Abstract
Obesity and diabetes are associated with higher cardiac vulnerability to ischemia–reperfusion (IR). The cardioprotective effect of regular exercise has been attributed to β3-adrenergic receptor (β3AR) stimulation and increased endothelial nitric oxide synthase (eNOS) activation. Here, we evaluated the role of the β3AR–eNOS pathway and NOS isoforms in exercise-induced cardioprotection of C57Bl6 mice fed with high fat and sucrose diet (HFS) for 12 weeks and subjected or not to exercise training during the last 4 weeks (HFS-Ex). HFS animals were more sensitive to in vivo and ex vivo IR injuries than control (normal diet) and HFS-Ex mice. Cardioprotection in HFS-Ex mice was not associated with increased myocardial eNOS activation and NO metabolites storage, possibly due to the β3AR–eNOS pathway functional loss in their heart. Indeed, a selective β3AR agonist (BRL37344) increased eNOS activation and had a protective effect against IR in control, but not in HFS hearts. Moreover, iNOS expression, nitro-oxidative stress (protein s-nitrosylation and nitrotyrosination) and ROS production during early reperfusion were increased in HFS, but not in control mice. Exercise normalized iNOS level and reduced protein s-nitrosylation, nitrotyrosination and ROS production in HFS-Ex hearts during early reperfusion. The iNOS inhibitor 1400 W reduced in vivo infarct size in HFS mice to control levels, supporting the potential role of iNOS normalization in the cardioprotective effects of exercise training in HFS-Ex mice. Although the β3AR–eNOS pathway is defective in the heart of HFS mice, regular exercise can protect their heart against IR by reducing iNOS expression and nitro-oxidative stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic heart disease is a leading cause of death worldwide. Heart reperfusion is the most effective way to limit infarct size; however, post-ischemic reperfusion is associated with detrimental effects, such as myocardial stunning, ventricular arrhythmias, microvascular dysfunction and cell death. The risk of heart disease and ischemic events increases greatly with metabolic disorders that are predictive of higher morbidity and mortality after acute coronary syndrome [25]. Metabolic disorders associated with obesity and type 2 diabetes are characterized by abdominal obesity, elevated fasting blood glucose and insulin, high serum triglycerides and low high density cholesterol. Obese diabetic subjects develop coronary atherosclerosis, a major cause of thrombus, and are also more vulnerable to ischemia reperfusion (IR) injuries [1, 20, 32]. Moreover, in these patients, cardioprotective strategies are less or not effective (for review see [9]). The underlying mechanisms are not fully understood, but hypercholesterolemia, hyperlipidemia [36], insulin-resistance [28] and increased oxidative/nitro-oxidative stress [45, 49] could affect the severity of IR injuries.
Exercise training is a well-described cardioprotective strategy in healthy subjects [38]. Moderate exercise training is also highly recommended in patients with metabolic disorders for the prevention and rehabilitation of cardiovascular diseases. In obese and type 2 diabetic subjects, exercise training has beneficial effects on blood hypertension, insulin resistance, diabetes, dyslipidemia and obesity. However, few studies investigated whether exercise training can modulate the metabolic disorder-induced vulnerability to IR [27] and restore cardioprotective signaling pathways [37]. In healthy animal models, exercise training protects the heart against IR injury by activating catecholaminergic-dependent β3-adrenergic receptors (β3AR) [7]. In the heart, activated β3AR mobilize endothelial NO synthase (eNOS) that increases nitric oxide (NO) production and the subsequent storage pool of NO metabolites [7, 12]. In healthy subjects, β3AR agonists, such as BRL37344, can mimic exercise beneficial effects and reduce the severity of IR injuries without exercise training [7, 12].
However, it is not known whether the β3AR–eNOS–NO pathway can limit the severity of IR injuries also in the heart of obese diabetic subjects. The aim of the present study was to address this question by using a mouse model of diet-induced obesity and type 2 diabetes and to investigate the effects of exercise and of the β3AR pathway in protecting the heart against IR injuries.
Methods
Detailed information on the methodology is available in Supplemental Information.
Animal model
All investigations conformed to the European Parliament Directive 2010/63/EU and were approved by the local research ethics committee (n. 00,322.03). Male C57BL/6 mice (8-week-old, Janvier Laboratories, France) were randomly assigned to the control group (Ctrl, n = 35) fed with a standard diet (A04, SAFE, France) or to the group that received a high fat/high sucrose (HFS) diet (230 HF, SAFE, France, completed with 10 % sucrose in drinking water; HFS group, n = 75) for 12 weeks to induce obesity and type 2 diabetes. After 8 weeks of HFS diet, HFS mice were randomly assigned to the sedentary (HFS, n = 45) or to the endurance exercise group (HFS-Ex, n = 30). The exercise program consisted in treadmill running once per day, five times a week for 4 weeks at 60 % of their maximal aerobic velocity (MAV).
Blood glucose, insulin and glucose tolerance test (IPGTT)
After 12 weeks of HFS diet, fasted blood glucose and blood insulin were measured. First, 50 µl blood was collected by using the tail-clip method to assess the fasting blood glucose level (Caresens® N, DinnoSante™) and blood insulin (Rat/Mouse Insulin ELISA kit, Millipore) according to the manufacturer’s instructions. Then, mice received an intraperitoneal injection of glucose solution (1.5 g/Kg) and blood glucose was measured at 20, 60 and 120 min after the injection.
In vivo ischemia reperfusion protocol
In vivo ischemia/reperfusion was performed as previously described [4]. Briefly, after anesthesia, mice were ventilated, a thoracotomy was performed to expose the interventricular coronary artery that was subsequently ligated for 45 min, followed by 90 min of reperfusion. Infarct size was determined by using the 2,3,5-Triphenyltetrazolium chloride (TTC) staining method. To assess iNOS role in heart sensitivity to IR injuries in HFS mice, some HFS mice received or not 10 mg/kg 1400 W, a potent iNOS inhibitor, by intraperitoneal injection (i.p.) 10 min prior ligation.
In vivo assessment of left intraventricular pressure
Mice were anesthetized with isoflurane, intubated and ventilated with a rodent ventilator (SAR 830 Bioseb) with 2 % isoflurane with a tidal volume of 0.2 mL and a breath rate of 160 min−1. Then, a thoracotomy was performed and a Millar pressure transducer (Millar Mikro-Tip®) was inserted in the apex of the left ventricle (LV). After 10 min of stabilization, an intravenous bolus injection of 5 µg/kg BRL37344 was performed and cardiac function was then monitored for 20 min.
