Abstract
Metabolic disturbance and mitochondrial dysfunction are a hallmark of diabetic cardiomyopathy (DC). Resistance exercise (RE) not only enhances the condition of healthy individuals but could also improve the status of those with disease. However, the beneficial effects of RE in the prevention of DC and mitochondrial dysfunction are uncertain. Therefore, this study investigated whether RE attenuates DC by improving mitochondrial function using an in vivo rat model of diabetes. Fourteen Otsuka Long-Evans Tokushima Fatty rats were assigned to sedentary control (SC, n = 7) and RE (n = 7) groups at 28 weeks of age. Long-Evans Tokushima Otsuka rats were used as the non-diabetic control. The RE rats were trained by 20 repetitions of climbing a ladder 5 days per week. RE rats exhibited higher glucose uptake and lower lipid profiles, indicating changes in energy metabolism. RE rats significantly increased the ejection fraction and fractional shortening compared with the SC rats. Isolated mitochondria in RE rats showed increase in mitochondrial numbers, which were accompanied by higher expression of mitochondrial biogenesis proteins such as proliferator-activated receptor-γ coactivator-1α and TFAM. Moreover, RE rats reduced proton leakage and reactive oxygen species production, with higher membrane potential. These results were accompanied by higher superoxide dismutase 2 and lower uncoupling protein 2 (UCP2) and UCP3 levels in RE rats. These data suggest that RE is effective at ameliorating DC by improving mitochondrial function, which may contribute to the maintenance of diabetic cardiac contractility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes (T2DM) is an important risk factor for heart failure, and the strong correlation between the two is well established. According to the Framingham Study, the risk of heart failure increases two- to fivefold in patients with diabetes, independent of hypertension or coronary artery disease [24]. Research has shown that complex interplay among changes in glucose tolerance and serum glucose and glycated hemoglobin levels are associated not only with systolic heart failure but also with the prevalence of diastolic dysfunction [60]. Although cardiovascular disease is still the main cause of death in patients with heart failure, the risk of death attributed to diabetic cardiomyopathy (DC) remains high. The incidence of DC is as high as 75% in asymptomatic patients [61] and is significantly associated with heart failure in diabetics. DC is a heart muscle disease with structural and functional abnormalities resulting from diabetes, independent of congenital, valvular, or hypertensive heart disease, alcoholism, or coronary artery atherosclerosis [14]. Moreover, DC indicates abnormal energy metabolism (higher fatty acid metabolism and lower glucose metabolism), thereby reducing energy production [35]. Although the pathology of DC and its relevant clinical phenotypes are heterogeneous, DC could be caused by disturbed myocardial metabolism [14].
Although the pathophysiology of DC and its relevant clinical phenotypes vary widely, it can be caused by disturbed myocardial metabolism [7]. The accumulation of myocardial triglycerides (TG) due to enhanced cardiac free fatty acid (FFA) uptake and decreased insulin-mediated glucose uptake in patients with T2DM increases cardiac oxygen consumption and mitochondrial uncoupling and dysfunction, subsequently inducing cardiomyocyte death and ventricular dysfunction [42]. Moreover, mitochondrial dysfunction caused by altered substrate utilization reduces mitochondrial respiration and adenosine triphosphate (ATP) production, leading to cardiac contractile dysfunction in DC [29]. Therefore, it would be therapeutically beneficial if the altered substrate metabolism of the failing heart in T2DM patients could be reversed. One well-known strategy for targeting metabolism is exercise.
Regular physical exercise improves cardiac function caused by obesity [50] and serves as an alternative for improving metabolism [47] and mitochondrial function, as well as in the treatment and prevention of cardiovascular disease [15]. The cardiovascular advantages of exercise are multifactorial and contain systemic metabolic effects, as well as beneficial changes within the myocardium [33]. It also improves mitochondrial metabolism through increased mitochondrial ATP synthesis by oxidative phosphorylation [49]. Recent evidences have demonstrated that aerobic exercise prevents cardiac dysfunction by apoptosis, fibrosis, and mitochondrial biogenesis in db/db mice [58]. In addition, Veeranki S et al. have revealed restoration of cardiac and mitochondrial function by aerobic exercise, leading to enhancing cardiac contraction in db/db mice [56]. Likewise, aerobic exercise has been recommended as an adjuvant therapy against DC. On the other hand, resistance exercise (RE) increases muscle strength [48] and improves one’s glucose tolerance and lipid profile due to an increase in muscle mass, even in patients with T2DM [10]. Previous studies have demonstrated that mitochondrial contents is enhanced by RE in patients with chronic kidney disease or T2DM [53]. Moreover, RE improves mitochondrial biogenesis by modulation of peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) in human skeletal muscle [11]. Recent study suggested that RE improves mitochondrial respiration by altering mitochondrial proteins and transcriptional expression in skeletal muscle [40]. Likewise, RE enhances health benefits via substrate metabolism and mitochondrial function, thereby enhancing energy production. Therefore, RE could be expected to be potential therapeutic strategy for treatment of T2DM, as well as aerobic exercise.
However, the underlying mechanisms of mitochondrial function in RE-induced DC have been well unknown. Moreover, the beneficial effects of aerobic exercise on mitochondrial function have been recognized for decades [43] and preventing against DC [56], but the effects of RE on cardiac mitochondrial function in T2DM individuals require clarification; in particular, its effects on improving cardiac metabolism and mitochondrial dysfunction in DC have not been fully elucidated.
Although the causal relationship between heart and mitochondrial function is difficult to prove, mitochondrial dysfunction is pivotal in patients with DC [12]. Therefore, we hypothesized that RE would prevent ventricular dysfunction in an advanced T2DM heart by preserving substrate metabolism and preventing mitochondrial dysfunction and examined this in an advanced diabetic animal model.
Materials and methods
Ethics statement
All experimental procedures were approved by the Institutional Review Board of Animals, Inje University College of Medicine (approval number: 2011-049). All surgery was performed under sodium pentobarbital anesthesia, and every effort was made to minimize animal suffering.
Animals
We obtained 4-week-old male Otsuka Long-Evans Tokushima Fatty (OLETF, n = 14) and control male Long-Evans Tokushima Otsuka (LETO, n = 7) rats from the Animal Center of Tokushima Research Institute (Otsuka Pharmaceutical, Tokushima, Japan) and maintained them until they reached 28 weeks of age, which is the typical age of onset of diabetes in OLETF rats. All rats were kept on a 12-h light/12-h dark cycle (lights on at 07:00 a.m.) at a controlled temperature (21–23 °C) with food and water available ad libitum.
