Abstract
Renal dysfunction is a frequent finding in patients with acute heart failure (AHF) and an important prognostic factor for adverse outcomes. Worsening of renal function occurs in 30–50 % of patients hospitalised for AHF, and is associated with increased mortality, prolonged hospital stay and increased risk of readmission. Likely mechanisms involved in the decrease in renal function include impaired haemodynamics and activation of neurohormonal factors, such as the renin–angiotensin–aldosterone system, the sympathetic nervous system and the arginine–vasopressin system. Additionally, many drugs currently used to treat AHF have a detrimental effect on renal function. Therefore, pharmacotherapy for AHF should carefully take into account any potential complications related to renal function. Serelaxin, currently in clinical development for the treatment of AHF is a recombinant form of human relaxin-2, identical in structure to the naturally occurring human relaxin-2 peptide hormone that mediates cardiac and renal adaptations during pregnancy. Data from both pre-clinical and clinical studies indicate a potentially beneficial effect of serelaxin on kidney function. In this review, we discuss the mechanisms and impact of impairment of renal function in AHF, and the potential benefits of new therapies, such as serelaxin, in this context.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with acute heart failure (AHF) frequently present with or have a history of renal impairment [1, 2] with a significant impact on outcomes. In a systematic review of 16 studies including 80,098 patients with heart failure (HF), 29 % of patients had moderate to severe renal impairment [3]. This is especially important as renal impairment, assessed using glomerular filtration rate (GFR), is a strong predictor of mortality in patients with both ischaemic and non-ischaemic HF (Fig. 1) [4, 5]. In contrast, lower left ventricular ejection fraction (LVEF) and greater clinical severity of the disease only moderately increase mortality risk [4].
Relationship between left ventricular ejection fraction (LVEF), glomerular filtration rate (GFR) and mortality. Reproduced with permission of Wolters Kluwer Health: Hillege et al. [4]. Data presented as quartiles and assessed using a multivariate proportional hazards regression model. eGFR estimated glomerular filtration rate, LVEF left ventricular ejection fraction
Renal impairment is also associated with an impact on other outcome measures. A retrospective analysis of 33,901 patients with AHF from a large managed care database from the United States of America showed that moderately reduced renal function (GFR <60 ml/min; Stages 3–5, as defined by the Chronic Kidney Disease (CKD)-Epidemiology Collaboration Group) was associated with a higher rate of all-cause readmissions (47 vs. 39 %), HF-related readmissions (31 vs. 21 %) and mortality at 6 months (14 vs. 9 %) when compared with patients with normal/mild reduction in GFR (≥60 ml/min; Stages 1–2) [6].
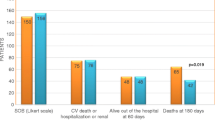
Moreover, worsening renal function (WRF) has been observed in 20–30 % of patients during hospitalisation for AHF [7, 8], and another 12 % of patients develop WRF following discharge [9, 10]. WRF is typically defined as an increase in serum creatinine of ≥0.3 mg/dl from the baseline value [11], although other measures such as reduced estimated GFR (≥25 %) and increased levels of plasma cystatin C (≥0.3 mg/l) and blood urea nitrogen (≥25 %) may also be indicative of WRF [12, 13]. Most recently, a precise terminology and definition of changes in renal function in AHF have been proposed, adapting the criteria of acute kidney injury (AKI) (Table 1) [14]. AKI and WRF are often recognised as distinct entities; AKI is indicative of renal injury, while WRF results from a functional decline in GFR, which may occur in the absence of AKI [14]. Monitoring clinical response(s) and measures of renal function may aid identification of true AKI in patients with AHF [14]. WRF probably reflects organ damage occurring during the acute phase and, in turn, impacts prognosis. For example, studies suggest that WRF is associated with increased duration of hospitalisation and readmission rates and might also be accompanied by increased mortality [3, 15–17]. Indeed, the recent RELAX-AHF study showed that WRF, defined by an increase in cystatin C of ≥0.3 mg/l at Day 2, was associated with increased 180-day mortality (Fig. 2) [18]. It is notable that many current treatments for AHF do not protect the kidney and some, such as diuretics, may even contribute to WRF [13, 19–21]. Clinically, the occurrence of WRF should not be considered in isolation and needs to be taken in the context of the broader profile of the presenting patient—Metra et al. [22] demonstrated that WRF has prognostic utility only in patients who also showed persistent signs of congestion, and not in those without congestion. Furthermore, in a separate analysis of the RELAX-AHF study data, greater incidences of the composite of cardiovascular mortality and/or rehospitalisation for HF or renal failure at Day 60, and cardiovascular mortality alone at Day 180, were observed in patients with both WRF (defined as an increase in serum creatinine of ≥0.3 mg/dl at Day 4/5) and poor diuretic response (lower than the median response; defined as weight loss per unit of diuretic dose) at Day 5, compared with patients with WRF and good diuretic response, as well as patients without WRF with both good and poor diuretic responses (interactions not significant) [23]. This suggests that a good diuretic response may be indicative of a lower risk of cardiovascular mortality and hospitalisation for HF or renal failure in patients with WRF.
Cumulative risk of all-cause death through Day 180 in the RELAX-AHF study. Patients subdivided by acute changes in renal function (cystatin C) from baseline to Day 2. Reproduced with permission of Elsevier open access licence: Metra et al. [18]
In this review, we consider the mechanisms and impact of renal impairment and WRF in AHF, and the potential benefits of new therapies, such as serelaxin, in this context.
