Abstract
Purpose
Prolonged atrial conduction and inhomogeneous sinus impulse propagation may play a role in the initiation and maintenance of atrial tachyarrhythmias. Such a process could be reflected in inter-lead P-wave duration differences known as “P-wave dispersion” (PWD). Abnormal PWD may be related to obstructive sleep apnea syndrome (OSAS). A meta-analysis of the available publications was conducted.
Methods
A MEDLINE, Web of Science, and Google Scholar search from 2000 to 2021 was performed. The keywords used for search were apnea AND “P wave dispersion.” Case-control studies and surveys were selected as long as they included healthy subjects and subjects with diagnosed OSAS who did not have any other major health problems. PWD values and correlations between apnea-hypopnea indices (AHI) and PWD were used as outcome measures.
Results
Ten studies met the inclusion creteria, encompassing 773 patients with OSAS and 347 healthy controls. The mean ages of the patients with OSAS ranged from 6.9 to 58.8 years. The estimated average Hedges’s g standardized mean difference in PWD values was equal to 1.883 (95% CI: 1.140 to 2.626, p < 0.001). The estimated average Fisher r-to-z transformed correlation coefficient between AHI and PWD was equal to 0.530 (95% CI: 0.075 to 0.985, p = 0.0225). Meta-regression analysis failed to find statistically significant correlations between the effect sizes and the mean age, male proportion, and the body mass index in the OSAS groups.
Conclusion
OSAS is associated with increased PWD, which may predispose to atrial tachyarrythmias.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Much interest has been raised in potential risk of cardiac arrhythmias in patients with sleep-disordered breathing, in particular patients diagnosed with obstructive sleep apnea syndrome (OSAS). Four percent of middle-aged men and 2% of women are affected by sleep apnea; up to 5% of adults may have undiagnosed OSAS [1]. OSAS is also not uncommon in pediatric population: in children, the estimated prevalence of OSAS is 1 to 3% [2,3,4].
Overnight polysomnography is considered as “the gold standard” in diagnosing OSAS. This method enables identification of the respiratory events and classification of their severity by the number of apneas and hypopneas per hour of sleep. In the adults, apnea-hypopnea indices (AHI) equal to 5, 15, and 30 are considered as the threshold of mild, moderate, and severe OSAS, respectively [5]. In children, these cut-points are suggested to be 1.5, 5, and 10, respectively [6].
OSAS is considered to be a risk factor of cardiovascular pathology including systemic and pulmonary hypertension, coronary artery disease, congestive heart failure, and cardiac arrhythmias [7]. Previous studies have revealed a relationship between the severity of OSAS and the frequency of atrial fibrillation (AF) [8]. AF has received much attention as an arrhythmia associated with OSAS [9]. The links between OSAS and AF may be multifactorial [10, 11]. Physiological changes may result in electrical and structural re-modeling that may in turn serve as a substrate to the origins of AF [12].
Known indirect marker for such electrical re-modeling is the prolonged atrial conduction time, represented by increased maximum P-wave duration. Atrial conduction time can be evaluated by measuring the maximum P-wave duration (Pmax) and/or P-wave dispersion (PWD), the difference between the maximum and the minimum P-wave duration, on the surface electrocardiogram (ECG). Increased Pmax and PWD indicate prolonged atrial conduction, inhomogeneous propagation of sinus impulses considered to be the risk factors for AF [13]. PWD is calculated by subtracting the minimum P-wave duration from the maximum in any of the 12 ECG leads during sinus rhythm. Conventionally, the P-wave onset is determined as the initial deflection from the isoelectric baseline defined by the T-P segment; the P-wave offset is defined as the junction of the end of the P wave and its return to baseline [14]. Manual measurement of the P-wave duration is usually performed with a hand-held caliper with the ECG rate increased to 50 mm/s and the ECG voltage to 1 mV/cm. However, digital measurement has been proved to be more accurate and preferable [15, 16].
