williamson SJ, Jones PA, Buchman VL (2008) Absence of alpha-synuclein affects dopamine metabolism and synaptic markers in the striatum of aging mice. Neurobiol Aging. Online 19 Dec 2008, doi:10.1016/j.neurobiolaging.2008.1011.1001
American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders. Text revision, 4th edn. American Psychiatric Association, Washington, DC
Beach TG, Adler CH, Sue LI, Peirce JB, Bachalakuri J, Dalsing-Hernandez JE, Lue LF, Caviness JN, Connor DJ, Sabbagh MN, Walker DG (2008) Reduced striatal tyrosine hydroxylase in incidental Lewy body disease. Acta Neuropathol 115:445–451
Bennett MC (2005) The role of alpha-synuclein in neurodegenerative diseases. Pharmacol Ther 105:311–331
Bernstein HG, Krell D, Baumann B, Danos P, Falkai P, Diekmann S, Henning H, Bogerts B (1998) Morphometric studies of the entorhinal cortex in neuropsychiatric patients and controls: clusters of heterotopically displaced lamina II neurons are not indicative of schizophrenia. Schizophr Res 33:125–132
Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112:389–404
Braak H, Bohl JR, Muller CM, Rub U, de Vos RA, Del Tredici K (2006) Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov Disord 21:2042–2051
Dao-Castellana MH, Paillere-Martinot ML, Hantraye P, Attar-Levy D, Remy P, Crouzel C, Artiges E, Feline A, Syrota A, Martinot JL (1997) Presynaptic dopaminergic function in the striatum of schizophrenic patients. Schizophr Res 23:167–174
DelleDonne A, Klos KJ, Fujishiro H, Ahmed Z, Parisi JE, Josephs KA, Frigerio R, Burnett M, Wszolek ZK, Uitti RJ, Ahlskog JE, Dickson DW (2008) Incidental Lewy body disease and preclinical Parkinson disease. Arch Neurol 65:1074–1080
Dickson DW, Fujishiro H, DelleDonne A, Menke J, Zeshan A, Klos KJ, Josephs KA, Frigerio R, Burnett M, Parisi JE, Ahlskog JE (2008) Evidence that incidental Lewy body disease is pre-symptomatic Parkinson’s disease. Acta Neuropathol 115:437–444
Falkai P, Schneider-Axmann T, Honer WG (2000) Entorhinal cortex pre-alpha cell clusters in schizophrenia: quantitative evidence of a developmental abnormality. Biol Psychiatry 47:937–943
Fearnley JM, Lees AJ (1991) Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain 114(Pt 5):2283–2301
Galvin JE (2006) Interaction of alpha-synuclein and dopamine metabolites in the pathogenesis of Parkinson’s disease: a case for the selective vulnerability of the substantia nigra. Acta Neuropathol 112:115–126
Gibb WR (1986) Idiopathic Parkinson’s disease and the Lewy body disorders. Neuropathol Appl Neurobiol 12:223–234
Gibb WR, Lees AJ (1988) The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 51:745–752
Haroutunian V, Religa D, Laudon H, Stycznska M, Winblad B, Naslund J (2003) Amyloid-beta pathology in Alzheimer’s disease and schizophrenia. Online Abstract Viewer/Itinerary Planner Washington, DC. Society for Neuroscience, Program No. 447.442
Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P, Bramon-Bosch E, Valmaggia L, Johns L, Broome M, McGuire PK, Grasby PM (2009) Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry 66:13–20
Jakob H, Beckmann H (1986) Prenatal developmental disturbances in the limbic allocortex in schizophrenics. J Neural Transm 65:303–326
Jeannotte AM, McCarthy JG, Redei EE, Sidhu A (2008) Desipramine modulation of alpha-, gamma-synuclein, and the norepinephrine transporter in an animal model of depression. Neuropsychopharmacology. Online 17 Sept 2008, doi:10.1038/npp.2008.1146
Jellinger KA, Gabriel E (1999) No increased incidence of Alzheimer’s disease in elderly schizophrenics. Acta Neuropathol 97:165–169
Jellinger KA (2004) Lewy body-related alpha-synucleinopathy in the aged human brain. J Neural Transm 111:1219–1235
Jellinger KA (2007) Morphological substrates of parkinsonism with and without dementia: a retrospective clinico-pathological study. J Neural Transm Suppl:91–104