Mechanisms underlying the development of renal impairment and worsening of renal function in AHF
Renal and cardiac dysfunction are closely related, forming the basis of the concept of the cardiorenal syndrome, which has been described in detail elsewhere [24]. The complex cross-talk between the two organs is important for the control of blood pressure, sodium and water excretion, arterial perfusion, tissue oxygenation and extracellular fluid balance [24, 25]. Thus, dysfunction of one organ may adversely affect the function of the other organ.
Renal impairment in patients presenting with AHF
It is likely that much of the renal impairment observed in patients presenting with AHF, including de novo AHF and decompensation in patients with chronic HF (CHF), is caused by comorbidities or risk factors, such as hypertension, diabetes mellitus, obesity, smoking or renal atherosclerosis [26, 27]. Kidney damage through the activation of inflammatory cytokines has been observed in patients with atherosclerotic disease [26]. Hypertension and diabetes are associated primarily with glomerular damage, which may be caused by impaired renal autoregulation increasing renal sensitivity to fluctuations in blood pressure and leading to impaired GFR. Disease progression is associated with the occurrence of glomerulosclerosis, interstitial fibrosis and the loss of nephrons. These conditions lead to water and sodium retention resulting in the upregulation of compensatory pathways, such as the renin–angiotensin–aldosterone system (RAAS), the sympathetic nervous system and activation of the calcium-parathyroid axis [28, 29]. Additionally, the loss of nephrons contributes to exacerbation of existing hypertension and to pressure and volume overload [29]. A consequence of the upregulation of compensatory pathways is the release of profibrotic factors, such as galectin-3 and tumour growth factor-β (TGF-β), which may contribute to the development of additional cardiovascular complications [29].
During an AHF event, systemic vasoconstriction and decreased cardiac function can lead to increased cardiac pre- and afterload and increased left ventricular filling pressures [10, 30]. This, in turn, leads to congestion and hypoperfusion, with neurohormonal activation, inflammation, oxidative stress and haemodynamic abnormalities, leading to organ dysfunction, including renal dysfunction [31–33]. Impaired renal function contributes to water and sodium retention and further impacts on cardiac function as described above [10].
Renal impairment and WRF during AHF can therefore be considered to be part of a multifactorial process which involves several mechanisms (Fig. 3) [34]. The worsening may result from haemodynamic or neurohormonal disturbances occurring during the AHF episode [10, 24].
Mechanisms involved in the impairment of renal function associated with heart failure. Reproduced by permission of Oxford University Press and the American College of Cardiology Foundation: Metra et al. [34]
Haemodynamic disturbances
Several regulatory mechanisms control renal blood flow and the glomerular filtration pressure through vasoconstriction and vasodilation of the afferent and/or efferent arterioles, allowing the kidney to maintain GFR and renal blood flow under various physiological conditions. However, the kidney is susceptible to haemodynamic changes, such as reduced cardiac output, frequent drops in systolic blood pressure and elevated central venous pressure, all of which are observed in AHF [33–36]. Reduced cardiac output results in systemic and renal hypoperfusion, leading to the release of renin by juxtaglomerular cells in the afferent arterioles [15]. As a consequence, the RAAS is activated and the sympathetic nervous system is stimulated by angiotensin II. This leads to sodium and water retention, volume expansion, increased systemic vascular resistance and ventricular remodelling [37]. Additionally, an increase in central venous pressure (backward HF) leads to an elevated glomerular efferent arteriole pressure, and is accompanied by a reduction in the glomerular filtration pressure gradient, fall in GFR, and also sodium and water retention [11, 38–40]. Reduced renal function also impacts the clearance of metabolic waste products such as uric acid, a by-product of purine metabolism [41]. Uric acid serves as a good indicator of oxidative stress and tissue damage. Elevated levels may indirectly cause endothelial dysfunction and impair regulation of vascular tone. Additionally, increased levels of uric acid are associated with inflammation, morphological and functional changes in the glomeruli and renal arteriole and increased salt-sensitivity—hyperuricemic or salt-sensitive kidney-dependent hypertension.
Neurohormonal activation
Initially in patients with HF, over-activity of the sympathetic nervous system with elevated levels of circulating catecholamines (in particular, epinephrine and norepinephrine) and RAAS hormones (angiotensin II and aldosterone), represents a useful compensatory mechanism for maintaining homoeostasis of the circulatory flow in the context of declining cardiac function [11, 34, 42, 43]. However, with progression of HF, maladaptive changes occur with excessive increase of these hormones, which leads to vasoconstriction and volume dysregulation. This, in turn, results in an increase of peripheral vascular resistance and a further deterioration of LV function, initiating a vicious cycle. Although vasoconstriction is accompanied by a simultaneous increase in vasodilating hormones, such as natriuretic peptides, prostacyclin and nitric oxide, the effect is not strong enough to offset the overall detrimental increase in peripheral vascular resistance [11, 34, 44].