RE training
We divided the 28-week-old OLETF rats (n = 14) into two groups: sedentary control (SC, n = 7) and RE (n = 7). The rats in the RE group performed corresponding exercises 5 days per week for 12 weeks after 3 days of familiarization. RE rats exercised by climbing a 1-m grid ladder inclined at 85°, as reported previously [26]. During the familiarization phase, RE rats performed ten repetitions of ladder climbing without any extra weight. During the training phase, RE rats underwent progressive RE with weights attached to their tails. The rats repeatedly climbed the ladder 20 times during each session, until they showed signs of fatigue. Each time the rats reached the top of the ladder, they were allowed to rest for 1 min. At the beginning of the training phase, the rats performed the exercise with weights attached to their tails that were 10% of their body weight; the weight was subsequently increased by 10% of the rat’s body weight every 2 weeks (Fig. 1a).
RE improves glucose tolerance and lipid profiles in T2DM. a Following RE for 12 weeks, which were divided into SC and RE groups and exercised after development of T2DM. b, c IPGTT and AUCglucose before RE. d Fasting glucose level. e, f IPGTT and AUCglucose after 12 weeks of RE. g Representative image of measurement of epicardial fat tissue (EFT) in LETO, SC, and RE rats, respectively. h Comparison of EFT between experimental rats. i–k Serum total cholesterol (TC), triglyceride (TG), and free fatty acids (FFA), respectively. The red arrows indicate the area of EFT. Values are mean ± SEM, n = 7 per group). *P < 0.05 vs. LETO rats, # P < 0.05 vs. SC rats (color figure online)
Glucose tolerance test
OLETF rats are characterized by abnormal glucose tolerance and they develop overt T2DM at approximately 20–28 weeks of age [51]. To confirm abnormal glucose tolerance, we performed intraperitoneal glucose tolerance tests (IPGTT) on the OLETF and LETO rats at 28 weeks of age and again after the 12-week experiment. The IPGTT consisted of injecting 1 g/kg of glucose intraperitoneally after a 16 h fast, followed by blood samples obtained from the tail vein 30, 60, 90, and 120 min post-injection. Blood glucose levels were measured by Accu-check (Hoffmann la Roche, Ltd., Basel, Switzerland). The area under the curve for the IPGTT (AUCglucose): glucose concentration × time curve was calculated by the trapezoidal rule [28].
Echocardiography
The rats were anesthetized for transthoracic echocardiography by inhaling 2% isoflurane in 100% oxygen. Echocardiographic studies were performed using the VIVID 7 Dimension System (General Electric-Vingmed Ultrasound, Horton, Norway). Images were obtained with high spatial and temporal resolution using a 10S transducer (5.5–12 MHz). The transducer was placed directly on the chest wall. A complete two-dimensional M-mode echocardiogram was obtained according to the standards of the American Society of Echocardiography. The following data were recorded: left ventricular (LV) internal dimension in diastole and systole (LVIDd and LVIDs), LV volume in diastole and systole (LVEDV and LVESV), ejection fraction (EF), and LV fractional shortening (FS). Epicardial fat thickness (EFT) was used to assess the echocardiography from the right ventricular free wall at the end of systole, as described by Shim et al. [52].
Serum biochemistry and heart and skeletal muscle weight
After 12 weeks of exercise or the observational period, the rats were anesthetized with sodium pentobarbital (100 mg/kg, intraperitoneally) and heparin (300 IU/mL/kg, intraperitoneally) and killed. Hearts and skeletal muscles were removed from the animals and weighed. Blood samples were collected from the rats’ hearts and placed in serum separator tubes. The serum was collected after centrifugation at 3000×g for 20 min and stored at − 80 °C until analysis. To optimize accuracy, we submitted the samples to a certified commercial reference laboratory (Ewon Reference Laboratory, Seoul, Korea) for the biochemical analyses, including measurements of glucose, total cholesterol (TC), triglyceride (TG), and free fatty acids (FFAs).
Electron microscopy
To determine the mitochondrial morphology, including number, size, and shape, the hearts were sliced and fixed in a 2.5% glutaraldehyde in PBS solution at 4 °C overnight, and then fixed with 1% osmium tetroxide in PBS for 2 h. The sliced hearts were double stained with uranyl acetate and lead citrate. Mitochondrial morphology was observed using an electron microscope (× 6000 and × 30,000 images) [20], and the number of mitochondria was calculated using the program ImageJ (NIH software).
Mitochondrial isolation
Using a medium-fitting glass Teflon Potter–Elvehjem homogenizer, the cardiac tissues were manually homogenized in mitochondrial isolation buffer (MIB buffer; 250 mM sucrose, 0.5 mM Na2EDTA, 10 mM Tris, and 0.1% BSA at pH 7.4), and the homogenate was clarified by centrifugation two times at 1000×g for 5 min at 4 °C. The supernatant was centrifuged twice at 10,000×g for 10 min, and the mitochondrial pellets were collected and diluted with three volumes of MIB buffer [55].
Mitochondrial respiration
Mitochondria oxygen consumption rates were measured using an Oroboros Oxyrgaph-2K (Oroboros Instruments, Innsbruck, Austria). This involved the use of a 2.1-ml chamber at 37 °C in an air-saturated (200 nmol O2/mL) respiration medium (MiR05) that consisted of 0.5 mM ethylene glycol tetraacetic acid, 3 mM MgCl2 6H2O, 10 mM KH2PO4, 20 mM HEPES, 110 mM sucrose 60 mM K-lactobionate, and 0.1% BSA at pH 7.1. State 4 respiration rates were examined with 1 mM glutamate and 0.5 mM malate as respiration substrates. State 3 active respiration rates were determined in the presence of 1 mM ADP. Proton leakage was assessed by the difference in oxygen consumption rates between oligomycin (2.5 μM) and antimycin A (2.5 μM), which is the amount of oxygen consumed by a proton leak [41]. Oxygen consumption was expressed as pmol O2/min/mg of mitochondrial protein.
Mitochondrial ROS level
Isolated mitochondria were double stained with MitoTracker Red (0.5 μM; excitation/emission 550/590 nm) and dichlorodihydrofluorescein (DCF) diacetate (10 μM; excitation/emission 488/535 nm). H2O2 levels were examined by changes in DCF fluorescence at 525 nm using flow cytometry. Mean values were analyzed by CellQuest (ver. 5.2; DB CellQuest™ Pro).
Mitochondria membrane potential
Isolated mitochondria were stained for 30 min with 0.1 μM tetramethylrhodamine ethyl ester (excitation/emission 564/580 nm) at room temperature and measured by flow cytometry to detect mitochondrial membrane potential. Mean values were analyzed by CellQuest (ver. 5.2; DB CellQuest™ Pro).
Measurement of ATP levels
Mitochondrial ATP was measured by the mitochondrial ToxGlo™ assay (Promega, Madison, WI, USA) according to the manufacturer’s protocol. Isolated cardiac mitochondria were plated at 1 μg/100 μL/well in white and clear-bottomed 96-well culture plates. The assay solution (100 μL) was added to the plate, and the plate was incubated at room temperature for 30 min. Luminescence was measured using a luminometer (Molecular Device, Sunnyvale, CA, USA) [23].