It would be logical to assume that sleep-disordered breathing, particularly OSAS, may be associated with increased PWD. This assumption is supported by studies that show that increased Pmax and PWD values are associated with OSAS in adults [17]. However, research in the field is limited. Even less is known about the possible link between OSAS and PWD in children.
To address the issue, a study was performed with the goal of conducting a systematic review and meta-analysis of relevant publications on the associations between OSAS and PWD. The primary goals were to see if patients with OSAS had higher PWD than otherwise healthy people, and if there was a link between AHI and PWD.
Materials and methods
Search strategy for identification of studies
A MEDLINE search from January 2000 to December 2021 at PubMed (NLM), Web of Science, and Google Scholar was performed using the PRISMA reporting guidelines [18]. The keywords used for search were apnea AND “P wave dispersion.” Textbooks were searched for additional data. Bibliographies of review articles and systematic reviews, as well as retrieved articles, were also examined for candidate publications. The abstracts of the retrieved papers were further considered for their relevance, and the candidate papers were read in full. Case-control studies, as well as the surveys that included comparisons between the patients with OSAS and healthy subjects groups were included in the analysis. As a part of the studies, the patients must have been evaluated for the PWD. The “outcome measures” were either the values of PWD or correlations between AHI and PWD. The population of interest included either children or adults. Studies in patients with known major anomalies were not included into analysis. PSGs and 12-lead surface ECGs were performed in all subjects. The authors of all included studies described the methods of measurement and the number of respiratory events and PWD. For each included study, confounding factors, such as gender and age of the subjects, were also recorded.
Methods of the review
Data extraction
Pertinent articles were reviewed using preset inclusion criteria. The reviewer was un-blinded to the source of publication or authors at each step during study selection. Only one article was chosen in cases of multiple publications of the same material. Decisions on study inclusion and use of the variables were based on the methods sections of the studies. Details on the methods used for evaluation of the OSAS, number of patients who entered the studies, their age and gender, AHI, and body-mass index (BMI) as well as PWD values were extracted.
Statistics
The analysis was performed using the Hedges’s standardized mean difference between the values of PWD in the patients with OSAS and the control subjects with 95% confidence intervals (CI) and Fisher r-to-z transformation of correlation coefficient between AHI and PWD with 95% CI as the effect size measures [19]. The Hedges’s values 0.2, 0.5, and 0.8 defined small, moderate, and large effect sizes, respectively, while correlations equal to 0.1, 0.3, and 0.5 were considered to be small, medium, and large effect sizes, respectively [20]. Heterogeneity of the studies was expected because of various designs, settings, and patients covered by the studies, as well as discrepancies in the approaches to identification of the OSAS severity in children and adults. Hense, a random-effects model was used. Two subgroups of outcome measures (mean PWD values with standard deviations (SD) and correlations between PWD and AHI) were defined. When the original findings on PWD values were presented as the median (interquartile range), they were converted to the means and SD according to suggested procedure [21] using Deep Meta Tool v.1.0 software. When the original data were presented within certain subgroups of the OSA severity, the summary data from the subgroups were calculated to recreate the data for the study as a whole according to specified procedure [19, 22], and then these summary data were used to compute the effect size and variance. The amount of heterogeneity (i.e., tau2) was calculated. In addition, the Q-test for heterogeneity and the I2 statistic were reported. In case any amount of heterogeneity was detected (i.e., tau2 > 0, regardless of the results of the Q-test), a prediction interval for the true outcomes was also calculated using CMA Prediction Interval software [23]. Studentized residuals and Cook’s distances were used to examine whether studies might be outliers and/or influential in the model. Outliers were defined as studies with a studentized residual greater than the 100 × (1 − 0.05/(2 × k))th percentile of a standard normal distribution. Studies with a Cook’s distances greater than the median plus six times the interquartile range of Cook’s distances were considered influential. Publication bias was estimated using funnel plot. To check for funnel plot asymmetry, the rank correlation and regression tests with the standard error of the observed outcomes as predictor were used. If the evidence of the funnel plot asymmetry and missing studies were found, the Duval and Tweedie “Trim and Fill” method was used to impute missing publications. To evaluate the effects of potential moderators, such as age and sex, on the measurements in consideration, a meta-regression analysis was performed in that the mean age or the percent of male subjects in the SDB group served as the explanatory variables. Method of moments, also known as the DerSimonian and Laird method, was used in meta-regression computations. Comprehensive meta-analysis (CMA) software version 2.0 was used [24].