The effect of current pharmacological therapy for AHF on renal function
Current treatment guidelines for AHF recommend the use of diuretics, vasodilators and natriuretic peptides as well as other pharmacological agents [45, 46]. However, many of these agents have a profound impact on renal function (Table 2) [34, 45, 46]. For example, loop diuretics have been implicated in WRF, via mechanisms of arterial underfilling, activated tubuloglomerular feedback with vasoconstriction of the vas afferens and neurohormonal activation [34, 46]. They may impair the kidneys’ ability to excrete and dilute urine. They are further associated with an immediate decrease of GFR and an increase in serum creatinine [22, 34]. Vasopressin-2 receptor antagonists have potentially beneficial long-term effects; however, they may impair renal function by arterial underfilling [34]. Vasodilators, such as nitrates, and natriuretic peptides, such as nesiritide, cause a drop in blood pressure and hypotension; treatment with vasodilators can also lead to an increase in serum creatinine [34, 47]. Nesiritide may provide early relief of dyspnoea in patients with AHF, and while early analyses indicated possible concerns regarding renal impairment, analysis of the ASCEND-HF study reported no increase in the incidence of WRF (defined as an increase of serum creatinine of >0.3 mg/dl and a change of ≥25 %) with nesiritide vs. placebo [48, 49]. Not all agents for the treatment of AHF have been reported to worsen renal function. Intravenous inotropic agents that increase cardiac output and renal perfusion may decrease creatinine levels and reduce the need for diuretics [34, 45]; however, the use of inotropic agents is limited due to possible induction of myocardial ischaemia and arrhythmia, and concerns regarding increased mortality [45, 50, 51]. In addition, treatment with levosimendan, an inodilator, has been shown to improve renal blood flow and/or GFR in patients with AHF, when compared with placebo [52] and dobutamine [53]. Reduced serum creatinine levels have also been reported following levosimendan treatment [52].
Interestingly, non-pharmacological treatment of AHF may also influence renal function. Ultrafiltration, which is recommended for the treatment of patients with AHF who are resistant to diuretic therapy [45, 46], was associated with increased creatinine levels in patients with AHF, persistent congestion and worsened renal function (defined as an increase in serum creatinine of ≥0.3 mg/dl), when compared with stepped pharmacological therapy, which included the use of diuretics, vasodilators and inotropic agents [54].
There has been some debate that short-term changes in renal function (particularly when assessed using serum creatinine levels) are less related to outcomes than deterioration of kidney function that occurs over longer time periods. In theory, the latter are more likely to be related to neurohormonal activation leading to nephron loss, renal fibrosis and permanent renal impairment [34]. However, a recent study in patients with AHF, included in the ADHERE registry and linked to Medicare claims in the USA, suggests that both transient and persistent WRF, assessed using serum creatinine and defined as any increase in serum creatinine ≥0.3 mg/dl from admission, are associated with significantly higher 90-day post-admission mortality compared with no WRF [55]. Furthermore, transient increases in serum creatinine have been shown to be related to longer cumulative length of hospital stay and higher costs than either no WRF or persistent WRF in 55,436 patients with AHF included in a large database [56]. These new findings suggest that the WRF events observed with some current therapies for AHF are clinically relevant.
Therefore, key treatment goals in patients with AHF are the amelioration of symptoms, the improvement of haemodynamics and the preservation of organ function in order to improve short- and long-term outcomes [34, 45]. Given that current treatment strategies for AHF may contribute to WRF, it is important that the therapeutic considerations also account for their potential impact on short- and long-term renal function.
Serelaxin: potential benefits on renal function
Serelaxin is a recombinant form of human relaxin-2, a naturally occurring peptide hormone that mediates cardiac and renal adaptations during pregnancy [57]. Studies in both animals and humans have shown that serelaxin receptors are located in the heart tissue, blood vessels and the kidneys [57–60]. Binding of serelaxin to its G-protein-coupled receptors (known as relaxin family peptide or RFXP receptors) initiates multiple signalling pathways with systemic and renal haemodynamic effects [61]. Binding of serelaxin to the RFXP1 receptor on endothelial cells has been shown to mediate systemic arterial and renal vasorelaxation via the release of nitric oxide (NO) (the term vasorelaxation is used to differentiate serelaxin from classical vasodilators such as nitrates, which mediate systemic vasodilation, while the vascular effects of serelaxin appear to be specific to certain vascular beds [62]) [63]. In pre-clinical studies, serelaxin has been shown to have positive effects on pulmonary congestion and the symptoms of AHF. Furthermore, anti-inflammatory, anti-oxidant, anti-apoptotic cell death, anti-fibrotic and pro-angiogenic effects have been reported, all of which may contribute to organ protection [64–73].
These studies suggested that serelaxin has beneficial effects in the kidneys, protecting them from damage and remodelling. In an animal study in rats, serelaxin was found to increase both GFR and effective renal plasma flow [74]. Additionally, serelaxin treatment attenuated vasoconstriction initiated by medical angiotensin II [74]. Similarly, in another study in rats, serelaxin increased GFR as well as impacting renal circulation (by causing renal vasorelaxation and hyperfiltration) and osmoregulation (by reducing plasma osmolality and sodium concentration) [74]. A third study of long-term administration of serelaxin to rats demonstrated increases in GFR and effective renal plasma flow, with a decrease in effective renal vascular resistance [75]. Additionally, a significant decrease in glomerular and tubular collagen deposition was observed [75].
Subsequently, renal protective effects of serelaxin have been observed in humans. A study in healthy volunteers treated with serelaxin (0.2 µg/kg bolus followed by 0.5 µg/kg/h infusion for 4 h) demonstrated an increase of 47 % in renal blood flow when compared with baseline levels (p < 0.0001) within 30 min [76]. Furthermore, in a phase II study in patients with CHF, a 24-h infusion of serelaxin 30 µg/kg/day led to a significant increase in renal plasma flow (time-weighted average ratio to baseline in serelaxin-treated patients was 1.31 as compared with 1.13 in placebo-treated patients; p = 0.004) [77]. In both studies, the increase in renal plasma flow was not associated with a change in GFR. In addition, in the patients with CHF treated with serelaxin, there was a small reduction in the filtration fraction as compared with placebo (time-weighted average change from baseline, 1.20 in serelaxin-treated patients and 1.44 in placebo-treated patients; p = 0.0004), suggesting a reduction in intraglomerular pressure, which may preserve renal function [77]. Serelaxin may mediate these improvements in renal function via direct vasorelaxation of afferent and efferent renal vessels, through activation of the endothelial type B receptor and release of NO [63, 78]. Significant reductions in serum creatinine were observed in another open-label, single-centre, pilot study in patients with CHF treated with serelaxin (30 µg/kg/day for 8 h; p < 0.05 vs. baseline) [79]. This study also showed trends towards an increase in the cardiac index as well as a decrease in pulmonary wedge pressure and circulating N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels, the latter findings being consistent with decreased cardiac stress [79]. Consequently, the beneficial effects of serelaxin treatment on renal function may result, in part, from improved decongestion.
