Abstract
To determine nitrogen (N) fate and environmental impact of applying anaerobic digestion slurry (ADS) to rice paddy (Oryza sativa L.), a field experiment was established using three treatments based on contrasting N application rate. The ADS (with ammonium-N accounting for >80 % of total N) treatment at a conventional application rate of 270 kg N ha−1 was compared to a negative control (no N fertilizer) and a positive control of urea applied at 270 kg N ha−1. The N budget showed the following distribution of applied N from ADS and urea: 41.3 ± 5.1 % for ADS and 36.6 ± 4.4 % for urea recovered by the rice plant (including straw, grain, and root), 16.4 ± 3.7 % for ADS and 7.4 ± 1.8 % for urea lost via ammonia volatilization, 0.26 ± 0.15 % for ADS and 0.15 ± 0.12 % for urea lost by direct N2O emission, 1.9 ± 0.5 % for ADS and 2.3 ± 0.8 % for urea leached downward, 0.70 ± 0.15 % for ADS and 0.67 ± 0.12 % for urea discharged with floodwater drainage, and 39.4 ± 8.4 % for ADS and 53.0 ± 9.1 % for urea retained by soil or lost by N2 emission. Compared to urea application, ADS application impacts the environment mainly through gaseous N losses rather than water N losses. ADS application had a positive impact on rice grain yield and reduced chemical fertilizer use. Considering the wide distribution of paddy fields and the ever-increasing quantities of ADS, ADS may serve as a valuable N source for rice cultivation, although mitigating ammonia and N2O losses should be further investigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Excessive reactive nitrogen (N) in the environment is of increasing concern worldwide as it induces a greenhouse effect, acid rain, nitrate pollution in groundwater, and eutrophication of surface water (Galloway et al. 2004; Yan et al. 2011). Fertilization in agricultural systems has been recognized as one of the main sources of excessive reactive N in water and atmospheric environments (Galloway et al. 2004; Giles 2005). Therefore, when an alternative source of N (such as anaerobically digested slurry) is available to widely supplement or replace a common N fertilizer source (such as urea) for crop cultivation, caution should be given to the availability of N to crops as well as environmental impacts.
As a liquid by-product in anaerobic digestion of organic waste, anaerobically digested slurry (ADS), is rich in N and offers a beneficial N source for crop cultivation (Arthurson 2009). Field application of ADS may reduce greenhouse gas emissions (Amon et al. 2006; Meijide et al. 2007) and mitigate water pollution from direct discharge of ADS (Cho et al. 2000; Lu et al. 2012) to surface waters. However, application of ADS to farmland is still not widespread, and knowledge concerning the fate of N applied as ADS over the course of a growing season is limited (Ghoneim et al. 2008; Lu et al. 2012). Due to the increasing popularity of anaerobic digestion to reduce organic waste and produce renewable energy in the form of biogas, the availability of ADS has dramatically increased in many parts of the world (Abraham et al. 2007; Arthurso 2009; Yu et al. 2010; Zhou et al. 2012). In China alone, about 600 million m3 of ADS was generated in 2010 (Yu et al. 2010; Lu et al. 2012). Therefore, increasing use of ADS for crop cultivation is a promising way for consuming the increasing quantities of ADS and preventing adverse environmental impacts (Hou et al. 2007;Palm 2008; Arthurson 2009). Paddy field is the largest irrigated farming system globally and the most important source of food in Asia (Zhao et al. 2009; Zhou et al. 2011; Lu et al. 2012); thus, it provides a broad opportunity for the beneficial use and disposal of ADS. Referring to the average total N concentration in ADS (∼650 mg l−1) reported by Yu et al. (2010) and (Lu et al. (2012), currently available volume of ADS offers a potential N source that corresponds to ∼40 % of total urea applied quantity for rice cultivations in China (i.e., 1.88 million Mg, Xing and Zhu 2000). However, before ADS can be effectively and widely used for rice cultivation, there is an urgent need to determine the fate of ADS N in a paddy field system to assess the availability of ADS N to rice plant and the potential environmental impacts.
Although the general mechanisms of N transformations and losses in conventional paddy fields are well known (Galloway et al. 2004; Giles 2005; Yan et al. 2011), the fate of N from ADS has not been rigorously evaluated. The special properties of ADS, such as strong reduction potential, strong alkalinity, dominance of NH4 +, and high content of organic matter (Hou et al. 2007; Sänger et al. 2010; Lu et al. 2012), appreciably alter N transformation pathways in ADS applied to paddy fields. Previous studies indicated that N lost by ammonia volatilization from ADS was much higher than from chemical fertilizer in paddy fields (Hou et al. 2007; Sunaga et al. 2009). A nitrogen-15-labeled experiment found that rice grain, straw, and roots had a lower uptake percentage but higher percentage of soil N retention from ADS than from chemical fertilizer (Ghoneim 2008; Ghoneim et al. 2012). For ADS applied paddy field, N2O emission was significantly higher than for chemical fertilizer (Moeller and Stinner 2009; Senbayram et al. 2009). A chamber incubation experiment also indicated N2O emission from a flooded soil was increased by ADS application compared with mineral N application (Sänger et al. 2011). However, other studies found that there were no significant differences in either N2O emission or nitrate concentration in drainage water between the ADS and chemical fertilizer applied to paddy fields (Sawamoto et al. 2010; Win et al. 2010; Sasada et al. 2011). Similarly, a recent study suggested that ADS application did not increase N losses to surface water and groundwater compared with urea application (Lu et al. 2012). In general, current studies examining ADS application to paddy fields have restricted investigations to a single N cycling process. Little quantitative information is available concerning the distribution of N from ADS that is lost to water and atmospheric pathways, recovered by the rice plant, or retained in the soil.
