Abstract
The incidence and mortality associated with acute heart failure (AHF) remain high despite tremendous progress in the treatment of chronic heart failure. Novel approaches to AHF management are needed to improve outcomes. Serelaxin, a recombinant form of human relaxin-2, is a vasoactive peptide hormone with hemodynamic and neurohormonal effects. Serelaxin’s major clinical trials (Pre-RELAX-AHF and RELAX-AHF) demonstrate efficacy in improving heart failure symptoms, decreasing hospital length of stay and reducing morality at 180 days. The AHF patients included in these trials represent a specific and prevalent cohort, briefly those who present with dyspnea, elevated natriuretic peptide levels, systolic blood pressures greater than 125 mmHg, and mild-to-moderate renal insufficiency. Early serelaxin treatment is associated with less end-organ damage and improved outcomes. Serelaxin holds promise as a novel therapeutic for the treatment of AHF and two large additional Phase III trials are underway to confirm and extend these prior results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute heart failure (AHF) is a prevalent cardiovascular syndrome and is the most common reason for admission for Medicare beneficiaries [1]. Hospitalization for AHF independently predicts a poor prognosis [2, 3]. Approximately 1,000,000 hospitalizations for AHF in the United States occur annually and total direct costs of heart failure exceed $60 billion [4, 5]. Once hospitalized, nearly one-third will die or be re-hospitalized within 90 days after discharge and one-year mortality approaches 30 %.
Dyspnea, a complex symptom exacerbated by venous congestion in the pulmonary parenchyma, is the most commonly reported AHF symptom. Up to half of patients continue to have dyspnea after 24 h of treatment, and 25 % still have symptoms by discharge [6]. Persistent dyspnea and end-organ dysfunction, including myocardial damage, worsening renal function, and hepatic impairment, are independent predictors of mortality in patients with AHF [7••, 8, 9]. In addition to volume overload, vascular dysfunction with increased tone is also a driver of the pathophysiology for AHF exacerbations, particularly in patients with elevated systolic blood pressure [10].
Pharmacologic therapy for AHF with elevated systolic blood pressure comprises primarily intravenous loop diuretics and vasodilators. Intravenous loop diuretics remain the mainstay of treatment for acute symptomatic relief. However, there is a lack of strong clinical data to support a beneficial effect of diuretics (or any other AHF therapy) on mortality [11]. While some retrospective studies suggest that higher doses of diuretics are associated with increased mortality [12], recent prospective data suggest that higher diuretic doses lead to better relief of dyspnea and more weight and fluid volume loss [13, 14].
Although nitrovasodilators have long been used in AHF, the evidence supporting their use is limited [15]. No data convincingly demonstrate any symptomatic, morbidity, or mortality benefits with nitrovasodilators [16]. Nitroglycerin tolerance and tachyphylaxis occurs in upwards of 30 % of patients within the first 24 h [17, 18]. Nitroprusside also has limited data in regards to its impact on outcomes. Furthermore, concerns of thiocyanate toxicity and the perceived necessity for invasive monitoring have limited its use.
As vasoconstriction, vascular congestion, and elevated left ventricular filling pressures continue to be key therapeutic targets in AHF, multiple novel pharmacologic therapeutic trials have been designed in an effort to target these mechanisms. Unfortunately, favorable Phase II data were unable to be replicated in larger trials of nesiritide, tezosentan, milrinone, or levosimendan [19–22]. The high morbidity and mortality from AHF combined with the limited pharmacologic armamentarium to improve symptoms or outcomes highlight the continued unmet need for new AHF therapies.
Serelaxin is a novel recombinant form of human relaxin-2, a peptide hormone believed to mediate cardiovascular and renal adaptations to pregnancy in humans. Serelaxin’s pleiotropic effects have demonstrated a beneficial role in treating patients with AHF. RELAX-AHF (RELAXin in Acute Heart Failure), a Phase III trial comparing intravenous serelaxin to placebo, showed improved AHF symptoms, reduced worsening of heart failure, decreased hospital length of stay, and improved 180-day both all-cause and CV survival for those treated with serelaxin [23••]. Additional Phase III trials (RELAX-AHF-2; RELAX-AHF-ASIA) are currently being conducted to confirm these initial findings and to further evaluate serelaxin’s efficacy for patients with AHF.
