Abstract
Prolonged P-wave duration, indicating atrial conduction delay, is a marker of left atrial abnormality and is reported as a potent precursor of atrial fibrillation (AF). Several studies have shown that obstructive sleep apnea (OSA) is associated with AF. We evaluated the relationship between OSA and prolonged P-wave duration. Consecutive subjects who underwent overnight polysomnography and showed a normal sinus rhythm, had no history of AF or ischemic heart disease, and showed no evidence of heart failure were enrolled. Apnea–hypopnea index (AHI) is defined as the number of apnea and hypopnea events per hour of sleep. P-wave duration was determined on the basis of the mean duration of three consecutive beats in lead II from a digitally stored electrocardiogram. A total of 250 subjects (middle-aged, predominantly male, mildly obese, with a mean P-wave duration of 106 ms) were enrolled. In addition to age, male gender, body mass index (BMI), hypertension, dyslipidemia, and uric acid and creatinine levels, AHI (r = 0.56; P < 0.001) had significant univariable relationship with P-wave duration. Multivariate regression analysis showed that age, BMI, male gender, and AHI (partial correlation coefficient, 0.47; P < 0.001) were significantly independently correlated to P-wave duration. Severity of OSA is significantly associated with delayed atrial conduction time. Obstructive sleep apnea may lead to progression of atrial remodeling as an AF substrate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
P-wave duration on an electrocardiogram is generally accepted as a reliable and noninvasive indicator of atrial conduction [1]. Prolonged P-wave duration, which indicates delayed atrial conduction, is associated with some degree of atrial enlargement [2, 3], abnormality in left atrial function [4], and subsequently with significant atrial arrhythmias, such as atrial fibrillation (AF) [5, 6]. According to a recent National Health and Nutrition Examination Survey, P-wave prolongation may be a predictor of long-term all-cause and cardiovascular mortality [7].
By contrast, obstructive sleep apnea (OSA) is a highly prevalent disorder associated with an increased risk of cardiovascular disease [8–11]. In such relationships between OSA and cardiovascular disease, several pathophysiological effects of OSA are observed, including enhanced cardiac wall stress associated with exaggerated intrathoracic pressure oscillations [12, 13], elevated sympathetic nerve activity [14], activated systemic inflammation [15], increased production of reactive oxygen species [16], and vascular endothelial dysfunction [17]. In addition to hypertension [18] and left ventricular diastolic dysfunction [19, 20], both of which are frequently observed in patients with OSA, the pathophysiological effects of OSA may directly cause left atrial overload and left atrial remodeling, leading to P-wave prolongation.
Several reports have demonstrated the relationship between P-wave prolongation and the presence or severity of OSA. However, these studies involved a limited sample size and were conducted in Western countries [21–23]. Thus, no data are available for the Asian population involving a large sample of subjects who have undergone sleep study.
The aim of this study was to determine whether a strong relationship exists between P-wave duration and OSA severity in a large population in Japan.
Subjects and methods
Subjects
Subjects with a normal sinus rhythm on electrocardiogram who had undergone an attended overnight sleep study in the Sleep Center of the Toranomon Hospital (Tokyo, Japan) from January to May 2007 were enrolled in the study if the following criteria were met: (1) age ≥20 years and (2) normal sinus rhythm, where every P-wave was positive in leads I, II, and aVF with uniform morphologies, every QRS complex was preceded by a P-wave, and every P-wave was followed by a QRS. The following subjects were excluded from the study: (1) subjects with a history of AF/flutter or sodium-channel blocker administration, (2) subjects with a pacemaker or implantable cardioverter-defibrillator, (3) subjects with evidence of heart failure, (4) subjects with history of ischemic heart disease, (5) subjects with significant valvular heart disease, (6) subjects with severe chronic pulmonary disease, (7) subjects on dialysis, or (8) subjects with central sleep apnea.
Informed consent to participate in the study was obtained from all patients. The study complied with the Declaration of Helsinki and was conducted according to the ethics policies of the involved institutions.