The objective of this study was to explore the N fate and environmental impacts associated with ADS fertilized paddy fields over the course of a rice growing season under field conditions. The N inputs from irrigation water, precipitation, residual load in paddy field before rice seedling transplanting and fertilizer (ADS or urea), as well as N outputs by floodwater drainage, leaching, rice plant uptake, NH3 volatilization, and N2O emission were quantified. In addition, the N input through biological N fixation was considered since biological N2 fixation accounts for a considerable rate of N input to paddy fields during the rice growing season (Xue et al. 2011). The different impacts of ADS and urea on water and atmospheric N pathways, rice plant N recovery efficiency, and biomass production were assessed. Because there is no direct method for denitrification measurements in paddy soil (Zhao et al. 2009), N budgets were calculated to bracket N fluxes to soil retention and N2 emission.
Material and methods
Experimental design
A paddy field experiment was conducted between March and November 2010 in Jiaxing region, Zhejiang Province, China (130° 83′ N, 120° 84′ E), which is within the catchment of Taihu Lake. The area has a subtropical monsoon climate with an average annual ambient temperature of 16.4 °C and an average annual rainfall of 1,200 mm with 550 mm during the rice growing season. The soil is a gleyed paddy soil (clay loam, mesic Mollic Endoaquepts) with the following characteristics in the top 20-cm layer: organic matter 2.3–2.5 %, total N 3.0–3.1 g kg−1, available N by alkaline hydrolysis 124–127 mg kg−1, available phosphorus by bicarbonate extraction 4.5–7.0 mg kg−1, total potassium 1,010–1,141 mg kg−1, and soil bulk density 1.2 g cm−3. The anaerobically digested slurry (ADS) originated from a biogas production plant at a pig breeding farm. The ADS was stored in a pond for 7 to 10 days near the experimental site before application. The ADS contained relatively high concentrations of nutrients and oxidizable organic matter as measured by manganese oxidation (Table 1).
Nine plots, each 4 × 5 m, were separated by 1 m deep concrete board, and all plots were bordered by a 0.40 m deep trench (Fig. 1). The plots were installed 3 months prior to commencing the experiment—this was to ensure soil stabilization and minimize disturbance effects. To be comparable among all plots, the same water amount was irrigated to each plot during the rice growing season according to local agronomic practices. All plots received the same quantity of irrigated water by water meters (Fig. 1). Consistent with local agronomic practices, all plots were drained 42 days after rice transplanting and allowed to dry for 10 days to control non-productive tillering. This was followed by surface drainage 120 days after rice transplanting to prepare for harvest.
Experimental setup for nitrogen balance in a paddy field fertilized with anaerobically digested slurry (ADS). IM is an inlet with a water meter for calculating the ADS or water irrigation quantity in each plot, OM is an outlet with a water meter for calculating the floodwater discharge quantity in each plot, WLR is a floodwater level recorder, and PPS is a pipe with a ceramic cup for percolation water sampling
The experiment had three treatments based on input N loadings from chemical fertilizer or ADS for the period of one growing season (Table 2). The treatments were conducted in triplicate, and plots were selected in a randomized complete block design. All treatments were dosed to a level of 39.3 kg P ha−1 with combinations of superphosphate or P from ADS (Table 2). The positive control (NF) had urea and KCl added such that they equaled the concentration of the ADS. The ADS treatment (NADS) contained 35.1 kg K ha−1 and 270 kg N ha−1. Consistent with local agronomic practices, the nutrient supply from ADS or chemical fertilizers was applied in three increments at 10-day intervals (2 July, 12 July, and 22 July) to each plot at an applied quantity ratio of 5:2:3. All chemical fertilizers were evenly dispersed by hand, and all ADS were applied by irrigation with flexible pipes in each plot. Rice (Oryza sativa L.) seedlings (25 days old) were transplanted at 200 × 200 mm spacing (480 plants per plot) on 2 July 2010. The rice was harvested on 3 November 2010.