The early initiation of intravenous therapies is associated with decreased markers of end-organ damage and improved outcomes [7••, 24]. This impetus to put the “acute” back into AHF treatment requires the emergency room physician and admitting physician to thoughtfully and comfortably deploy evidence-based therapeutics. This article will review serelaxin’s pharmacologic mechanism of action, clinical data on hemodynamic and renal effects, clinical efficacy trial results, and its role in the treatment of AHF.
Mechanism of Action
The peptide hormone relaxin was initially discovered in 1926 as a reproductive hormone [25•]. Plasma levels of relaxin increase in the first trimester as the mother’s cardiovascular and renal demands increase during gravidity. The increased systemic vasodilation compensates for the increased blood volume, allows for increased nutritional delivery to the placenta, and provides additional blood flow to the renal system accommodating the expanded requirements for metabolic waste clearance. In aggregate, this results in a 20 % increase in cardiac output, a 30 % increase in arterial compliance, a 20 % decrease in systemic vascular resistance, and a 45 % increase in renal blood flow [26–28]. These physiologic changes would be desirable attributes in a therapeutic agent for AHF. Relaxin has been identified as both a trigger and a mediator for these beneficial physiologic changes observed in pregnancy.
Primary effects of relaxin are mediated by multiple mechanisms leading to the relaxation of vascular tone and downregulation of pathologic myocardial remodeling. Relaxin achieves vasodilation through activation of endothelin-B (ET-B) receptor [29, 30]. Relaxin also activates salutary matrix metalloproteinases, decreasing the progression of myocardial fibrosis [31]. The subsequent reduction in arterial stiffness, afterload, and left ventricular work may give biological plausibility for the longer term mortality benefits seen in serelaxin’s Phase II and Phase III trials [23•, 32, 33].
Acute Hemodynamic and Renal Effects of Serelaxin
The immediate hemodynamic effects of serelaxin were evaluated in a Phase I safety and tolerability study [34•]. Sixteen patients with stable, chronic heart failure (CHF) underwent invasive hemodynamic monitoring during Swan-Ganz catheterization following intravenous administration of serelaxin. Doses evaluated ranged from 10 to 960 µg/kg/day. Lower doses of serelaxin (10–100 µg/kg/day) had more profound effects on right atrial pressure, pulmonary capillary wedge pressure (PCWP), pulmonary artery pressure, and NT-pro BNP than higher doses. The mean PCWP change from baseline was −5.0 + 2.6 mmHg, −3.5 ± 3.7 mmHg, 0.33 ± 2.6 mmHg, and −0.17 ± 2.7 mmHg at 30, 100, 480, and 960 µg/kg/day, respectively. Low-dose infusion rates had less of an effect on systemic vascular tone (SVR) and cardiac index (CI), while higher doses had a slightly higher effect. Beyond the hemodynamic activity observed in this small study, the varied dose responses should be interpreted with caution. Differences in baseline hemodynamic status between groups or patient variability in a small-sample size study may have influenced the cardiovascular response to serelaxin where lower doses produce more venous vasodilatation with a subsequent decrease in PCWP and higher doses may produce more arterial vasodilation, leading to lower SVR and higher CI [33]. Others have suggested that this effect is the result of negative cooperativity at the receptor level.
A larger hemodynamic study was later conducted to examine the central hemodynamic effects of serelaxin compared to placebo administered within 48 h of presentation to the hospital in 71 patients with AHF [35•]. Hemodynamic measurements were recorded at baseline and over a 20-hour infusion of 30 µg/kg/day. In patients with AHF, serelaxin significantly decreased PCWP by 2.4 mmHg (p = 0.0042), lowered mean pulmonary artery pressure by 3.9 mmHg (p = 0.0002), and reduced SVR by 134 dynes × s/cm5 (p = 0.05) compared to placebo.