Results
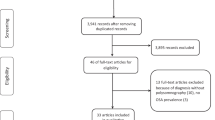
A total of 697 records met initial search criteria. Fourteen duplicate records were removed before screening. Ten studies covering the period from 2009 to 2019 and including 773 patients with OSAS and 347 healthy control subjects reporting the values of the PWD met the inclusion criteria (Fig. 1). The mean ages of the participants from the OSAS groups in the included studies ranged from 6.9 to 58.8 years. One study was performed in children (OSAS group: 77 patients, median age 82.8 months, 58% males, mean AHI equal to 3.2; control group: 44 children). The remaining 9 studies were carried out in adults (OSAS group: 696 patients, median age 49 years, mean AHI equal to 43.6, mean body mass index equal to 30.7; control group 303 subjects). Proportions of male subjects in the OSAS groups ranged between 33 and 79%. Table 1 is the summary of the studies included in the analysis.
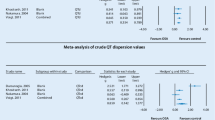
A total of k = 10 studies were included in the analysis of the PWD mean differences between the OSAS and the healthy control subjects. Table 2 summarizes the obtained values of PWD in patients from the OSAS and control groups across these studies. The Hedges’s g standardized mean differences ranged from 0.011 to 5.997, and all estimates were positive. The estimated average Hedges’s g standardized mean difference was equal to 1.883 (95% CI: 1.140 to 2.626). Therefore, the average outcome differed significantly from zero (z = 4.968, p < 0.0001), and the effect size was large (Fig. 2). The values of the Q-test were indicative that the true outcomes were heterogeneous (Q(9) = 218.243, p < .0001, tau2 = 1.346, I2 = 95.876%). A 95% prediction interval for the true outcomes in all comparable populations falls in the interval −0.93 to 4.70. It means that in some studies, the true outcome might in fact be negative. An examination of the studentized residuals found that one study (Metwally et al.) [31] had a value larger than ± 2.8070. This study might be a potential outlier. According to the Cook’s distances, this same study (Metwally et al.) [31] could be considered as overly influential. Potential funnel plot asymmetry was confirmed by both the rank correlation and the regression tests (p = 0.0091 and p < 0.0001, respectively). The “Trim and Fill” method based on a random effects model was used to look for missing studies. The method suggested that 2 studies to the right side of the mean effect were missing, and the imputed point estimate was 2.32852 (95% CI: 1.30827, 3.34877).
The test for subgroup differences (adults vs. children) indicates that there is no statistically significant subgroup effect: Q(1) = 0.179, p = .672, suggesting that age category does not modify the effect of OSAS and that the effect size is consistent between adults and children. However, a smaller number of studies (only one study on children) and participants contributed data to the “Children” subgroup than to the “Adults” subgroup, meaning that the analysis may not be able to detect subgroup differences.
A meta-analysis covering 9 studies comprising only adult patients was further performed. The estimated average Hedges’s g standardized mean difference was equal to 1.829 (95% CI: 1.046 to 2.612), and these figures did not significantly differ from the total estimates covering 10 studies covering both adults and children.