A recent double-blind, multicentre phase II study in 71 patients with AHF evaluated the haemodynamic effects of serelaxin treatment and assessed the impact on renal function in 34 patients as compared with placebo (n = 37) [80]. Treatment with serelaxin resulted in an 11 % increase in creatinine clearance as compared with a 21 % decrease in the placebo-treated group during the treatment period. Both groups of patients had similar creatinine clearance rates at baseline. Treatment with serelaxin resulted in an increase of 20 % from baseline in creatinine clearance values; in the placebo-treated group, creatinine clearance decreased by 24 % from baseline, indicating a significant treatment difference of 39 %. Urine flow rates decreased in both treatment groups as compared with baseline and were deemed related to the study design by the authors as patients received intravenous loop diuretic administration 4 h prior to treatment initiation and after 8 h of treatment.
The largest study to date, RELAXin in AHF (RELAX-AHF), was a phase III, multicentre, randomised, double-blind, placebo-controlled study designed to investigate the efficacy and safety of serelaxin in the treatment of AHF in patients with mild-to-moderate renal insufficiency [defined as estimated (e)GFR 30–75 ml/min/1.73 m2] [81]. Patients included in the RELAX-AHF study were hospitalised for AHF with systolic blood pressure >125 mmHg, increased levels of NT-proBNP, and were randomised within 16 h of presentation to treatment with either serelaxin (30 μg/kg/day as a 48-h intravenous infusion) or placebo in addition to treatment with standard of care [81]. The study primary endpoints were assessment of dyspnoea improvement from baseline in the visual analogue scale area under the curve (VAS AUC) to Day 5 and the proportion of patients with moderate or marked dyspnoea improvement measured by the Likert scale. In the study, serelaxin treatment improved the VAS AUC primary dyspnoea endpoint as compared with placebo (p = 0.007), but had no effect on the second primary endpoint as assessed by the Likert scale (p = 0.70) [81]. Serelaxin was associated with significant reductions in early (in-hospital) worsening of heart failure (p < 0.001 as compared with placebo), signs and symptoms of congestion, initial length of hospital stay and duration of intensive care treatment [81]. In serelaxin-treated patients, cardiovascular and all-cause mortality at Day 180 were significantly reduced by 37 % at 6 months [81]. Additionally, serelaxin treatment was associated with a significant reduction in the use of loop diuretics and fewer patients treated with serelaxin had adverse events related to renal impairment as compared with placebo [81].
Interestingly, a reduced incidence of WRF (defined as increases in serum creatinine and plasma cystatin C at Day 2 of ≥0.3 mg/dl and ≥0.3 mg/l, respectively) as well as lower levels of biomarkers that are indicative of renal dysfunction, specifically tubular necrosis [creatinine and cystatin C (markers of GFR) and urea (general marker of renal function)] were observed in serelaxin-treated patients compared to placebo (Fig. 4) [18]. This effect on biomarkers of renal dysfunction may help to explain the underlying mechanisms for improved mortality in RELAX-AHF. The observed reduction in 180-day cardiovascular and all-cause mortality in the serelaxin group was more pronounced in the subgroup of patients with moderate renal impairment (GFR <60 ml/min/1.73 m2) (Table 3) [81, 82]. Taken together, these data from pre-clinical and clinical serelaxin studies provide further support for the protective effects of serelaxin in the kidneys.
Change from baseline in biomarkers of renal dysfunction in patients treated with serelaxin or placebo in the RELAX-AHF study. Reproduced with permission of Elsevier open access licence: Metra et al. [18]. a–c *p < 0.05, **p < 0.005, and ***p < 0.001 by 2-sided, 2-sample t-test for serelaxin versus placebo. d *p < 0.05, **p < 0.005, and ***p < 0.001 versus placebo by repeated measures analysis of variance with adjustment for baseline value. BUN blood urea nitrogen
Serelaxin: ongoing studies and current status
Serelaxin was approved by the Ministry of Health in Russia in 2014; additional data were requested by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) Committee for Medicinal Products for Human Use (CHMP) following initial submissions for approval [83]. An additional Phase III trial, RELAX-AHF 2, began in September 2013 to further determine the effects of serelaxin on cardiovascular mortality in 6375 patients with AHF [84]. The results of the RELAX-AHF-2 trial are expected in 2016 [84]. Further studies in patients with AHF are ongoing, including the geographically specific RELAX-AHF-EU and RELAX-AHF-Asia studies [85, 86]. These studies include endpoints assessing effects on renal function that will further inform understanding of the effects of serelaxin [84–86].
Conclusions
Renal impairment and WRF during hospitalisation are common in patients presenting with AHF. Impairment of renal function is associated with adverse outcomes and should be considered as part of patient management. Current therapies for the treatment of AHF patients do not include renal protective measures. Rather, some drugs may even contribute to WRF. Interestingly, serelaxin exhibits potentially beneficial effects on mortality and symptom amelioration and on renal function in patients with AHF. Thus, serelaxin has the potential not only to act as a potent treatment in AHF, but also to prevent WRF.