A recent prospective randomized control trial assessed the renal hemodynamic effects of serelaxin in patients with symptomatic CHF, impaired renal function, and an SBP > 110 mmHg [36•]. Intravenous serelaxin infused at 30 μg/kg per day for 24 h increased renal plasma flow by 29 %, compared with 14 % on placebo (p = 0.04), whereas glomerular filtration rate did not differ significantly during 8–24 h. Notably, filtration fraction increased by 36 % with serelaxin and 62 % with placebo (p = 0.002), identifying less of a diuretic and natriuretic effect than previously thought. Together, the combination of an increased renal blood flow, unchanged glomerular filtration rate, and decreased filtration fraction suggests that serelaxin may have primarily a direct renal vasorelaxant effect and that it reduces intraglomerular pressures, providing a potential mechanism for a renoprotective effect.
Clinical Efficacy
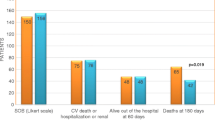
Pre-RELAX-AHF, a Phase II dose-finding study, assessed the impact of intravenous serelaxin on multiple clinical outcomes along with safety, to identify a potential dose or doses to study further [32]. In this placebo-controlled, double-blind, parallel-group study, 234 patients who presented with dyspnea, congestion on chest radiograph, elevated natriuretic peptide concentrations, mild-to-moderate renal dysfunction, and a systolic blood pressure greater than 125 mmHg were randomized within 16 h of presentation to receive one of four doses of serelaxin (10, 30, 100, or 250 µg/kg/day) or placebo for 48 h. The results identified multiple signals of benefit in the serelaxin-treated patients. Dyspnea, as assessed by a moderate or marked improvement on the Likert scale, rapidly improved in 40 % of patients over the first 24 h and was sustained at 14 days. Positive signals toward decreased loop diuretic requirements, worsening of heart failure, 60-day cardiovascular (CV) death, and readmission and 180-day mortality were also observed. While all groups demonstrated benefits, these effects were most pronounced in the group receiving relaxin 30 µg/kg/day. These salutary trends led to the development of the Phase III RELAX-AHF trial.
The RELAXin in Acute Heart Failure (RELAX-AHF) trial was designed to test the efficacy of serelaxin, with the primary outcome of dyspnea relief [23••]. This prospective, randomized, double-blind, placebo-controlled, parallel-group trial compared 48-h continuous infusion of serelaxin (30 µg/kg/day) with placebo in patients presenting to the hospital with AHF in the first 16 h. With similar enrollment criteria to the Pre-RELAX-AHF study, a targeted approach was undertaken to select a specific type of patient with AHF. The inclusion and exclusion criteria applied a lower limit to the patient’s enrollment blood pressure to avoid hypotension and to select for patients most likely to mechanistically benefit from relaxin. While one review of large AHF registries suggests that 60–80 % of AHF patients would meet blood pressure targets in this study, a separate analysis of international registries suggests that patients potentially eligible for RELAX-AHF represent 20 % of patients with AHF with most being excluded due to the renal insufficiency inclusion criterion [37, 38]. The primary clinical end point of dyspnea relief was evaluated through two primary end points: moderate to marked improvement of patient-reported dyspnea using the Likert scale at 6, 12, and 24 h time points and the change in area under the curve (AUC) in patient-reported scores on the visual analogue scale (VAS) from baseline to Day 5. Although both were included as co-primary end points, earlier studies suggest that the VAS may better reflect changes in dyspnea over the treatment timeline and a poor correlation between VAS and the Likert scale [39, 40]. Overall, patients treated with serelaxin experienced mild improvements in the measures of dyspnea based on the VAS primary outcome, decreased rates of early worsening of heart failure events through Day 5 [12.2 vs. 6.7 %, HR 0.52, (0.36, 0.79)], shortened hospital length of stay (10.5 vs. 9.6 days, p = 0.039), and a 37 % reduction in cardiovascular and all-cause mortality at 180 days (p = 0.028). There were no significant effects on the other primary end point of dyspnea using the Likert scale (27 vs. 26 %, p = 0.70), nor cardiovascular deaths or readmissions to the hospital for heart failure or renal failure at 60 days (13.2 vs. 13.0 %, p = 0.89).