Western blot analysis
Heart tissues were homogenized in RIPA lysis buffer with protein and phosphatase inhibitor cocktail. The lysates were centrifuged at 16,000×g for 15 min at 4 °C. Protein concentrations were determined by the Bradford protein assay (Bio-Rad, Hercules, CA, USA), and 30 μg protein was loaded per lane onto 10% SDS polyacrylamide gels. The gels were transferred to nitrocellulose membranes (Whatman, Freiburg, Germany) and incubated with specific antibodies against glucose transporter 4 (GLUT4), superoxide dismutase 2 (SOD2), peroxisome proliferator-activated receptor alpha (PPARα), beta-tubulin (Cell Signaling Technology, Danvers, MA, USA), peroxisome proliferator-activated receptor gamma coactivator (PGC) 1α, carnitine palmitoyltransferase 1α (CPT-1α), mitochondrial transcription factor A (TFAM) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), pyruvate dehydrogenase E1-alpha (PDH E1α), or uncoupling proteins (UCP) 2 and 3 (Abcam, San Francisco, CA, USA). Western blot analysis was performed using these antibodies and an Ab Signal™ Western blotting detection kit (AbClon, Seoul, Korea). Blots were visualized with an LAS-3000 Plus imager (Fuji Photo Film, Tokyo, Japan) and analyzed using ImageJ.
Statistical analysis
All data are presented as the means ± standard error of mean (SEM). The statistical analyses were performed with SPSS 23.0 (IBM Corp., Armonk, NY, USA) using a one-way analysis of variance (ANOVA). If the ANOVA indicated a difference, Duncan’s post-hoc test was performed. Statistical significance was set at a P value < 0.05.
Results
Effect of RE on body and heart weights of OLETF rats
Table 1 presents the body weight and food intake data of the three groups before and after the 12-week exercise period. The OLETF rats (both SC and RE) showed a significantly higher body weight compared to that of the LETO rats before RE. Mean daily food intake at baseline was lower in LETO rats than that in OLETF rats. After 12 weeks of RE, the weights of the RE rats were decreased significantly, while those of the SC rats had not changed significantly. In addition, the mean daily food intake of the SC rats was higher than that of LETO rats, but similar to RE rats. Heart weight was higher in SC and RE rats than in LETO rats, and the heart/body weight ratio of the RE rats was significantly higher than those of the LETO and SC rats. However, the LV mass index (LV mass/body weight, LVMI) and RWT, an indicator of cardiac hypertrophy [25], did not differ among groups. RE did not attenuate cardiac hypertrophy as calculated by the heart/body weight ratio and LVMI in OLETF rats. Although the weights of the soleus and gastrocnemius muscles were similar between the SC and RE rats, muscle weight and body weight were higher in the RE than in the SC rats. These data indicate that RE only reduces body weight, not involved in attenuating cardiac hypertrophy.
RE improves glucose tolerance and lipid profiles in T2DM
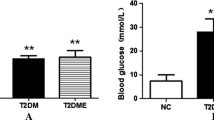
Impaired glucose tolerance was defined as a maximum blood glucose level of at least 300 mg/dL or a blood glucose level of at least 200 mg/dL after 120 min [51]. Blood glucose levels were measured every 30 min for 2 h after glucose injection before (Fig. 1b) and after (Fig. 1e) the 12-week test period. The glucose levels of the OLETF rats (392.50 ± 25.60 mg/dL at 60 min and 354.67 ± 29.56 mg/dL at 120 min) were consistently higher than those of the LETO rats (191.67 ± 7.18 mg/dL at 60 min and 174.17 ± 8.01 mg/dL at 120 min) before the 12-week test period. The AUCglucose was also higher in the OLETF rats compared with the LETO rats (Fig. 1c). These differences between the OLETF and LETO rats remained the same after the 12-week test period, but the rats who underwent RE showed significantly attenuated levels of glucose and AUCglucose compared with the SC rats (Fig. 1d–f).
The epicardial fat thickness (EFT) measurement during transthoracic echocardiography was higher in SC rats compared to LETO rats (Fig. 1g, h). However, the EFT was lower in RE rats compared to SC rats and similar to that in LETO rats. The SC rats had higher fasting levels of TC, TG, and FFA than the LETO rats, and the RE rats were closer to the LETO rats than the SC rats in terms of those levels (Fig. 1i–k). These results suggest that RE not only enhances glucose tolerance but also decreases lipid metabolism, leading to altering energy metabolism.
RE enhances cardiac contraction in T2DM
Next, we investigated the effect of cardiac contraction on RE-induced T2DM hearts. The LVIDd and LVEDV were not different between the groups (Fig. 2a, d). However, the LVIDs and LVESV in the RE rats were lower than those in the SC rats (Fig. 2b, e), resulting in higher FS and EF values in RE rats compared to SC rats (Fig. 2c, f). The SC and RE rats exhibited a decreased heart rate (HR) compared to the LETO rats and no difference was detected between the SC and RE rats (Fig. 2g), indicating that RE affects the inotropic response more than the chronoscopic response in the diabetic heart.
RE enhances cardiac contraction in T2DM. a–c Comparison of left ventricular internal dimension in diastole (LVIDd) and systole (LVIDs), fractional shortening (FS) between experimental rats, respectively. d–f Comparison of LV end of volume in diastole (LVEDV) and systole (LVESV) and ejection fraction (EF) between experimental rats, respectively. g Heart rate (HR). Values are mean ± SEM, n = 7 per group). *P < 0.05 vs. LETO rats, # P < 0.05 vs. SC rats
RE regulates energy metabolism in T2DM hearts
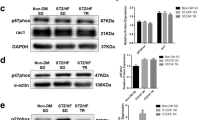
To determine whether RE can regulate glucose and fat metabolism-related gene expression in the T2DM heart, GLUT4, PDH E1α, CPT-1α, and PPARα were quantified by Western blotting (Fig. 3a). The hearts from SC rats showed lower expression of GLUT4 and PDH E1α compared with LETO rats, while the hearts from RE rats expressed higher levels of these proteins than SC rats (Fig. 3b, c). The increased expression of CPT-1α and PPARα in SC rats compared with LETO rats was significantly reversed in RE rats (Fig. 3d, e). These observations suggest that RE modulates glucose and fat metabolism in T2DM hearts.
RE regulates energy metabolism in T2DM hearts. a The expression of proteins related to glucose (GLUT4 and PDH E1α) and fat (CPT-1α and PPARα) metabolism were evaluated by western blotting. b–e The relative ratio of proteins, including GLUT4, PDH E1α, CPT-1α, and PPARα to β-tubulin levels were determined by image J. Values are mean ± SEM (n = 4 per group). *P < 0.05 vs. LETO rats, # P < 0.05 vs. SC rats
RE alters mitochondrial morphology in T2DM hearts
In SC rats, the sarcomeres had a collapsed appearance and the mitochondria were bizarrely shaped with poorly defined cristae (Fig. 4a). There were also fewer mitochondria compared with the LETO rats (Fig. 4b). However, the mitochondria from RE rats did not show these changes, and significantly, more mitochondria were observed than in SC rats. Damaged mitochondria were higher in SC rats, but RE rats showed significantly lower than SC rats (Fig. 4c). These results were accompanied by higher expression of mitochondrial biogenesis-related genes, including PGC-1α and TFAM [44] (Fig. 4d–f). These results reveal that RE attenuates aberrant mitochondrial morphology in response to change mitochondrial biogenesis.