Sleep study
All subjects underwent an overnight attended sleep study using a digital polygraph (SomnoStar α Sleep System; Sensor Medics, Yorba Linda, CA, USA) at our sleep laboratory; the definitions and scoring methods used have been described previously [24–26]. The apnea–hypopnea index (AHI) was used to evaluate OSA severity. The AHI is calculated as the total number of apnea and hypopnea events divided by total sleep time, expressed as the number of events per hour. Obstructive sleep apnea was diagnosed if the AHI was >5 events/h, and OSA was further subdivided as mild (5 ≤ AHI < 15 events/h), moderate (15 ≤ AHI < 30 events/h), and severe (AHI ≥ 30 events/h). We defined patients with central sleep apnea as those having an AHI of ≥15 events/h, of which more than 50 % were central events [27].
Electrocardiogram and measurements of P-wave duration
On the same day as the sleep study, a standard 12-lead electrocardiogram was obtained using the same electrocardiogram machine (PageWriter Touch; Philips, Amsterdam, the Netherlands) for all subjects at 25 mm/s with 1 mV/cm standardization after supine rest for at least 5 min. Data were stored digitally on the server in our hospital.
Next, P-wave duration was measured manually by an experienced observer who was blinded to polysomnographic data by using software for the digital caliper (Hakarun, version 0.7.0; Onegland, Shizuoka, Japan) with fourfold magnification. In this study, P-wave duration was obtained as the mean duration of three consecutive beats in lead II; each measurement included identifying the initiation of P-wave onset and its termination at P-wave offset. P-wave onset was identified as the junction between the T–P isoelectric line and the beginning of the positive deflection, and offset was identified as the junction between the end of the P-wave deflection and the PR segment. To determine the intraobserver reproducibility, the coefficients of variation (CV) were obtained from measurements repeated three times on different days in 10 patients with a random selection. The average CV was 2.2 %.
Left ventricular hypertrophy (LVH) was defined using Sokolow–Lyon voltage criteria (i.e., S-wave voltage in V1 plus the greater of R wave in lead V5 or V6, >3.5 mV) [28].
Blood samples and other data
Venous blood samples were obtained early in the morning after overnight fasting, immediately following the sleep study. Height and weight were also measured at this time. Blood pressure and heart rate were measured on the same day as the sleep study. Blood pressure was measured using a sphygmomanometer with an appropriately sized cuff after subjects had been rested for at least 5 min. Three consecutive measurements were carried out and the mean of the last two values was recorded.
Hypertension was defined as systolic blood pressure of ≥140 mmHg, diastolic blood pressure of ≥90 mmHg, or treatment with antihypertensive medications. Diabetes mellitus was defined as fasting plasma glucose level of ≥126 mg/dl, hemoglobin A1c level of ≥6.5 %, or treatment with antidiabetic medication (oral hypoglycemic drugs or insulin injections). Dyslipidemia was defined as serum total cholesterol level of ≥220 mg/dl, high-density lipoprotein cholesterol level of <50 mg/dl, triglycerides level of ≥150 mg/dl, or treatment with drugs for dyslipidemia. A habitual drinker was defined as one who ingested alcohol at least three times per week at the time of sleep study. A current smoker was defined as one who smoked at the time of the sleep study or had quit smoking within 1 year before the sleep study.
Statistical analysis
Data are expressed as mean ± standard deviation (SD) or median (interquartile range) for continuous variables, or numbers and percentages for categorical variables. Correlations between P-wave duration and clinical parameters were analyzed. To identify factors that correlated independently with P-wave duration, we conducted multivariable regression analysis, which included P-wave duration as the dependent variable and variables regarded as significant (i.e., P < 0.05) in the univariable analyses as the independent variables. Statistical analyses were performed using a statistical software package (StatView, version 5.0, for Windows; SAS Institute, Cary, NC, USA), and P < 0.05 was considered statistically significant.
Results
A total of 250 subjects were enrolled in the study. Characteristics of all subjects are shown in Table 1. Subjects were generally middle-aged, predominantly male, and mildly obese. Of these, 41 % had hypertension, 8 % had diabetes mellitus, 12 % were current smokers, and 41 % were habitual drinkers. The mean P-wave duration of all subjects was 106 ± 11 ms. Nine percent of subjects had P-wave durations of ≥120 ms, while normal P-wave duration in lead II was considered to be <120 ms [29].
Sleep study data are summarized in Table 2. Among the 250 subjects, 22 (9 %) did not have OSA, 38 (15 %) had mild OSA, 62 (25 %) had moderate OSA, and 128 (51 %) had severe OSA.
