Abstract
Lewy bodies, the histologic hallmark of Parkinson’s disease (PD), are detected in the brains of about 10% of clinically normal people over the age of 60 years. When Lewy bodies are found in normal individuals, the process is sometimes referred to as incidental Lewy body disease (iLBD). The distribution of Lewy bodies in iLBD is similar to the distribution in PD, but neuronal populations vulnerable to Lewy bodies do not show significant neuronal loss in iLBD. It remains unknown if Lewy bodies in this setting represent pre-symptomatic PD or an age-related change unrelated to PD. To address this question we identified cases of iLBD and used a marker for dopaminergic and noradrenergic neurons, tyrosine hydroxylase (TH), to determine if there were changes similar to those found in PD. TH immunoreactivity in the striatum and the epicardial nerve fibers was decreased in iLBD compared to normal controls, but not to the same extent as in PD. The findings suggest that iLBD is preclinical PD and that the lack of symptoms is due to subthreshold pathology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuronal loss and α-synuclein immunoreactive Lewy bodies in vulnerable neuronal populations, such as the substantia nigra, are the histopathologic hallmarks of Parkinson’s disease (PD) [23, 30]. Lewy bodies are also detected in the brains of clinically normal people. It has been known for several decades that as many as 8–12% of normal individuals over age 60 years have Lewy bodies in brainstem nuclei with routine histologic methods, which is often termed coincidental or incidental Lewy body disease (iLBD) [19, 22]. In prospective studies of normal aging using modern immunohistochemical methods the frequency is the same [6, 34, 35]. When cases with dementia are also included the frequency increases to over 30% [31]. The affected brainstem nuclei in asymptomatic elderly individuals are the same as those affected in PD [17]. It remains unknown if Lewy bodies in this setting represent pre-symptomatic PD or an age-related change unrelated to PD, but the frequency of iLBD is approximately ten times the frequency of clinical PD [18].
Analysis of the topography of Lewy bodies in a large cross-sectional study led Braak and co-workers to hypothesize that the neurodegenerative process in PD may begin in the lower brainstem and the anterior olfactory nucleus [12, 16]. In this staging scheme, midbrain dopaminergic and basal forebrain cholinergic neurons, which are implicated in motor and cognitive dysfunction characteristic of PD, are affected only at a middle stage of PD, while the end stage is characterized by involvement of cortical neurons [12]. Anatomical regions not specifically addressed in the original staging scheme are the spinal cord and autonomic nervous system, yet both are known to be affected in PD [44, 45], and more recent iterations of the staging scheme incorporate these areas, as well [13, 14]. Several studies have noted Lewy bodies or α-synuclein immunoreactive neurites in the spinal cord and autonomic nervous system in iLBD [8, 21, 33], suggesting that spinal cord and autonomic nervous system may be affected at a relatively early stage of PD, if indeed iLBD is pre-symptomatic PD.
In PD, Lewy bodies are accompanied by neuronal loss and gliosis, as well as evidence of degeneration of distal nerve termini in target regions of vulnerable neurons; the best studied being the nigrostriatal dopaminergic neurons. Specifically, there is neuronal loss and gliosis in the pars compacta of the substantia nigra and evidence from both clinical imaging methods and postmortem pathologic studies of degeneration of dopaminergic nerve termini in the striatum [4, 11, 15, 16, 24, 28].
If the staging scheme proposed by Braak is correct, non-motor, non-dopaminergic symptoms should precede motor symptoms. In particular, one might predict that early PD may be characterized by autonomic dysfunction, olfactory dysfunction and sleep disorders, given the role that lower brainstem monoaminergic nuclei, as well as spinal and enteric ganglia have in these processes. It is of interest that epidemiologic studies indicate that autonomic symptoms may precede clinical PD by as much as 12 years [1, 2]. Constipation is a common problem in PD, and it can antedate extrapyramidal symptoms by years [1]. Investigation of the gastrointestinal tract in PD reveals Lewy bodies and α-synuclein neurites in the enteric plexus [13, 46]. A sleep disorder that may be a harbinger of PD is rapid eye movement sleep behavior disorder (RBD), another condition that may antedate PD by decades [42]. The RBD syndrome appears to have its anatomic origins within lower brainstem nuclei that are consistently affected in PD [9] Olfactory dysfunction is common in PD [27], and it may precede overt motor symptoms [7]. A recent report has indicated that both RBD and olfactory dysfunction may be associated with preclinical PD [43].