References
Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J (2007) High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail 13(6):422–430
Maggioni AP, Dahlstrom U, Filippatos G, Chioncel O, Crespo LM, Drozdz J, Fruhwald F, Gullestad L, Logeart D, Fabbri G, Urso R, Metra M, Parissis J, Persson H, Ponikowski P, Rauchhaus M, Voors AA, Nielsen OW, Zannad F, Tavazzi L (2013) EURObservational Research Programme: regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot). Eur J Heart Fail 15(7):808–817
Smith GL, Lichtman JH, Bracken MB, Shlipak MG, Phillips CO, DiCapua P, Krumholz HM (2006) Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol 47(10):1987–1996
Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A, Hampton JR, van Veldhuisen DJ (2000) Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation 102(2):203–210
Smilde TD, Hillege HL, Navis G, Boomsma F, de Zeeuw D, van Veldhuisen DJ (2004) Impaired renal function in patients with ischemic and nonischemic chronic heart failure: association with neurohormonal activation and survival. Am Heart J 148(1):165–172
Swindle J, Chan W, Johnson KW, Becker L, Blauer-Peterson C, Riedel A (2013) Renal impairment in acute heart failure: insights from a managed care database. Circulation 128:A12097
Forman DE, Butler J, Wang Y, Abraham WT, O’Connor CM, Gottlieb SS, Loh E, Massie BM, Rich MW, Stevenson LW, Young JB, Krumholz HM (2004) Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol 43(1):61–67
Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M, Yancy CW, Califf RM, Stevenson LW, Hill JA (2008) Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol 51(13):1268–1274
Blair JE, Pang PS, Schrier RW, Metra M, Traver B, Cook T, Campia U, Ambrosy A, Burnett JC Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Konstam MA, Gheorghiade M (2011) Changes in renal function during hospitalization and soon after discharge in patients admitted for worsening heart failure in the placebo group of the EVEREST trial. Eur Heart J 32(20):2563–2572
Gheorghiade M, Pang PS (2009) Acute heart failure syndromes. J Am Coll Cardiol 53(7):557–573
Carubelli V, Metra M, Lombardi C, Bettari L, Bugatti S, Lazzarini V, Dei CL (2012) Renal dysfunction in acute heart failure: epidemiology, mechanisms and assessment. Heart Fail Rev 17(2):271–282
Dupont M, Shrestha K, Singh D, Finucan M, Tang WH (2013) Lack of concordance in defining worsening renal function by rise in creatinine vs rise in cystatin C. Congest Heart Fail 19(4):E17–E21
Klein L, Massie BM, Leimberger JD, O’Connor CM, Pina IL, Adams KF Jr, Califf RM, Gheorghiade M (2008) Admission or changes in renal function during hospitalization for worsening heart failure predict postdischarge survival: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF). Circ Heart Fail 1(1):25–33
Damman K, Tang WH, Testani JM, McMurray JJ (2014) Terminology and definition of changes renal function in heart failure. Eur Heart J 35(48):3413–3416
Damman K, Valente MA, Voors AA, O’Connor CM, van Veldhuisen DJ, Hillege HL (2014) Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J 35(7):455–469
Kociol RD, Greiner MA, Hammill BG, Phatak H, Fonarow GC, Curtis LH, Hernandez AF (2010) Long-term outcomes of medicare beneficiaries with worsening renal function during hospitalization for heart failure. Am J Cardiol 105(12):1786–1793
Testani JM, McCauley BD, Chen J, Shumski M, Shannon RP (2010) Worsening renal function defined as an absolute increase in serum creatinine is a biased metric for the study of cardio-renal interactions. Cardiology 116(3):206–212
Metra M, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF Jr, Dorobantu MI, Grinfeld L, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Prescott MF, Edwards C, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin T, Teerlink JR (2013) Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the Relaxin in Acute Heart Failure (RELAX-AHF) development program: correlation with outcomes. J Am Coll Cardiol 61(2):196–206
Butler J, Forman DE, Abraham WT, Gottlieb SS, Loh E, Massie BM, O’Connor CM, Rich MW, Stevenson LW, Wang Y, Young JB, Krumholz HM (2004) Relationship between heart failure treatment and development of worsening renal function among hospitalized patients. Am Heart J 147(2):331–338
Llorens P, Miro O, Herrero P, Martin-Sanchez FJ, Jacob J, Valero A, Alonso H, Perez-Dura MJ, Noval A, Gil-Roman JJ, Zapater P, Llanos L, Gil V, Perello R (2014) Clinical effects and safety of different strategies for administering intravenous diuretics in acutely decompensated heart failure: a randomised clinical trial. Emerg Med J 31(9):706–713
Rossignol P, Dobre D, McMurray JJ, Swedberg K, Krum H, van Veldhuisen DJ, Shi H, Messig M, Vincent J, Girerd N, Bakris G, Pitt B, Zannad F (2014) Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF). Circ Heart Fail 7(1):51–58
Metra M, Davison B, Bettari L, Sun H, Edwards C, Lazzarini V, Piovanelli B, Carubelli V, Bugatti S, Lombardi C, Cotter G, Dei CL (2012) Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ Heart Fail 5(1):54–62