Water quality and quantity monitoring
Floodwater from each plot was collected as a composite of seven to ten subsamples. Composite samples were collected at 1–3-day intervals after each N application. Thereafter, samples were collected at 10- to 28-day intervals. The pH was measured at the time of collection using a HYDROLab Multi-Parameter Data Sonde 5 (Hach Company, USA). Two water meters were installed to measure irrigation water volume and the water volume discharged after 42 and 120 days (Fig. 1). Nine 1-m-length plastic rulers were set in each plot for measuring floodwater level at 3- to 15-day intervals. Percolating water samples were collected at 3–10-day intervals using modified PVC pipes (3 cm diameter) that had a soft plug sealing the top and a ceramic cup at the base (Fig. 1). Each plot had three separate monitoring points, and a manual vacuum pump was used to remove percolating water from each cup. To obtain the percolating water quantity during the rice growing season, a regression model for predicting daily water percolation (P i , millimeters) for paddy fields with clay loam soil in the Taihu Lake region was adopted (Shi et al. 2010):
where h i is the floodwater level at the ith day (millimeters). During the drying period, daily percolation rates were predicted as:
where H is the rice main root length (meters) and t i is the duration day number after floodwater drainage. The average percolation rates were calculated from average rates for the entire rice growing season with daily values interpolated for intervals between measurements. Daily rainfall (provided by local weather bureau), irrigation inputs, floodwater level, and percolation rates for each treatment are shown in Fig. 2.
Rainfall, irrigation, floodwater, and percolation water levels in paddy fields during a rice growing season under different treatments. 0 kg N ha−1 from urea or anaerobically digested slurry (ADS) (CK), 270 kg N ha−1 from urea (NF), and ADS (NADS). Vertical bars above and below each point are standard errors. Shadow zone denotes the 10-day drying period
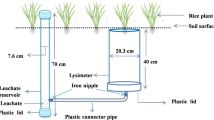
Floodwater and percolating water samples were collected in 1.5-l and 0.1-l polyethylene bottles, respectively, and then acidified with 1 M H2SO4 solution (15 ml H2SO4 per floodwater sample and 2 ml H2SO4 per percolating water sample). Samples were stored on ice, and chemical analyses were performed within 4 h after sampling. Total nitrogen (TN) concentration was determined spectrophotometrically after persulfate digestion (Ferree and Shannon 2001). Samples were made alkaline with 0.5 M NaOH and digested at 120 to 124 C with 0.15 M persulfate for 30 min. Samples were measured at 220 and 275 nm using a spectrophotometer (Shanghai Spectrum Instruments Co., Ltd, China). Samples for NH4 +-N and NO3 −-N analyses were all filtered through a prerinsed, nylon membrane filter (0.45 μm). NH4 +-N concentration was determined using an Astoria Analyzer System (AAS, Brown Rupee CO. Ltd., Germany). Nitrate concentration was determined using a colorimetric assay measured at 220 and 275 nm (Shanghai Spectrum Instruments Co., Ltd, China) (Ferree and Shannon 2001). The average TN, NH4 +-N, and NO3 −-N concentrations in percolating waters were calculated from averaging daily concentrations throughout the rice growing season with daily concentrations interpolated for intervals between measurements.
Gas emission sampling and analysis
Ammonia volatilization was measured in triplicate using modified capture chambers (Fig. 3) as described by Wang et al. (2004). The chamber was made of a PVC tube (15 cm internal diameter and 30 cm high). Two pieces of round sponge (15 cm in diameter and 2 or 3 cm in thickness) were put into each chamber after they were moistened with 15 or 20 ml of phosphate-glycerol solution (dilute 50 ml analytical phosphate and 40 ml glycerol to 1,000 ml with pure water). Since the volume of the solution accounted for <5 % of the sponge volume, the sponge was still ventilative after being moistened. The sponge inside the chamber absorbs NH3 volatilized from soil, and the top sponge was for absorbing NH3 from the ambient air. Glycerol was used to absorb moisture from the air in or outside the chamber and to prevent the sponges from drying. To prevent interferences from rainfall and other factors, a plastic frame with a sealed bottom and hollowed round was reversely covered on the PVC tube with a distance of 8 cm between the plastic frame bottom and the top sponge (Fig. 3). Ammonia in the phosphate-glycerol solution in each sponge inside the vented chamber was extracted with 1,000 ml 1 M KCl after 60 min of oscillation. Ammonium concentration (C, milligrams per liter) in the KCl extract solution was measured as per water quality analysis. Ammonia volatilization flux (AV, grams N per square meter per day) from the soil was estimated by the following formula:
where A is the cross-section area (square meters) of the round chamber. To avoid the suppressive effect of the chamber on NH3 volatilization, each capture chamber was relocated to a different site within each plot for replicate sampling. The capture duration for each sampling was 24 h commencing between 11:00 and 12:00 AM and with 1–15-day intervals between measurements. The average NH3 volatilization fluxes were calculated from averaging daily NH3 fluxes throughout the period of rice growing with daily fluxes interpolated for intervals between measurements.