Imaging of sympathetic nerve fibers in the heart is possible with 125I-meta-iodobenzylguanidine (MIBG) scintography [25, 38, 40]. These studies show that cardiac denervation occurs early in PD. In a recent case report, a patient with abnormal MIBG cardiac scintography had Lewy bodies in autonomic ganglia [36]. Another study has documented abnormalities of MIBG cardiac scintography in patients with RBD [37].
If iLBD is preclinical PD, it is predicted that there should be measurable differences between normals with and without Lewy bodies, but that the changes in iLBD would be less than those found in PD. One means of assessing the impact of Lewy body pathology on dopaminergic and sympathetic noradrenergic neurons is to assess the integrity of these neurons in their target regions. For this purpose we used immunohistochemistry for tyrosine hydroxylase (TH) [26]. It is well established that TH is the rate-limited enzyme in dopamine and norepinephrine synthesis and that immunostaining for TH can be used to study these systems [4, 16, 26]. In this report we describe evidence that iLBD has features consistent with preclinical PD in that there are measurable decreases in TH immunoreactivity in both the autonomic nervous system in the heart and the dopaminergic nerve termini in the striatum compared to in normals without Lewy bodies, but that the degree of decrease is less than that found in PD.
Materials and methods
Case material
Cases of iLBD were identified from a series of clinically normal individuals who had been followed at the Mayo Clinic and subsequently had autopsy, with tissue available for further study from the Mayo Clinic Rochester Tissue Registry. All iLBD and normal control cases were 60 years of age or older at death without clinical evidence of parkinsonism, tremor, dementia or any other central nervous system disorder. Cases were only included if they had medical record documentation of several medical contacts during the last 5 years of life and postmortem delays of less than 24 h. Inclusion and exclusion criteria are described in detail in a previous report [33]. The clinical histories were reviewed blinded to pathologic findings to capture cases that were neurologically normal and who had at least one clinical examination within 1 year of death. Cases of PD were also identified from the Tissue Registry of the Mayo Clinic. They met similar inclusion and exclusion criteria, except that they all had clinically probable PD [29] and had been followed in the movement disorder clinic.
Tissue blocks were prepared from archival formalin-fixed brain, spinal cord and heart tissue and embedded in paraffin. Not all regions were available on every case. Alzheimer type pathology was assessed with thioflavin-S fluorescent microscopy, and a Braak neurofibrillary tangle (NFT) stage was assigned to each case. Senile plaques were also assessed with thioflavin-S fluorescent microscopy and scored on a 4-point scale: 0 = none; 1 = sparse non-neuritic plaques; 2 = moderate to many diffuse plaques; 3 = moderate to many plaques, with at least some being neuritic in type. To detect iLBD cases, sections of cingulate gyrus, basal forebrain, midbrain, pons and medulla were screened for α-synuclein pathology. Immunohistochemistry for α-synuclein employed a polyclonal antibody (NACP98; 1:3000) after antigen retrieval in 80% formic acid and steam treatment. The sections were processed in batches to assure uniformity in a DAKO Autostainer with the EnVision HRP-rabbit system. The chromogen was 3,3’-diaminobenzidine.
Striatal TH immunoreactivity
Immunofluorescence staining for TH was performed following pretreatment of sections with 5 mM proteinase K. Sections were blocked in all-purpose blocking serum and incubated overnight at 4°C with a polyclonal antibody to TH (1:600, Affinity Bioreagents, Golden, CO, USA). The secondary antibody was conjugated to a fluorochrome (1:300; Alexa Fluor568; Invitrogen Corp, Carlsbad, CA, USA). Sections were immersed in a solution of 1% Sudan Black B to block autofluorescence. Consistent and relatively uniform TH immunoreactivity was detected in these paraffin-embedded archival tissue samples, apparently unaffected by tissue thickness and other factors that have plagued studies with other antibodies to TH [3].