RE alters mitochondrial morphology in T2DM hearts. a Representative image of mitochondrial morphologies with transmission electron microscopy (× 6000 and × 30,000 images) of hearts from LETO, SC, and RE rats, respectively. b Quantification of cardiac mitochondrial number relative to total cellular area. c Quantification of damaged mitochondrial number relative to total mitochondrial number and represents as a fold to LETO. d–f PGC-1α and TFAM protein expressions were evaluated by western blotting. M, mitochondria; S, sarcomere; LETO, white line (× 6000); 1 μm, white line (× 30,000); 200 nm. Values are mean ± SEM (n = 4–7 per group). *P < 0.05 vs. LETO rats, # P < 0.05 vs. SC rats
RE improves mitochondrial function in T2DM hearts
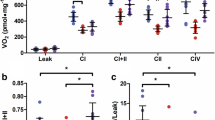
We confirmed that RE ameliorates abnormal mitochondrial biogenesis in T2DM hearts. Therefore, we next investigated whether RE improves mitochondrial function in T2DM hearts. State 4 respiration (Fig. 5a), but not state 3 respirations (Fig. 5b), by mitochondria from SC rat hearts significantly increased compared to LETO rats; however, the hearts from the RE rats showed attenuated increases in state 4 respiration (Fig. 5a). Despite the lack of a difference in state 3 respiration between the experimental groups, the respiratory control ratio (RCR) was higher in mitochondria from RE rats compared to those from SC (Fig. 5c). The membrane potential was lower in mitochondria from SC rats, which were improved in RE rats compared to SC rats (Fig. 5d). These observations agree with the finding of increased ATP levels in RE rats (Fig. 5e). Mitochondria from SC rats showed higher levels of ROS compared to those from LETO rats. However, the mitochondrial ROS levels in RE rats were significantly lower compared to those in SC rats (Fig. 5f). These results were accompanied by higher expression of SOD2 in RE rats (Fig. 5h, i). Proton leak, as represented by different oxygen consumption rates between oligomycin and antimycin A [41], was higher in SC rats and was significantly decreased in RE rats (Fig. 5g). These results are related to the lower expression of UCP2 and 3 in RE rats (Fig. 5j, k). These results suggest that RE enhances energy metabolism and oxidative phosphorylation, thereby enhancing energy efficiency.
RE improves mitochondrial function in T2DM hearts. Oxygen consumption rate in a state 4 and b state 3. c Relative control ratio (RCR) of mitochondrial respiration. d Mitochondrial membrane potential. e ATP level. f Reactive oxygen species (ROS) level. g Proton leak. h Representative Western blots and quantification of superoxide dismutase (SOD) 2, uncoupling proteins (UCP) 2 and 3 in the hearts from each group. i–k The relative ratio of SOD2, UCP2 and 3 to β-tubulin levels were determined by ImageJ. l Schematic diagram of the proposed protective mechanism of RE in diabetic cardiomyopathy. Values are mean ± SEM (n = 4–7 per group). *P < 0.05 vs. LETO rats, # P < 0.05 vs. SC rats
Discussion
In this study, we examined the effects of RE for 12 weeks on OLETF rat hearts within an established diabetic range of hyperglycemia at the age of 28 weeks. We observed significant differences in physiological and mitochondrial functions, including the fasting blood levels of glucose, TC, TG, and FFA, between SC and RE rats. RE prevented the higher EFT and systolic dysfunction in diabetic rat hearts. Electron microscopic (EM) studies revealed that RE also preserved mitochondrial morphology and number. The beneficial effects were also observed in the mitochondrial function study, as reflected by the enhanced oxidative phosphorylation level, higher membrane potential, decreased ROS level, and increased SOD2 level. These results demonstrate the protective effects of RE on systolic and mitochondrial dysfunction with altered substrate metabolism in OLETF rats, even in an established diabetic stage.
The pathophysiology of DC remains controversial. One explanatory mechanism may be a change in fuel metabolism by the heart, particularly fatty acid (FA) metabolism. PPARα, which is primarily expressed in locations with high capacity for FA oxidation such as heart and skeletal muscle [16], plays a pivotal role in FA metabolism by regulating mitochondrial transport (CPT-1) [9]. Previous studies have demonstrated that rats with DC have higher PPARα and CPT-1 expression levels, which may be related to abnormal glucose and FA metabolism [45]. Moreover, a disturbance in mitochondrial uncoupling caused by the intramyocardial accumulation of TG induces mitochondrial dysfunction in DC [4]. The OLETF rats already showed marked hyperglycemia compatible with the animal model of T2DM at the beginning of RE in our experiment [51]. However, the RE rats showed significantly improved plasma glucose as evidenced by the IPGTT, TC, TG, and FFA results. These improved glucose metabolism and lipid profiles were accompanied by higher expression of glucose metabolic proteins (GLUT4 and PDH E1α) in the hearts of RE rats [42]. Epicardial fat deposits are greater in obese patients with T2DM compared with those in non-diabetic obese subjects and are positively correlated with myocardial TG content as measured by proton magnetic resonance spectroscopy. In addition, myocardial TG content is independently associated with decreased stroke volume [17]. In line with these findings, our results suggest that manipulating myocardial metabolism from non-esterified FA to glucose uptake by RE is supported by the increased GLUT4 and decreased CPT-1α and PPARα expression, which parallel decreased epicardial fat depots and improved LV function. Although the RE rats did not reverse their heart/body weight and LVMI compared with the SC rats (Table 1), RE prevented the increases in LVIDs and LVESV, resulting in an increased FS and EF (Fig. 2). We speculate that RE rats showed increases in heart/body weight compared to SC or LETO rats. Restoration of EF and FS without increasing the LVESV indicates improved cardiac function in RE rats compared to that in SC rats.