Safety
Serelaxin has demonstrated a strong safety profile in both the Phase II and Phase III clinical trials. To date, no studies have identified an increased rate of serious adverse events in patients receiving serelaxin compared to placebo. While there were significantly greater decreases from baseline in systolic blood pressure during infusion of serelaxin compared to placebo (on the order of 4–6 mmHg difference), hypotension-related adverse events were comparable between groups (5 vs. 4 %, p = 0.78). Importantly, patients treated with serelaxin had lower rates of events related to renal impairment compared with serelaxin (9 vs. 6 %, nominal p = 0.03) and overall similar adverse events [23••].
Clinical Implications
If confirmed by ongoing Phase III studies, serelaxin would be one of the only AHF therapeutic agents to provide both an improvement in immediate AHF symptom relief and a mortality benefit. The AHF literature is littered with failed attempts to improve symptoms or outcomes [14, 20–22]. Serelaxin’s Phase II and Phase III trials utilized robust inclusion criteria to target a unique but highly prevalent phenotype of AHF associated with increased vasoconstriction as evidenced by an elevated systolic blood pressure, vascular congestion, and renal impairment. The aforementioned markers are all suggestive of neurohormonal and inflammatory cascade activation. Importantly, the RELAX-AHF program targeted patients early in their hospitalization.
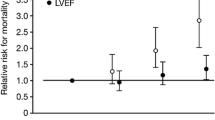
Early intervention, as performed in Pre-RELAX-AHF and RELAX-AHF, potentially minimizes the deleterious neurohormonal cascade seen in AHF and limits end-organ damage. Conversely, without early and effective intervention during the acute exacerbation, organs may undergo further injury. Unsurprisingly, organ injury in AHF carries prognostic significance [7••]. Additionally, current standard-of-care interventions for AHF, including diuretics and inotropes, frequently exacerbate end-organ injury [41]. Pre-RELAX-AHF and RELAX-AHF enrolled patients within 16 h, a significantly narrower window compared to prior AHF trials which had enrollment cutoffs of 24–48 h. In a post hoc analysis of RELAX-AHF, changes in markers of cardiac (high-sensitivity cardiac troponin T), renal (creatinine and cystatin C), and hepatic (aspartate transaminase and alanine transaminase) damage and of decongestion (N-terminal pro-brain natriuretic peptide) at Day 2 and worsening heart failure during admission were associated with 180-day mortality. Serelaxin administration improved these markers, consistent with the prevention of organ damage [7••].
Serelaxin’s benefits extend to both patients with heart failure with preserved ejection fraction (HFpEF) (≥50 %) and heart failure with reduced ejection fraction (HFrEF) (<50 %) [42]. Approximately 50 % of patients admitted with AHF are individuals with HFpEF [37, 40]. No pharmacological therapeutics have been previously studied in the HFpEF population with AHF. In RELAX-AHF, serelaxin induced a similar degree of dyspnea relief in HFpEF and HFrEF patients by VAS-AUC through Day 5 [42]. No differences were encountered in the effect of serelaxin on short- or long-term outcome between HFpEF and HFrEF patients including cardiovascular death or hospitalization.
Conclusion: Current and Future Uses
In February 2014, the FDA’s Cardiovascular and Renal Advisory Committee (CDRAC) voted unanimously against recommendation for approval of serelaxin due to only a single efficacy trial and reservations that the mortality benefit was an exploratory outcome.
The RELAX-AHF-2 trial (clinicaltrial.gov identifier NCT01870778) was initiated prior to the FDA meeting, designed to assess 180-day morality and worsening heart failure at five days. Although an event-driven trial, the planned sample size is approximately 6800 patients. Clinical trial results are anticipated for release in 2017. RELAX-AHF-ASIA (clinicaltrial.gov identifier NCT02007720) is also underway to assess efficacy regarding heart failure symptoms in at least eight Asian countries.