Fluorescent image analysis was performed using an Olympus BX50 microscope with an Olympus DP70 digital camera. For each case five non-overlapping images were captured from the putamen at high magnification. Images were taken from areas of the putamen that did not include internal capsule, fascicles of corticobulbar and corticospinal tracts or external capsule. Images were captured for the mid-putamen at a level that included the anterior commissure in the same section. The images were converted to monochrome gray scale and subsequently analyzed blinded to clinical and pathologic information using MetaMorph software (Molecular Devices, Sunnyvale, CA, USA), which measures pixel intensity after individual threshold adjustments were made to maximize signal-to-noise ratio. TH immunoreactivity was expressed as the ratio of immunoreactive pixels to the total pixels of the entire field. The final value for each case was the average of five fields.
Heart TH immunoreactivity
A previous anatomical study indicated that the greatest TH-immunoreactive nerve fiber density was in the anterior wall of the left ventricle [32] and therefore this region was sampled. Sections from the anterior left ventricle near the anterior descending artery were embedded in paraffin and analyzed as recently reported [20]. Given the retrospective nature of this study, heart tissue was not available on all cases. Immunohistochemistry for TH used the same antibody as above and immunoperoxidase methods similar to those used to detect α-synuclein.
Using a light microscope (Olympus BX41) TH-immunoreactive nerve fibers in the epicardium were semi-quantitatively analyzed blinded to clinical and pathological diagnoses. All fascicles in the epicardium in a given section were scored. A score was assigned as follows: 0 = absent or nearly absent; 1 = sparse; 2 = moderate; 3 = abundant. The number of fascicles varied from case to case (average: 22 ± 10). The average of the scores for all observed epicardial nerve bundles was used for statistical analyses.
Assessment of neuronal loss
Sections of midbrain, pons and medulla were scanned with Aperio ScanScope CS (Aperio Technologies, Vista, CA, USA), which permitted scanning the entire slide containing the regions of interest (ventrolateral tier of the pars compacta, locus ceruleus and dorsal motor nucleus of the vagus). The regions of interest were identified and delineated using ImageScope software, and the area was calculated and expressed in μm2. All neurons in the region of interest, which had to have a nucleus in the plane of section, were then counted. The number of neurons was divided by the area to give an estimate of neuronal density for the nucleus. Given the retrospective and archival nature of the tissue samples, it was not possible to take into account tissue shrinkage. Consequently, estimates of neuronal density must be interpreted with caution.
Statistical methods
Statistical analyses were performed utilizing SigmaStat for Windows Version 3.0.1a (Systat Software, Inc. Point Richmond, CA, USA). Differences between normal, iLBD and PD that were normally distributed were assessed using one way repeated measures analysis of variance, followed by pairwise comparisons using Tukey Test. Chi-square analysis was used to compare sex differences and Mann–Whitney Rank Sum test to compare Braak PD stages. Correlations between pathologic variables were assessed with Spearman Rank Order Correlation analysis. A P < 0.05 was considered significant.
Results
Table 1 summarizes the clinical and pathologic features of iLBD compared to normals and PD. The PD cases had average disease duration of clinically overt parkinsonism of about 12 years, while controls and iLBD cases had no neurologic disorders. The iLBD cases (N = 12; six men) ranged in age from 62 to 95 years of age (mean 78 ± 11 years) and were similar in age and not significantly different in sex distribution compared to the normal controls (N = 17; 12 men; 77 ± 7 years; range: 67–97 years) and PD cases (N = 16; 14 men; 78 ± 6 years; range 66–91 years). Given the strict inclusion and exclusion criteria employed in this study, the cases and controls tended to have minimal or no Alzheimer type pathology, with average Braak NFT stages of II. The number of senile plaques was variable, but none of the cases had neuritic plaques in association cortices indicative of Alzheimer’s disease. The three groups (iLBD, PD and normal controls) did not differ in any of these parameters.