Emerging studies have shown the role of mitochondria in causing DC. Mitochondrial dysfunction and decreased mitochondrial biogenesis brought about by oxidative stress due to ROS can result in a vicious cycle of ROS and mitochondrial dysfunction, leading to the progression and development of diseases such as DC [12]. Therefore, we postulated that RE could attenuate these changes in diabetic OLETF rats. Mitochondrial biogenesis is related to mitochondrial antioxidant defense, oxidative capacity, and regulation of mitochondrial morphology [5] and is mainly regulated by PGC-1α [45]. It has also been found that T2DM is associated with decreased expression of PGC-1α [36], and the altered levels could affect mitochondrial ultrastructure in disease models [31]. Previous studies have demonstrated that ultrastructural alterations in mitochondria reflect their dysfunction in the oocytes of women with diabetes [59]. The EM examination in our study revealed fewer mitochondria and damaged cristae in SC, but not in RE rat hearts. We also observed higher expression of mitochondrial PGC-1α and TFAM in RE rats compared to that in SC rats, suggesting that RE-induced mitochondrial biogenesis prevented impaired mitochondrial structure in diabetic hearts [5]. Our results indicate that mitochondria from OLETF rat hearts processed less oxygen with lower ATP levels, increased ROS, and increased depolarization of the membrane potential, whereas RE training tended to reverse these abnormalities, which could help prevent mitochondrial dysfunction in OLETF rat hearts. Taken together with improved mitochondrial biogenesis, these results show that RE exhibited improved mitochondrial efficiency, which might be associated with higher cardiac contractility in T2DM hearts.
After identifying improved the mitochondria biogenesis and efficiency in RE-induced T2DM hearts, we then examined whether RE further enhanced mitochondrial function and mitochondrial uncoupling in T2DM hearts. UCPs are mitochondrial carrier proteins located in the mitochondrial inner membrane, which increase with enhanced FA metabolism. It has been demonstrated that FA-induced activation of UCP2 and UCP3 through PPARα increases FA oxidation and reduces cardiac efficiency in DC [2]. Although the decrease in the expression of UCP2 and UCP3 with RE in our experiment seemed to be associated with substrate switching from FAs to glucose, it was also related to decreased ROS production in T2DM hearts. Because superoxide is a major mitochondrial ROS elicited by uncoupling from FA influx [13], attenuated expression of UCPs in RE rat hearts was partly associated with decreased mitochondrial state 4 respiration and higher RCR, decreased proton leak, and altered mitochondrial membrane potential [6]. Moreover, SOD2 regulates UCP expression by modulating superoxide radical/anion concentrations, and UCP may be part of a negative feedback mechanism related to SOD2. In addition, mitochondrial SOD2 (manganese-dependent SOD), which effectively removes mitochondrial ROS [37], decreased in the hearts from SC rats, but increased in response to RE. This is consistent with previous reports, in which diminished SOD2 due to oxidative stress was reversed by exercise in an animal model [39], supporting the argument that RE may prevent oxidative stress by catalyzing superoxide ion via higher SOD2 expression in RE rat hearts. However, it is unclear from our study whether the reduced level of ROS was caused by increased ROS scavenging or decreased ROS generation because we did not specifically address the significant sources of extra-mitochondrial ROS, such as NADPH oxidase (NOX) [1]. One study showed that swimming attenuates the increase in isoproterenol (ISO)-induced myocardial expression of NOX4 messenger RNA (mRNA) and protein and NOX2 mRNA as well as antioxidant enzymes (SOD1, SOD2, and catalase) in adenosine monophosphate-activated protein kinase (AMPK)α2+/+ mice, but not in AMPKα−/− mice, indicating that exercise attenuates ISO-induced cardiac fibrosis by AMPK-dependent reduced ROS generation (NOX) and increased ROS scavenging (MnSOD) [32].
RE improves insulin sensitivity, glucose tolerance, and the lipid profile by elevating muscle mass, as skeletal muscle is an important factor in resting metabolic rate [8]. Moreover, a decreased muscle mass (e.g., sarcopenia) is associated with heart failure, which is improved by RE [46]. In our experiments, 12 weeks of RE increased the ratio of the soleus muscle and gastrocnemius muscle weight to body weight as well as the ratio of heart/body weight compared to those in SC rats, although RE rats tended to have higher soleus muscle (P = 0.08) and gastrocnemius muscle (P = 0.08) weights than did SC rats (Table 1). The effect of RE on the diabetic heart exerted by improving glucose tolerance and lipid profiles may be related to peripheral muscle mass with systemic effects, in accordance with previous reports of the beneficial effect of muscle mass on T2DM-induced sarcopenia [30].
Numerous clinical trials using angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and mineralocorticoid-receptor antagonists showed positive outcomes and proposed data for the current guidelines to treat non-diabetic patients with heart failure [34]. However, diabetes-specific trials or guidelines are limited. In addition, the effects of glucose-lowering medications except metformin on LV dysfunction in patients with advanced DC are limited because of weight gain or aggravated edema (i.e., thiazolidinediones) [27] or increased hospitalization for worsening heart failure (saxagliptin, a dipeptidyl peptidase 4 inhibitor) [54]. Although the beneficial effects of exercise on glycemic control in T2DM, weight loss, blood pressure, vascular function, and dyslipidemia have been reported in humans, the effects of exercise on myocardial function have been less well studied [21]. This paucity of evidence might be associated with the difficulty of maintaining rigorous exercise.
