Abstract
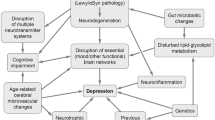
Depression is one of the most frequent neuropsychiatric symptoms in corticobasal degeneration (CBD), a rare, sporadic, and late-onset progressive neurodegenerative disorder of unknown etiology. It is clinically characterized by a levodopa-poorly responsible akinetic-rigid syndrome, apraxia, limb dystonia, cognitive, mood, behavioral, and language disorders. This 4-repeat (4R) tauopathy is morphologically featured by asymmetric frontoparietal atrophy, neuronal loss, and gliosis in cortex and subcortex including substantia nigra, ballooned/achromatic neurons with filamentous 4R tau aggregates in cortex and striatum, widespread thread-like structures, pathognomonic "astroglial plaques", "tufted astrocytes", and numerous "coiled bodies" (in astrocytes and oligodendroglia) in cerebral white matter. CBD is non-specific, as pathologically proven cases include several clinical phenotypes. Pubmed and Google Scholar were systematically analyzed until October 2023, with focus on the prevalence, clinical manifestation, neuroimaging data, and treatment options of depression in CBD. Its prevalence is about 30–40% which is more frequent than in most other atypical parkinsonian syndromes. Depression usually does not correlate with motor and other clinical parameters, suggesting different pathophysiological mechanisms. Asymmetric atrophy and hypometabolism of frontoparietal cortical areas are associated with disruption of fronto-subcortical circuits, nigrostriatal dopaminergic, and cholinergic deficiency. Since no specific neuroimaging, neuropathological, or biomarker studies of depression in CBD are available, its pathobiological mechanisms and pathogenesis are poorly understood. Antidepressive therapy may be useful, but is often poorly tolerated. Depression in CBD, like in other parkinsonian syndromes, may be related to multi-regional patterns of cerebral disturbances and complex pathogenic mechanisms that deserve further elucidation as a basis for early diagnosis and adequate treatment to improve the quality of life in this fatal disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depression, a highly prevalent psychiatric disorder and common cause of disability worldwide, is commonly associated with neurodegenerative diseases, Alzheimer (AD), Parkinson (PD) and Huntington disease, amyotrophic lateral sclerosis or atypical parkinsonian syndromes, with a prevalence of 23–50%. Over the past decades, some of the pathophysiological and molecular mechanisms that contribute to these co-morbidities have been elucidated and indicate, despite presenting distinct features, several similarities between the pathobiological mechanisms that lead to depression and neurodegeneration (Galts et al. 2019; Gupta et al. 2023). The possible overlapping mechanisms between corticobasal degeneration (CBD) (Gibb et al. 1989) and co-morbid depression are largely unknown, and deserve a critical discussion.
CBD, previously described as corticodentatonigral degeneration with achromatic neurons (Rebeiz et al. 1968), is a rare, sporadic and late-onset neurodegenerative disorder with progressive fatal course. This 4-repeat (4R) tauopathy, morphologically characterized by pathological tau aggregation in neurons and glia in widespread cortical and subcortical regions (Dickson et al. 2002), is a clinically and neuropathologically heterogenous syndrome, with levodopa-poorly responsive akinetic rigidity and multiple focal, often asymmetric symptoms (apraxia, aphasia, "alien limb syndrome", dystonia), executive dysfunction, depression, and various cognitive and behavioral impairments (Armstrong et al. 2013; Jellinger 2023b; Litvan et al. 1998). The alien hand or limb syndrome is a phenomenon in which one hand/limb is not under mind control and acts as if it has a mind of its own (Lewis-Smith et al. 2020).
CBD is a comparatively rare atypical parkinsonian syndrome, with an estimated prevalence of 2.05–2.3/100,000/year, compared with 4.28–7.1/100,000/year for progressive supranuclear palsy (PSP), another 4R tauopathy with frequent clinical and pathological similarities (Swallow et al. 2022), and an incidence of 0.4/100,000 person-years. The peak prevalence rate of CBD is in the age group 70–79 years, with higher incidence in men than in women (Stang et al. 2020), and a disease duration from 5 to 7 (range 2.5–12.5) years (Wenning et al. 1998).
Neuropathology of CBD shows focal, frequently asymmetric atrophy of frontoparietal cortex and corpus callosum, depigmented substantia nigra (SN), severe neuronal loss and gliosis in atrophic cortical and subcortical areas including SN. Histological hallmarks, but not specific lesions are “ballooned” (achromatic) neurons containing 4R tau-positive neurofibrillary tangles in affected cortices that are chemically and ultrastructurally identical to those in PSP (Dickson et al. 2011) but different from those in AD (Tatsumi et al. 2014; Uchihara 2014). Other lesions are small spherical cytoplasmatic “corticobasal” bodies in SN and pathognomonic “astroglial plaques” (tau-positive and, thus, different from neuritic plaques in AD) in neocortex and striatum, and "tufted astrocytes" (densely packed 4R tau-positive fibers) in astroglial processes, "coiled bodies" (coil-like tau-positive structures) in oligodendroglia and astrocytes in frontal cortex and striatum but uncommon in brainstem (Ling et al. 2016), and thread-like (tau-positive) structures in oligodendroglia in cerebral white matter. The presence of "astrocytic plaques" and tufted astrocytes differentiates CBD from PSP, with distal-dominant aggregation of "astrocytic plaques" and pretangles in CBD versus their proximal-dominant aggregation in PSP (Yoshida 2014).