Voors AA, Davison BA, Teerlink JR, Felker GM, Cotter G, Filippatos G, Greenberg BH, Pang PS, Levin B, Hua TA, Severin T, Ponikowski P, Metra M (2014) Diuretic response in patients with acute decompensated heart failure: characteristics and clinical outcome—an analysis from RELAX-AHF. Eur J Heart Fail 16(11):1230–1240
Ronco C, Haapio M, House AA, Anavekar N, Bellomo R (2008) Cardiorenal syndrome. J Am Coll Cardiol 52(19):1527–1539
McCullough PA, Haapio M, Mankad S, Zamperetti N, Massie B, Bellomo R, Berl T, Anker SD, Anand I, Aspromonte N, Bagshaw SM, Bobek I, Cruz DN, Daliento L, Davenport A, Hillege H, House AA, Katz N, Maisel A, Mebazaa A, Palazzuoli A, Ponikowski P, Ronco F, Shaw A, Sheinfeld G, Soni S, Vescovo G, Zanco P, Ronco C, Berl T (2010) Prevention of cardio-renal syndromes: workgroup statements from the 7th ADQI consensus conference. Nephrol Dial Transplant 25(6):1777–1784
Cleland JG, Carubelli V, Castiello T, Yassin A, Pellicori P, Antony R (2012) Renal dysfunction in acute and chronic heart failure: prevalence, incidence and prognosis. Heart Fail Rev 17(2):133–149
House AA, Anand I, Bellomo R, Cruz D, Bobek I, Anker SD, Aspromonte N, Bagshaw S, Berl T, Daliento L, Davenport A, Haapio M, Hillege H, McCullough P, Katz N, Maisel A, Mankad S, Zanco P, Mebazaa A, Palazzuoli A, Ronco F, Shaw A, Sheinfeld G, Soni S, Vescovo G, Zamperetti N, Ponikowski P, Ronco C (2010) Definition and classification of cardio-renal syndromes: workgroup statements from the 7th ADQI consensus conference. Nephrol Dial Transplant 25(5):1416–1420
Bidani AK, Griffin KA, Epstein M (2012) Hypertension and chronic kidney disease progression: why the suboptimal outcomes? Am J Med 125(11):1057–1062
Tumlin JA, Costanzo MR, Chawla LS, Herzog CA, Kellum JA, McCullough PA, Ronco C (2013) Cardiorenal syndrome type 4: insights on clinical presentation and pathophysiology from the eleventh consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contrib Nephrol 182:158–173
Biolo A, Fisch M, Balog J, Chao T, Schulze PC, Ooi H, Siwik D, Colucci WS (2010) Episodes of acute heart failure syndrome are associated with increased levels of troponin and extracellular matrix markers. Circ Heart Fail 3(1):44–50
Tsutsui H, Kinugawa S, Matsushima S (2011) Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol 301(6):H2181–H2190
Guazzi M, Gatto P, Giusti G, Pizzamiglio F, Previtali I, Vignati C, Arena R (2013) Pathophysiology of cardiorenal syndrome in decompensated heart failure: role of lung–right heart–kidney interaction. Int J Cardiol 169(6):379–384
Damman K, Navis G, Smilde TD, Voors AA, van der Bij W, van Veldhuisen DJ, Hillege HL (2007) Decreased cardiac output, venous congestion and the association with renal impairment in patients with cardiac dysfunction. Eur J Heart Fail 9(9):872–878
Metra M, Cotter G, Gheorghiade M, Dei CL, Voors AA (2012) The role of the kidney in heart failure. Eur Heart J 33(17):2135–2142
Uthoff H, Breidthardt T, Klima T, Aschwanden M, Arenja N, Socrates T, Heinisch C, Noveanu M, Frischknecht B, Baumann U, Jaeger KA, Mueller C (2011) Central venous pressure and impaired renal function in patients with acute heart failure. Eur J Heart Fail 13(4):432–439
Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL (2009) Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol 53(7):582–588
Gheorghiade M, De LL, Fonarow GC, Filippatos G, Metra M, Francis GS (2005) Pathophysiologic targets in the early phase of acute heart failure syndromes. Am J Cardiol 96(6A):11G–17G
Dini FL, Demmer RT, Simioniuc A, Morrone D, Donati F, Guarini G, Orsini E, Caravelli P, Marzilli M, Colombo PC (2012) Right ventricular dysfunction is associated with chronic kidney disease and predicts survival in patients with chronic systolic heart failure. Eur J Heart Fail 14(3):287–294
Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WH (2009) Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 53(7):589–596
Shamseddin MK, Parfrey PS (2009) Mechanisms of the cardiorenal syndromes. Nat Rev Nephrol 5(11):641–649
Kanbay M, Segal M, Afsar B, Kang DH, Rodriguez-Iturbe B, Johnson RJ (2013) The role of uric acid in the pathogenesis of human cardiovascular disease. Heart 99(11):759–766
Lymperopoulos A (2013) Physiology and pharmacology of the cardiovascular adrenergic system. Front Physiol 4:240
Dzau VJ, Colucci WS, Hollenberg NK, Williams GH (1981) Relation of the renin–angiotensin–aldosterone system to clinical state in congestive heart failure. Circulation 63(3):645–651
Shah BN, Greaves K (2010) The cardiorenal syndrome: a review. Int J Nephrol 2011:920195
McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A (2012) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 33(14):1787–1847
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL (2013) 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 128(16):e240–e327