Using a static chamber (40 × 40 × 110 cm dimensions) for each plot, gas samples for N2O emission were collected between 07:00 and 11:00 AM with 2–20-day intervals (Cai et al. 1997; Xing et al. 2002; Sunaga et al. 2009; Zhou et al 2009; Win et al. 2010; Sänger et al. 2011). An electric fan was fixed below the ceiling of the chamber. When gas samples were collected, the chamber was placed 5–10 cm into the soil, and the electric fan was used to mix the gas. The gas inside the chamber was withdrawn with a 50-ml syringe at 1, 11, 21, and 31 min and immediately transferred into an evacuated vial with a volume of 10 ml (La-pha-pack ®GmbH, Germany). The vial was sealed with a butyl rubber septum and covered by a protective plastic cap. The N2O concentration was analyzed by a gas chromatograph (GC-12A Shimadzu, Kyoto, Japan) equipped with a 63Ni electron capture detector. The interfering O2 contained in the injected air sample (1.0 ml) was separated by a pre-column (Porapac N, 1.0 m) and vented by using a ten-port valve. The pre-column and the main column (Porapac Q, 3.0 m) were flushed with 30 ml min−1 of 5 % CH4 in Ar carrier gas at 85 °C. N2O flux (F, micrograms N per square meter per hour) was calculated according to (Xing et al. 2002):
where ρ is the density of N2O at standard state, V is the effective volume of chamber (cubic meters), A indicates the area from which N2O was emitted into the chamber (square meters), △C/△t represents the rate of accumulation in parts per billion by volume per hour, and T is temperature in °C. The average N2O fluxes were calculated by averaging daily N2O fluxes throughout the rice growing season with daily concentrations interpolated for intervals between measurements.
Soil sampling and analysis
Composite soil samples (seven subsamples per plot) were collected from the top 20-cm soil layer prior to planting of rice seedlings and after the rice harvest. Soil samples were air-dried, milled, and passed through a 2-mm sieve. Soil organic matter content was measured by K2Cr2O7-H2SO4 oxidation in an oil bath at 180 °C for 5 min and then titrated with a FeSO4 solution (Nelson and Sommers 1982). Total N was determined by dry combustion at 960 °C (Nitrogen Analyzer and Rapid N Cube; Elementar Analysensysteme GmbH, Germany) (Chen and Shrestha 2012).
Plant sampling and analysis
Six rice seedlings were selected randomly on the transplanting date (2 July). From each plot, three rice plants with soil pillar (25 cm in depth and 15 cm in diameter) were randomly sampled on the 44th day after rice transplanting, and six rice plants with soil pillar (50 cm in depth and 20 cm in diameter) were randomly harvested on the 120th day after rice transplanting. The roots were immersed in purified water in the laboratory for 2 h and then rinsed with purified water to remove soil bonded to the roots. The cleaned roots for rice sampled on the 44th day were laid on a glass pane with a fixed plastic ruler to measure the main root length (H). The sampled rice seedlings and harvested grain, straw, and roots on the 120th day were dried at 70 °C to constant weight to determine their dry weight. Three clusters of dried rice seedlings and rice grain, straw, and roots were sampled for each plot, then milled with their hulls (JNMJ3, Taizhou Grain Instrument, Zhejiang, China) and sieved (0.5-mm sieve) prior to N analyses. Nitrogen contents for the whole rice seedling and the harvested grain, straw, and roots were measured as described in the “Soil sampling and analysis” section.
Nitrogen budget calculation
The N budget for the rice growing season in each plot can be expressed by:
where N au, N i, N p, N b, and N f were TN inputs from ADS or urea application, irrigation water, precipitation, background in paddy field soil prior to planting the rice seedlings, and biological fixation, respectively. Biological N fixation rate was adopted from a summary for paddy fields at N fertilizer application rates of 0–270 kg ha−1 per season in the Taihu Lake region (Xue et al. 2011). N U, N A, N N, N L, and N D were TN outputs from plant uptake (including grain, straw, and roots), NH3 volatilization, N2O emission, leaching, and drainage, respectively. N p was cumulatively estimated by multiplying rainfall and TN concentration during the experimental period, with data provided by the local Environmental Protection Bureau. Both N A and N N were estimated by multiplying the average NH3 volatilization flux by the rice growing season duration and the average N2O emission flux by the rice growing season duration, respectively. N L was estimated by multiplying weighted-average TN concentration and weighted-average percolating water quantity for the duration of the rice growing season. The “balance,” which is the difference between total inputs and measured outputs, represents the measurement and estimation errors and the unaccounted sinks (e.g., N2 from denitrification and change in soil storage).
To identify the fate of applied N from urea or ADS, the difference method (Zhao et al. 2009) was adopted to calculate the difference in N uptake, NH3 volatilization, N2O emission, leaching, drainage, and “balance” between the plots receiving N from urea and ADS and the control (no N addition). The calculated difference in each pathway represents the contributed N quantity from urea or ADS. Correlation analysis, regression analysis, and analysis of variance (ANOVA) were performed using SPSS statistical software (version 16.0, SPSS Inc. Chicago, USA).