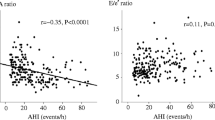
As shown in Table 3, age (r = 0.39; P < 0.001), male gender (r = 0.29; P < 0.001), BMI (r = 0.25; P < 0.001), hypertension (r = 0.29; P < 0.001), dyslipidemia (r = 0.14; P = 0.014), uric acid level (r = 0.25; P < 0.001), and creatinine level (r = 0.20; P = 0.002) exhibited significant univariable relationships with P-wave duration. In addition, AHI showed a significant positive correlation with P-wave duration (r = 0.56; P < 0.001) (Fig. 1). Multivariate regression analysis, which included P-wave duration as the dependent variable and age, male gender, BMI, hypertension, dyslipidemia, uric acid and creatinine levels, and AHI as independent variables, age (partial correlation coefficient, 0.32; P < 0.001), BMI (partial correlation coefficient, 0.13; P = 0.038), male gender (partial correlation coefficient, 0.23; P < 0.001), and AHI (partial correlation coefficient, 0.47; P < 0.001) were significantly independently correlated to P-wave duration (Table 4).
Discussion
This study revealed several important findings, thus providing insights into the relationship between atrial arrhythmias and OSA. We found that the severity of OSA is significantly and directly associated with atrial conduction time; the greater the AHI, the longer the P-wave duration. This direct relationship was independent of other well-known correlations to P-wave duration such as age, gender, BMI, and hypertension [3, 30–32]. Furthermore, we observed a relationship between atrial conduction time and OSA in the Japanese population. These results suggested a close relationship between OSA and prolonged P-wave duration, a marker of left atrial abnormality, in clinical subjects and in the Asian population.
P-wave duration corresponds to the time required for complete depolarization of both right and left atria, and prolonged P-wave duration indicates an intra-atrial and interatrial conduction delay. Electrophysiological evaluation by Kistler et al. [30] revealed that aging is associated with regional conduction velocity slowing in the atrium and increasing P-wave duration. Ariyarajah et al. [2] reported that left atrial size is directly related to prolonged P-wave duration. P-wave duration was shown to fluctuate depending on fluid volume and left atrial pressure, and volume overload or increased left atrial pressure can lead to prolonged P-wave duration [33]. In a large population-based study (Atherosclerosis Risk in Communities study), P-wave duration in lead II was independently associated with an increased risk of AF incidence (hazard ratio (HR) per 1 SD, 1.80; 95 % confidence interval (95 % CI), 1.49–2.20) in multivariate analysis [5]. In the Framingham Heart Study, maximum P-wave duration was reported to be correlated with long-term AF risk (HR per 1 SD, 1.12; 95 % CI 1.00–1.25; adjusted for age and gender) in the elderly population [6]. More recently, Magnani et al. [7] showed in the National Health and Nutrition Examination Survey that P-wave duration in lead II is significantly associated with cardiovascular mortality (HR per 1 SD, 1.13; 95 % CI 1.04–1.23) and all-cause mortality (HR per 1 SD, 1.06; 95 % CI 1.00–1.13) after multivariable adjustment for cardiovascular risk factors. Therefore, prolonged P-wave duration is now recognized as a marker of left atrial abnormality, a potent precursor of AF, and a risk factor of long-term mortality.
There are several potential mechanisms linking OSA and prolonged P-wave duration. Faggiano et al. [34] showed a positive correlation between P-wave duration and left atrial pressure. Obstructive sleep apnea was shown to be associated with left ventricular diastolic dysfunction [19, 20], which can result in impaired left ventricular filling and increased left atrial pressure. Atrial fibrosis, an element of atrial conduction disturbance [35], can be caused by mechanical stretch [36, 37], which may increase because of exaggerated intrathoracic pressure oscillations in addition to the pathophysiology of OSA described above. Kim et al. [38] recently demonstrated that increased left ventricular afterload can lead to left atrial fibrosis and reduced regional conduction velocity in the left atrium. Elevated sympathetic nerve activity or endothelial dysfunction, a downstream pathophysiology of OSA, can result in increased arterial stiffness, which increases left ventricular afterload [39]. Levels of inflammation markers or oxidative stress markers were elevated in patients with OSA but decreased after continuous positive airway pressure therapy [15, 16]. Increasing evidence has supported the influence of inflammation or oxidative stress in the pathogenesis of atrial injury [40]. On the other hand, Oliveira et al. [41] showed that AHI is independently associated with an increase in maximum left atrial volume by using real-time three-dimensional echocardiography, which leads to P-wave prolongation on an electrocardiogram [2, 3].