CBD is a clinically and morphologically heterogenous disorder, as the major clinical features of pathologically proven forms include at least four phenotypes (Armstrong et al. 2013) that encompass: (1) corticobasal syndrome (CBS), (2) frontal behavioral–spatial syndrome (FBS), (3) nonfluent/agrammatic variant of primary progressive aphasia (nfvPPA), and (4) PSP–Richardson's syndrome (CBD–RS) (Shimohata et al. 2015) (Table 1). Since the characteristic clinical features were explained by a distinct topographical distribution of degenerative lesions, the term "corticobasal syndrome" was coined (Cordato et al. 2001), referring to the shared clinical features of the various morphological presentations. The term CBD was limited to the neuropathological presentation, while CBS referred to a clinical phenotype presenting with specific signs and symptoms. CBS, a clinical syndrome characterized by a combination of akinetic-rigidity, bradykinesia, dystonia, dysphagia, ideomotor apraxia, myoclonus, cortical sensory loss, cognitive, behavioral, mood, and language impairments, is associated with a series of pathologies beyond CBD. In a cohort of CBS patients, CBD (24%), PSP, frontotemporal lobar degeneration (FTLD), Lewy body dementia (DLB), and other pathologies, such as TDP-43 encephalopathy, cerebrovascular disorders, argyrophilic grain disease, another 4R tauopathy with temporal predilection, or co-morbid AD pathology (present in 59% of CBS cases) were found (Koga et al. 2022; Sainouchi et al. 2022; Shir et al. 2023). In a small series of 4R tauopathies, however, no cases following the CBD criteria were found (Yokota et al. 2023). The well-described PSP-like Richardson's syndrome (about 25% of sporadic CBD cases), with early-onset postural instability, falls, axial rigidity, dysphagia, motor disability, vertebral gaze palsy, pseudobulbar palsy, and cognitive dysfunction (Williams et al. 2005), is associated with more severe neuronal loss in medial SN and higher tau burden in medulla and cerebellum, hindbrain-predominant tau pathology, while PSP-RS shows greater tau burden in frontoparietal cortices and putamen, i.e., forebrain-accentuated tau pathology (Kouri et al. 2011). Other CBD variants manifest as predominant cognitive, dysexecutive-behavioral, language-impaired or other atypical syndromes (Armstrong et al. 2013; Koga et al. 2022; Parmera et al. 2022). Since a detailed description of the neuropathology of CBD, its clinical and morphological phenotypes has been published recently (Jellinger 2023b; Koga et al. 2022), they will not be presented in this brief overview that will concentrate on depression in CBD/CBS, based on systematic literature research in PubMed and Google Scholar until October 2023.
Diagnostic principles and epidemiology
Depression is common in CBD, but usually does not correlate with any other motor, cognitive or behavioral presentation (Litvan et al. 1998). Like in PD and other parkinsonian disorders, the clinical diagnosis of depression should be based on standard critical criteria according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), which includes depressed mood, loss of energy, decreased ability to concentrate, irritability, inappropriate feeling of guilt, loss or gain of appetite, insomnia or hypersomnia, pessimism about future, recurrent thoughts of death and others (American Psychiatric Association 2013). The Beck Depression Inventory II (BDI-II) is a reliable and valid tool for the assessment of depression in CBD, and there is a correlation between higher BDI-II scores and lower quality of life, irrespective of motor and cognitive symptoms (Cuoco et al. 2021). Self-reporting of depressive symptoms uses the Geriatric Depression Scale that includes five symptoms (dysphoria, hopelessness, withdrawal, worry, and cognitive concerns) and accurately predicted depression at a rate between 70 and 84% (Shdo et al. 2020).
CBD/CBS patients show a higher frequency of depressive disorders compared with PD (global frequency 30.7%) (Aarsland et al. 2001; Chendo et al. 2022; Jellinger 2022; Wenning et al. 1998), multiple system atrophy (MSA) (mean frequency 38–41%) (Köllensperger et al. 2010), and PSP (27–59%, median 30%) (Bower et al. 2021; Campagnolo et al. 2023; Jellinger 2023a). The prevalence of depression in CBD varied: 22% (Geda et al. 2007), 77% (Litvan et al. 1998), or 54% (Belvisi et al. 2018). The prevalence of suicidal and death ideation (SDI) in patients with CBD was 29.2% and those with PSP 27.2%, which was significantly higher than that in patients with PD before and after propensity score matching (p < 0.05). Multivariate analysis indicated that higher non-motor symptoms and depression were independently associated with the occurrence of SDI in patients with CBS/CBD (p < 0.05) (Ou et al. 2020).
CBD depression and other symptoms
Like in many other neurodegenerative diseases, CBD can manifest as depression in the prodromal phase, before the onset of core symptoms (Tateno et al. 2023). Patients with CBD (and PSP) usually have depression early in their disease by showing hopelessness as one of the primary features in their depressive symptom profiles. Many patients with CBD report levels of hopefulness 2.5 standard deviation higher than the average healthy older adult (PSP around 3 SDs) (Shdo et al. 2020). The ENGENE-PSP Study Group in a majority of relevant studies reported elevated depression in CBS (Gerstenecker et al. 2013) and experienced depression rather than apathy, whereas the latter was predominant in PSP (Bruns and Josephs 2013).