Results
Nitrogen dynamics in floodwater and percolating water
In the 7 days after ADS application, TN and NH4 +-N concentrations in floodwater decreased rapidly (Fig. 4a). Ammonium-N accounted for more than 80 % of TN in ADS floodwater. Due to the kinetics of the urea hydrolysis reaction, NH4 +-N concentrations peaked in the second day after each urea application and then decreased rapidly over the following 5–6 days. The TN and NH4 +-N concentrations in floodwater after the first urea or ADS application were both higher than after subsequent applications (P < 0.05) since it received the highest N application rate. Unlike TN and NH4 +-N, NO3 –-N concentration in floodwaters of the NF and NADS treatments displayed random time delays after each ADS or urea application with concentrations ranging from 0.1 to 3.7 and 0.1 to 4.5 mg l−1, respectively (Fig. 4a). On the 12th day after the third N application (i.e., 42 days after rice transplanting), concentrations of TN, NH4 +-N, and NO3 –-N in the drainage water were similar between NF and NADS treatments (P > 0.05; Fig. 4a). All plots were then dried for 10 days, after which they were reflooded with surface water. Between reflooding and drainage for harvest on 3 November, concentrations of all N forms in floodwater were constant and low (<2 mg l−1) and similar between NF and NADS treatments (P > 0.05).
Variations in total N (TN), NH4 +-N, and NO3 −-N concentrations in floodwater (a) and percolation water (b) during a rice growing season under different treatments. 0 kg N ha−1 from urea or anaerobically digested slurry (ADS) (CK), 270 kg N ha−1 from urea (NF), and ADS (NADS). Arrows indicate ADS or fertilizer application. Vertical bars above and below each point are standard errors. Shadow zone denotes the 10-day drying period
Unlike N in floodwater, TN, NH4 +-N, and NO3 −-N concentrations in percolating water displayed random in peak concentrations with time delays after each ADS or urea application (Fig. 4b). The TN, NH4 +-N, and NO3 −-N concentrations in NADS treatments ranged between 2.1 and 5.5 mg l−1, 0.34 and 1.34 mg l−1, and between 0.17 and 1.69 mg l−1, respectively. In NF treatment plots, the TN, NH4 +-N, and NO3 −-N concentrations ranged between 1.9 and 6.6 mg l−1, 0.17 and 1.16 mg l−1, and between 0.25 and 1.78 mg l−1, respectively. There were no significant differences in average N concentrations between NF and NADS treatments, but there were significant differences (P < 0.05) between N concentrations in NF (mean ± SD; TN 4.01 ± 1.47 mg l−1, NH4 +-N 0.53 ± 0.22 mg l−1, NO3 −-N 0.83 ± 0.28 mg l−1) and CK (TN 2.23 ± 1.13 mg l−1, NH4 +-N 0.49 ± 0.11 mg l−1, NO3 −-N 0.58 ± 0.17 mg l−1) treatments and between NADS (TN 3.62 ± 1.29 mg l−1, NH4 +-N 0.79 ± 0.22 mg l−1, NO3 −-N 0.73 ± 0.33 mg l−1) and CK treatments.
Ammonia volatilization and N2O emission
After each ADS application, NH3 volatilization fluxes increased, peaked, and then decreased rapidly (Fig. 5a). After 3 days, NH3 volatilization fluxes approached background values (i.e., similar to CK plots). In contrast, NH3 volatilization fluxes in NF treatments approached peak values in the second day after each urea application and then decreased rapidly. Ammonia emission was affected primarily by the timing of N application. There were strong correlations between daily average NH3 volatilization fluxes (y, grams N per square meter per day) and NH4 +-N concentrations (x, milligrams per liter) in floodwater of NF (y = 0.18x + 0.76; R 2 = 0.67, n = 23, P < 0.01) and NADS (y = 0.12x + 1.62; R 2 = 0.85, n = 23, P < 0.01) treatments. During the rice growing season, average NH3 volatilization fluxes followed: NADS (3.98 ± 0.46 g N m−2 day−1) > NF (2.03 ± 0.35 g N m−2 day−1) > CK (0.44 ± 0.15 g N m−2 day−1; P < 0.05). However, there were no significant correlations between NH3 volatilization and pH value in floodwaters for ADS and urea treatments (P > 0.05), with average pH value of 8.6 ± 1.2 in NADS, 8.5 ± 1.3 in NF, and 8.4 ± 1.0 in CK.
Variations of ammonia volatilization fluxes (a) and N2O emission fluxes (b) from paddy field during a rice growing season under different treatments. 0 kg N ha−1 from urea or anaerobically digested slurry (ADS) (CK), 270 kg N ha−1 from urea (NF), and ADS (NADS). Arrows indicate ADS or fertilizer application. Vertical bars above and below each point are standard errors. Shadow zone denotes the 10-day drying period
Patterns for temporal variations of N2O emission fluxes were quite different from those of NH3 volatilization fluxes (Fig. 5b). The emission of N2O was sporadic and pulse-like and showed no spikes following each urea or ADS application. There were no significant correlations between N2O emission fluxes and NO3 –-N concentrations in floodwater for each treatment (P > 0.05). In contrast, peak N2O fluxes were observed at the beginning of the disappearance of the floodwater layer from each treatment. There were also large variations in average N2O fluxes among triplicates (indicated as high standard deviations in Fig. 5b) for each treatment. Statistical analysis showed significant differences in average N2O flux among the three treatments during the growing season: NADS (50.2 ± 28.9 μg N m−2 h−1) > NF (40.1 ± 26.4 μg N m−2 h−1) > CK (26.7 ± 15.3 μg N m−2 h−1; P < 0.05).