Isolated heart perfusion and global myocardial ischemia/reperfusion protocol
After anesthesia (60 mg/kg sodium pentobarbital, i.p.) and the total loss of consciousness, hearts were isolated and mounted on a Langendorff apparatus, perfused with Krebs solution (118 mMNaCl; 5.3 mMKCl; 1.2 mM MgSO4; 2.5 mM NaHCO3; 10 mM glucose; 2.25 mM CaCl2; 0.5 mM pyruvate; 0.5 mM EDTA) at 37 °C, and submitted to global no-flow ischemia and reperfusion, as described [8]. Hearts were paced at a rate of 420 beats/min (Low voltage stimulator, BSL MP35 SS58L, 3V) and a non-compliant balloon was inserted in the LV to monitor the LV pressure all along the procedure. Infarct size was determined by TTC staining [8]. To assess the role of the β3AR–eNOS–NO pathway in cardioprotection, some hearts were perfused with 0.1 µM BRL37344, a selective β3AR agonist, five min before and after ischemia (Fig. 2a). Finally, the role of oxidative stress during IR was evaluated by treating HFS mice with an amphiphilic spin-trap derived from phenyl-butyl-nitrone (0.5 mg/kg LPBNAH by i.p.) for five consecutive days before the IR procedure.
Antioxidant enzyme activity
Cardiac superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT) activity were assessed as previously described [30].
Nitrite and protein S-nitrosylation measurement
Quantification of LV nitrite and protein S-nitrosylation (SNO) were determined as previously described [8].
Nitrotyrosine measurements
The level of 3-nitrotyrosine-modified proteins in LV homogenates was determined with an ELISA assay kit (Abcam) according to the manufacturer’s instructions.
Measurement of total reactive oxygen species production
Reactive oxygen species (ROS) production after 10 min of reperfusion was measured by electron paramagnetic resonance (EPR) as described [10].
Western blot analysis
Proteins were separated by gel electrophoresis and transferred onto PVDF membranes. Then, membranes were blocked in 5 % milk, 10 % milk or 3 % bovine serum albumin (BSA), depending of the antibody, in Tris-Buffered Saline solution with 0.05 % Tween-20. Primary antibodies were: anti-mouse eNOS (1/1000, 1 % milk, BD Biosciences), anti-mouse eNOS-Pser1177 (1/1000, 1 % milk, BD Biosciences), anti-rabbit β1AR (1/1000, 3 % BSA, Santa Cruz), anti-rabbit β2AR (1/500, 3 % BSA, Santa Cruz), anti-chicken β3AR (1/2000, 5 % milk, Abcam), anti-rabbit caspase 3 (1/500, 5 % milk, Cell Signaling), anti-rabbit AKT-Pser473 (1/500, 5 % BSA, Cell Signaling), anti-rabbit AKT (1/1000, 5 % BSA, Cell Signaling), anti-rabbit GAPDH (1/3000, 3 % BSA, Santa Cruz) and anti-rabbit tubulin (1/3000, 3 % BSA, Santa Cruz).
Statistical analysis
Data were expressed as the mean ± SEM. For comparison of multiple experimental conditions, analysis of variance (ANOVA) or repeated ANOVA were used, followed by the Bonferroni adjusted t test. For assessing the difference between values, the Student’s t test was used. A value of p < 0.05 was considered statistically significant.
Results
The HFS diet induces obesity and type 2 diabetes in mice
At the end of the 12 weeks of HFS diet, body weight (+30 %) and the percentage of adipose tissue (four times) were significantly higher in HFS mice than in Ctrl mice (Table 1). Moreover, HFS mice were diabetic. Blood glucose measured after overnight fasting was markedly higher in HFS than Ctrl mice (Table 1) and remained higher throughout the 2-h IPGTT, as indicated by the higher area under the curve (Table 1). Insulin level measured after overnight fasting also was significantly more elevated in HFS than in Ctrl mice (Table 1). Accordingly, the Homeostatic Model Assessment of Insulin Resistance score (HOMA-IR) was higher in HFS than in Ctrl animals (Table 1). These results indicate that HFS mice are a reliable obese diabetic mouse model. Moreover, the efficiency of exercise training in the HFS-Ex group was confirmed by the higher maximal aerobic velocity in HFS-Ex mice than in sedentary HFS animals (HFS: 28 ± 1 m.min−1; HFS-Ex: 37 ± 2 m.min−1; p < 0.05). Exercise training stabilized body weight (HFS: 42.9 ± 1.4 g; HFS-Ex: 36.8 ± 3.6 g; p < 0.05). This weight difference was explained by the lower percentage of adipose tissue in HFS-Ex mice (HFS: 22.2 ± 0.3 %; HFS-Ex: 19.1 ± 0.3 %; p < 0.05). Exercise training also reduced the difference between Ctrl and HFS mice concerning fasting blood glucose but the difference between HFS and HFS-Ex mice did not reach significance (HFS: 164.4 ± 8.7 mg dL−1; HFS-Ex: 142.9 ± 12.6 mg dL−1; p = 0.11). Finally, the glycemic response to IPGTT in HFS mice was not altered by our exercise training program (HFS: 25,767 ± 2763 AUC; HFS-Ex: 28,498 ± 1870 AUC; NS).