Magnitude of hopelessness may help to diagnostically classify CBS (and PSP) patients in the earliest stages of disease (Shdo et al. 2020). At the initial visit, the mean BDI scores for CBD were 13.4 ± 98.4 points, compared to 10.4 ± 7.4 points for PD, 12.6 ± 7.5 points for MSA, and 13.7 ± 7.7 points for PSP. The severity of depressive symptoms at initial visit for CBD was 23% mild, 12% moderate and 7% severe, which was higher than for PSP and PD patients. A comparison of the cumulative incidence of newly diagnosed depressive symptoms over 2 and 3 years follow-up detected 45% of patients with depressive symptoms among CBD patients after 24 and 36 months compared to 61% and 78% of PSP patients, and the lowest percentage in the PD group (Almeida et al. 2017).
As recently described, most if not all CBD patients, in addition to depressive symptoms, develop cognitive impairment and behavioral abnormalities, while symptoms of depression, also being the earliest presenting evidence of CBD (similar to PD and PSP), are often overlooked due to clinical overlap between these conditions (Ahmad et al. 2023; Bruns and Josephs 2013; Jellinger 2023b).
Neuroimaging and neuropathological findings
CBD patients show asymmetric frontoparietal atrophy contralateral to the clinically affected regions, affection of the perirolandic cortex and atrophy of putamen and anterior corpus callosum (Illán-Gala et al. 2022). They are characterized by early degeneration of brain regions related to reward and emotion, e.g., basal ganglia and other subcortical structures (Gardner et al. 2013; Lee et al. 2011). One CBD patient with depression showed atrophy of the right parietal lobe and decreased blood flow in parietal lobes and posterior cingulate gyrus (Tarakita et al. 2017).
SPECT studies showed significantly decreased regional cerebral blood flow in frontoparietal cortex and thalamus opposite to the predominantly affected side (Huang et al. 2007; Lu et al. 1998). A meta-analysis reported gray matter loss in frontal, temporal and occipital lobes, bilateral thalamus and middle frontal gyrus, atrophy in frontomedian/midcingulate cortex, and premotor/supplementary motor area seemed to be specific for CBD (Albrecht et al. 2017), but cortical rather than thalamic atrophy was a major imaging correlate in CBD (Whitwell et al. 2010).
Earlier studies reported reduced 18F-6-fluorodopa uptake in striatum and significantly depressed oxygen metabolism in posterior frontal lobe, temporal and inferior parietal cortices (Sawle et al. 1991). FDG-PET revealed hypometabolism in frontoparietal and occipital cortices with involvement of ipsilateral striatum and thalamus (Franceschi et al. 2020; Pardini et al. 2019). CBS showed cholinergic deficits in thalamus, frontal, parietal and occipital cortices (Hirano et al. 2010). Like other atypical parkinsonian syndromes, CBD is characterized by both pre- and postsynaptic deficiencies, with reduction of striatal dopamine synthesis, storage and release (Nikolaus et al. 2007) and reduced dopamine transporter availability in putamen and thalamus (Mille et al. 2017). Other biochemical deficits include a marked loss of noradrenaline and serotonin due to the involvement of locus coeruleus and raphe nuclei (Litvan et al. 1998). CBD patients with negative PiB-PET (demonstrating β amyloid deposition) showed hypometabolism in posterior temporoparietal areas, thalamus and brainstem (Parmera et al. 2021).
Functional MR studies in CBD revealed increased connectivity between default mode network, dorsal attention and mood networks, and decreased connectivity within these networks (Whiteside et al. 2023).
Whereas there are many network studies related to movement, language and cognitive disorders, apraxia, alien limb, etc., demonstrating dysfunctions of subcortical projections to premotor and supplementary motor circuits (Garraux et al. 2000) and indicating the importance of disturbed functional connectivity over structural lesions (Ballarini et al. 2020; Jo et al. 2021; Tetreault et al. 2020), to the best of our knowledge, no neuroimaging and network studies related to depression in CBD are available.
Neuropathological data of CBD associated with depression exist only in two case reports. A woman aged 65 years presenting with forgetfulness, depression and violent actions, but no limb apraxia died 12 years after onset of symptoms. Neuropathology showed circumscribed atrophy of bilateral frontal lobe, caudate nucleus and SN, neuronal loss, status spongiosus and ballooned neurons in cortex, neuron loss and gliosis in dorsomedial thalamus, nucleus basalis of Meynert, SN, subthalamic nucleus hippocampus and pontine tegmentum. Neurofibrillary tangles were present in these areas as were numerous argyrophilic and tau-positive threads in cerebral white matter. In addition to these findings corresponding to CBD and those for PSP, widespread iron deposition throughout the CNS were a unique finding not being reported in the literature to date (Mizuno et al. 2002). The second was a male aged 52 years, who presented with visual problems, falls, dysarthria, eyelid apraxia, bradykinesia, neck rigidity, and depression; death at age 55. Neuropathology showed diffuse tau lesions in subcortical white matter, ballooned neurons in cingulate cortex, neurofibrillary tangles in hippocampus, entorhinal cortex and nucleus basalis of Meynert, many tau-positive threads in gray and white matter astrocytic plaques, pretangles and sparse oligodendroglial coiled bodies (Bayram et al. 2020) (case 1). Thus, with the exception of iron deposition in the first case, neuropathology in both patients with depression corresponded to classical CBD.