Rice plant biomass production and nitrogen uptake
Among rice biomass components, roots contained substantially lower biomass than straw and grain for each treatment (Fig. 6). Applying urea or ADS significantly increased the rice straw, grain, and root biomass relative to the control (P < 0.05). Although there were no significant differences (P > 0.05) in straw and root biomass between the urea and ADS treatments, there was a significant difference (P < 0.05) in the grain biomass between them. The grain yield from the ADS treatment was 12.4 % higher than the urea treatment on average.
Harvested rice grain, straw, and root dry biomass under different treatments. 0 kg N ha−1 from urea or anaerobically digested slurry (ADS) (CK); 270 N ha−1 from urea (NF), and ADS (NADS). Columns with the same letter are not significantly different using ANOVA (P > 0.05). Error bars are standard errors
Similar to biomass measurements, the content of N in roots was lower than that in straw and grain components for each treatment (Table 3). Applying urea or ADS significantly increased N content in rice straw, grain, and roots relative to the control treatment (P < 0.05). Although there were no significant differences (P > 0.05) in N content by straw and roots between the urea and ADS treatments, there was a significant difference (P < 0.05) in N content by grain between them. Nitrogen content in grain was 15.3 % higher in the ADS treatment compared to the urea treatment.
Nitrogen budget and fate of applied nitrogen
The N budget for each treatment is shown in Table 3. Among all the determined sink items, assimilation by plants was the most important, accounting for 61.2 ± 3.4 % of total input N in NF and 64.8 ± 4.1 % in NADS. Of total N inputs, 7.2 ± 1.2 % (NF) and 14.1 ± 1.8 % (NADS) were lost via ammonia volatilization; 0.34 ± 0.24 % (NF) and 0.42 ± 0.26 % (NADS) were lost via direct N2O emission; 3.6 ± 1.4 % (NF) and 3.3 ± 1.7 % (NADS) were leached downward; and 0.9 ± 0.1 % (NF) and 0.9 ± 0.1 % (NADS) were lost via drainage. Finally, the “balance” for N was calculated to be 26.9 ± 6.1 % in NF and 16.4 ± 5.2 % in NADS. In contrast, the “balance” for the control was negative, indicating that the CK plots consumed N from soil N and organic matter reserves to produce the rice plant biomass and measured N losses. This result was consistent with the decrease of total N and organic matter levels in the 0–20-cm soil depth of the CK plots (Fig. 7). Although the differences were not significant, soil TN and organic matter levels in CK plots displayed a decreasing trend when comparing post-harvest to pre-plant levels. In contrast, the positive N balances for NF and NADS treatments indicated that the 270 kg N ha−1 application rate was higher than rice plant demand. Therefore, soil total N and organic matter levels both in NF and NADS treatments displayed an increasing trend (Fig. 7), although there were no significant differences (P > 0.05).
Paddy soil total nitrogen (TN) and organic matter contents before rice seedling transplanting and after rice harvesting under different treatments. 0 kg N ha−1 from urea or anaerobically digested slurry (ADS) (CK); 270 kg N ha−1 from urea (NF), and ADS (NADS). The same capital (between before rice seedling transplanting and after rice harvesting) and small (among the treatments) letters are not significantly different using ANOVA (P > 0.05). Error bars are standard errors
Differences in N uptake and environmental losses between the treatments receiving N and the control (no N addition) were calculated to address the fates of applied N (Table 4). Of the N added as urea or ADS, rice plant (including straw, grain, and roots) N uptake accounted for 36.6 ± 4.4 and 41.3 ± 5.1 %, respectively. The percentages of N lost through gaseous and water pathways were: NH3 volatilization 16.4 ± 3.7 % (NADS) vs 7.4 ± 1.8 % (NF); direct N2O emission 0.15 ± 0.12 % (NADS) vs 0.26 ± 0.15 % (NF); nitrogen leaching 2.3 ± 0.8 % ( NF) vs 1.9 ± 0.5 % (NADS); and N discharged with floodwater drainage 0.67 ± 0.12 % (NF) vs 0.70 ± 0.15 % (NADS). Finally, 53.0 ± 9.1 and 39.4 ± 8.4 % of applied N was associated with unaccounted sinks (e.g., soil pool and N2 denitrification losses) for the NF and NADS treatments, respectively.