A series of meta-regression analyses were performed to check for possible association between mean patients age; proportion of male patients in the OSAS groups; mean BMI in the OSAS groups, on the one hand; and the PWD mean differences between the OSAS and the healthy control subjects, on the other. Estimated effect was diminishing with advancing patient age in the OSAS groups; however, this relationship was not statistically significant (b = −0.020, Qmodel(1) = 0.56, p = .46). Estimated effect was increasing with higher proportion of male patients in the OSAS groups; however, this association was insignificant (b = 0.041, Qmodel(1) = 2.34, p = .13). Likewise, estimated effect was increasing with higher values of BMI in patients with OSAS, although this association was insignificant (b = 0.144, Qmodel(1) = 1.18, p = .28).
Another outcome considered by this meta-analysis was correlation between AHI and PWD. A total of k = 5 studies with available data were included in this analysis. The observed Fisher r-to-z transformed correlation coefficients ranged from 0 to 1.4722. The majority of estimates (80%) were positive. The estimated average Fisher r-to-z transformed correlation coefficient was equal to 0.530 (95% CI: 0.075 to 0.985). Therefore, the average outcome differed significantly from zero (z = 2.2815, p = 0.0225), and the effect size was large. Calculated aggregated correlation coefficient was equal to 0.477 (95% CI: 0.155 to 0.7070 (Fig. 3). According to the Q-test, the true outcomes were heterogeneous (Q(4) = 36.0983, p < .0001, tau2 = 0.2472, I2 = 93.0314%). A 95% prediction interval for the true outcomes was from −0.5457 to 1.6053. Therefore, in some studies, the true outcome might in fact be negative. An examination of the studentized residuals revealed that one study (Bayir et al.) [26] had a value larger than ± 2.5758. This study might be a potential outlier. The values of the Cook’s distances did not found any studies that could be considered as overly influential. The regression test indicated funnel plot asymmetry (p = .0022). However, the rank correlation test did not (p = 0.2333). The “Trim and Fill” method based on a random effects model was used to look for missing studies. The method suggested that one study to the right side of the mean effect was missing, and the imputed point estimate was 0.58097 (95% CI: 0.22246, 0.80107). A meta-analysis covering 4 studies comprising only adult patients was further performed. The calculated aggregated correlation coefficient was equal to 0.573 (95% CI: 0.268 to 0.774), and these figures did not significantly differ from the total estimates covering 5 studies that comprised both adults and children.
Discussion
Atrial fibrillation is a common arrhythmia. It is caused by focal activation and multiple randomly reentrant wavelets that propagate, become extinct, or fractionate within the atrial tissue. The longest duration of right atrial electrograms, the maximum number of fragmented deflections, and the repetitive atrial firing zone have all been found to have significant correlations with P-wave duration measured from a single-surface ECG lead. Prolonged intra- and interatrial conduction time can be identified by a prolongation of the surface P-wave duration. This raises the question of whether inhomogeneous atrial conduction can be identified by a variation in P-wave duration between different surface ECG leads [15]. PWD is a relatively new addition to the field of noninvasive electrocardiology. It is defined as the difference between the longest and shortest P-wave durations recorded from multiple different surface ECG leads [13]. PWD was assumed to have a normal value of 29 ± 9 ms. The authors refer to a maximum PWD value of 36 ms, and PWD greater than 40 ms indicates the presence of heterogeneous electrical activity in various regions of the atrium, which may cause atrial tachyarrhythmias, particularly AF [33]. The PWD has gotten more attention and has been studied in a variety of clinical settings, including cardiovascular and non-cardiovascular diseases [14].
This meta-analysis was aimed at evaluation of possible effect of OSAS on PWD. The main finding of this study was that there was a statistically significant increase in PWD in patients with a confirmed diagnosis of OSAS, with a large effect size. The “dose-effect” response substantiated the potential causal inference of OSAS on increased PWD, in that a statistically significant correlation was found between AHI and PWD values across the studies included in the meta-analysis, with a large effect size.
Increased PWD is not only limited to clinical cases of OSAS. Increase in PWD values was induced with simulated respiratory disturbances in the healthy subjects using Mueller maneuver and inspiration through a threshold load. This may imply that intrathoracic pressure swings may lengthen intra- and interatrial conduction time, thereby increasing P-wave duration and PWD [34]. Increased PWD has also been reported in children with respiratory disturbances due to adenotonsillar hypertrophy [35].