Biomarkers
Clinical features that can predict the underlying pathology of CBS remain unclear. Using AD-related biomarkers (Aβ, tau) may help distinguish tauopathies from AD, but remain challenging for CBD (and PSP). Antemortem tau-PET uptake corresponded with quantitatively assessed 4R tau burden in basal ganglia, precentral cortex, operculum and supplementary motor areas, often showing an asymmetrical pattern (Ali et al. 2018; Koga et al. 2023; McMillan et al. 2016). Cerebrospinal fluid (CSF) analysis showed AD-specific pattern with reduction of Aβ-42, increased total and phospho-tau, as well as increased neurofilament light chain, all these findings, however, being non-specific (Jabbari et al. 2020; Koga et al. 2022); while CSF levels of soluble amyloid protein precursor α (sAPPα) were significantly lower in CBS with depression (and PSP) than in AD (Tang et al. 2020). Plasma ptau 217 differentiated CBS from other 4R-tauopathy-associated syndromes (VandeVrede et al. 2023). CSF biomarkers need to be validated in larger samples of pathologically confirmed cases.
Treatment options
There are currently no disease-modifying treatment for CBD (or PSP) and no approved pharmacological or paramedical treatments that are effective in controlling or relieving their symptoms. The use of most pharmacological treatment options is based on experience in other disorders, from non-randomized historical controls or small case series. Poor response to levodopa forms part of the consensus criteria of CBD (Armstrong et al. 2013). Antidepressants may be useful for depression but are often poorly tolerated due to adverse effects (Lamb et al. 2016). In the absence of an effective drug treatment to target the underlying cause of the disease, management should focus on optimizing quality of life, reducing symptoms, and assisting patients with their activities of daily living, which needs a multidisciplinary cooperation of neurologists, physiotherapists, occupational and language therapists, and palliative care specialists.
Conclusions and outlook
The pattern of cortical and subcortical neuropathological lesions in CBD should predict a specific clinical profile in this rare 4R tauopathy, which is characterized by a combination of akinesia/rigidity, limb dystonia, apraxia, language problems, cognitive, executive, behavioral impairment, and depression, the latter often being the early evidence of CBD, which usually may not correlate with most of the other clinical parameters. Patients with CBD show a specific dysexecutive syndrome, likely due to degeneration of prefrontal cortex and basal ganglia with disruption of fronto-subcortical networks, asymmetric praxia disorders, related to premotor and parietal lobe lesions and reduced connections of related neuronal networks, cognitive and behavioral changes, related to asymmetric prefronto-parieto-limbic atrophies and network dysfunctions. This complex syndrome is frequently associated with and complicated by severe depression that is particularly important due to its negative impact on the course of the disease. In comparison to depression in PD, the complex pathogenic mechanisms of which have been widely elucidated by modern neuroimaging and neuropathological studies (see Jellinger 2022), almost no functional imaging, neurochemical, and neuropathological studies about the pathomechanisms of depression in CBD are available. In addition to the well-documented depletion of the nigrostriatal dopaminergic functions, involvement of the noradrenergic and serotonergic systems that are generally associated with depressive symptoms, limited neuroimaging studies suggest a multi-regional degeneration with atrophy and hypometabolism in frontal cortex, thalamus and basal ganglia with dysfunction of prefronto-subcortical and other mood-related circuits. Despite available standard diagnostic criteria, depression in CBD as well as in other atypical parkinsonian syndromes is still underdiagnosed and difficult to be treated. No specific fluid biomarkers are currently available, since the existing ones are non-specific, and no controlled studies have investigated the treatment of depression and other neuropsychiatric symptoms (Berardelli et al. 2019). Prospective assessment and validation of depressive signs and symptoms in CBD will be improved by the combined use of modern neuroimaging data and specific fluid biomarkers. Furthermore, neuropathological assessment is required for elucidation of the pathobiological mechanisms underlying the morphological and biochemical substrate of co-morbid depression and its interrelationship with other symptoms in CBD. For this purpose, it will be necessary to conduct prospective clinicopathological cohort studies to get more insight into the molecular mechanisms of depression in CBD as a basis for an adequate treatment to improve the quality of life in this devastating and hitherto untreatable disease.