To our knowledge, there have been three reports evaluating the relationship between P-wave duration and OSA severity. Can et al. [21] reported that AHI was positively correlated with P-wave duration (r = 0.44, P = 0.002) in 67 subjects without comorbid cardiovascular disease. Among all variables assessed in the study, only AHI showed a significant correlation with P-wave duration; however, multivariate analysis was not performed. Baranchuk et al. [22] reported that age and AHI >25 were independent related factors for P-wave duration in 180 unselected subjects, including patients with heart failure or ischemic heart disease. P-wave duration was positively correlated with AHI (r = 0.20, P = 0.009), but this relationship was modest compared with that of the present study. The difference between the study by Baranchuk et al. and our study might be caused by the inclusion of patients with cardiovascular disease in the former study. Cagirci et al. [23] investigated P-wave duration in 126 subjects without cardiovascular disease; the subjects were classified into three groups according to AHI (namely, AHI <5, 5 ≤ AHI < 30, and AHI >30). P-wave duration was significantly longer in the more severe OSA group, but further evaluation of P-wave duration, including linear regression, was not performed. The finding that P-wave duration is associated with OSA severity is consistent with the three previous studies as well as with the present study. However, the previous studies focused on P-wave dispersion to a greater extent than on P-wave duration, whereas the Framingham Heart Study revealed no significant associations between P-wave dispersion and incidence of AF or mortality [6]. This is the first study to demonstrate an independent linear correlation of AHI to P-wave duration in lead II, which appears to be a simpler index than P-wave dispersion. Furthermore, all three studies involved data from Western countries. Therefore, the present study is the first to be conducted in the Asian population and included the largest number of subjects.
In addition, P-wave duration was manually determined using a magnifying lens in the studies by Can et al. and Cagirci et al., and semi-automatically determined using caliper software for scanned data in the study by Baranchuk et al. For manual measurement of P-wave duration, hand-held caliper measurements were reported to be less accurate than digital measurements [42]. We conducted on-screen measurements using digital calipers to increase the accuracy of our data.
Limitations
Our study has several limitations. Because the present study was cross-sectional, a cause–effect relationship between OSA and prolonged P-wave duration cannot be construed. Structural or functional information regarding the left atrium appears to be important for clarifying the mechanism between OSA and delayed atrial conduction. However, we did not obtain this information in the present study. The present study was not population-based. The low proportion of no or mild OSA subjects can lead to decreased generalizability of the results in the present study. Another limitation of this study included the potential for residual confounding by unmeasured or unidentified factors, such as drug exposure and duration of the disorder, among others. Whereas P-wave durations were used as the maximum value obtained from 12 leads in most studies, P-wave duration in the present study was measured from a single lead (lead II). Lead II nearly always yields longer P-wave durations than any other lead [43].
Conclusions
Severity of OSA is significantly correlated with prolonged P-wave duration. This suggests that OSA may cause left atrial overload and left atrial remodeling, leading to adverse effects, including increased risk of AF development. Since a cause–effect relationship between OSA and prolonged P-wave duration has not been demonstrated, further studies to examine whether baseline P-wave duration is a risk factor for AF incidence and whether effective treatment for OSA can shorten P-wave duration are necessary.