Exercise training protects the heart of HFS mice by a mechanism independent of eNOS and NO metabolite storage
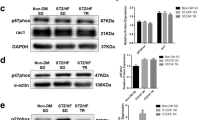
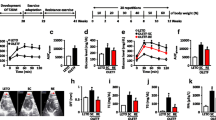
In our conditions, HFS mice were more vulnerable to in vivo IR than Ctrl animals (Fig. 1a). Indeed, although the extent of ischemia was similar between groups (Fig. 1a, middle panel), infarct size was bigger in HFS than in Ctrl animals (Fig. 1a, right panel). These results were confirmed in isolated hearts after ex vivo IR. Specifically, recovery of LV developed pressure (LVDevP) during post-ischemic reperfusion was lower in HFS than in Ctrl animals (Fig. 1b) and recovery of myocardial perfusion, indexed by coronary flow, tended to be lower in HFS animals (Fig. 1c). Finally, infarct size was higher in HFS than in Ctrl hearts by 31 % (Fig. 1d). Exercise training prevented the deleterious effect of obesity on cardiac vulnerability to IR. In vivo, the extent of ischemia was comparable between HFS and HFS-Ex mice (Fig. 1a, middle panel). However, compared with HFS mice, infarct size in HFS-Ex animals was significantly reduced to the size observed in the Ctrl group (Fig. 1a, right panel). Exercise cardioprotective effect was also observed in isolated hearts. The recovery of LVDevP (Fig. 1b) and coronary flow (Fig. 1c) was higher and infarct size was smaller in HFS-Ex hearts, than in HFS hearts (Fig. 1d). It has been reported that exercise-induced cardioprotection mainly depends on eNOS [7, 8]. Activation of eNOS is regulated by its homodimerization and the balance between phosphorylation of the activation site (serine 1177) and the inhibitory site (threonine 495). Consistently with previous reports [7, 8], our exercise training protocol increased eNOS phosphorylation on ser1177 in healthy mice (Suppl Fig. 1a) and reduced infarct size (Suppl Fig. 1b). In basal conditions, eNOS concentration was increased in HFS hearts (Fig. 2a). The level of eNOS-Pser1177 relative to total eNOS was higher in HFS hearts by 136 %, which could suggest increased activation (Fig. 2a). However, the level of eNOS-Pthr495 also was increased by 146 % in HFS mice compared with Ctrl mice (Fig. 2a). Conversely, eNOS dimer/monomer ratio was decreased in HFS hearts compared with Ctrl, suggesting eNOS uncoupling and, potentially, generation of superoxide anion (O2 −) instead of NO (Fig. 2b). Exercise training activated eNOS in healthy mice (Suppl Fig. 1a). In HFS-Ex mice, exercise training reduced both eNOS-Pthr495 and eNOS-Pser1177 levels (Fig. 2a) and had no effect on eNOS dimerization (Fig. 2b). Altogether, these results indicate that exercise training in HFS mice did not activate eNOS. It has been recently reported that nNOS plays a key role in the beneficial effect of exercise training on the heart antioxidant properties [40] and in some cardioprotective strategies [29, 48]. The level of nNOS was reduced in HFS hearts compared with Ctrl (Fig. 2c). Exercise training did not normalize nNOS expression in HFS-Ex heart and even exacerbated this reduction (Fig. 2c). As NO availability is critical during IR and is considered to be a main trigger of exercise-induced cardioprotection [7], NO metabolite storage was analyzed by measuring nitrite content in LV homogenates. HFS diet increased nitrite concentration (Fig. 2d) compared with Ctrl. Moreover, exercise training did not have any effect on LV nitrite concentration (Fig. 2d). Altogether, these results indicate that the beneficial effect of exercise training on IR injuries in HFS-Ex animals are not related to an increase in eNOS activation, nNOS expression and NO metabolites storage, differently from what observed in healthy animals [7, 8].
Exercise training reduces heart vulnerability to ischemia reperfusion in a mouse model of obesity and type 2 diabetes. a Left panel transverse heart sections from Ctrl, HFS and HFS-Ex mice after in vivo regional ischemia reperfusion. The area at risk is indicated by the absence of Evans blue staining and the remaining ischemic viable myocardium is stained in red by TTC staining; middle panel, quantification of the area at risk after in vivo regional ischemia (40 min) and reperfusion (85 min) in Ctrl, HFS and HFS-Ex mice; right panel, effect of HFS diet with or without exercise training on the cardiac infarct size (expressed relative to the area at risk) after in vivo regional ischemia (40 min) and reperfusion (85 min). b Left panel left ventricular (LV) pressure trace in hearts mounted on a Langendorff apparatus during ischemia/reperfusion; right panel, quantification of left ventricular developed pressure recovery after 30 min of post-ischemic reperfusion in Ctrl, HFS and HFS-Ex hearts. c Effect of HFS diet with or without exercise training on coronary flow recovery after 30 min of post-ischemic reperfusion. d Effect of HFS diet with or without exercise training on cardiac infarct size after 2 h of post-ischemic reperfusion with TTC staining. *p < 0.05 HFS vs Ctrl, #p < 0.05 HFS-Ex vs HFS. In vivo experiments: Ctrl, n = 9; HFS: n = 7; HFS-Ex: n = 8; Ex-vivo experiments: n = 6 in each group
Exercise training does not increase eNOS activation and NO metabolites in the heart of HFS mice. a eNOS expression, phosphorylation at Ser1177 (eNOS-Pser1177) and Thr495 (eNOS-Pthr495) analyzed by western blotting in hearts of Ctrl, HFS and HFS-Ex mice. eNOS is expressed relative to GAPDH content and eNOS-Pser1177 and eNOS-Pthr495 relative to total eNOS content. b eNOS dimer/monomer ratio analyzed by detecting SDS-resistant eNOS dimers using low-temperature SDS-PAGE in hearts of Ctrl, HFS and HFS-Ex mice. c nNOS expression analyzed by western blotting in hearts of Ctrl, HFS and HFS-Ex mice. nNOS is expressed relative to tubulin content. d Nitrite concentration in hearts of Ctrl, HFS and HFS-Ex mice. *p < 0.05 HFS vs Ctrl, #p < 0.05 HFS-Ex vs HFS. n = 5 in each group
Stimulation of β3 adrenergic receptors is not associated with eNOS activation and cardioprotection in HFS mice
Activation of eNOS and increased NO metabolite storage by exercise is regulated by adrenergic-dependent activation of β3AR [7]. Therefore, we evaluated in vivo whether intravenous injection of epinephrine in HFS mice could modulate eNOS-Pser1177. Epinephrine increased eNOS-Pser1177 level in Ctrl mice, but not in HFS mice (Suppl Fig. 2a, b). As adrenergic stress during exercise is mediated by the eNOS-NO pathway after β3AR activation, we next focused on the β3AR–eNOS pathway. To explore the functionality of the β3AR–eNOS pathway in HFS mice, we evaluated the in vivo effect of BRL37344, a potent and selective β3AR agonist, on LV developed pressure (LVDevP). As previously reported [43], LVDevP tended to decrease in Ctrl mice after BRL37344 treatment. Conversely, it significantly increased in HFS mice (Fig. 3a) 5 and 10 min after BRL37344 injection (Fig. 3a). The β3AR-eNOS pathway induces eNOS activation in exercised healthy rodents [2] and also reduces heart injuries during IR [12, 18]. To determine whether β3AR activation during IR could protect the heart of HFS mice, isolated Langendorff hearts were perfused with BRL37344, 5 min before and after ischemia. In Ctrl mice, β3AR activation reduced the infarct size by 22 % (Fig. 3b) and tended to increase coronary flow recovery (p = 0.08, Suppl Fig. 3), in line with previous report [2]. Conversely, BRL37344 did not have any protective effect on infarct size and coronary flow recovery in HFS hearts (Fig. 3b). Activation of the eNOS-NO pathway by β3AR is mediated by AKT [12]. The level of AKT phosphorylated at Ser 473 (AKT-Pser473; activating phosphorylation) was similar in untreated HFS and Ctrl mice (Suppl Fig. 4). Conversely, BRL37344 perfusion increased AKT-Pser473 level only in Ctrl mice (Suppl Fig. 4). In agreement with this result, the levels of eNOS-Pser1177 and of nitrite significantly increased in Ctlr hearts and decreased in HFS hearts perfused with BRL37344 compared with untreated hearts (Fig. 3c, d). Finally, β1AR and β2AR expression levels (assessed by western blot analysis) were comparable in HFS and Ctrl hearts (Suppl Fig. 5), whereas β3AR level was reduced in the myocardium of HFS and HFS-Ex mice (Fig. 3e). This result could explain the lack of BRL37344 beneficial effect in HFS hearts. Taken together, these findings indicate that the β3AR-eNOS-NO pathway is defective in HFS mice and, therefore, cannot mediate the cardioprotective effect induced by exercise in HFS-Ex mice.