Discussion
Impacts of urea and ADS on nitrogen lost through water pathways
During the 7 days after each urea or ADS application, the N concentrations in the floodwater were relatively high, especially for TN and NH4 +-N (Fig. 4a). This creates a potential for higher surface water pollution resulting from the discharge of this floodwater from the paddy field. However, N concentrations rapidly decreased through NH3 volatilization (Fig. 5a), denitrification (Fig. 5b), soil adsorption (Fig. 7), and rice plant uptake (Table 3). Nitrogen concentrations were similar between ADS and urea treatments 9 to 10 days after each N application. Thus, the discharged floodwaters 42 and 120 days after rice transplanting resulted in similar N loss loads between ADS and urea applications (Table 4), indicating that there was no elevated risk of N loss to surface water from ADS application. To avoid surface water pollution resulting from ADS application, it is necessary to prevent floodwater overflow from the plot during the 7 days after each ADS application, which can be achieved by maintaining enough height of plot bunds to fully contain the floodwaters.
In the percolating water, there were no increasing trends in N concentrations following each urea or ADS application (Fig. 4b), suggesting that N in percolating water is not directly related to increased floodwater N concentrations following N application to the paddy field (Zhu et al. 2003). Moreover, there was no significant difference in TN leaching loads between the urea and ADS fertilized paddy fields (Tables 3 and 4). The percentages of TN leaching load of the total applied N from urea and ADS obtained in this study are both comparable with those estimated by 15N isotopic technique in the available literature (Table 4). Application of ADS created no significant elevated risk to groundwater quality compared with urea-fertilized paddy fields. The low losses of TN (3–4 % of applied N) were related to soil hydrologic conditions affecting the site. These conditions included: (1) the presence of a hard pan at the bottom of the topsoil (about 20 cm depth) limited floodwater N infiltration into the subsoil (Lu et al. 2012), (2) NO3 −-N was transformed into nitrogen gas (N2) or N2O by denitrifying bacteria under saturated paddy conditions (Chantigny et al. 2008), and (3) the limited mobility of NH4 +-N within the soil profile prevented N loss from percolating waters (Zhou et al. 2009) since NH4 +-N is the dominant N form in floodwater (Fig. 4a).
Impacts of urea and ADS on nitrogen lost through gaseous pathways
During the rice growing season, loss via NH3 volatilization represented 16.4 ± 3.7 % of applied ADS N, and it was much higher than that of applied urea N (7.4 ± 1.8 %), which is comparable with that reported in the available literature using 15N isotopic technique for ADS and urea-fertilized paddy fields, respectively (Table 4). The observed significant differences in NH3 volatilization proportion appear to be mainly related to the higher NH4 +-N concentration in floodwaters treated with ADS (6.0 ± 3.1 mg l−1) as compared to those treated with urea (3.2 ± 2.0 mg l−1; Fig. 4a). Although other factors, such as pH values in floodwater and air temperature, also influence NH3 volatilization (Zhou et al. 2009; Sommer and Hutchings 2001), results suggest that the NH4 +-N concentration in the floodwater is a dominant factor in our experimental field conditions, i.e., the floodwater keeping at alkaline and little change in the temperature. Regression analysis indicated that 67.3and 85.3 % of the variance in NH3 volatilization fluxes could be explained by the NH4 +-N concentration in floodwater for the urea and ADS treatments, respectively. As a result, ADS application dramatically increases the NH3 volatilization loss to the atmosphere compared to urea application. Therefore, in practice, mitigating NH3 volatilization is necessary to limit N losses from paddy fields following ADS application. Previous studies have indicated that adding wood vinegar or increasing floodwater levels can reduce NH3 volatilization after ADS application in a cost-efficient manner (Win et al. 2009, 2010). However, it should be pointed out that high NH3 volatilization flux will increase atmospheric N deposition rate since NH3 volatilization is an important source of atmospheric N (Hou et al. 2007). Therefore, the proportion of N loss through NH3 volatilization is speculated to decrease when ADS is widely applied in paddy fields for a region.
There were both no apparent temporal trend in N2O emission fluxes after each urea or ADS application (Fig. 5b) and no significant correlation between N2O emission fluxes and N concentrations in floodwater, indicating that there was no significant effect of N form or concentration in floodwater (Breitenbeck et al. 1980; Granli and Bøckman 1994), but there might have significant effect of soil temperature and redox potential (Xu et al. 1997; Xing et al. 2002) on N2O emission. Distinct emission peaks appeared for each treatment during the dry down periods (from 42 to 52 days after rice seedling transplanting) may be ascribed to the release of trapped N2O in the soil solution and/or optimum conditions for the production of N2O and its release at that time (Cai et al. 1997; Xing et al. 2002). However, there might be no significant N2O emissions after final drainage prior to harvest due to the lack of available N for nitrifiers and denitrifiers (Xu et al. 1997; Xing et al. 2002; Ding et al. 2011). Future studies may improve the results by increasing N2O emission observations at the rice late growth stage, considering the sparse observations obtained during this period in this study (Fig. 5b). Direct N2O emission accounted for about 0.26 ± 0.15 % of applied ADS N, and it was significantly higher than that for urea treatments (0.15 ± 0.12 %, which falls within the reported ranges in the available literature using 15N isotopic technique, Table 4). Due to the strong reducing potential and high organic matter content of ADS (Table 1), it is speculated that N2O emission was increased due to higher microbial activity that provided electrons for the denitrification reaction (Zhou et al. 2009 and 2012; Sänger et al. 2011). Considering the global warming potential of N2O, mitigating N2O emission must be addressed to minimize the greenhouse gas impact of widespread application of ADS to paddy fields. Increasing floodwater level (Cai et al. 1997) or adding nitrification inhibitor to floodwaters (Xu et al. 2002; Ding et al. 2011) may suppress N2O emission following ADS application.