The normal cases had no Lewy bodies, while iLBD cases had a small number of Lewy bodies in one or multiple brain regions; some had sparse Lewy bodies in the limbic or temporal cortices. The average Braak PD stage for iLBD was 2.7 ± 0.3. In contrast, PD cases had more numerous Lewy bodies in all regions and had a significantly higher Braak PD stage (4.4 ± 0.3; mean ± standard error of the mean).
The neuronal densities in iLBD were less than in normal controls in the three regions studied (dorsal motor nucleus of the vagus, locus ceruleus and substantia nigra); but this reached statistical significance only in the locus ceruleus. As expected, neuronal densities in all three regions were significantly reduced in PD compared to both iLBD and normals. When all cases were considered together, there was strong significant inverse correlation (P < 0.001) between Braak PD stage and neuronal densities in the three regions (dorsal motor nucleus, r = −61; locus ceruleus, r = −0.75, substantia nigra, r = −0.62). These correlations were significant in the dorsal motor nucleus and locus ceruleus when considering only cases with Lewy bodies (dorsal motor nucleus, r = −45; locus ceruleus), with a trend (P = 0.07) for the substantia nigra (r = −0.41).
Striatal TH
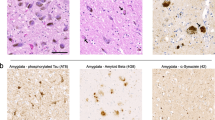
Immunofluorescence for TH revealed a dense plexus of fine, sometimes beaded, neurites in the putamen (Fig. 1). The immunoreactivity was noticeably decreased in most PD cases, while some iLBD cases had more subtle decrease. The origin of these fibers in the putamen is the dopaminergic neurons of the pars compacta of the substantia nigra, which were severely depleted in PD and much less so in iLBD (Fig. 2 and Table 1). Immunohistochemistry for TH was significantly decreased in the terminal neuronal processes of dopaminergic nigrostriatal neurons in the striatum in PD compared to both iLBD and controls. The TH immunoreactivity in iLBD was less than in normals, but this did not reach statistical significance (Fig. 2 and Table 1). When all cases were considered together, there were strong correlations (P < 0.001) between striatal TH immunoreactivity and both substantia nigra neuronal density and Braak PD stage (substantia nigra neuronal density, r = 0.74; Braak PD stage, r = −0.89). Statistically significant correlations (P < 0.01) were also detected for striatal TH immunoreactivity when considering only cases with Lewy bodies (substantia nigra neuronal density, r = 0.80; Braak PD stage, r = −0.60).
Comparison of iLBD to age-matched normals and PD patients with respect to substantia nigra neuronal density (a), TH immunofluorescence in the putamen (b) and TH immunohistochemistry in the epicardial nerve fibers (c). In all measures iLBD is intermediate between normals and PD, and TH immunoreactivity in both striatum and epicardium are statistically different from normals. Box plots show median and 25th and 75th percentiles, with whiskers showing 5th and 95th percentiles. Superimposed are point plots for all data
Epicardial sympathetic fiber TH
Immunohistochemistry for TH revealed diffuse staining of epicardial nerve fibers (Fig. 1). The immunoreactivity was decreased in a larger proportion of fibers in PD than in iLBD, while most fibers in normals had at least some TH immunoreactivity. The origin of these fibers is the post-ganglionic sympathetic neurons of the stellate ganglia. Given that the heart tissue was obtained from archives of a general autopsy service, sympathetic ganglia were not available for study; thus, it was impossible to assess the integrity of the cells of origin. Nevertheless, epicardial TH immunohistochemistry showed a significant decrease in both PD and iLBD compared to normal controls (Fig. 2 and Table 1). The decreases in iLBD were intermediate between normals and PD, but not statistically different from PD. Heart tissue was not available on all cases, particularly for normal controls. When all cases were considered together, there was a strong correlation between cardiac TH immunoreactivity and Braak PD stage (r = −0.75, P < 0.001). A statistically significant correlation (P < 0.01) was also detected for cardiac TH immunoreactivity and Braak PD stage when considering only cases with Lewy bodies (r = −0.61).