References
American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (ACC/AHA/HRS Writing Committee to Develop Data Standards on Electrophysiology) (2006) ACC/AHA/HRS 2006 key data elements and definitions for electrophysiological studies and procedures: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (ACC/AHA/HRS Writing Committee to Develop Data Standards on Electrophysiology). Circulation 114:2534–2570
Ariyarajah V, Mercado K, Apiyasawat S, Puri P, Spodick DH (2005) Correlation of left atrial size with P-wave duration in interatrial block. Chest 128:2615–2618
Dagli N, Karaca I, Yavuzkir M, Balin M, Arslan N (2008) Are maximum P wave duration and P wave dispersion a marker of target organ damage in the hypertensive population? Clin Res Cardiol 97:98–104
Goyal SB, Spodick DH (2001) Electromechanical dysfunction of the left atrium associated with interatrial block. Am Heart J 142:823–827
Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC Jr (2009) Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke 40:1204–1211
Magnani JW, Johnson VM, Sullivan LM, Gorodeski EZ, Schnabel RB, Lubitz SA, Levy D, Ellinor PT, Benjamin EJ (2011) P wave duration and risk of longitudinal atrial fibrillation in persons ≥60 years old (from the Framingham Heart Study). Am J Cardiol 107:917–921
Magnani JW, Gorodeski EZ, Johnson VM, Sullivan LM, Hamburg NM, Benjamin EJ, Ellinor PT (2011) P wave duration is associated with cardiovascular and all-cause mortality outcomes: the National Health and Nutrition Examination Survey. Heart Rhythm 8:93–100
Bradley TD, Floras JS (2009) Obstructive sleep apnoea and its cardiovascular consequences. Lancet 373:82–93
Naito R, Sakakura K, Kasai T, Dohi T, Wada H, Sugawara Y, Kubo N, Yamashita S, Narui K, Ishiwata S, Ohno M, Ako J, Momomura S (2012) Aortic dissection is associated with intermittent hypoxia and re-oxygenation. Heart Vessels 27:265–270
Saruhara H, Takata Y, Usui Y, Shiina K, Hashimura Y, Kato K, Asano K, Kawaguchi S, Obitsu Y, Shigematsu H, Yamashina A (2012) Obstructive sleep apnea as a potential risk factor for aortic disease. Heart Vessels 27:166–173
Dohi T, Narui K, Kasai T, Takaya H, Inoshita A, Maeno K, Kasagi S, Ishiwata S, Ohno M, Yamaguchi T, Momomura S (2011) Effects of olmesartan on blood pressure and insulin resistance in hypertensive patients with sleep-disordered breathing. Heart Vessels 26:603–608
Buda AJ, Pinsky MR, Ingels NB Jr, Daughters GT 2nd, Stinson EB, Alderman EL (1979) Effect of intrathoracic pressure on left ventricular performance. N Engl J Med 301:453–459
Zamagni M, Sforza E, Boudewijns A, Petiau C, Krieger J (1996) Respiratory effort. A factor contributing to sleep propensity in patients with obstructive sleep apnea. Chest 109:651–658
Somers VK, Dyken ME, Clary MP, Abboud FM (1995) Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 96:1897–1904
Yokoe T, Minoguchi K, Matsuo H, Oda N, Minoguchi H, Yoshino G, Hirano T, Adachi M (2003) Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation 107:1129–1134
Alonso-Fernández A, García-Río F, Arias MA, Hernanz A, de la Peña M, Piérola J, Barceló A, López-Collazo E, Agustí A (2009) Effects of CPAP on oxidative stress and nitrate efficiency in sleep apnoea: a randomised trial. Thorax 64:581–586
Ip MS, Tse HF, Lam B, Tsang KW, Lam WK (2004) Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med 169:348–353
Fletcher EC, DeBehnke RD, Lovoi MS, Gorin AB (1985) Undiagnosed sleep apnea in patients with essential hypertension. Ann Intern Med 103:190–195
Arias MA, García-Río F, Alonso-Fernández A, Mediano O, Martínez I, Villamor J (2005) Obstructive sleep apnea syndrome affects left ventricular diastolic function: effects of nasal continuous positive airway pressure in men. Circulation 112:375–383
Baguet JP, Barone-Rochette G, Lévy P, Vautrin E, Pierre H, Ormezzano O, Pépin JL (2010) Left ventricular diastolic dysfunction is linked to severity of obstructive sleep apnoea. Eur Respir J 36:1323–1329
Can I, Aytemir K, Demir AU, Deniz A, Ciftci O, Tokgozoglu L, Oto A, Sahin A (2009) P-wave duration and dispersion in patients with obstructive sleep apnea. Int J Cardiol 133:e85–e89
Baranchuk A, Parfrey B, Lim L, Morriello F, Simpson CS, Hopman WM, Redfearn DP, Fitzpatrick M (2011) Interatrial block in patients with obstructive sleep apnea. Cardiol J 18:171–175