Stimulation of β3-adrenergic receptors with BRL37344 does not activate eNOS in the heart of HFS mice. a Left panel representative trace of the effect of β3-adrenergic receptor stimulation by BRL37344 on the left intra-ventricular pressure; right panel, quantification of the changes in LV developed pressure 1, 5, 10 and 15 min after a bolus injection of BRL37344 (5 µg kg−1, iv) to stimulate β3-adrenergic receptors. b Effect of β3-adrenergic receptor stimulation on cardiac infarct size after 2 h of post-ischemic reperfusion; TTC staining of Ctrl and HFS hearts. c Effect of BRL37344 on eNOS phosphorylation at serine 1177 (eNOS-Pser1177) analyzed by western blotting in LV homogenates from Ctrl and HFS mice. eNOS-Pser1177 is expressed relative to total eNOS content. d Nitrite concentration in LV homogenates from Ctrl and HFS after treatment with BRL37344 or not. e β3 adrenergic receptor (β3AR) expression was analyzed by western blotting in LV homogenates of Ctrl, HFS and HFS-Ex mice. β3AR is expressed relative to GAPDH content. *p < 0.05 HFS vs Ctrl; £p < 0.05 BRL37344-treated vs non-treated. n = 5 in each group
Exercise training cardioprotection in HFS mice is mediated by iNOS
Cardiac vulnerability to IR could be modulated by inducible NOS (iNOS) [29, 40, 48], although its role during IR remains controversial. Mild iNOS upregulation is beneficial in late cardiac pre-conditioning [15], whereas severe and/or chronic iNOS upregulation can be detrimental [14, 29]. The high nitrite level observed in HFS mice (Fig. 2d) could not be explained by higher eNOS activation and nNOS expression. Therefore, we evaluated the effect of the HFS diet with or without exercise training on iNOS expression. The level of iNOS was increased by 95 % in HFS hearts compared with Ctrl (Fig. 4a). As high iNOS level is classically associated with nitrosative stress [13], we assessed nitrosative stress by measuring the level of protein S-nitrosylation and nitrotyrosination (an index of peroxynitrite formation). Protein S-nitrosylation tended to be higher (Fig. 4b) and protein nitrotyrosination (Fig. 4c) was significantly higher in HFS mice than in Ctrl. Exercise training normalized iNOS content in HFS-Ex hearts to the level observed in Ctrl hearts (Fig. 4a) and reduced protein s-nitrosylation (Fig. 4b) and nitrotyrosination (Fig. 4c). As ROS and activated caspase 3 mediate the deleterious effects of iNOS during IR [46, 48], we measured ROS production and caspase 3 activation after 10 min of reperfusion. HFS mice, but not HFS-Ex animals, produced more ROS than Ctrl mice (Fig. 4d). Similarly, active caspase 3 level was higher in HFS hearts, whereas in HFS-Ex hearts its level was comparable to that of Ctrl mice (Fig. 4e). Finally, to further explore iNOS involvement in the protective effects of exercise training in HFS hearts, we blocked iNOS in vivo by treating HFS mice with the potent and specific iNOS inhibitor 1400 W, 10 min before ischemia. Blocking iNOS in vivo before IR markedly reduced heart vulnerability in HFS mice to the level observed in Ctrl animals (Ctrl mice infarct size: 45.7 ± 1.8 % of area at risk; HFS + 1400 W mice: 36.2 ± 4.2 % of area at risk; NS). Indeed, although the extent of ischemia (area at risk) was similar between groups (Fig. 4f, middle panel), infarct size was smaller in treated than untreated HFS mice (Fig. 4f, right panel). Altogether, these results highlight the key role of iNOS and nitro-oxidative stress in the higher vulnerability of HFS heart to IR, and strongly suggest that exercise-induced cardioprotection in HFS-Ex mice is mediated by iNOS normalization and subsequent decrease nitro-oxidative stress.
Exercise protects the heart of HFS mice through iNOS/nitro-oxidative stress normalization. a iNOS expression analyzed by western blotting in hearts of Ctrl, HFS and HFS-Ex mice. iNOS content is expressed relative to tubulin level. b Protein S-nitrosylation analyzed by western blotting using the switch assay method with the iodoTMT reagents in LV homogenates of Ctrl, HFS and HFS-Ex mice. c Level of 3-nitrotyrosine-modified proteins determined by ELISA assay. d Total ROS production measured by electron paramagnetic resonance in fresh frozen LV of Ctrl, HFS and HFS-Ex mice after 10 min of post-ischemic reperfusion. e Caspase 3 activation measured by western blotting in LV homogenates of Ctrl, HFS and HFS-Ex mice after 10 min of post-ischemic reperfusion. Cleaved caspase 3 (active form) is expressed relative to total caspase 3 content. f In vivo regional ischemia (40 min) and reperfusion (85 min) performed in HFS mice treated or not with the iNOS inhibitor 1400 W (10 mg/kg, i.p.) 10 min before ischemia. Left panel, representative images of transverse heart sections. The area at risk is demarcated by the absence of Evans blue staining and the remaining ischemic viable myocardium is stained in red by TTC staining; middle panel, average area at risk expressed relative to the LV area; right panel, infarct size, expressed relative to the area at risk.*p < 0.05 HFS vs Ctrl; #p < 0.05 HFS-Ex vs HFS; $p < 0.05 HFS + 1400 W vs HFS. Biochemical assay: n = 5 in each group; in vivo experiments: HFS + vehicle, n = 7; HFS + 1400 W: n = 9
Discussion
Activation of the β3AR signaling pathway has been recently linked to various cardioprotective strategies against ischemic myocardial injuries, including the use of nebivolol [2], BRL37344 [7, 12] and exercise training [7]. This effect has been mainly explained by eNOS activation [2, 7]. However, the role of the β3AR pathway in cardioprotection of obese diabetic mice has never been investigated. Here, we show, using a diet-induced obese diabetic mouse model (HFS mice), that the β3AR-eNOS signaling pathway is deficient in HFS mice and thus cannot protect the heart against IR. However, exercise still constitutes a cardioprotective strategy against IR damage in HFS mice mainly through iNOS expression normalization and nitro-oxidative stress reduction.