Discussion
The present results, as well as previous results from studies of the spinal cord in iLBD [14, 33], indicate that Lewy bodies that are found in clinically asymptomatic elderly individuals are not confined to the brainstem. In fact the Braak PD stage, which was based on the distribution rather than the density of Lewy bodies in the present study, was only marginally different in iLBD compared to PD (median PD stage 3 vs. stage 4 for PD). On the other hand, the density of Lewy bodies in most areas was significantly greater in PD than in iLBD (not shown). The intermediate degree of pathology between normals and PD suggests that iLBD represents preclinical PD, rather than a nonspecific aging phenomenon. An alternative hypothesis that cannot be dispelled given the available evidence is that individuals with iLBD have inherent deficiencies in nigrostriatal dopaminergic and cardiac sympathetic innervation, as well as lower populations of neurons in vulnerable brainstem nuclei, and that these observations are unrelated to Lewy body pathology; but this seems unlikely.
The evidence of nigrostriatal dopaminergic TH immunoreactivity intermediate between normal and PD fits with previous studies that showed reduced nigral neuronal counts in iLBD compared to normal controls [41]. A recent study using an enzyme-linked immunoassay to measure TH immunoreactivity has also shown decreases in striatal TH in iLBD, similar to the results in the present study based upon image analytic methods [5]. In the present study, we did not detect statistically significant neuronal loss in iLBD cases in the dorsal motor nucleus of the vagus or substantia nigra compared to normals, but in all regions the average neuronal density tended to be lower, and in the locus ceruleus it reached statistical significance. The neuronal density measure used in the present study may not be the most accurate reflection of absolute neuronal counts, but given the retrospective nature of the analysis, it was impossible to perform counting with an unbiased stereologic method. The findings in the present study suggests that striatal dopaminergic deficiency occurs relatively early in the disease process at a time when there is not yet significant neuronal loss in the substantia nigra. This might indicate that initial pathology in the nigrostriatal dopaminergic pathway is in the distal termini of neurons projecting from the substantia nigra.
If indeed iLBD represents pre-symptomatic PD, the present results suggest that pathology in target zones of dopaminergic nerve fibers in the striatum and sympathetic nerve fibers in the heart are apparently below a threshold that would be symptomatic. The average decrease in TH in the striatum was about 30% of control levels. This level of reduction is less than the minimum decrease of 50% required for symptomatic PD, which is based upon calculations derived from imaging dopamine with positron emission tomography [10, 24, 28]. The subthreshold decreases in iLBD may account for the absence of parkinsonian symptoms. In contrast to iLBD, the PD cases exceeded this threshold (nearly 80% lower than normals), as might be expected in patients with moderately advanced to advanced PD.
In the PD-staging scheme proposed by Braak and co-workers the earliest pathological changes in the central nervous system are in the caudal brainstem, with absence of pathology in the substantia nigra. In the present study, some iLBD cases with stages 1–2 had decreased density of TH-immunoreactive nerve fibers in the epicardium. This result fits with a recent study by Orimo and co-workers who reported that 6 of 11 cases with PD Braak stage 2 had cardiac sympathetic denervation [39]. To the best of our knowledge, the partial cardiac sympathetic denervation detected in iLBD was not associated with cardiac dysfunction, although several iLBD cases (as well as PD cases) had cardiac arrhythmias. Given the prevalence of arrhythmias in the elderly, this finding was not statistically significant. In addition, none of the iLBD cases had clinical evidence of PD, as documented by careful review of the medical records, providing further evidence that cardiac sympathetic denervation occurs before symptomatic parkinsonism, at least in some patients.