Abbreviations
- 4R:
-
4-Repeat
- AD:
-
Alzheimer disease
- CBD:
-
Corticobasal degeneration
- CBS:
-
Corticobasal syndrome
- CSF:
-
Cerebrospinal fluid
- PD:
-
Parkinson disease
- PSP:
-
Progressive supranuclear palsy
- SN:
-
Substantia nigra
References
Aarsland D, Litvan I, Larsen JP (2001) Neuropsychiatric symptoms of patients with progressive supranuclear palsy and Parkinson’s disease. J Neuropsychiatry Clin Neurosci 13:42–49
Ahmad MH, Rizvi MA, Ali M, Mondal AC (2023) Neurobiology of depression in Parkinson’s disease: Insights into epidemiology, molecular mechanisms and treatment strategies. Ageing Res Rev 85:101840
Albrecht F, Bisenius S, Morales Schaack R, Neumann J, Schroeter ML (2017) Disentangling the neural correlates of corticobasal syndrome and corticobasal degeneration with systematic and quantitative ALE meta-analyses. NPJ Parkinsons Dis 3:12
Ali F, Whitwell JL, Martin PR, Senjem ML, Knopman DS, Jack CR, Lowe VJ, Petersen RC, Boeve BF, Josephs KA (2018) [(18)F] AV-1451 uptake in corticobasal syndrome: the influence of beta-amyloid and clinical presentation. J Neurol 265:1079–1088
Almeida L, Ahmed B, Walz R, De Jesus S, Patterson A, Martinez-Ramirez D, Vaillancourt D, Bowers D, Ward H, Okun MS, McFarland NR (2017) Depressive symptoms are frequent in atypical parkinsonian disorders. Mov Disord Clin Pract 4:191–197
American, Psychiatric, Association, Association AP (2013) Diagnostic and Statistical Manual of Mental Disorders, 5th edn. American Psychiatric Publishing, Washington, DC
Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, Boxer AL, Dickson DW, Grossman M, Hallett M, Josephs KA, Kertesz A, Lee SE, Miller BL, Reich SG, Riley DE, Tolosa E, Troster AI, Vidailhet M, Weiner WJ (2013) Criteria for the diagnosis of corticobasal degeneration. Neurology 80:496–503
Ballarini T, Albrecht F, Mueller K, Jech R, Diehl-Schmid J, Fliessbach K, Kassubek J, Lauer M, Fassbender K, Schneider A, Synofzik M, Wiltfang J, Otto M, Schroeter ML (2020) Disentangling brain functional network remodeling in corticobasal syndrome - A multimodal MRI study. Neuroimage Clin 25:102112
Bayram E, Dickson DW, Reich SG, Litvan I (2020) Pathology-proven corticobasal degeneration presenting as Richardson’s syndrome. Mov Disord Clin Pract 7:267–272
Belvisi D, Berardelli I, Suppa A, Fabbrini A, Pasquini M, Pompili M, Fabbrini G (2018) Neuropsychiatric disturbances in atypical parkinsonian disorders. Neuropsychiatr Dis Treat 14:2643–2656
Berardelli I, Belvisi D, Pasquini M, Fabbrini A, Petrini F, Fabbrini G (2019) Treatment of psychiatric disturbances in hypokinetic movement disorders. Expert Rev Neurother 19:965–981
Bower SM, Weigand SD, Ali F, Clark HM, Botha H, Stierwalt JA, Whitwell JL, Josephs KA (2021) Depression and apathy across different variants of progressive supranuclear palsy. Mov Disord Clin Pract 9:212–217
Bruns MB, Josephs KA (2013) Neuropsychiatry of corticobasal degeneration and progressive supranuclear palsy. Int Rev Psychiatry 25:197–209
Campagnolo M, Weis L, Fogliano C, Cianci V, Garon M, Fiorenzato E, Carecchio M, Ferreri F, Bisiacchi P, Antonini A, Biundo R (2023) Clinical, cognitive, and morphometric profiles of progressive supranuclear palsy phenotypes. J Neural Transm 130:97–109
Chendo I, Silva C, Duarte GS, Prada L, Vian J, Quintão A, Voon V, Ferreira JJ (2022) Frequency of depressive disorders in Parkinson’s disease: a systematic review and meta-analysis. J Parkinsons Dis 12:1409–1418
Cordato NJ, Halliday GM, McCann H, Davies L, Williamson P, Fulham M, Morris JG (2001) Corticobasal syndrome with tau pathology. Mov Disord 16:656–667
Cuoco S, Cappiello A, Abate F, Tepedino MF, Erro R, Volpe G, Pellecchia MT, Barone P, Picillo M (2021) Psychometric properties of the Beck Depression Inventory-II in progressive supranuclear palsy. Brain Behav 11:e2344
Dickson DW, Bergeron C, Chin SS, Duyckaerts C, Horoupian D, Ikeda K, Jellinger K, Lantos PL, Lippa CF, Mirra SS, Tabaton M, Vonsattel JP, Wakabayashi K, Litvan I (2002) Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol 61:935–946
Dickson DW, Hauw JJ, Agid Y, Litvan I (2011) Progressive supranuclear palsy and corticobasal degeneration. In: Dickson DW, Weller RO (eds) Neurodegeneration: the molecular pathology of dementia and movement disorders, 2nd edn. Blackwell Publishing Ltd., Oxford, pp 135–155
Franceschi AM, Clifton M, Naser-Tavakolian K, Ahmed O, Cruciata G, Bangiyev L, Clouston S, Franceschi D (2020) ((18)F)-Fluorodeoxyglucose positron emission tomography/magnetic resonance imaging assessment of hypometabolism patterns in clinical phenotypes of suspected corticobasal degeneration. World J Nucl Med 20:176–184