Serelaxin, a recombinant peptide of the naturally occurring hormone relaxin, has demonstrated symptomatic efficacy in treating AHF. Serelaxin is also associated with decreased end-organ damage, reductions in the incidence of worsening heart failure, shorter length of stay, and decreased 180-day CV mortality. This novel therapeutic is well tolerated and has no increase in serious adverse events compared to placebo to date. Two large-scale clinical trials are underway to confirm serelaxin’s effects on symptoms, worsening heart failure, and 180-day CV mortality.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Desai AS, Stevenson LW. Rehospitalization for heart failure: predict or prevent? Circulation. 2012;126(4):501–6. doi:10.1161/circulationaha.112.125435.
Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154(2):260–6. doi:10.1016/j.ahj.2007.01.041.
Solomon SD, Wang D, Finn P, Skali H, Zornoff L, McMurray JJ, et al. Effect of candesartan on cause-specific mortality in heart failure patients: the Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation. 2004;110(15):2180–3. doi:10.1161/01.cir.0000144474.65922.aa.
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. doi:10.1161/cir.0000000000000152.
Voigt J, Sasha John M, Taylor A, Krucoff M, Reynolds MR, Michael Gibson C. A reevaluation of the costs of heart failure and its implications for allocation of health resources in the United States. Clin Cardiol. 2014;37(5):312–21.
Metra M, O’Connor CM, Davison BA, Cleland JG, Ponikowski P, Teerlink JR, et al. Early dyspnoea relief in acute heart failure: prevalence, association with mortality, and effect of rolofylline in the PROTECT Study. Eur Heart J. 2011;32(12):1519–34. doi:10.1093/eurheartj/ehr042.
•• Metra M, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, et al. Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the Relaxin in Acute Heart Failure (RELAX-AHF) development program: correlation with outcomes. J Am Coll Cardiol. 2013;61(2):196–206. doi:10.1016/j.jacc.2012.11.005. This secondary analysis of the RELAX-AHF trial showed that serelaxin administration was associated with a reduction of biomarkers indicative of myocardial (troponin T), hepatic (aminotransferases) and renal (cystatin-C) injury, providing pathophysiological support for the mortality benefit observed in the serelaxin treated group (see Ref. 23).
Metra M, Cotter G, Gheorghiade M, DeiCas L, Voors AA. The role of the kidney in heart failure. Eur Heart J. 2012;33(17):2135–42. doi:10.1093/eurheartj/ehs205.
van der Westhuizen ET, Halls ML, Samuel CS, Bathgate RA, Unemori EN, Sutton SW, et al. Relaxin family peptide receptors–from orphans to therapeutic targets. Drug Discov Today. 2008;13(15–16):640–51. doi:10.1016/j.drudis.2008.04.002.
Cotter G, Metra M, Milo-Cotter O, Dittrich HC, Gheorghiade M. Fluid overload in acute heart failure—re-distribution and other mechanisms beyond fluid accumulation. Eur J Heart Fail. 2008;10(2):165–9. doi:10.1016/j.ejheart.2008.01.007.
Cotter OM, Sasimangalam AN, Arumugham PS, Kaluski E, Weatherley B, Cotter G. Diuretics—a panacea for acute heart failure? Different formulations, doses, and combinations. Heart Fail Monit. 2008;6(1):9–19.
Hasselblad V, Gattis Stough W, Shah MR, Lokhnygina Y, O’Connor CM, Califf RM, et al. Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE trial. Eur J Heart Fail. 2007;9(10):1064–9. doi:10.1016/j.ejheart.2007.07.011.
Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, et al. Diuretic strategies in patients with acute decompensated heart failure. N E J Med. 2011;364(9):797–805. doi:10.1056/NEJMoa1005419.
Felker GM, Mentz RJ. Diuretics and ultrafiltration in acute decompensated heart failure. J Am Coll Cardiol. 2012;59(24):2145–53. doi:10.1016/j.jacc.2011.10.910.
Wakai A, McCabe A, Kidney R, Brooks SC, Seupaul RA, Diercks DB, et al. Nitrates for acute heart failure syndromes. Cochrane Database Syst Rev. 2013;8:Cd005151. doi:10.1002/14651858.CD005151.pub2.
Alexander P, Alkhawam L, Curry J, Levy P, Pang PS, Storrow AB, et al. Lack of evidence for intravenous vasodilators in ED patients with acute heart failure: a systematic review. Am J Emerg Med. 2015;33(2):133–41. doi:10.1016/j.ajem.2014.09.009.