Involvement of the peripheral autonomic nervous system is not taken into consideration in the original Braak PD staging scheme, but central autonomic nuclei in the caudal brainstem are proposed as targets of some of the earliest pathology [12]. It is becoming increasingly clear that the peripheral autonomic nervous system is affected early in the disease course and often before parkinsonian signs. Previous studies of iLBD cases have also documented peripheral autonomic involvement [8, 13, 21]. These results support previous notions that α-synuclein pathology may involve spinal cord and peripheral autonomic nervous system at about the same time that Lewy bodies are initially detected in the medulla. It is worth noting, however, that in a number of individuals with iLBD, α-synuclein pathology was detected simultaneously in multiple regions of central and peripheral nervous systems. Such cases might suggest that rather than a step-wise progression, α-synuclein pathology may be a multicentric process from it earliest stages.
There are several possible explanations for the intermediate values we found for iLBD between normals and PD. First, iLBD may represent a disorder that would have eventually progressed to PD had the person lived longer. On the other hand, if the rate of degeneration in iLBD is much slower than in PD, it is possible that within the maximum human lifespan it might never be symptomatic. Second, it may reflect a progressive process that was aborted and essentially a static finding. Third, it may reflect a single factor in a disorder requiring multiple converging factors to become clinically manifest.
References
Abbott RD, Petrovitch H, White LR, Masaki KH, Tanner CM, Curb JD, Grandinetti A, Blanchette PL, Popper JS, Ross GW (2001) Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology 57:456–462
Abbott RD, Ross GW, Petrovitch H, Tanner CM, Davis DG, Masaki KH, Launer LJ, Curb JD, White LR (2007) Bowel movement frequency in late-life and incidental Lewy bodies. Mov Disord [Epub ahead of print]
Beach TG, Tago H, Nagai T, Kimura H, McGeer PL, McGeer EG (1987) Perfusion-fixation of the human brain for immunohistochemistry: comparison with immersion-fixation. J Neurosci Methods 19:183–192
Beach TG, Walker DG, Sue LI, Newell A, Adler CC, Joyce JN (2004) Substantia nigra Marinesco bodies are associated with decreased striatal expression of dopaminergic markers. J Neuropathol Exp Neurol 63:329–337
Beach TG, Adler CH, Sue LI, Peirce JB, Bachalakuri J, Dalsing-Hernandez JE, Lue LF, Caviness JN, Connor DJ, Sabbagh MN, Walker DG (2008) Reduced striatal tyrosine hydroxylase in incidental Lewy body disease. Acta Neuropathol [Epub ahead of print]
Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS (2006) Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 66:1837–1844
Berendse HW, Booij J, Francot CM, Bergmans PL, Hijman R, Stoof JC, Wolters EC (2001) Subclinical dopaminergic dysfunction in asymptomatic Parkinson’s disease patients’ relatives with a decreased sense of smell. Ann Neurol 50:34–41
Bloch A, Probst A, Bissig H, Adams H, Tolnay M (2006) Alpha-synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol 32:284–295
Boeve BF, Dickson DW, Olson EJ, Shepard JW, Silber MH, Ferman TJ, Ahlskog JE, Benarroch EE (2007) Insights into REM sleep behavior disorder pathophysiology in brainstem-predominant Lewy body disease. Sleep Med 8:60–64
Booij J, Bergmans P, Winogrodzka A, Speelman JD, Wolters EC (2001) Imaging of dopamine transporters with [123I]FP-CIT SPECT does not suggest a significant effect of age on the symptomatic threshold of disease in Parkinson’s disease. Synapse 39:101–108