Until now, the beneficial effects of RE on diabetes-related heart disease have been mainly limited to early-stage diabetes animal models; few studies have investigated the effect of RE on DC. This is the first report to investigate the effects of RE on the relationships between mitochondrial dysfunction and systolic dysfunction in an animal model of DC with an advanced stage of hyperglycemia and dyslipidemia. However, this study had some limitations that should be mentioned. First, cardiomyopathy has been categorized as restrictive (preserved EF) or dilated (reduced EF) according to the clinical presentation, and phenotype-specific mechanisms have been proposed [14]. The phenotype of our animal model was similar to dilated cardiomyopathy in that it also exhibited increased LVIDs and LVESV with reduced FS and EF. However, we did not confirm diastolic dysfunction by assessing mitral inflow with Doppler-based velocities, which is known as an early sign of DC preceding the systolic dysfunction in patients with diabetes because of rapid heart rates and angle dependency. We could not exclude the possibility of bias in the echocardiographic parameters caused by different HRHRs between experimental animals (363.65 ± 15.28 vs. 284.03 ± 13.55 vs. 276.14 ± 6.14 bpm, in LETO, SC, and RE rats, respectively, P < 0.05). However, the variation in HR was within 100 bpm for a set of experiments. Second, we did not explore the effects of RE on FA metabolism in hepatic, peripheral muscles, or adipose tissues because we used systolic dysfunction after long-standing hyperglycemia in OLETF rats. Although the attenuation of hyperglycemia by RE could affect fuel metabolism in the diabetic heart, it is possible that the direct effects of resistance RE on hepatic and peripheral tissues by improving insulin resistance is associated with the systemic effects [22]. Third, we are curious to know whether the effect of RE lasts for several weeks after stopping the exercise. After long-term exercise, cessation of exercise (detraining) is characterized by loss of exercise-induced adaptations [38]. One study reported that stopping exercise after long-term wheel running exercise maintained the body weight reduction with a higher energy expenditure in diabetic animal models. Although we did not examine the effect of stopping exercise after long-term RE, we expect the effects of RE, as well as aerobic exercise, to continue. Fourth, we did not confirm the transcriptional levels of molecules demonstrated in Figs. 3a and 4c. However, we had examined the alteration of genes by mRNA microarray experiment. In our experimental data, although protein levels were significantly differenced, there were no significant difference of mRNA levels including SLC2A4 (GLUT4), PDHA1 (PDH E1α), CPT1A (CPT-1a), PPARA (PPARα), PPARGC1A (PGC-1α), and TFAM (TFAM) between groups (Fig. S1). It was well known that the mismatch of mRNA and protein levels was at same time point [57], due to firstly, there are complicated post-transcriptional mechanisms (transcription and translocation). Secondly, proteins may be different half-lives. And finally, there are errors in protein and mRNA experiments [18]. In this context, we may not definitely explain the mismatch of mRNA levels and protein levels of selected molecules; however, we cautiously hypothesize that diabetes condition could alter the rate of protein synthesis and degradation of muscle proteins selectively and further regulate the functions of proteins [3]. Fifth, we did not perform the HE staining, fibrosis, inflammation, and vascularity of cardiac tissues between groups. However, we analyzed microarray data and we found that a number of fibrosis- and inflammation-related genes including LTA4H, CYSLTR1, FPR1, TLR3, and HDAC2 were increased in SC rats, whereas these genes were decreased by RE (Fig. S2). Our results suggest that chronic RE can mediate cardiac fibrosis and inflammation in diabetic cardiomyopathy. Sixth, although we did not evaluate the strength of RE, we can calculate the strength of exercise by using potential energy formula. In physics, potential energy is the stored energy of position possessed by a body by virtue of its position relative to other factors [57]. The potential energy formula is given by the following:
Where m indicates mass in kilograms, g is gravity as 9.8 m/s2, and h is the height of object. In our experiment, RE rats were trained for20 times on a 1-m ladder with an added weight percentage relative to their weight. At the beginning of the training phase, the rats performed the exercise with weights that were 10% of their body weight attached to their tails; the weight was subsequently increased in increments of 10% relative to the rat’s body weight every 2 weeks. Using potential energy formula, we can analyze potential energy in our RE models as a measure of energy expenditure; Energy (J) = m (% of body weight) × g × h (ladder height) (Fig. S3). Last, high-intensity exercise in our protocol could have increased the risk of acute hyperglycemia in the diabetic model [19]. Thus, we trained RE rats to climb at a lower intensity compared to other studies, which may have used a higher intensity. Our RE protocol is the first attempt to examine exercise in a T2DM animal model.
In conclusion, our results show that RE ameliorates not only hyperglycemia and dyslipidemia but also mitochondrial biogenesis in an established diabetic stage and that RE improves mitochondrial function, thereby enhancing cardiac contractility in DC (Fig. 5l). This prospective observational study shows that RE improved cardiac energetics by modulating mitochondria and provides direct evidence that impaired mitochondrial biogenesis and uncoupling in T2DM hearts is reversed by RE. In turn, modulation of PGC-1α, UCP2, and UCP3 activation by RE may be a therapeutic target for preventing T2DM. This mechanism may contribute to shift energy utilization and enhance cardiac energy efficiency in T2DM hearts.
References
Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J (2010) Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res 106:1253–1264. https://doi.org/10.1161/CIRCRESAHA.109.213116
An D, Rodrigues B (2006) Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol 291:H1489–H1506. https://doi.org/10.1152/ajpheart.00278.2006
Ashford AJ, Pain VM (1986) Effect of diabetes on the rates of synthesis and degradation of ribosomes in rat muscle and liver in vivo. J Biol Chem 261:4059–4065
Battiprolu PK, Gillette TG, Wang ZV, Lavandero S, Hill JA (2010) Diabetic cardiomyopathy: mechanisms and therapeutic targets. Drug Discov Today Dis Mech 7:e135–e143. https://doi.org/10.1016/j.ddmec.2010.08.001
Benard G, Rossignol R (2008) Ultrastructure of the mitochondrion and its bearing on function and bioenergetics. Antioxid Redox Signal 10:1313–1342. https://doi.org/10.1089/ars.2007.2000
Bosetti F, Baracca A, Lenaz G, Solaini G (2004) Increased state 4 mitochondrial respiration and swelling in early post-ischemic reperfusion of rat heart. FEBS Lett 563:161–164. https://doi.org/10.1016/S0014-5793(04)00294-7
Brahma MK, Pepin ME, Wende AR (2017) My sweetheart is broken: role of glucose in diabetic cardiomyopathy. Diabetes Metab J 41:1–9. https://doi.org/10.4093/dmj.2017.41.1.1
Braith RW, Stewart KJ (2006) Resistance exercise training: its role in the prevention of cardiovascular disease. Circulation 113:2642–2650. https://doi.org/10.1161/CIRCULATIONAHA.105.584060
Burri L, Thoresen GH, Berge RK (2010) The role of PPARalpha activation in liver and muscle. PPAR Res 2010. https://doi.org/10.1155/2010/542359