Publication Committee for the VMAC Investigators (Vasodilatation in the Management of Acute CHF), et al. Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;287(12):1531–40.
Fung HL, Bauer JA. Mechanisms of nitrate tolerance. Cardiovasc Drugs Ther. 1994;8(3):489–99.
Felker GM, Benza RL, Chandler AB, Leimberger JD, Cuffe MS, Califf RM, et al. Heart failure etiology and response to milrinone in decompensated heart failure: results from the OPTIME-CHF study. J Am Coll Cardiol. 2003;41(6):997–1003.
McMurray JJ, Teerlink JR, Cotter G, Bourge RC, Cleland JG, Jondeau G, et al. Effects of tezosentan on symptoms and clinical outcomes in patients with acute heart failure: the VERITAS randomized controlled trials. JAMA. 2007;298(17):2009–19. doi:10.1001/jama.298.17.2009.
Mebazaa A, Nieminen MS, Packer M, Cohen-Solal A, Kleber FX, Pocock SJ, et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE Randomized Trial. JAMA. 2007;297(17):1883–91. doi:10.1001/jama.297.17.1883.
O’Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365(1):32–43. doi:10.1056/NEJMoa1100171.
•• Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet (Lond Engl). 2013;381(9860):29–39. doi:10.1016/s0140-6736(12)61855-8. The first Phase III clinical trial to demonstrate a significant clinical benefit on short-term outcomes (dyspnea releif and reduction in worsening heart failure) and suggest a decrease (37 %) in both cardiovascular and all-cause mortality in patients hospitalized for acute heart failure treated with serelaxin.
Peacock WFT, Fonarow GC, Emerman CL, Mills RM, Wynne J. Impact of early initiation of intravenous therapy for acute decompensated heart failure on outcomes in ADHERE. Cardiology. 2007;107(1):44–51. doi:10.1159/000093612.
• Bathgate RA, Halls ML, van der Westhuizen ET, Callander GE, Kocan M, Summers RJ. Relaxin family peptides and their receptors. Physiol Rev. 2013;93(1):405–80. doi:10.1152/physrev.00001.2012. An excellent review detailing relaxin’s mechanism of action and targets for pharmcologic intervention.
Baylis C. Relaxin may be the “elusive” renal vasodilatory agent of normal pregnancy. Am J Kidney Dis. 1999;34(6):1142–4; discussion 4-5. doi:10.1016/s0272-6386(99)70024-7.
Jeyabalan A, Shroff SG, Novak J, Conrad KP. The vascular actions of relaxin. Adv Exp Med Biol. 2007;612:65–87. doi:10.1007/978-0-387-74672-2_6.
Schrier RW, Durr JA. Pregnancy: an overfill or underfill state. Am J Kidney Dis. 1987;9(4):284–9.
Bogzil AH, Ashton N. Relaxin-induced changes in renal function and RXFP1 receptor expression in the female rat. Ann N Y Acad Sci. 2009;1160:313–6. doi:10.1111/j.1749-6632.2008.03797.x.
Dschietzig T, Richter C, Bartsch C, Laule M, Armbruster FP, Baumann G, et al. The pregnancy hormone relaxin is a player in human heart failure. FASEB J. 2001;15(12):2187–95. doi:10.1096/fj.01-0070com.
Samuel CS, Unemori EN, Mookerjee I, Bathgate RA, Layfield SL, Mak J, et al. Relaxin modulates cardiac fibroblast proliferation, differentiation, and collagen production and reverses cardiac fibrosis in vivo. Endocrinology. 2004;145(9):4125–33. doi:10.1210/en.2004-0209.
Teerlink JR, Metra M, Felker GM, Ponikowski P, Voors AA, Weatherley BD, et al. Relaxin for the treatment of patients with acute heart failure (Pre-RELAX-AHF): a multicentre, randomised, placebo-controlled, parallel-group, dose-finding phase IIb study. Lancet (Lond Engl). 2009;373(9673):1429–39. doi:10.1016/s0140-6736(09)60622-x.