The marked autonomic imbalance that occurs in OSAS is one of the potential mechanisms linking increased PWD and OSAS [36]. The vagal predominance in conjunction with bradyarrhythmias at the end of apneas may alter the atria’s conduction properties. Previous research found that autonomic tone influenced P-wave durations [37]. Other factors directly related to OSAS (repetitive hypoxemia, systemic inflammation, fluctuations in intrathoracic hemodynamic, left ventricular diastolic dysfunction from pulmonary hypertension, increased atrial stretch, and left atrial dilatation) may all lead to prolonged intra- and interatrial conduction time and inhomogeneous propagation of sinus impulses, as reflected by increased Pmax and PWD [36, 38]. These factors have been linked to the pathophysiological mechanisms that contribute to the development of AF in adults with OSAS [39, 40].
It is possible that children with OSAS will have abnormal atrial conduction, as evidenced by increased PWD. Own findings do not contradict that suggestion, since no statistically significant differences in the effects of OSAS on PWD were found between children and adult subgroups. When the mean age of the OSAS group patients was included into the model as a covariant, meta-regression analysis failed to find statistically significant influence of that parameter on the effect size. However, it is of interest that the estimated value of the regression coefficient was indicative on diminishing estimated effect of OSAS on PWD with advancing patient age, meaning that in children the effect may potentially be even more pronounced than in adults. A smaller number of studies and participants that contributed data to the “Children” subgroup than to the “Adults” subgroup in this meta-analysis prevent from making definite judgments, and previous studies in adult patients were indicative that PWD was higher in older subjects [41].
Since overweight and obese patients have increased risk of OSAS, of special interest seems potential interplay between OSAS, body mass index (BMI), and PWD. Several studies have looked at the relationship between P wave indices and obesity, and it has been found that people who are obese have significantly longer P wave indices than people who are not [42]. Even after controlling for other clinical variables, BMI was found to be moderately related to PWD [42]. It is worth noting that a decrease in P wave indices has been observed following weight loss [43]. After inclusion of the mean BMI in the OSAS group into the model as a covariant, the estimated value of the meta-regression coefficient was positive, thus implying growing PWD with increasing BMI in the patients with OSAS across the studies covered by this meta-analysis. However, this association was statistically insignificant. Probably, it was the breathing disturbance related to the excessive weight that influences PWD the most, rather than the excessive weight itself.
OSAS has been reported to occur more commonly in male patients. Therefore, an attempt was made to evaluate a possible modifying effect of patients’ gender on the association between OSAS and PWD by inclusion of the percent of male subjects in the OSAS groups across the studies into the meta-regression model as a covariant. This covariant did not significantly contribute to the model, although positive value of the estimated regression coefficient precluded increasing effect with increasing proportion of the males in the OSAS group.
This study has some advantages. It was based, like any meta-analysis, on a systematic review of the literature, allowing for a conclusive synthesis of accumulating scientific evidence. By combining data from independent studies, it was possible to include 773 patients with OSAS and 347 healthy control subjects, improving statistical precision. The high methodological quality of the included studies, as well as the strict eligibility criteria, ensured the validity.
The study has several limitations that should be acknowledged. First, the relationship between PWD and true inhomogeneity of sinus impulse propagation is debatable. However, despite the conceptual difficulties, and despite the fact that there is a clear publication bias toward positive findings, the large number of studies showing some meaningful results with PWD should not be dismissed lightly [44]. Another limitation is that measuring the P wave and its dispersion is not strictly standardized. The studies are hampered by the fundamental limitations of using PWD measurements. The accuracy of the P-wave length and dispersion measurements has been limited by difficulties in determining the onset and offset of the P-wave. The lability of P-wave characteristics, as well as their circadian behavior, may also cause measurement imprecision [45]. The effect of these potential inevitable discrepancies was minimized by the means of using standardized mean difference as a measure of the effect size and by using random effect model in this meta-analysis. We must also acknowledge high heterogeneity of the effects across the studies that entered this meta-analysis and the revealed signs of publication bias. Although appropriate statistical procedures were performed enabling to take into account these problems, the findings, nevertheless, should be interpreted cautiously.