Booij J, Speelman JD, Horstink MW, Wolters EC (2001) The clinical benefit of imaging striatal dopamine transporters with [123I]FP-CIT SPET in differentiating patients with presynaptic parkinsonism from those with other forms of parkinsonism. Eur J Nucl Med 28:266–272
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Braak H, de Vos RA, Bohl J, Del Tredici K (2006) Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett 396:67–72
Braak H, Sastre M, Bohl JR, de Vos RA, Del Tredici K (2007) Parkinson’s disease: lesions in dorsal horn layer I, involvement of parasympathetic and sympathetic pre- and postganglionic neurons. Acta Neuropathol 113:421–429
Brooks DJ, Frey KA, Marek KL, Oakes D, Paty D, Prentice R, Shults CW, Stoessl AJ (2003) Assessment of neuroimaging techniques as biomarkers of the progression of Parkinson’s disease. Exp Neurol 184(Suppl 1):S68–S79
DelleDonne A, Tsuboi Y, Uchikado H, Ahmed Z, Mash DC, Dickson DW (2006) Tyrosine hydroxylase expression in the nigrostriatal pathway in Lewy body disease with and without dementia. Mov Disord 21(Suppl 15):S553
Del Tredici K, Rub U, De Vos RA, Bohl JR, Braak H (2002) Where does Parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol 61:413–426
Foltynie T, Matthews FE, Ishihara L, Brayne C (2006) The frequency and validity of self-reported diagnosis of Parkinson’s disease in the UK elderly: MRC CFAS cohort. BMC Neurol 6:29
Forno LS (1969) Concentric hyalin intraneuronal inclusions of Lewy type in the brains of elderly persons (50 incidental cases): relationship to parkinsonism. J Am Geriatr Soc 17:557–575
Fujishiro H, Frigerio R, Burnett M, Klos KJ, Josephs JA, DelleDonne A, Parisi JE, Ahlskog JE, Dickson DW (2008) Cardiac sympathetic denervation correlates with clinical and pathologic stages of Parkinson’s disease. Mov Disord 23 (in press)
Fumimura Y, Ikemura M, Saito Y, Sengoku R, Kanemaru K, Sawabe M, Arai T, Ito G, Iwatsubo T, Fukayama M, Mizusawa H, Murayama S (2007) Analysis of the adrenal gland is useful for evaluating pathology of the peripheral autonomic nervous system in Lewy body disease. J Neuropathol Exp Neurol 66:354–362
Gibb WR, Lees AJ (1988) The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 51:745–752
Gibb WR, Lees AJ (1989) The significance of the Lewy body in the diagnosis of idiopathic Parkinson’s disease. Neuropathol Appl Neurobiol 15:27–44
Guttman M, Burkholder J, Kish SJ, Hussey D, Wilson A, DaSilva J, Houle S (1997) [11C]RTI-32 PET studies of the dopamine transporter in early dopa-naive Parkinson’s disease: implications for the symptomatic threshold. Neurology 48:1578–1583
Hamada K, Hirayama M, Watanabe H, Kobayashi R, Ito H, Ieda T, Koike Y, Sobue G (2003) Onset age and severity of motor impairment are associated with reduction of myocardial 123I-MIBG uptake in Parkinson’s disease. J Neurol Neurosurg Psychiatry 74:423–426
Hattori T (1993) Conceptual history of the nigrostriatal dopamine system. Neurosci Res 16:239–262
Hawkes CH, Shephard BC, Daniel SE (1997) Olfactory dysfunction in Parkinson’s disease. J Neurol Neurosurg Psychiatry 62:436–446
Hilker R, Schweitzer K, Coburger S, Ghaemi M, Weisenbach S, Jacobs AH, Rudolf J, Herholz K, Heiss WD (2005) Nonlinear progression of Parkinson disease as determined by serial positron emission tomographic imaging of striatal fluorodopa F 18 activity. Arch Neurol 62:378–382
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Hughes AJ, Daniel SE, Blankson S, Lees AJ (1993) A clinicopathologic study of 100 cases of Parkinson’s disease. Arch Neurol 50:140–148
Jellinger KA (2004) Lewy body-related alpha-synucleinopathy in the aged human brain. J Neural Transm 111:1219–1235
Kawano H, Okada R, Yano K (2003) Histological study on the distribution of autonomic nerves in the human heart. Heart Vessels 18:32–39