O’Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF Jr, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalan R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Mendez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM (2011) Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med 365(1):32–43
Sackner-Bernstein JD, Skopicki HA, Aaronson KD (2005) Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation 111(12):1487–1491
van Deursen V, Hernandez AF, Stebbins A, Hasselblad V, Ezekowitz JA, Califf RM, Gottlieb SS, O’Connor CM, Starling RC, Tang WH, McMurray JJ, Dickstein K, Voors AA (2014) Nesiritide, renal function, and associated outcomes during hospitalization for acute decompensated heart failure: results from the Acute Study of Clinical Effectiveness of Nesiritide and Decompensated Heart Failure (ASCEND-HF). Circulation 130(12):958–965
O’Connor CM, Gattis WA, Uretsky BF, Adams KF Jr, McNulty SE, Grossman SH, McKenna WJ, Zannad F, Swedberg K, Gheorghiade M, Califf RM (1999) Continuous intravenous dobutamine is associated with an increased risk of death in patients with advanced heart failure: insights from the Flolan International Randomized Survival Trial (FIRST). Am Heart J 138(1 Pt 1):78–86
Tacon CL, McCaffrey J, Delaney A (2012) Dobutamine for patients with severe heart failure: a systematic review and meta-analysis of randomised controlled trials. Intensive Care Med 38(3):359–367
Fedele F, Bruno N, Brasolin B, Caira C, D’Ambrosi A, Mancone M (2014) Levosimendan improves renal function in acute decompensated heart failure: possible underlying mechanisms. Eur J Heart Fail 16(3):281–288
Yilmaz MB, Yalta K, Yontar C, Karadas F, Erdem A, Turgut OO, Yilmaz A, Tandogan I (2007) Levosimendan improves renal function in patients with acute decompensated heart failure: comparison with dobutamine. Cardiovasc Drugs Ther 21(6):431–435
Bart BA, Goldsmith SR, Lee KL, Givertz MM, O’Connor CM, Bull DA, Redfield MM, Deswal A, Rouleau JL, LeWinter MM, Ofili EO, Stevenson LW, Semigran MJ, Felker GM, Chen HH, Hernandez AF, Anstrom KJ, McNulty SE, Velazquez EJ, Ibarra JC, Mascette AM, Braunwald E (2012) Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 367(24):2296–2304
Krishnamoorthy A, Greiner MA, Sharma PP, DeVore AD, Johnson KW, Fonarow GC, Curtis LH, Hernandez AF (2014) Transient and persistent worsening renal function during hospitalization for acute heart failure. Am Heart J 168(6):891–900
Palmer JB, Friedman HS, Waltman Johnson K, Navaratnam P, Gottlieb SS (2014) Association of worsening renal function with length of stay and costs in patients hospitalized with acute heart failure. J Card Fail 20(Suppl.):S50–S51
Teichman SL, Unemori E, Dschietzig T, Conrad K, Voors AA, Teerlink JR, Felker GM, Metra M, Cotter G (2009) Relaxin, a pleiotropic vasodilator for the treatment of heart failure. Heart Fail Rev 14(4):321–329
Hsu SY, Nakabayashi K, Nishi S, Kumagai J, Kudo M, Sherwood OD, Hsueh AJ (2002) Activation of orphan receptors by the hormone relaxin. Science 295(5555):671–674
Kohsaka T, Min G, Lukas G, Trupin S, Campbell ET, Sherwood OD (1998) Identification of specific relaxin-binding cells in the human female. Biol Reprod 59(4):991–999
Novak J, Parry LJ, Matthews JE, Kerchner LJ, Indovina K, Hanley-Yanez K, Doty KD, Debrah DO, Shroff SG, Conrad KP (2006) Evidence for local relaxin ligand-receptor expression and function in arteries. FASEB J 20(13):2352–2362
Díez J (2014) Serelaxin: a novel therapy for acute heart failure with a range of hemodynamic and non-hemodynamic actions. Am J Cardiovasc Drugs 14:275–285
Jelinic M, Leo CH, Post Uiterweer ED, Sandow SL, Gooi JH, Wlodek ME, Conrad KP, Parkington H, Tare M, Parry LJ (2014) Localization of relaxin receptors in arteries and veins, and region-specific increases in compliance and bradykinin-mediated relaxation after in vivo serelaxin treatment. FASEB J 28(1):275–287
McGuane JT, Debrah JE, Sautina L, Jarajapu YP, Novak J, Rubin JP, Grant MB, Segal M, Conrad KP (2011) Relaxin induces rapid dilation of rodent small renal and human subcutaneous arteries via PI3 kinase and nitric oxide. Endocrinology 152(7):2786–2796
Du XJ, Bathgate RA, Samuel CS, Dart AM, Summers RJ (2010) Cardiovascular effects of relaxin: from basic science to clinical therapy. Nat Rev Cardiol 7(1):48–58
Teichman SL, Unemori E, Teerlink JR, Cotter G, Metra M (2010) Relaxin: review of biology and potential role in treating heart failure. Curr Heart Fail Rep 7(2):75–82
Danielson LA, Kercher LJ, Conrad KP (2000) Impact of gender and endothelin on renal vasodilation and hyperfiltration induced by relaxin in conscious rats. Am J Physiol Regul Integr Comp Physiol 279(4):R1298–R1304
Boehnert MU, Hilbig H, Armbruster FP (2005) Relaxin as an additional protective substance in preserving and reperfusion solution for liver transplantation, shown in a model of isolated perfused rat liver. Ann N Y Acad Sci 1041:434–440
Boehnert MU, Armbruster FP, Hilbig H (2009) Relaxin as a protective substance in the preserving solution for liver transplantation: spectrophotometric in vivo imaging of local oxygen supply in an isolated perfused rat liver model. Ann N Y Acad Sci 1160:320–321