Conclusion
With all limitations in mind, and with the understanding that more research in the field is needed, the findings of this meta-analysis suggest that OSAS is associated with increased P-wave dispersion across all age groups, which may predispose to atrial tachyarrhythmias in patients with sleep-disordered breathing.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Young T, Peppard PE, Gottlieb DJ (2002) Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 165(9):1217–1239
Ali NJ, Pitson DJ, Stradling JR (1993) Snoring, sleep disturbance, and behaviour in 4-5 year olds. Arch Dis Child 68(3):360–366
Tarasiuk A, Greenberg-Dotan S, Simon-Tuval T, Freidman B, Goldbart AD, Tal A, Reuveni H (2007) Elevated morbidity and health care use in children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 175(1):55–61
Schwengel DA, Sterni LM, Tunkel DE, Heitmiller ES (2009) Perioperative management of children with obstructive sleep apnea. Anesth Analg 109(1):60–75
Quan SF, Gillin JC, Littner MR, Shepard JW (1999) Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. editorials. Sleep (New York, NY) 22(5):662–689
Mitchell RB (2007) Adenotonsillectomy for obstructive sleep apnea in children: outcome evaluated by pre-and postoperative polysomnography. Laryngoscope 117(10):1844–1854
Young T, Skatrud J, Peppard PE (2004) Risk factors for obstructive sleep apnea in adults. Jama 291(16):2013–2016
Todd K, McIntyre WF, Baranchuk A (2010) Obstructive sleep apnea and atrial fibrillation. Nat Sci Sleep 2:39
Wann LS, Curtis Anne B, January Craig T, Ellenbogen Kenneth A, Lowe James E, Estes NAM, Page Richard L, Ezekowitz Michael D, Slotwiner David J, Jackman Warren M, Stevenson William G, Tracy Cynthia M, Fuster V, Rydén Lars E, Cannom David S, Le Heuzey J-Y, Crijns Harry J, Lowe James E, Curtis Anne B et al (2011) 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (updating the 2006 guideline). J Am Coll Cardiol 57(2):223–242. https://doi.org/10.1016/j.jacc.2010.10.001
Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ (2008) Sleep apnea and cardiovascular disease: an American heart association/American college of cardiology foundation scientific statement from the American heart association council for high blood pressure research professional education committee, council on clinical cardiology, stroke council, and council on cardiovascular nursing in collaboration with the national heart, lung, and blood institute national center on sleep disorders research (national institutes of health). J Am Coll Cardiol 52(8):686–717
Bauters F, Rietzschel ER, Hertegonne KBC, Chirinos JA (2016) The link between obstructive sleep apnea and cardiovascular disease. Curr Atheroscler Rep 18(1):1
Baranchuk A, Parfrey B, Lim L, Morriello F, Simpson CS, Hopman WM, Redfearn DP, Fitzpatrick M (2011) Interatrial block in patients with obstructive sleep apnea. Cardiol J 18(2):171–175
Dilaveris PE, Gialafos JE (2001) P-wave dispersion: a novel predictor of paroxysmal atrial fibrillation. Ann Noninvasive Electrocardiol 6(2):159–165
Magnani JW, Johnson VM, Sullivan LM, Lubitz SA, Schnabel RB, Ellinor PT, Benjamin EJ (2010) P-wave indices: derivation of reference values from the Framingham Heart Study. Ann Noninvasive Electrocardiol 15(4):344–352. https://doi.org/10.1111/j.1542-474X.2010.00390.x
Okutucu S, Aytemir K, Oto A (2016) P-wave dispersion: what we know till now? JRSM Cardiovasc Dis 5:2048004016639443. https://doi.org/10.1177/2048004016639443