Teichman SL, Unemori E, Teerlink JR, Cotter G, Metra M. Relaxin: review of biology and potential role in treating heart failure. Curr Heart Fail Rep. 2010;7(2):75–82. doi:10.1007/s11897-010-0010-z.
• Dschietzig T, Teichman S, Unemori E, Wood S, Boehmer J, Richter C, et al. Intravenous recombinant human relaxin in compensated heart failure: a safety, tolerability, and pharmacodynamic trial. J Cardiac Fail. 2009;15(3):182–90. doi:10.1016/j.cardfail.2009.01.008. This paper describes the first important clinical study of serelaxin in patients with heart failure.
• Ponikowski P, Mitrovic V, Ruda M, Fernandez A, Voors AA, Vishnevsky A, et al. A randomized, double-blind, placebo-controlled, multicentre study to assess haemodynamic effects of serelaxin in patients with acute heart failure. Eur Heart J. 2014;35(7):431–41. doi:10.1093/eurheartj/eht459. A more contemporary hemodynamic study of serelaxin patients with acute heart failure.
• Voors AA, Dahlke M, Meyer S, Stepinska J, Gottlieb SS, Jones A, et al. Renal hemodynamic effects of serelaxin in patients with chronic heart failure: a randomized, placebo-controlled study. Circ Heart Fail. 2014;7(6):994–1002. doi:10.1161/circheartfailure.114.001536. An elegant renal hemodynamic study, demonstrating beneficial renal effects of serelaxin in patients with chronic heart failure.
Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol. 2009;53(7):557–73. doi:10.1016/j.jacc.2008.10.041.
Wang TS, Hellkamp AS, Patel CB, Ezekowitz JA, Fonarow GC, Hernandez AF. Representativeness of RELAX-AHF clinical trial population in acute heart failure. Circ Cardiovasc Qual Outcomes. 2014;7(2):259–68. doi:10.1161/circoutcomes.113.000418.
Allen LA, Metra M, Milo-Cotter O, Filippatos G, Reisin LH, Bensimhon DR, et al. Improvements in signs and symptoms during hospitalization for acute heart failure follow different patterns and depend on the measurement scales used: an international, prospective registry to evaluate the evolution of measures of disease severity in acute heart failure (MEASURE-AHF). J Cardiac Fail. 2008;14(9):777–84. doi:10.1016/j.cardfail.2008.07.188.
Pang PS, Collins SP, Sauser K, Andrei AC, Storrow AB, Hollander JE, et al. Assessment of dyspnea early in acute heart failure: patient characteristics and response differences between Likert and visual analog scales. Acad Emerg Med. 2014;21(6):659–66. doi:10.1111/acem.12390.
Parissis JT, Farmakis D, Nieminen M. Classical inotropes and new cardiac enhancers. Heart Fail Rev. 2007;12(2):149–56. doi:10.1007/s10741-007-9014-5.
Filippatos G, Teerlink JR, Farmakis D, Cotter G, Davison BA, Felker GM, et al. Serelaxin in acute heart failure patients with preserved left ventricular ejection fraction: results from the RELAX-AHF trial. Eur Heart J. 2014;35(16):1041–50. doi:10.1093/eurheartj/eht497.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Tyler declares no conflict of interest. Dr. Pang is or has been over the past year a consultant for Janssen, Medtronic, Novartis, Trevena, sc Pharmaceuticals, Cardioxyl, Roche Diagnostics, and Relypsa and received honoraria from Palatin Technologies and Research Support from Roche and Novartis. Dr. Teerlink has received research grants and consulting fees from Amgen, Bayer, Cardio3 Bioscience, Cytokinetics, Mast Therapeutics, Medtronic, Novartis, Relypsa, St. Jude, Trevena, and ZS Pharma.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the authors.
Additional information
This article is part of the Topical collection on Heart Failure.
Rights and permissions
About this article
Cite this article
Tyler, J.M., Pang, P.S. & Teerlink, J.R. Serelaxin in the Treatment of Acute Heart Failure. Curr Emerg Hosp Med Rep 4, 213–218 (2016). https://doi.org/10.1007/s40138-016-0114-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40138-016-0114-1