Collino M, Rogazzo M, Pini A, Benetti E, Rosa AC, Chiazza F, Fantozzi R, Bani D, Masini E (2013) Acute treatment with relaxin protects the kidney against ischaemia/reperfusion injury. J Cell Mol Med 17(11):1494–1505
Samuel CS, Zhao C, Bathgate RA, Du XJ, Summers RJ, Amento EP, Walker LL, McBurnie M, Zhao L, Tregear GW (2005) The relaxin gene-knockout mouse: a model of progressive fibrosis. Ann N Y Acad Sci 1041:173–181
Samuel CS, Cendrawan S, Gao XM, Ming Z, Zhao C, Kiriazis H, Xu Q, Tregear GW, Bathgate RA, Du XJ (2011) Relaxin remodels fibrotic healing following myocardial infarction. Lab Invest 91(5):675–690
Sasser JM, Molnar M, Baylis C (2011) Relaxin ameliorates hypertension and increases nitric oxide metabolite excretion in angiotensin II but not N(omega)-nitro-l-arginine methyl ester hypertensive rats. Hypertension 58(2):197–204
Segal MS, Sautina L, Li S, Diao Y, Agoulnik AI, Kielczewski J, McGuane JT, Grant MB, Conrad KP (2012) Relaxin increases human endothelial progenitor cell NO and migration and vasculogenesis in mice. Blood 119(2):629–636
Danielson LA, Sherwood OD, Conrad KP (1999) Relaxin is a potent renal vasodilator in conscious rats. J Clin Invest 103(4):525–533
Danielson LA, Welford A, Harris A (2006) Relaxin improves renal function and histology in aging Munich Wistar rats. J Am Soc Nephrol 17(5):1325–1333
Smith MC, Danielson LA, Conrad KP, Davison JM (2006) Influence of recombinant human relaxin on renal hemodynamics in healthy volunteers. J Am Soc Nephrol 17(11):3192–3197
Voors AA, Dahlke M, Meyer S, Stepinska J, Gottlieb SS, Jones A, Zhang Y, Laurent D, Slart RH, Navis GJ (2014) Renal hemodynamic effects of serelaxin in patients with chronic heart failure: a randomized, placebo-controlled study. Circ Heart Fail 7:994–1002
Conrad KP, Shroff SG (2011) Effects of relaxin on arterial dilation, remodeling, and mechanical properties. Curr Hypertens Rep 13(6):409–420
Dschietzig T, Teichman S, Unemori E, Wood S, Boehmer J, Richter C, Baumann G, Stangl K (2009) Intravenous recombinant human relaxin in compensated heart failure: a safety, tolerability, and pharmacodynamic trial. J Card Fail 15(3):182–190
Ponikowski P, Mitrovic V, Ruda M, Fernandez A, Voors AA, Vishnevsky A, Cotter G, Milo O, Laessing U, Zhang Y, Dahlke M, Zymlinski R, Metra M (2014) A randomized, double-blind, placebo-controlled, multicentre study to assess haemodynamic effects of serelaxin in patients with acute heart failure. Eur Heart J 35(7):431–441
Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF Jr, Dorobantu MI, Grinfeld LR, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin TM, Metra M (2013) Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet 381(9860):29–39
Metra M, Ponikowski P, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Hua TA, Severin T, Unemori E, Voors AA, Teerlink JR (2013) Effects of serelaxin in subgroups of patients with acute heart failure: results from RELAX-AHF. Eur Heart J 34(40):3128–3136
Novartis Pharmaceuticals (2015). http://www.novartis.com/downloads/newsroom/corporate-fact-sheet/2a_Pharmaceuticals_EN.pdf. Accessed 19 Feb 2015
Clinicaltrials.gov. NCT01870778 (2015) Efficacy, safety and tolerability of serelaxin when added to standard therapy in AHF. https://clinicaltrials.gov/ct2/show/NCT01870778. Accessed 19 Feb 2015
Clinicaltrials.gov. NCT02064868 (2014) Effect of serelaxin versus standard of care in acute heart failure (AHF) patients (RELAX-AHF-EU) http://www.clinicaltrials.gov/ct2/show/NCT02064868?term=NCT02064868&rank=1. Accessed 19 Feb 2015
Clinicaltrials.gov. NCT02007720 (2014) Efficacy, Safety and tolerability of sexelaxin when added to standard therapy in AHF. http://www.clinicaltrials.gov/ct2/show/NCT02007720?term=NCT02007720&rank=1. Accessed 19 Feb 2015
Acknowledgments
We thank Minal Kotecha of CircleScience, an Ashfield Company, part of UDG Healthcare plc, for providing writing assistance, which was funded by Novartis Pharma AG, Basel, Switzerland. The authors did not receive any funding grants to support the development of this manuscript.
Conflict of interest
Roland E Schmieder: Received Speakers Bureau honoraria and is an advisory board member for Novartis. His University Hospital has also received grants from Novartis. Veselin Mitrovic: Employed by Kerckhoff-Klinik Forschungsgesellschaft mbH, has received grant/research support from Bayer and Novartis; honoraria from Bayer, Novartis and GlaxoSmithKline and is a consultant for Cardiorentis and a Board Member for Daichi Sankyo. Christian Hengstenberg: Received speakers bureau honoraria from AstraZeneca, Boehringer, Edwards, Novartis, and Symetis; Advisory Board member for Novartis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmieder, R.E., Mitrovic, V. & Hengstenberg, C. Renal impairment and worsening of renal function in acute heart failure: can new therapies help? The potential role of serelaxin. Clin Res Cardiol 104, 621–631 (2015). https://doi.org/10.1007/s00392-015-0839-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-015-0839-y