Okutucu S, Oto A (2017) P-wave dispersion in different clinical situations: expanding list with resembling mechanisms. JRSM Cardiovasc Dis 6:2048004017720369. https://doi.org/10.1177/2048004017720369
Yagmur J, Yetkin O, Cansel M, Acikgoz N, Ermis N, Karakus Y, Tasolar H (2012) Assessment of atrial electromechanical delay and influential factors in patients with obstructive sleep apnea. Sleep Breath 16(1):83–88. https://doi.org/10.1007/s11325-010-0477-6
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Moher D (2021) Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol 134:103–112. https://doi.org/10.1016/j.jclinepi.2021.02.003
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR (2021) Introduction to meta-analysis, 2nd edn. Wiley, Chichester
Ellis PD (2010) The essential guide to effect sizes: statistical power, meta-analysis, and the interpretation of research results. Cambridge University Press, Cambridge
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5(1):1–10
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (2019) Cochrane handbook for systematic reviews of interventions, 2nd edn. Wiley, Chichester
Borenstein M, Higgins JP, Hedges LV, Rothstein HR (2017) Basics of meta-analysis: I(2) is not an absolute measure of heterogeneity. Res Synth Methods 8(1):5–18. https://doi.org/10.1002/jrsm.1230
Borenstein M, Hedges L, Higgins J, Rothstein H (2005) Comprehensive meta-analysis version 2. Biostat, Englewood
Arslan BO, Fırat H, Ardıç S (2017) Evaluation of P wave dispersion, QT dispersion and P wave amplitude in patients with obstructive sleep apnea syndrome. J Turk Sleep Med 4(3):84
Bayir PT, Demirkan B, Bayir O, Duyuler S, Firat H, Guray U, Guray Y, Tatar EC (2014) Impact of continuous positive airway pressure therapy on atrial electromechanical delay and P-wave dispersion in patients with obstructive sleep apnea. Ann Noninvasive Electrocardiol 19(3):226–233. https://doi.org/10.1111/anec.12106
Cagirci G, Cay S, Gulsoy KG, Bayindir C, Vural MG, Firat H, Kilic H, Yeter E, Akdemir R, Ardic S (2011) Tissue Doppler atrial conduction times and electrocardiogram interlead P-wave durations with varying severity of obstructive sleep apnea. J Electrocardiol 44(4):478–482. https://doi.org/10.1016/j.jelectrocard.2011.03.009
Can I, Aytemir K, Demir AU, Deniz A, Ciftci O, Tokgozoglu L, Oto A, Sahin A (2009) P-wave duration and dispersion in patients with obstructive sleep apnea. Int J Cardiol 133(3):e85–e89. https://doi.org/10.1016/j.ijcard.2007.11.037
Çiçek D, Gökay S, Lakadamyali H, Sapmaz I, Muderrisoglu H (2012) Effect of obstructive sleep apnea on heart rate, heart rate recovery and QTc and P-wave dispersion in newly diagnosed untreated patients. Am J Med Sci 344(3):180–185
Kraikriangsri C, Khositseth A, Kuptanon T (2019) P-wave dispersion as a simple tool for screening childhood obstructive sleep apnea syndrome. Sleep Med 54:159–163. https://doi.org/10.1016/j.sleep.2018.09.032
Metwally M, Roshdy S, Ghany MA, Abd El Razik A (2014) P wave dispersion and severity of obstructive sleep apnea syndrome. Egypt J Chest Dis Tuberc 63(1):141–144. https://doi.org/10.1016/j.ejcdt.2013.11.018
Russo V, Di Meo F, Rago A, Mosella M, Molino A, Russo MG, Nigro G (2016) Impact of continuous positive airway pressure therapy on atrial electromechanical delay in obesity-hypoventilation syndrome patients. J Cardiovasc Electrophysiol 27(3):327–334. https://doi.org/10.1111/jce.12879