Animal models with metabolic disorders, such as obesity, metabolic syndrome or type 2 diabetes, are more sensitive to ischemic stress and less or not responsive to cardioprotective strategies [9, 39]. Accordingly [7], we found that in healthy mice, β3AR-eNOS pathway stimulation protected the heart against IR and was associated with eNOS phosphorylation on its activation site (eNOS-Pser1177). Conversely, in HFS mice, the β3AR agonist BRL37344 did not protect the heart during IR and eNOS-Pser1177 level was not increased by BRL37344, exercise training or intravenous injection of epinephrine. To the best of our knowledge, there is no data available on the effect of type 2 diabetes on β3AR level in the heart. However, in line with our results, in ob/ob mice β3AR mRNA level is decreased by 78 % in cardiac myocytes compared with wild type mice [22]. Conversely, in a rat model of type 1 diabetes, obtained by a single injection of streptozotocin, β3AR expression was increased [5]. However, in type 1 (or insulin-dependent) diabetes, which results from the destruction of insulin-producing pancreatic cells, hyperglycemia is not associated with hyperinsulinemia. In our model, HFS-induced type 2 diabetes was associated with fasting hyperinsulinemia. As insulin can reduce β3AR mRNA expression in a dose-dependent manner in adipocytes [10, 16], we can hypothesize that such mechanism could be involved in β3AR reduction in the heart of HFS mice. Further studies are needed to confirm this hypothesis.
As the β3AR-eNOS pathway is defective in HFS animals, all potential cardioprotective strategies that target this receptor [7, 12] are certainly not effective in this model. Indeed, exercise training could not normalize β3AR expression in HFS mice. Moreover, exercise training and epinephrine injection did not lead to increased eNOS-Pser1177 level in HFS mice. As a consequence, the storage pool of NO metabolites, such as cardiac nitrite content and SNO level, was not modified by exercise in HFS mice, differently from what we previously observed in healthy animals [19]. Similar results were obtained in β3AR-deficient mice, in which eNOS-Pser1177 and NO metabolite storage pool did increase following exercise training, thus resulting in the loss of cardioprotection [7]. However, although exercise did not activate the β3AR-eNOS-NO pathway, it still reduced heart vulnerability to IR in HFS-Ex mice.
Exercise training could regulate the expression/activity of other NOS isoforms. For instance, higher nNOS expression [2] or activation [35, 44] is associated with β3AR-dependent cardioprotection. In addition, nNOS plays an essential role against eNOS uncoupling [21]. However, nNOS was reduced in HFS hearts and exercise did not prevent this effect, in line with the decreased β3AR expression and reduced eNOS dimerization in these hearts. Therefore, it is very unlikely that nNOS might contribute to the protective effect of exercise training against IR injuries in HFS mice. Although eNOS activation and nNOS expression were reduced in HFS hearts, NO metabolite storage was increased. These results fit well with higher iNOS levels, as classically reported in diabetic hearts [41, 42] and also in our model. Indeed, iNOS produces large amounts of NO, up to 100-fold higher than the normal levels observed in cardiac cells. This leads to high levels of nitro-oxidative stress in a variety of cell types and tissues, as detected in the heart of our obese diabetic mice. Moreover, iNOS-derived high NO level could promote mitochondrial ROS production during IR [48], as observed in the heart of HFS mice during early reperfusion. Many studies reported that mild increase of iNOS expression contribute to cardioprotection during IR [26, 47]. Indeed, ischemic pre-conditioning is associated with selective iNOS upregulation [47] and iNOS expression in cardiac myocytes protects against IR [26]. These results provide evidence for a key role of iNOS in the cardioprotection linked to ischemic pre-conditioning. Although iNOS role in limiting IR injuries in healthy rodent is clearly acknowledged, its function in animal models with metabolic disorders is not. Differences regarding the chronicity and level of iNOS expression may explain the discrepancies between healthy animals and mice with metabolic disorders. Indeed, it is recognized in the literature that mild iNOS upregulation could be beneficial against IR injuries, whereas high iNOS levels are deleterious [6]. Ischemic pre-conditioning increases only acutely and modestly iNOS total content in the ischemic-reperfused region [6, 47]. Conversely, chronic high iNOS expression in animals with chronic metabolic disorders is associated with increased nitro-oxidative stress [46], increased ROS production [49] and consequently higher heart vulnerability to IR [19, 29]. Therefore, our finding that normalization of iNOS levels, which are increased by 95 % in HFS hearts compared with Ctrl, by exercise or by administration of the iNOS inhibitor 1400 W reduces HFS heart vulnerability to IR clearly constitute a major finding of this work. This emphasizes iNOS key role in heart vulnerability to IR in mice with metabolic disorders.
As nitro-oxidative stress is the result of the reaction between ROS and NO, the beneficial effects of exercise training could also be partly explained by the higher antioxidant properties of HFS-Ex hearts. Indeed, before the discovery of the eNOS pathway role in cardioprotection [7, 8], exercise-induced cardioprotection was mainly attributed to the increased myocardial antioxidant capacity [38]. Here, we found that exercise training in HFS mice results in higher cardiac activities of catalase (CAT) and glutathione peroxidase (GPx), with no effect on superoxide dismutase (Suppl Fig. 6a). Thus, the exercise-induced higher antioxidant activities could prevent the reaction between ROS and NO to form peroxynitrite and also ROS scavenging during IR. Excessive ROS production activates apoptosis in the heart [17], while increased antioxidant defenses reduce apoptosis activation during IR [11, 34]. Thus, targeting the oxidative stress pathway may also be of importance in obese diabetic mice, as indicated by the finding that HFS mice treated with a ROS scavenger (LPBNAH) for 5 days are less sensitive to IR (Suppl Fig. 6b). This is in agreement with previous results showing that in animals with metabolic disorders, antioxidant treatments can reduce post-IR cardiac injuries [24, 33], while pre- and post-conditioning agents are less or not effective [25].