Cauza E, Hanusch-Enserer U, Strasser B, Ludvik B, Metz-Schimmerl S, Pacini G, Wagner O, Georg P, Prager R, Kostner K, Dunky A, Haber P (2005) The relative benefits of endurance and strength training on the metabolic factors and muscle function of people with type 2 diabetes mellitus. Arch Phys Med Rehabil 86:1527–1533. https://doi.org/10.1016/j.apmr.2005.01.007
Deldicque L, Atherton P, Patel R, Theisen D, Nielens H, Rennie MJ, Francaux M (2008) Effects of resistance exercise with and without creatine supplementation on gene expression and cell signaling in human skeletal muscle. J Appl Physiol (1985) 104:371–378. https://doi.org/10.1152/japplphysiol.00873.2007
Duncan JG (2011) Mitochondrial dysfunction in diabetic cardiomyopathy. Biochim Biophys Acta 1813:1351–1359. https://doi.org/10.1016/j.bbamcr.2011.01.014
Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD (2002) Superoxide activates mitochondrial uncoupling proteins. Nature 415:96–99. https://doi.org/10.1038/415096a
Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Kuhl U, Maisch B, McKenna WJ, Monserrat L, Pankuweit S, Rapezzi C, Seferovic P, Tavazzi L, Keren A (2008) Classification of the cardiomyopathies: a position statement from the European Society of Cardiology working group on myocardial and pericardial diseases. Eur Heart J 29:270–276. https://doi.org/10.1093/eurheartj/ehm342
Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB 3rd, Kligfield PD, Krumholz HM, Kwong RY, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR Jr, Smith SC Jr, Spertus JA, Williams SV, Anderson JL (2012) 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 126:e354–e471. https://doi.org/10.1161/CIR.0b013e318277d6a0
Fruchart JC, Staels B, Duriez P (2001) The role of fibric acids in atherosclerosis. Curr Atheroscler Rep 3:83–92
Gaborit B, Kober F, Jacquier A, Moro PJ, Cuisset T, Boullu S, Dadoun F, Alessi MC, Morange P, Clement K, Bernard M, Dutour A (2012) Assessment of epicardial fat volume and myocardial triglyceride content in severely obese subjects: relationship to metabolic profile, cardiac function and visceral fat. Int J Obes 36:422–430. https://doi.org/10.1038/ijo.2011.117
Greenbaum D, Colangelo C, Williams K, Gerstein M (2003) Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol 4:117. https://doi.org/10.1186/gb-2003-4-9-117
Guelfi KJ, Jones TW, Fournier PA (2005) The decline in blood glucose levels is less with intermittent high-intensity compared with moderate exercise in individuals with type 1 diabetes. Diabetes Care 28:1289–1294
Ha MW, Ma R, Shun LP, Gong YH, Yuan Y (2005) Effects of allitridi on cell cycle arrest of human gastric cancer cells. World J Gastroenterol 11:5433–5437
Hare JL, Hordern MD, Leano R, Stanton T, Prins JB, Marwick TH (2011) Application of an exercise intervention on the evolution of diastolic dysfunction in patients with diabetes mellitus: efficacy and effectiveness. Circ Heart Fail 4:441–449. https://doi.org/10.1161/CIRCHEARTFAILURE.110.959312
Jahng JW, Song E, Sweeney G (2016) Crosstalk between the heart and peripheral organs in heart failure. Exp Mol Med 48:e217. https://doi.org/10.1038/emm.2016.20
Jeong SH, Kim HK, Song IS, Lee SJ, Ko KS, Rhee BD, Kim N, Mishchenko NP, Fedoryev SA, Stonik VA, Han J (2014) Echinochrome A protects mitochondrial function in cardiomyocytes against cardiotoxic drugs. Mar Drugs 12:2922–2936. https://doi.org/10.3390/md12052922
Kannel WB, Hjortland M, Castelli WP (1974) Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol 34:29–34
Kim HK, Youm JB, Lee SR, Lim SE, Lee SY, Ko TH, Long le T, Nilius B, Won du N, Noh JH, Ko KS, Rhee BD, Kim N, Han J (2012) The angiotensin receptor blocker and PPAR-gamma agonist, telmisartan, delays inactivation of voltage-gated sodium channel in rat heart: novel mechanism of drug action. Pflugers Arch 464:631–643. https://doi.org/10.1007/s00424-012-1170-3
Kim JY, Choi MJ, So B, Kim HJ, Seong JK, Song W (2015) The preventive effects of 8 weeks of resistance training on glucose tolerance and muscle fiber type composition in Zucker rats. Diabetes Metab J 39:424–433. https://doi.org/10.4093/dmj.2015.39.5.424
Lago RM, Singh PP, Nesto RW (2007) Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet 370:1129–1136. https://doi.org/10.1016/S0140-6736(07)61514-1
Larsen MO, Rolin B, Wilken M, Carr RD, Gotfredsen CF (2003) Measurements of insulin secretory capacity and glucose tolerance to predict pancreatic beta-cell mass in vivo in the nicotinamide/streptozotocin Gottingen minipig, a model of moderate insulin deficiency and diabetes. Diabetes 52:118–123
Lee TW, Bai KJ, Lee TI, Chao TF, Kao YH, Chen YJ (2017) PPARs modulate cardiac metabolism and mitochondrial function in diabetes. J Biomed Sci 24:5. https://doi.org/10.1186/s12929-016-0309-5
Leenders M, Verdijk LB, van der Hoeven L, Adam JJ, van Kranenburg J, Nilwik R, van Loon LJ (2013) Patients with type 2 diabetes show a greater decline in muscle mass, muscle strength, and functional capacity with aging. J Am Med Dir Assoc 14:585–592. https://doi.org/10.1016/j.jamda.2013.02.006
Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy JO, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP (2005) PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol 3:e101. https://doi.org/10.1371/journal.pbio.0030101
Ma X, Fu Y, Xiao H, Song Y, Chen R, Shen J, An X, Shen Q, Li Z, Zhang Y (2015) Cardiac fibrosis alleviated by exercise training is AMPK-dependent. PLoS One 10:e0129971. https://doi.org/10.1371/journal.pone.0129971
Mann N, Rosenzweig A (2012) Can exercise teach us how to treat heart disease? Circulation 126:2625–2635. https://doi.org/10.1161/CIRCULATIONAHA.111.060376
McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik K, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A (2012) [ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012]. Turk Kardiyoloji Dernegi arsivi: Turk Kardiyoloji Derneginin yayin organidir 40 Suppl 3:77–137
Miki T, Yuda S, Kouzu H, Miura T (2013) Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Fail Rev 18:149–166. https://doi.org/10.1007/s10741-012-9313-3
Moreno-Santos I, Perez-Belmonte LM, Macias-Gonzalez M, Mataro MJ, Castellano D, Lopez-Garrido M, Porras-Martin C, Sanchez-Fernandez PL, Gomez-Doblas JJ, Cardona F, de Teresa-Galvan E, Jimenez-Navarro M (2016) Type 2 diabetes is associated with decreased PGC1alpha expression in epicardial adipose tissue of patients with coronary artery disease. J Transl Med 14:243. https://doi.org/10.1186/s12967-016-0999-1
Motoori S, Majima HJ, Ebara M, Kato H, Hirai F, Kakinuma S, Yamaguchi C, Ozawa T, Nagano T, Tsujii H, Saisho H (2001) Overexpression of mitochondrial manganese superoxide dismutase protects against radiation-induced cell death in the human hepatocellular carcinoma cell line HLE. Cancer Res 61:5382–5388
Mujika I, Padilla S (2000) Detraining: loss of training-induced physiological and performance adaptations. Part II: long term insufficient training stimulus. Sports Med 30:145–154