Galts CPC, Bettio LEB, Jewett DC, Yang CC, Brocardo PS, Rodrigues ALS, Thacker JS, Gil-Mohapel J (2019) Depression in neurodegenerative diseases: common mechanisms and current treatment options. Neurosci Biobehav Rev 102:56–84
Gardner RC, Boxer AL, Trujillo A, Mirsky JB, Guo CC, Gennatas ED, Heuer HW, Fine E, Zhou J, Kramer JH, Miller BL, Seeley WW (2013) Intrinsic connectivity network disruption in progressive supranuclear palsy. Ann Neurol 73:603–616
Garraux G, Salmon E, Peigneux P, Kreisler A, Degueldre C, Lemaire C, Destée A, Franck G (2000) Voxel-based distribution of metabolic impairment in corticobasal degeneration. Mov Disord 15:894–904
Geda YE, Boeve BF, Negash S, Graff-Radford NR, Knopman DS, Parisi JE, Dickson DW, Petersen RC (2007) Neuropsychiatric features in 36 pathologically confirmed cases of corticobasal degeneration. J Neuropsychiatry Clin Neurosci 19:77–80
Gerstenecker A, Duff K, Mast B, Litvan I (2013) Behavioral abnormalities in progressive supranuclear palsy. Psychiatry Res 210:1205–1210
Gibb WR, Luthert PJ, Marsden CD (1989) Corticobasal degeneration. Brain 112(Pt 5):1171–1192
Gupta R, Advani D, Yadav D, Ambasta RK, Kumar P (2023) Dissecting the relationship between neuropsychiatric and neurodegenerative disorders. Mol Neurobiol 60:6476–6529
Hirano S, Shinotoh H, Shimada H, Aotsuka A, Tanaka N, Ota T, Sato K, Ito H, Kuwabara S, Fukushi K, Irie T, Suhara T (2010) Cholinergic imaging in corticobasal syndrome, progressive supranuclear palsy and frontotemporal dementia. Brain 133:2058–2068
Huang KJ, Lu MK, Kao A, Tsai CH (2007) Clinical, imaging and electrophysiological studies of corticobasal degeneration. Acta Neurol Taiwan 16:13–21
Illán-Gala I, Nigro S, VandeVrede L, Falgàs N, Heuer HW, Painous C, Compta Y, Martí MJ, Montal V, Pagonabarraga J, Kulisevsky J, Lleó A, Fortea J, Logroscino G, Quattrone A, Perry DC, Gorno-Tempini ML, Rosen HJ, Grinberg LT, Spina S, La Joie R, Rabinovici GD, Miller BL, Rojas JC, Seeley WW, Boxer AL (2022) Diagnostic accuracy of magnetic resonance imaging measures of brain atrophy across the spectrum of progressive supranuclear palsy and corticobasal degeneration. JAMA Netw Open 5:e229588
Jabbari E, Holland N, Chelban V, Jones PS, Lamb R, Rawlinson C, Guo T, Costantini AA, Tan MMX, Heslegrave AJ, Roncaroli F, Klein JC, Ansorge O, Allinson KSJ, Jaunmuktane Z, Holton JL, Revesz T, Warner TT, Lees AJ, Zetterberg H, Russell LL, Bocchetta M, Rohrer JD, Williams NM, Grosset DG, Burn DJ, Pavese N, Gerhard A, Kobylecki C, Leigh PN, Church A, Hu MTM, Woodside J, Houlden H, Rowe JB, Morris HR (2020) Diagnosis across the spectrum of progressive supranuclear palsy and corticobasal syndrome. JAMA Neurol 77:377–387
Jellinger KA (2022) The pathobiological basis of depression in Parkinson disease: challenges and outlooks. J Neural Transm 129:1397–1418
Jellinger KA (2023a) Pathomechanisms of depression in progressive supranuclear palsy. J Neural Transm 130:1049–1056
Jellinger KA (2023b) Pathomechanisms of cognitive and behavioral impairment in corticobasal degeneration. J Neural Transm 130:1509–1522
Jo S, Oh JS, Cheong EN, Kim HJ, Lee S, Oh M, Kim JS, Chung SJ, Lee CS, Kwon M, Kang D, Lee JH (2021) FDG-PET patterns associated with ideomotor apraxia and imitation apraxia in patients with corticobasal syndrome. Parkinsonism Relat Disord 88:96–101
Koga S, Josephs KA, Aiba I, Yoshida M, Dickson DW (2022) Neuropathology and emerging biomarkers in corticobasal syndrome. J Neurol Neurosurg Psychiatry 93:919–929
Koga S, Metrick MA 2nd, Golbe LI, Santambrogio A, Kim M, Soto-Beasley AI, Walton RL, Baker MC, De Castro CF, DeTure M, Russell D, Navia BA, Sandiego C, Ross OA, Vendruscolo M, Caughey B, Dickson DW (2023) Case report of a patient with unclassified tauopathy with molecular and neuropathological features of both progressive supranuclear palsy and corticobasal degeneration. Acta Neuropathol Commun 11:88
Köllensperger M, Geser F, Ndayisaba JP, Boesch S, Seppi K, Ostergaard K, Dupont E, Cardozo A, Tolosa E, Abele M, Klockgether T, Yekhlef F, Tison F, Daniels C, Deuschl G, Coelho M, Sampaio C, Bozi M, Quinn N, Schrag A, Mathias CJ, Fowler C, Nilsson CF, Widner H, Schimke N, Oertel W, Del Sorbo F, Albanese A, Pellecchia MT, Barone P, Djaldetti R, Colosimo C, Meco G, Gonzalez-Mandly A, Berciano J, Gurevich T, Giladi N, Galitzky M, Rascol O, Kamm C, Gasser T, Siebert U, Poewe W, Wenning GK (2010) Presentation, diagnosis, and management of multiple system atrophy in Europe: final analysis of the European multiple system atrophy registry. Mov Disord 25:2604–2612