Aytemir K, Ozer N, Atalar E, Sade E, Aksoyek S, Ovunc K, Oto A, Ozmen F, Kes S (2000) P wave dispersion on 12-lead electrocardiography in patients with paroxysmal atrial fibrillation. Pacing Clin Electrophysiol 23(7):1109–1112. https://doi.org/10.1111/j.1540-8159.2000.tb00910.x
Gaisl T, Wons AM, Rossi V, Bratton DJ, Schlatzer C, Schwarz EI, Camen G, Kohler M (2016) Simulated obstructive sleep apnea increases P-wave duration and P-wave dispersion. PLoS One 11(4):e0152994. https://doi.org/10.1371/journal.pone.0152994
Kocabas A, Salman N, Ekici F, Cetin I, Akcan FA (2014) Evaluation of cardiac functions and atrial electromechanical delay in children with adenotonsillar hypertrophy. Pediatr Cardiol 35(5):785–792. https://doi.org/10.1007/s00246-013-0853-7
Roche F, Thanh Xuong AN, Court-Fortune I, Costes F, Pichot V, Duverney D, Vergnon JM, Gaspoz JM, Barthelemy JC (2003) Relationship among the severity of sleep apnea syndrome, cardiac arrhythmias, and autonomic imbalance. Pacing Clin Electrophysiol 26(3):669–677
Tükek T, Akkaya V, Demirel Ş, Sözen AB, Kudat H, Atılgan D, Özcan M, Güven Ö, Korkut F (2000) Effect of Valsalva maneuver on surface electrocardiographic P-wave dispersion in paroxysmal atrial fibrillation. Am J Cardiol 85(7):896–899
Niroumand M, Kuperstein R, Sasson Z, Hanly PJ (2001) Impact of obstructive sleep apnea on left ventricular mass and diastolic function. Am J Respir Crit Care Med 163(7):1632–1636
Maan A, Mansour M, Anter E, Patel VV, Cheng A, Refaat MM, Ruskin JN, Heist EK (2015) Obstructive sleep apnea and atrial fibrillation: pathophysiology and implications for treatment. Cri Pathw Cardiol 14(2):81–85
Magnani JW, Williamson MA, Ellinor PT, Monahan KM, Benjamin EJ (2009) P wave indices: current status and future directions in epidemiology, clinical, and research applications. Circ Arrhythm Electrophysiol 2(1):72–79
Turhan H, Yetkin E, Sahin O, Yasar AS, Senen K, Atak R, Sasmaz H, Cehreli S (2003) Comparison of P-wave duration and dispersion in patients aged > or =65 years with those aged < or =45 years. J Electrocardiol 36(4):321–326. https://doi.org/10.1016/j.jelectrocard.2003.08.002
Kosar F, Aksoy Y, Ari F, Keskin L, Sahin I (2008) P-wave duration and dispersion in obese subjects. Ann Noninvasive Electrocardiol 13(1):3–7
Duru M, Seyfeli E, Kuvandik G, Kaya H, Yalcin F (2006) Effect of weight loss on P wave dispersion in obese subjects. Obesity 14(8):1378–1382
Perez-Riera AR, de Abreu LC, Barbosa-Barros R, Grindler J, Fernandes-Cardoso A, Baranchuk A (2016) P-wave dispersion: an update. Indian Pacing Electrophysiol J 16(4):126–133. https://doi.org/10.1016/j.ipej.2016.10.002
Dilaveris P, Tousoulis D (2017) P-wave dispersion measurement: methodological considerations. Indian Pacing Electrophysiol J 17(3):89. https://doi.org/10.1016/j.ipej.2017.03.001
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants performed by the author.
Consent to participate
This type of study does not require informed consent.
Conflict of interest
The author declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kelmanson, I.A. Increased P-wave dispersion in patients with obstructive sleep apnea syndrome: a meta-analysis. Sleep Breath 27, 291–301 (2023). https://doi.org/10.1007/s11325-022-02630-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-022-02630-1