Naderi R, Mohaddes G, Mohammadi M, Ghaznavi R, Ghyasi R, Vatankhah AM (2015) Voluntary exercise protects heart from oxidative stress in diabetic rats. Adv Pharm Bull 5:231–236. 10.15171/apb.2015.032
Porter C, Reidy PT, Bhattarai N, Sidossis LS, Rasmussen BB (2015) Resistance exercise training alters mitochondrial function in human skeletal muscle. Med Sci Sports Exerc 47:1922–1931. https://doi.org/10.1249/MSS.0000000000000605
Reily C, Mitchell T, Chacko BK, Benavides G, Murphy MP, Darley-Usmar V (2013) Mitochondrially targeted compounds and their impact on cellular bioenergetics. Redox Biol 1:86–93. https://doi.org/10.1016/j.redox.2012.11.009
Rijzewijk LJ, van der Meer RW, Lamb HJ, de Jong HW, Lubberink M, Romijn JA, Bax JJ, de Roos A, Twisk JW, Heine RJ, Lammertsma AA, Smit JW, Diamant M (2009) Altered myocardial substrate metabolism and decreased diastolic function in nonischemic human diabetic cardiomyopathy: studies with cardiac positron emission tomography and magnetic resonance imaging. J Am Coll Cardiol 54:1524–1532. https://doi.org/10.1016/j.jacc.2009.04.074
Rowe GC, El-Khoury R, Patten IS, Rustin P, Arany Z (2012) PGC-1alpha is dispensable for exercise-induced mitochondrial biogenesis in skeletal muscle. PLoS One 7:e41817. https://doi.org/10.1371/journal.pone.0041817
Safdar A, Little JP, Stokl AJ, Hettinga BP, Akhtar M, Tarnopolsky MA (2011) Exercise increases mitochondrial PGC-1alpha content and promotes nuclear-mitochondrial cross-talk to coordinate mitochondrial biogenesis. J Biol Chem 286:10605–10617. https://doi.org/10.1074/jbc.M110.211466
Schupp M, Kintscher U, Fielitz J, Thomas J, Pregla R, Hetzer R, Unger T, Regitz-Zagrosek V (2006) Cardiac PPARalpha expression in patients with dilated cardiomyopathy. Eur J Heart Fail 8:290–294. https://doi.org/10.1016/j.ejheart.2005.09.003
Selig SE, Carey MF, Menzies DG, Patterson J, Geerling RH, Williams AD, Bamroongsuk V, Toia D, Krum H, Hare DL (2004) Moderate-intensity resistance exercise training in patients with chronic heart failure improves strength, endurance, heart rate variability, and forearm blood flow. J Card Fail 10:21–30
Seo DY, Lee S, Figueroa A, Kwak YS, Kim N, Rhee BD, Ko KS, Bang HS, Baek YH, Han J (2012) Aged garlic extract enhances exercise-mediated improvement of metabolic parameters in high fat diet-induced obese rats. Nutr Res Pract 6:513–519. https://doi.org/10.4162/nrp.2012.6.6.513
Seo DY, Lee SR, Kim N, Ko KS, Rhee BD, Han J (2014) Humanized animal exercise model for clinical implication. Pflugers Arch 466:1673–1687. https://doi.org/10.1007/s00424-014-1496-0
Seo DY, Lee SR, Kim N, Ko KS, Rhee BD, Han J (2016) Age-related changes in skeletal muscle mitochondria: the role of exercise. Integr Med Res 5:182–186. https://doi.org/10.1016/j.imr.2016.07.003
Seo DY, Lee SR, Kwak HB, Seo KW, McGregor RA, Yeo JY, Ko TH, Bolorerdene S, Kim N, Ko KS, Rhee BD, Han J (2016) Voluntary stand-up physical activity enhances endurance exercise capacity in rats. Korean J Physiol Pharmacol 20:287–295. https://doi.org/10.4196/kjpp.2016.20.3.287
Shim CY, Song BW, Cha MJ, Hwang KC, Park S, Hong GR, Kang SM, Lee JE, Ha JW, Chung N (2014) Combination of a peroxisome proliferator-activated receptor-gamma agonist and an angiotensin II receptor blocker attenuates myocardial fibrosis and dysfunction in type 2 diabetic rats. J Diabetes Investig 5:362–371. https://doi.org/10.1111/jdi.12153
Shim IK, Cho KI, Kim HS, Heo JH, Cha TJ (2015) Impact of gender on the association of epicardial fat thickness, obesity, and circadian blood pressure pattern in hypertensive patients. J Diabetes Res 2015:924539. https://doi.org/10.1155/2015/924539
Sparks LM, Johannsen NM, Church TS, Earnest CP, Moonen-Kornips E, Moro C, Hesselink MK, Smith SR, Schrauwen P (2013) Nine months of combined training improves ex vivo skeletal muscle metabolism in individuals with type 2 diabetes. J Clin Endocrinol Metab 98:1694–1702. https://doi.org/10.1210/jc.2012-3874
Suh S, Seo GH, Jung CH, Kim MK, Jin SM, Hwang YC, Lee BW, Kim JH (2015) Increased risk of hospitalization for heart failure with newly prescribed dipeptidyl peptidase-4 inhibitors and pioglitazone using the Korean Health Insurance Claims Database. Diabetes Metab J 39:247–252. https://doi.org/10.4093/dmj.2015.39.3.247
Thu VT, Kim HK, Long le T, Lee SR, Hanh TM, Ko TH, Heo HJ, Kim N, Kim SH, Ko KS, Rhee BD, Han J (2012) NecroX-5 prevents hypoxia/reoxygenation injury by inhibiting the mitochondrial calcium uniporter. Cardiovasc Res 94:342–350. https://doi.org/10.1093/cvr/cvs122
Veeranki S, Givvimani S, Kundu S, Metreveli N, Pushpakumar S, Tyagi SC (2016) Moderate intensity exercise prevents diabetic cardiomyopathy associated contractile dysfunction through restoration of mitochondrial function and connexin 43 levels in db/db mice. J Mol Cell Cardiol 92:163–173. https://doi.org/10.1016/j.yjmcc.2016.01.023
Vogel C, Marcotte EM (2012) Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet 13:227–232. https://doi.org/10.1038/nrg3185
Wang H, Bei Y, Lu Y, Sun W, Liu Q, Wang Y, Cao Y, Chen P, Xiao J, Kong X (2015) Exercise prevents cardiac injury and improves mitochondrial biogenesis in advanced diabetic cardiomyopathy with PGC-1alpha and Akt activation. Cell Physiol Biochem 35:2159–2168. https://doi.org/10.1159/000374021
Wang Q, Ratchford AM, Chi MM, Schoeller E, Frolova A, Schedl T, Moley KH (2009) Maternal diabetes causes mitochondrial dysfunction and meiotic defects in murine oocytes. Mol Endocrinol 23:1603–1612. https://doi.org/10.1210/me.2009-0033
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL (2013) 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 128:1810–1852. https://doi.org/10.1161/CIR.0b013e31829e8807
Zabalgoitia M, Ismaeil MF, Anderson L, Maklady FA (2001) Prevalence of diastolic dysfunction in normotensive, asymptomatic patients with well-controlled type 2 diabetes mellitus. Am J Cardiol 87:320–323
Funding
This work was supported by the grant from the Priority Research Centers Program and Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology (NRF-2010-0020224, NRF-2015R1A2A1A13001900) and Korean Diabetes Association (J.C.W., 2015F2). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All experimental procedures were approved by the Institutional Review Board of Animals, Inje University College of Medicine (approval number: 2011-049).
Electronic supplementary material
ESM 1
(DOCX 77 kb)
Rights and permissions
About this article
Cite this article
Ko, T.H., Marquez, J.C., Kim, H.K. et al. Resistance exercise improves cardiac function and mitochondrial efficiency in diabetic rat hearts. Pflugers Arch - Eur J Physiol 470, 263–275 (2018). https://doi.org/10.1007/s00424-017-2076-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-017-2076-x