Kouri N, Murray ME, Hassan A, Rademakers R, Uitti RJ, Boeve BF, Graff-Radford NR, Wszolek ZK, Litvan I, Josephs KA, Dickson DW (2011) Neuropathological features of corticobasal degeneration presenting as corticobasal syndrome or Richardson syndrome. Brain 134:3264–3275
Lamb R, Rohrer JD, Lees AJ, Morris HR (2016) Progressive supranuclear palsy and corticobasal degeneration: pathophysiology and treatment options. Curr Treat Options Neurol 18:42
Lee SE, Rabinovici GD, Mayo MC, Wilson SM, Seeley WW, DeArmond SJ, Huang EJ, Trojanowski JQ, Growdon ME, Jang JY, Sidhu M, See TM, Karydas AM, Gorno-Tempini ML, Boxer AL, Weiner MW, Geschwind MD, Rankin KP, Miller BL (2011) Clinicopathological correlations in corticobasal degeneration. Ann Neurol 70:327–340
Lewis-Smith DJ, Wolpe N, Ghosh BCP, Rowe JB (2020) Alien limb in the corticobasal syndrome: phenomenological characteristics and relationship to apraxia. J Neurol 267:1147–1157
Ling H, Kovacs GG, Vonsattel JP, Davey K, Mok KY, Hardy J, Morris HR, Warner TT, Holton JL, Revesz T (2016) Astrogliopathy predominates the earliest stage of corticobasal degeneration pathology. Brain 139:3237–3252
Litvan I, Cummings JL, Mega M (1998) Neuropsychiatric features of corticobasal degeneration. J Neurol Neurosurg Psychiatry 65:717–721
Lu CS, Ikeda A, Terada K, Mima T, Nagamine T, Fukuyama H, Kohara N, Kojima Y, Yonekura Y, Chen RS, Tsai CH, Chu NS, Kimura J, Shibasaki H (1998) Electrophysiological studies of early stage corticobasal degeneration. Mov Disord 13:140–146
McMillan CT, Irwin DJ, Nasrallah I, Phillips JS, Spindler M, Rascovsky K, Ternes K, Jester C, Wolk DA, Kwong LK, Lee VM, Lee EB, Trojanowski JQ, Grossman M (2016) Multimodal evaluation demonstrates in vivo (18)F-AV-1451 uptake in autopsy-confirmed corticobasal degeneration. Acta Neuropathol 132:935–937
Mille E, Levin J, Brendel M, Zach C, Barthel H, Sabri O, Bötzel K, Bartenstein P, Danek A, Rominger A (2017) Cerebral glucose metabolism and dopaminergic function in patients with corticobasal syndrome. J Neuroimaging 27:255–261
Mizuno Y, Ozeki M, Iwata H, Takeuchi T, Ishihara R, Hashimoto N, Kobayashi H, Iwai K, Ogasawara S, Ukai K, Shibayama H (2002) A case of clinically and neuropathologically atypical corticobasal degeneration with widespread iron deposition. Acta Neuropathol 103:288–294
Nikolaus S, Antke C, Kley K, Poeppel TD, Hautzel H, Schmidt D, Müller HW (2007) Investigating the dopaminergic synapse in vivo. I. Molecular imaging studies in humans. Rev Neurosci 18:439–472
Ou R, Wei Q, Hou Y, Zhang L, Liu K, Xu X, Gu X, Lin J, Jiang Z, Liu J, Song W, Cao B, Shang H (2020) Suicidal and death ideation in patients with progressive supranuclear palsy and corticobasal syndrome. J Affect Disord 276:1061–1068
Pardini M, Huey ED, Spina S, Kreisl WC, Morbelli S, Wassermann EM, Nobili F, Ghetti B, Grafman J (2019) FDG-PET patterns associated with underlying pathology in corticobasal syndrome. Neurology 92:e1121–e1135
Parmera JB, Coutinho AM, Aranha MR, Studart-Neto A, de Godoi Carneiro C, de Almeida IJ, Fontoura Solla DJ, Ono CR, Barbosa ER, Nitrini R, Buchpiguel CA, Brucki SMD (2021) FDG-PET patterns predict amyloid deposition and clinical profile in corticobasal syndrome. Mov Disord 36:651–661
Parmera JB, Oliveira MCB, Rodrigues RD, Coutinho AM (2022) Progressive supranuclear palsy and corticobasal degeneration: novel clinical concepts and advances in biomarkers. Arq Neuropsiquiatr 80:126–136
Rebeiz JJ, Kolodny EH, Richardson EP Jr (1968) Corticodentatonigral degeneration with neuronal achromasia. Arch Neurol 18:20–33
Sainouchi M, Tada M, Fitrah YA, Hara N, Tanaka K, Idezuka J, Aida I, Nakajima T, Miyashita A, Akazawa K, Ikeuchi T, Onodera O, Kakita A (2022) Brain TDP-43 pathology in corticobasal degeneration: Topographical correlation with neuronal loss. Neuropathol Appl Neurobiol 48:e12786
Sawle GV, Brooks DJ, Marsden CD, Frackowiak RS (1991) Corticobasal degeneration. A unique pattern of regional cortical oxygen hypometabolism and striatal fluorodopa uptake demonstrated by positron emission tomography. Brain 114(Pt 1B):541–556
Shdo SM, Ranasinghe KG, Sturm VE, Possin KL, Bettcher BM, Stephens ML, Foley JM, You SC, Rosen HJ, Miller BL, Kramer JH, Rankin KP (2020) Depressive symptom profiles predict specific neurodegenerative disease syndromes in early stages. Front Neurol 11:446
Shimohata T, Aiba I, Nishizawa M (2015) Criteria for the diagnosis of corticobasal degeneration. Brain Nerve 67:513–523