Klos KJ, Ahlskog JE, Josephs KA, Apaydin H, Parisi JE, Boeve BF, DeLucia MW, Dickson DW (2006) Alpha-synuclein pathology in the spinal cords of neurologically asymptomatic aged individuals. Neurology 66:1100–1102
Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, Smith GE, Dickson DW, Johnson KA, Petersen LE, McDonald WC, Braak H, Petersen RC (2003) Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol 62:1087–1095
Mikolaenko I, Pletnikova O, Kawas CH, O’Brien R, Resnick SM, Crain B, Troncoso JC (2005) Alpha-synuclein lesions in normal aging, Parkinson disease, and Alzheimer disease: evidence from the Baltimore Longitudinal Study of Aging (BLSA). J Neuropathol Exp Neurol 64:156–162
Mitsui J, Saito Y, Momose T, Shimizu J, Arai N, Shibahara J, Ugawa Y, Kanazawa I, Tsuji S, Murayama S (2006) Pathology of the sympathetic nervous system corresponding to the decreased cardiac uptake in 123I-metaiodobenzylguanidine (MIBG) scintigraphy in a patient with Parkinson disease. J Neurol Sci 243:101–104
Miyamoto T, Miyamoto M, Inoue Y, Usui Y, Suzuki K, Hirata K (2006) Reduced cardiac 123I-MIBG scintigraphy in idiopathic REM sleep behavior disorder. Neurology 67:2236–2238
Orimo S, Ozawa E, Nakade S, Sugimoto T, Mizusawa H (1999) (123)I-metaiodobenzylguanidine myocardial scintigraphy in Parkinson’s disease. J Neurol Neurosurg Psychiatry 67:189–194
Orimo S, Amino T, Itoh Y, Takahashi A, Kojo T, Uchihara T, Tsuchiya K, Mori F, Wakabayashi K, Takahashi H (2005) Cardiac sympathetic denervation precedes neuronal loss in the sympathetic ganglia in Lewy body disease. Acta Neuropathol 109:583–588
Orimo S, Takahashi A, Uchihara T, Mori F, Kakita A, Wakabayashi K, Takahashi H (2007) Degeneration of cardiac sympathetic nerve begins in the early disease process of Parkinson’s disease. Brain Pathol 17:24–30
Ross GW, Petrovitch H, Abbott RD, Nelson J, Markesbery W, Davis D, Hardman J, Launer L, Masaki K, Tanner CM, White LR (2004) Parkinsonian signs and substantia nigra neuron density in descendents elders without PD. Ann Neurol 56:532–539
Schenck CH, Bundlie SR, Mahowald MW (1996) Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology 46:388–393
Stiasny-Kolster K, Doerr Y, Moller JC, Hoffken H, Behr TM, Oertel WH, Mayer G (2005) Combination of ‘idiopathic’ REM sleep behaviour disorder and olfactory dysfunction as possible indicator for alpha-synucleinopathy demonstrated by dopamine transporter FP-CIT-SPECT. Brain 128:126–137
Wakabayashi K, Takahashi H (1997) The intermediolateral nucleus and Clarke’s column in Parkinson’s disease. Acta Neuropathol 94:287–289
Wakabayashi K, Takahashi H (1997) Neuropathology of autonomic nervous system in Parkinson’s disease. Eur Neurol 38(Suppl 2):2–7
Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F (1988) Parkinson’s disease: the presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol 76:217–221
Acknowledgments
This study is supported by the Morris K. Udall Center for Excellence in Parkinson’s Disease Research at Mayo Clinic (NIH P50-NS40256-09). The authors acknowledge Michael Oelkers and Allison Kendall for their efforts in acquiring tissue for these studies through the Mayo Tissue Registry. The assistance of Virginia Phillips, Linda Rousseau and Monica Casey-Castanedes for histologic and immunohistochemistry studies is also greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dickson, D.W., Fujishiro, H., DelleDonne, A. et al. Evidence that incidental Lewy body disease is pre-symptomatic Parkinson’s disease. Acta Neuropathol 115, 437–444 (2008). https://doi.org/10.1007/s00401-008-0345-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-008-0345-7