Finally, exercise-induced cardioprotection in HFS-Ex mice could also be explained by its effects on the lipid and glucose/insulin metabolism. Indeed, hyperlipidemia could be a potential contributor to heart sensitivity to IR [9]. However, considering that we performed IR also in isolated hearts, it does not seem to be a key factor. Nevertheless, we cannot exclude that chronic hyperlipidemia in HFS mice might have increased the intracellular accumulation of lipid droplets that could modulate heart sensitivity to IR [3, 23]. Insulin resistance also could partially explain the higher vulnerability to IR injuries of HFS mice [31]. The finding that HFS mice show high fasting blood glucose associated with hyperinsulinemia and altered response to IPGTT suggests that our model is also characterized by insulin-resistance. Moderate exercise training in HFS-Ex mice increased the maximal aerobic velocity and reduced obesity, but only slightly modulated fasting blood glucose level and had no effect on the IPGTT. Thus, HFS-Ex mice were less obese, but remained glucose intolerant. Although we cannot exclude that such minor correction of metabolic disorders could contribute to the exercise-mediated normalization of the heart sensitivity to IR in HFS mice, a major role seems unlikely.
In conclusion, we report that the β3AR-eNOS-NO signaling pathway is deficient in obese diabetic mice and thus cannot protect the heart against IR. However, exercise is still an effective cardioprotective strategy in HFS mice, butof the β3AR-eNOS-NO signaling pathway. Exercise in obese and diabetic mice reduces iNOS content and consequently nitro-oxidative stress and ROS production/caspase 3 activation during early reperfusion. This result emphasizes iNOS key role in the heart of obese diabetic mice during IR and the cardioprotective function of exercise training through iNOS/nitro-oxidative stress modulation in obese diabetic mice.
References
Alegria JR, Miller TD, Gibbons RJ, Yi QL, Yusuf S, Collaborative Organization of RheothRx Evaluation (CORE) Trial Investigators (2007) Infarct size, ejection fraction, and mortality in diabetic patients with acute myocardial infarction treated with thrombolytic therapy. Am Heart J 154:743–750. doi:10.1016/j.ahj.2007.06.020
Aragón JP, Condit ME, Bhushan S, Predmore BL, Patel SS, Grinsfelder DB, Gundewar S, Jha S, Calvert JW, Barouch LA, Lavu M, Wright HM, Lefer DJ (2011) β3-adrenoreceptor stimulation ameliorates myocardial ischemia-reperfusion injury via endothelial nitric oxide synthase and neuronal nitric oxide synthase activation. J Am Coll Cardiol 58:2683–2691. doi:10.1016/j.jacc.2011.09.033
Barba I, Chavarria L, Ruiz-Meana M, Mirabet M, Agulló E, Garcia-Dorado D (2009) Effect of intracellular lipid droplets on cytosolic Ca2+ and cell death during ischaemia-reperfusion injury in cardiomyocytes. J Physiol 587:1331–1341. doi:10.1113/jphysiol.2008.163311
Belaidi E, Thomas A, Bourdier G, Moulin S, Lemarié E, Levy P, Pépin JL, Korichneva I, Godin-Ribuot D, Arnaud C (2016) Endoplasmic reticulum stress as a novel inducer of hypoxia inducible factor-1 activity: its role in the susceptibility to myocardial ischemia-reperfusion induced by chronic intermittent hypoxia. Int J Cardiol 210:45–53. doi:10.1016/j.ijcard.2016.02.096
Birenbaum A, Tesse A, Loyer X, Michelet P, Andriantsitohaina R, Heymes C, Riou B, Amour J (2008) Involvement of β3-adrenoceptor in altered beta-adrenergic response in senescent heart: role of nitric oxide synthase 1-derived nitric oxide. Anesthesiology 109:1045–1053. doi:10.1097/ALN.0b013e31818d7e5a
Bolli R (2007) Preconditioning: a paradigm shift in the biology of myocardial ischemia. Am J Physiol Heart Circ Physiol 292:H19–H27. doi:10.1152/ajpheart.00712.2006
Calvert JW, Condit ME, Aragón JP, Nicholson CK, Moody BF, Hood RL, Sindler AL, Gundewar S, Seals DR, Barouch LA, Lefer DJ (2011) Exercise protects against myocardial ischemia-reperfusion injury via stimulation of β3-adrenergic receptors and increased nitric oxide signaling: role of nitrite and nitrosothiols. Circ Res 108:1448–1458. doi:10.1161/CIRCRESAHA.111.241117
Farah C, Kleindienst A, Bolea G, Meyer G, Gayrard S, Geny B, Obert P, Cazorla O, Tanguy S, Reboul C (2013) Exercise-induced cardioprotection: a role for eNOS uncoupling and NO metabolites. Basic Res Cardiol 108:389. doi:10.1007/s00395-013-0389-2
Ferdinandy P, Hausenloy DJ, Heusch G, Baxter GF, Schulz R (2014) Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev 66:1142–1174. doi:10.1124/pr.113.008300
Fève B, Elhadri K, Quignard-Boulangé A, Pairault J (1994) Transcriptional down-regulation by insulin of the β3-adrenergic receptor expression in 3T3-F442A adipocytes: a mechanism for repressing the cAMP signaling pathway. Proc Natl Acad Sci USA 91:5677–5681
French JP, Hamilton KL, Quindry JC, Lee Y, Upchurch PA, Powers SK (2008) Exercise-induced protection against myocardial apoptosis and necrosis: MnSOD, calcium-handling proteins, and calpain. FASEB J Off Publ Fed Am Soc Exp Biol 22:2862–2871. doi:10.1096/fj.07-102541
García-Prieto J, García-Ruiz JM, Sanz-Rosa D, Pun A, García-Alvarez A, Davidson SM, Fernández-Friera L, Nuno-Ayala M, Fernández-Jiménez R, Bernal JA, Izquierdo-Garcia JL, Jimenez-Borreguero J, Pizarro G, Ruiz-Cabello J, Macaya C, Fuster V, Yellon DM, Ibanez B (2014) β3 adrenergic receptor selective stimulation during ischemia/reperfusion improves cardiac function in translational models through inhibition of mPTP opening in cardiomyocytes. Basic Res Cardiol 109:422. doi:10.1007/s00395-014-0422-0