Shir D, Thu Pham NT, Botha H, Koga S, Kouri N, Ali F, Knopman DS, Petersen RC, Boeve BF, Kremers WK, Nguyen AT, Murray ME, Reichard RR, Dickson DW, Graff-Radford N, Josephs KA, Whitwell J, Graff-Radford J (2023) Clinicoradiologic and neuropathologic evaluation of corticobasal syndrome. Neurology 101:e289-e299
Stang CD, Turcano P, Mielke MM, Josephs KA, Bower JH, Ahlskog JE, Boeve BF, Martin PR, Upadhyaya SG, Savica R (2020) Incidence and trends of progressive supranuclear palsy and corticobasal syndrome: a population-based study. J Parkinsons Dis 10:179–184
Swallow DMA, Zheng CS, Counsell CE (2022) Systematic review of prevalence studies of progressive supranuclear palsy and corticobasal syndrome. Mov Disord Clin Pract 9:604–613
Tang W, Wang Y, Cheng J, Yao J, Yao YY, Zhou Q, Guan SH (2020) CSF sAPPalpha and sAPPbeta levels in Alzheimer’s disease and multiple other neurodegenerative diseases: a network meta-analysis. Neuromolecular Med 22:45–55
Tarakita N, Nishijima H, Yasui-Furukori N (2017) Levodopa-responsive depression associated with corticobasal degeneration: a case report. Neuropsychiatr Dis Treat 13:1107–1110
Tateno A, Nogami T, Sakayori T, Yamamoto K, Okubo Y (2023) Depression as a prodromal symptom of neurodegenerative diseases. J Nippon Med Sch 90:157–164
Tatsumi S, Uchihara T, Aiba I, Iwasaki Y, Mimuro M, Takahashi R, Yoshida M (2014) Ultrastructural differences in pretangles between Alzheimer disease and corticobasal degeneration revealed by comparative light and electron microscopy. Acta Neuropathol Commun 2:161
Tetreault AM, Phan T, Petersen KJ, Claassen DO, Neth BJ, Graff-Radford J, Albrecht F, Fliessbach K, Schneider A, Synofzik M, Diehl-Schmid J, Otto M, Schroeter ML, Darby RR (2020) Network localization of alien limb in patients with corticobasal syndrome. Ann Neurol 88:1118–1131
Uchihara T (2014) Pretangles and neurofibrillary changes: Similarities and differences between AD and CBD based on molecular and morphological evolution. Neuropathology 34:571–577
VandeVrede L, La Joie R, Thijssen EH, Asken BM, Vento SA, Tsuei T, Baker SL, Cobigo Y, Fonseca C, Heuer HW, Kramer JH, Ljubenkov PA, Rabinovici GD, Rojas JC, Rosen HJ, Staffaroni AM, Boeve BF, Dickerson BC, Grossman M, Huey ED, Irwin DJ, Litvan I, Pantelyat AY, Tartaglia MC, Dage JL, Boxer AL (2023) Evaluation of plasma phosphorylated tau217 for differentiation between Alzheimer disease and frontotemporal lobar degeneration subtypes among patients with corticobasal syndrome. JAMA Neurol 80:495–505
Wenning GK, Litvan I, Jankovic J, Granata R, Mangone CA, McKee A, Poewe W, Jellinger K, Ray Chaudhuri K, D’Olhaberriague L, Pearce RK (1998) Natural history and survival of 14 patients with corticobasal degeneration confirmed at postmortem examination. J Neurol Neurosurg Psychiatry 64:184–189
Whiteside DJ, Street D, Murley AG, Jones PS, Malpetti M, Ghosh BCP, Coyle-Gilchrist I, Gerhard A, Hu MT, Klein JC, Leigh PN, Church A, Burn DJ, Morris HR, Rowe JB, Rittman T (2023) Network connectivity and structural correlates of survival in progressive supranuclear palsy and corticobasal syndrome. Hum Brain Mapp 44:4239–4255
Whitwell JL, Jack CR Jr, Boeve BF, Parisi JE, Ahlskog JE, Drubach DA, Senjem ML, Knopman DS, Petersen RC, Dickson DW, Josephs KA (2010) Imaging correlates of pathology in corticobasal syndrome. Neurology 75:1879–1887
Williams DR, de Silva R, Paviour DC, Pittman A, Watt HC, Kilford L, Holton JL, Revesz T, Lees AJ (2005) Characteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson’s syndrome and PSP-parkinsonism. Brain 128:1247–1258
Yokota O, Miki T, Ishizu H, Haraguchi T, Kishimoto Y, Takenoshita S, Hara N, Miyashita A, Ikeuchi T, Terada S, Yamada N (2023) Four-repeat tauopathies and late-onset psychiatric disorders: etiological relevance or incidental findings? Neuropathology 43:51–71
Yoshida M (2014) Astrocytic inclusions in progressive supranuclear palsy and corticobasal degeneration. Neuropathology 34:555–570
Acknowledgements
The author thanks Mr. E. Mitter-Ferstl for secretarial and editorial work.
Funding
The study was funded by the Society for the Promotion of Research in Experimental Neurology, Vienna, Austria.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jellinger, K.A. The enigma of depression in corticobasal degeneration, a frequent but poorly understood co-morbidity. J Neural Transm 131, 195–202 (2024). https://doi.org/10.1007/s00702-023-02731-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-023-02731-5