Kazantsev AG, Kolchinsky AM (2008) Central role of alpha-synuclein oligomers in neurodegeneration in Parkinson disease. Arch Neurol 65:1577–1581
Klos KJ, Ahlskog JE, Josephs KA, Apaydin H, Parisi JE, Boeve BF, DeLucia MW, Dickson DW (2006) Alpha-synuclein pathology in the spinal cords of neurologically asymptomatic aged individuals. Neurology 66:1100–1102
Koziorowski D, Friedman A, Arosio P, Santambrogio P, Dziewulska D (2007) ELISA reveals a difference in the structure of substantia nigra ferritin in Parkinson’s disease and incidental Lewy body compared to control. Parkinsonism Relat Disord 13:214–218
Lopez OL, Becker JT, Sweet RA, Martin-Sanchez FJ, Hamilton RL (2006) Lewy bodies in the amygdala increase risk for major depression in subjects with Alzheimer disease. Neurology 67:660–665
Mirra SS, Gearing M, McKeel DW Jr, Crain BJ, Hughes JP, van Belle G, Heyman A (1994) Interlaboratory comparison of neuropathology assessments in Alzheimer’s disease: a study of the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). J Neuropathol Exp Neurol 53:303–315
Niizato K, Arai T, Kuroki N, Kase K, Iritani S, Ikeda K (1998) Autopsy study of Alzheimer’s disease brain pathology in schizophrenia. Schizophr Res 31:177–184
Nikolaus S, Antke C, Kley K, Poeppel TD, Hautzel H, Schmidt D, Muller HW (2007) Investigating the dopaminergic synapse in vivo. I. Molecular imaging studies in humans. Rev Neurosci 18:439–472
Norris EH, Giasson BI, Lee VM (2004) Alpha-synuclein: normal function and role in neurodegenerative diseases. Curr Top Dev Biol 60:17–54
Obi K, Akiyama H, Kondo H, Shimomura Y, Hasegawa M, Iwatsubo T, Mizuno Y, Mochizuki H (2008) Relationship of phosphorylated alpha-synuclein and tau accumulation to Abeta deposition in the cerebral cortex of dementia with Lewy bodies. Exp Neurol 210:409–420
Purohit DP, Davidson M, Perl DP, Powchik P, Haroutunian VH, Bierer LM, McCrystal J, Losonczy M, Davis KL (1993) Severe cognitive impairment in elderly schizophrenic patients: a clinicopathological study. Biol Psychiatry 33:255–260
Rapp MA, Schnaider-Beeri M, Grossman HT, Sano M, Perl DP, Purohit DP, Gorman JM, Haroutunian V (2006) Increased hippocampal plaques and tangles in patients with Alzheimer disease with a lifetime history of major depression. Arch Gen Psychiatry 63:161–167
Ross GW, Petrovitch H, Abbott RD, Nelson J, Markesbery W, Davis D, Hardman J, Launer L, Masaki K, Tanner CM, White LR (2004) Parkinsonian signs and substantia nigra neuron density in decendents elders without PD. Ann Neurol 56:532–539
Scarr E, Gray L, Keriakous D, Robinson PJ, Dean B (2006) Increased levels of SNAP-25 and synaptophysin in the dorsolateral prefrontal cortex in bipolar I disorder. Bipolar Disord 8:133–143
Siever LJ, Davis KL (2004) The pathophysiology of schizophrenia disorders: perspectives from the spectrum. Am J Psychiatry 161:398–413
Singleton AB (2005) Altered alpha-synuclein homeostasis causing Parkinson’s disease: the potential roles of dardarin. Trends Neurosci 28:416–421
Sweet RA, Hamilton RL, Butters MA, Mulsant BH, Pollock BG, Lewis DA, Lopez OL, DeKosky ST, Reynolds CFIII (2004) Neuropathologic correlates of late-onset major depression. Neuropsychopharmacology 29:2242–2250
World Health Organization (1992) The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. WHO, Geneva
Acknowledgments
The study was supported in part by the Society for the Support of Research in Experimental Neurology, Vienna, Austria. The author thanks Mrs. V. Rappelsberger for excellent laboratory work and Mr. E. Mitter-Ferstl, PhD, for secretarial and computer work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jellinger, K.A. Lewy body/α-synucleinopathy in schizophrenia and depression: a preliminary neuropathological study. Acta Neuropathol 117, 423–427 (2009). https://doi.org/10.1007/s00401-009-0492-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-009-0492-5