Gödecke A, Molojavyi A, Heger J, Flögel U, Ding Z, Jacoby C, Schrader J (2003) Myoglobin protects the heart from inducible nitric-oxide synthase (iNOS)-mediated nitrosative stress. J Biol Chem 278:21761–21766. doi:10.1074/jbc.M302573200
Guo Y, Sanganalmath SK, Wu W, Zhu X, Huang Y, Tan W, Ildstad ST, Li Q, Bolli R (2012) Identification of inducible nitric oxide synthase in peripheral blood cells as a mediator of myocardial ischemia/reperfusion injury. Basic Res Cardiol 107:253. doi:10.1007/s00395-012-0253-9
Guo Y, Stein AB, Wu WJ, Zhu X, Tan W, Li Q, Bolli R (2005) Late preconditioning induced by NO donors, adenosine A1 receptor agonists, and δ1-opioid receptor agonists is mediated by iNOS. Am J Physiol Heart Circ Physiol 289:H2251–H2257. doi:10.1152/ajpheart.00341.2005
Hadri KE, Charon C, Pairault J, Hauguel-De Mouzon S, Quignard-Boulangé A, Fève B (1997) Down-regulation of β3-adrenergic receptor expression in rat adipose tissue during the fasted/fed transition: evidence for a role of insulin. Biochem J 323(Pt 2):359–364
von Harsdorf R, Li PF, Dietz R (1999) Signaling pathways in reactive oxygen species-induced cardiomyocyte apoptosis. Circulation 99:2934–2941
Heusch G (2011) β3-adrenoceptor activation just says NO to myocardial reperfusion injury. J Am Coll Cardiol 58:2692–2694. doi:10.1016/j.jacc.2011.09.034
Hu A, Jiao X, Gao E, Koch WJ, Sharifi-Azad S, Grunwald Z, Ma XL, Sun JZ (2006) Chronic beta-adrenergic receptor stimulation induces cardiac apoptosis and aggravates myocardial ischemia/reperfusion injury by provoking inducible nitric-oxide synthase-mediated nitrative stress. J Pharmacol Exp Ther 318:469–475. doi:10.1124/jpet.106.102160
Huang JV, Lu L, Ye S, Bergman BC, Sparagna GC, Sarraf M, Reusch JEB, Greyson CR, Schwartz GG (2013) Impaired contractile recovery after low-flow myocardial ischemia in a porcine model of metabolic syndrome. Am J Physiol Heart Circ Physiol 304:H861–H873. doi:10.1152/ajpheart.00535.2012
Idigo WO, Reilly S, Zhang MH, Zhang YH, Jayaram R, Carnicer R, Crabtree MJ, Balligand JL, Casadei B (2012) Regulation of endothelial nitric-oxide synthase (NOS) S-glutathionylation by neuronal NOS: evidence of a functional interaction between myocardial constitutive NOS isoforms. J Biol Chem 287:43665–43673. doi:10.1074/jbc.M112.412031
Larson JE, Rainer PP, Watts VL, Yang R, Miller KL, Phan A, Barouch LA (2012) Dependence of β3-adrenergic signaling on the adipokine leptin in cardiac myocytes. Int J Obes 36:876–879. doi:10.1038/ijo.2011.137
Lei P, Baysa A, Nebb HI, Valen G, Skomedal T, Osnes JB, Yang Z, Haugen F (2013) Activation of Liver X receptors in the heart leads to accumulation of intracellular lipids and attenuation of ischemia-reperfusion injury. Basic Res Cardiol 108:323. doi:10.1007/s00395-012-0323-z
Lekli I, Szabo G, Juhasz B, Das S, Das M, Varga E, Szendrei L, Gesztelyi R, Varadi J, Bak I, Das DK, Tosaki A (2008) Protective mechanisms of resveratrol against ischemia-reperfusion-induced damage in hearts obtained from Zucker obese rats: the role of GLUT-4 and endothelin. Am J Physiol Heart Circ Physiol 294:H859–H866. doi:10.1152/ajpheart.01048.2007
Levantesi G, Macchia A, Marfisi R, Franzosi MG, Maggioni AP, Nicolosi GL, Schweiger C, Tavazzi L, Tognoni G, Valagussa F, Marchioli R, GISSI-Prevenzione Investigators (2005) Metabolic syndrome and risk of cardiovascular events after myocardial infarction. J Am Coll Cardiol 46:277–283. doi:10.1016/j.jacc.2005.03.062
Li Q, Guo Y, Ou Q, Cui C, Wu WJ, Tan W, Zhu X, Lanceta LB, Sanganalmath SK, Dawn B, Shinmura K, Rokosh GD, Wang S, Bolli R (2009) Gene transfer of inducible nitric oxide synthase affords cardioprotection by upregulating heme oxygenase-1 via a nuclear factor-{kappa}B-dependent pathway. Circulation 120:1222–1230. doi:10.1161/CIRCULATIONAHA.108.778688
Lund J, Hafstad AD, Boardman NT, Rossvoll L, Rolim NP, Ahmed MS, Florholmen G, Attramadal H, Wisløff U, Larsen TS, Aasum E (2015) Exercise training promotes cardioprotection through oxygen-sparing action in high fat-fed mice. Am J Physiol Heart Circ Physiol 308:H823–H829. doi:10.1152/ajpheart.00734.2014
Marso SP, Miller T, Rutherford BD, Gibbons RJ, Qureshi M, Kalynych A, Turco M, Schultheiss HP, Mehran R, Krucoff MW, Lansky AJ, Stone GW (2007) Comparison of myocardial reperfusion in patients undergoing percutaneous coronary intervention in ST-segment elevation acute myocardial infarction with versus without diabetes mellitus (from the EMERALD Trial). Am J Cardiol 100:206–210. doi:10.1016/j.amjcard.2007.02.080
Meyer G, André L, Kleindienst A, Singh F, Tanguy S, Richard S, Obert P, Boucher F, Jover B, Cazorla O, Reboul C (2015) Carbon monoxide increases inducible NOS expression that mediates CO-induced myocardial damage during ischemia-reperfusion. Am J Physiol Heart Circ Physiol 308:H759–H767. doi:10.1152/ajpheart.00702.2014
Meyer G, André L, Tanguy S, Boissiere J, Farah C, Lopez-Lauri F, Gayrard S, Richard S, Boucher F, Cazorla O, Obert P, Reboul C (2010) Simulated urban carbon monoxide air pollution exacerbates rat heart ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 298:H1445–H1453. doi:10.1152/ajpheart.01194.2009
Miki T, Itoh T, Sunaga D, Miura T (2012) Effects of diabetes on myocardial infarct size and cardioprotection by preconditioning and postconditioning. Cardiovasc Diabetol 11:67. doi:10.1186/1475-2840-11-67
Mozaffari MS, Schaffer SW (2008) Myocardial ischemic-reperfusion injury in a rat model of metabolic syndrome. Obes Silver Spring Md 16:2253–2258. doi:10.1038/oby.2008.356
Nduhirabandi F, Du Toit EF, Blackhurst D, Marais D, Lochner A (2011) Chronic melatonin consumption prevents obesity-related metabolic abnormalities and protects the heart against myocardial ischemia and reperfusion injury in a prediabetic model of diet-induced obesity. J Pineal Res 50:171–182. doi:10.1111/j.1600-079X.2010.00826.x