Cagirci G, Cay S, Gulsoy KG, Bayindir C, Vural MG, Firat H, Kilic H, Yeter E, Akdemir R, Ardic S (2011) Tissue Doppler atrial conduction times and electrocardiogram interlead P-wave durations with varying severity of obstructive sleep apnea. J Electrocardiol 44:478–482
Rechtschaffen A, Kales AA (1968) Manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. NIH Publication No. 204, US Government Printing Office, Washington, DC
The American Sleep Disorders Association Atlas Task Force (1992) EEG arousals: scoring rules and examples. Sleep 15:173–184
American Academy of Sleep Medicine Task Force (1999) Sleep-related breathing disorders in adults: recommendation for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 22:667–689
Kasai T, Narui K, Dohi T, Ishiwata S, Yoshimura K, Nishiyama S, Yamaguchi T, Momomura S (2005) Efficacy of nasal bi-level positive airway pressure in congestive heart failure patients with Cheyne–Stokes respiration and central sleep apnea. Circ J 69:913–921
Sokolow M, Lyon T (1949) The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J 37:161–186
Mirvis DM, Goldberger AL (2001) Electrocardiography. In: Braunwald E, Zipes DP, Libby P (eds) Heart disease: a textbook of cardiovascular medicine, 6th edn. WB Saunders, Philadelphia, pp 82–125
Kistler PM, Sanders P, Fynn SP, Stevenson IH, Spence SJ, Vohra JK, Sparks PB, Kalman JM (2004) Electrophysiologic and electroanatomic changes in the human atrium associated with age. J Am Coll Cardiol 44:109–116
Magnani JW, Johnson VM, Sullivan LM, Lubitz SA, Schnabel RB, Ellinor PT, Benjamin EJ (2010) P-wave indices: derivation of reference values from the Framingham Heart Study. Ann Noninvasive Electrocardiol 15:344–352
Seyfeli E, Duru M, Kuvandik G, Kaya H, Yalcin F (2006) Effect of obesity on P-wave dispersion and QT dispersion in women. Int J Obes (Lond) 30:957–961
Song J, Kalus JS, Caron MF, Kluger J, White CM (2002) Effect of diuresis on P-wave duration and dispersion. Pharmacotherapy 22:564–568
Faggiano P, D’Aloia A, Zanelli E, Gualeni A, Musatti P, Giordano A (1997) Contribution of left atrial pressure and dimension to signal-averaged P-wave duration in patients with chronic congestive heart failure. Am J Cardiol 79:219–222
Burstein B, Nattel S (2008) Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol 51:802–809
Kijima K, Matsubara H, Murasawa S, Maruyama K, Mori Y, Ohkubo N, Komuro I, Yazaki Y, Iwasaka T, Inada M (1996) Mechanical stretch induces enhanced expression of angiotensin II receptor subtypes in neonatal rat cardiac myocytes. Circ Res 79:887–897
Goette A, Staack T, Röcken C, Arndt M, Geller JC, Huth C, Ansorge S, Klein HU, Lendeckel U (2000) Increased expression of extracellular signal-regulated kinase and angiotensin-converting enzyme in human atria during atrial fibrillation. J Am Coll Cardiol 35:1669–1677
Kim SJ, Choisy SC, Barman P, Zhang H, Hancox JC, Jones SA, James AF (2011) Atrial remodeling and the substrate for atrial fibrillation in rat hearts with elevated afterload. Circ Arrhythm Electrophysiol 4:761–769
London GM, Marchais SJ, Guerin AP, Pannier B (2004) Arterial stiffness: pathophysiology and clinical impact. Clin Exp Hypertens 26:689–699
Van Wagoner DR (2008) Oxidative stress and inflammation in atrial fibrillation: role in pathogenesis and potential as a therapeutic target. J Cardiovasc Pharmacol 52:306–313
Oliveira W, Campos O, Bezerra Lira-Filho E, Cintra FD, Vieira M, Ponchirolli A, de Paola A, Tufik S, Poyares D (2008) Left atrial volume and function in patients with obstructive sleep apnea assessed by real-time three-dimensional echocardiography. J Am Soc Echocardiogr 21:1355–1361
Dilaveris P, Batchvarov V, Gialafos J, Malik M (1999) Comparison of different methods for manual P wave duration measurement in 12-lead electrocardiograms. Pacing Clin Electrophysiol 22:1532–1538
Spodick DH (2004) Unappreciated prevalence of interatrial block and associated consequences: a poorly perceived pandemic. Mayo Clin Proc 79:668–670
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maeno, Ki., Kasai, T., Kasagi, S. et al. Relationship between atrial conduction delay and obstructive sleep apnea. Heart Vessels 28, 639–645 (2013). https://doi.org/10.1007/s00380-012-0288-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-012-0288-8