Nistri S, Boccalini G, Bencini A, Becatti M, Valtancoli B, Conti L, Lucarini L, Bani D (2015) A new low molecular weight, MnII-containing scavenger of superoxide anion protects cardiac muscle cells from hypoxia/reoxygenation injury. Free Radic Res 49:67–77. doi:10.3109/10715762.2014.979168
Niu X, Watts VL, Cingolani OH, Sivakumaran V, Leyton-Mange JS, Ellis CL, Miller KL, Vandegaer K, Bedja D, Gabrielson KL, Paolocci N, Kass DA, Barouch LA (2012) Cardioprotective effect of β3 adrenergic receptor agonism: role of neuronal nitric oxide synthase. J Am Coll Cardiol 59:1979–1987. doi:10.1016/j.jacc.2011.12.046
Osipov RM, Bianchi C, Feng J, Clements RT, Liu Y, Robich MP, Glazer HP, Sodha NR, Sellke FW (2009) Effect of hypercholesterolemia on myocardial necrosis and apoptosis in the setting of ischemia-reperfusion. Circulation 120:S22–S30. doi:10.1161/CIRCULATIONAHA.108.842724
Pons S, Martin V, Portal L, Zini R, Morin D, Berdeaux A, Ghaleh B (2013) Regular treadmill exercise restores cardioprotective signaling pathways in obese mice independently from improvement in associated co-morbidities. J Mol Cell Cardiol 54:82–89. doi:10.1016/j.yjmcc.2012.11.010
Powers SK, Smuder AJ, Kavazis AN, Quindry JC (2014) Mechanisms of exercise-induced cardioprotection. Physiol Bethesda Md 29:27–38. doi:10.1152/physiol.00030.2013
Przyklenk K (2011) Efficacy of cardioprotective “conditioning” strategies in aging and diabetic cohorts: the co-morbidity conundrum. Drugs Aging 28:331–343. doi:10.2165/11587190-000000000-00000
Roof SR, Ho HT, Little SC, Ostler JE, Brundage EA, Periasamy M, Villamena FA, Györke S, Biesiadecki BJ, Heymes C, Houser SR, Davis JP, Ziolo MT (2015) Obligatory role of neuronal nitric oxide synthase in the heart’s antioxidant adaptation with exercise. J Mol Cell Cardiol 81:54–61. doi:10.1016/j.yjmcc.2015.01.003
Soliman H, Craig GP, Nagareddy P, Yuen VG, Lin G, Kumar U, McNeill JH, Macleod KM (2008) Role of inducible nitric oxide synthase in induction of RhoA expression in hearts from diabetic rats. Cardiovasc Res 79:322–330. doi:10.1093/cvr/cvn095
Song D, Kuo KH, Yao R, Hutchings SR, Pang CCY (2008) Inducible nitric oxide synthase depresses cardiac contractile function in Zucker diabetic fatty rats. Eur J Pharmacol 579:253–259. doi:10.1016/j.ejphar.2007.09.043
Tavernier G, Toumaniantz G, Erfanian M, Heymann MF, Laurent K, Langin D, Gauthier C (2003) β3-Adrenergic stimulation produces a decrease of cardiac contractility ex vivo in mice overexpressing the human β3-adrenergic receptor. Cardiovasc Res 59:288–296
Trappanese DM, Liu Y, McCormick RC, Cannavo A, Nanayakkara G, Baskharoun MM, Jarrett H, Woitek FJ, Tillson DM, Dillon AR, Recchia FA, Balligand JL, Houser SR, Koch WJ, Dell’Italia LJ, Tsai EJ (2015) Chronic β1-adrenergic blockade enhances myocardial β3-adrenergic coupling with nitric oxide-cGMP signaling in a canine model of chronic volume overload: new insight into mechanisms of cardiac benefit with selective β1-blocker therapy. Basic Res Cardiol 110:456. doi:10.1007/s00395-014-0456-3
Wang T, Mao X, Li H, Qiao S, Xu A, Wang J, Lei S, Liu Z, Ng KFJ, Wong GT, Vanhoutte PM, Irwin MG, Xia Z (2013) N-Acetylcysteine and allopurinol up-regulated the Jak/STAT3 and PI3K/Akt pathways via adiponectin and attenuated myocardial postischemic injury in diabetes. Free Radic Biol Med 63:291–303. doi:10.1016/j.freeradbiomed.2013.05.043
Wang XL, Liu HR, Tao L, Liang F, Yan L, Zhao RR, Lopez BL, Christopher TA, Ma XL (2007) Role of iNOS-derived reactive nitrogen species and resultant nitrative stress in leukocytes-induced cardiomyocyte apoptosis after myocardial ischemia/reperfusion. Apoptosis Int J Program Cell Death 12:1209–1217. doi:10.1007/s10495-007-0055-y
Wang Y, Guo Y, Zhang SX, Wu WJ, Wang J, Bao W, Bolli R (2002) Ischemic preconditioning upregulates inducible nitric oxide synthase in cardiac myocyte. J Mol Cell Cardiol 34:5–15. doi:10.1006/jmcc.2001.1482
Weidinger A, Müllebner A, Paier-Pourani J, Banerjee A, Miller I, Lauterböck L, Duvigneau JC, Skulachev VP, Redl H, Kozlov AV (2015) Vicious inducible nitric oxide synthase-mitochondrial reactive oxygen species cycle accelerates inflammatory response and causes liver injury in rats. Antioxid Redox Signal 22:572–586. doi:10.1089/ars.2014.5996
Yi W, Sun Y, Gao E, Wei X, Lau WB, Zheng Q, Wang Y, Yuan Y, Wang X, Tao L, Li R, Koch W, Ma XL (2011) Reduced cardioprotective action of adiponectin in high-fat diet-induced type II diabetic mice and its underlying mechanisms. Antioxid Redox Signal 15:1779–1788. doi:10.1089/ars.2010.3722
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the “Société Francophone du Diabète”, the “région Provence Alpes Côtes d’Azur”, the “Structure Fédérative de Recherche” TERSYS. SB was granted by the” Groupe de reflexion sur la Recherche Cardiovasculaire (GRRC)” for its experimental work conducted in Grenoble.
Conflict of interest
No potential conflicts of interest relevant to this article were reported.
Additional information
A. Kleindienst and S. Battault contributed equally to this work.
O. Cazorla, C. Reboul are senoir co-authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kleindienst, A., Battault, S., Belaidi, E. et al. Exercise does not activate the β3 adrenergic receptor–eNOS pathway, but reduces inducible NOS expression to protect the heart of obese diabetic mice. Basic Res Cardiol 111, 40 (2016). https://doi.org/10.1007/s00395-016-0559-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-016-0559-0