Impacts of urea and ADS on rice plant nitrogen uptake
Comparing rice plant biomass among all treatments clearly demonstrates that artificial N application was necessary to obtain a high rice plant biomass, especially for grain yield (Fig. 6). Applying ADS to the paddy fields increased total rice plant biomass, especially for the grain yield, which corroborated previous studies where ADS was applied as the primary N source for paddy fields (Zhou et al. 2009; Lu et al. 2012). Total N uptake by rice plants in ADS-treated plots was greater than in the urea-treated plots (Tables 3, 4), indicating that the combination of inorganic and organic nutrients may improve rice grain yield by improving plant nutrient-use efficiency (Li et al. 2009). Rice plant recovery of applied N from ADS (41.3 ± 5.1 %) and urea (36.6 ± 4.4 %) is comparable with that reported in the available literature using 15N isotopic technique for rice treated with ADS and urea, respectively (Table 4). Fertilizing with ADS notably improved the agricultural economics of rice grain production by lowering the need for commercial fertilizer (Fig. 6). In this study, ADS application reduced the chemical application of N (100 %), K (23 %), and P (44 %) compared to conventional rice cultivation practices (Table 2)—and this was achieved with an increase in grain yields. These lead to higher economic return for farmer, supporting increasing use of ADS for rice cultivation.
Impacts of urea and ADS on other nitrogen sinks
The calculated difference in each N pathway between plots receiving N and the control (no N addition) estimated that unaccounted for sinks represented 53.0 ± 9.1 and 39.4 ± 8.4 % of applied N from urea and ADS, respectively (Table 4). The primary unaccounted sinks include soil retention and N2 emission (Zhou et al. 2009, 2012). The role of soil retention as a sink for applied N from urea and ADS is supported by the increase in soil total N content after rice harvest (Fig. 7). As products of denitrification, higher N2O emission would also suggest higher N2 emission from ADS versus urea-amended paddy fields, which is supported by the results derived from 15N isotopic studies (Table 4). Previous studies relied on 15N isotopic technique reported that N loss via denitrification (i.e., N2 + N2O) in paddy fields accounted for 9.9–38.9 % of applied N from ADS (Zhou et al. 2012; Ghoneim et al. 2012), which is comparable to the percentage of N residual in soil of applied N from ADS after rice harvest (i.e., 13.2–34 %, Ghoneim 2008; Ghoneim et al. 2012; Zhou et al. 2011, 2012). Further study is required to address the contributions of soil N retention and N2 emissions to the N balance following ADS application to paddy fields. Referring to the previously reported results using 15N isotopic technique from the available literature (Table 4), it also appears that the contributions of soil retention and N2 emission to the unaccounted N balance (53.0 ± 9.1 %) associated with urea fertilizer application are roughly comparable to each other. Certainly, the measurement and estimation errors associated with the N balance contribute to the unaccounted proportions of applied N from urea and ADS as well as the unknown term indicated in Table 4 from the available literature.
It should be pointed out that N distributions in different pathways mentioned above are strongly related to soil microorganisms associated with N cycle, such as ammonia-oxidizing bacteria, nitrite-oxidizing bacteria, N fixation bacteria, and denitrifying microorganisms (Abubaker 2012). These soil microorganisms are easily altered in activities and diversities by changing ambient properties, such as pH, heavy metal, and chloric compounds (Nannipieri et al. 2003; Abubaker 2012). Therefore, high pH value (>8.0) (Hou et al. 2007; Lu et al. 2012), low heavy metal contents (Sänger et al. 2010; Lu et al. 2012), and possibly contained chloric compounds in ADS (Arthurson 2009) or from other chemical fertilizer (such as KCl) are speculated to have a potential for further altering N fates in paddy fields, requiring long-term monitoring to determine if there were negative effects on soil microorganisms.
Conclusion
There were distinct differences in the fate of applied N from urea (NF) and anaerobically digested slurry (NADS) to paddy fields. At the conventional application rate of 270 kg N ha−1 during the rice growing season, rice plant (including straw, grain, and roots) recovery was 36.6 ± 4.4 and 41.3 ± 5.1 % for urea and ADS, respectively. Of the applied N, N loss through NH3 volatilization in NADS was 16.4 ± 3.7 %, which was higher than that in NF (7.4 ± 1.8 %). Direct N2O emission was 0.26 ± 0.15 % in NADS, which was also higher than in NF (0.15 ± 0.12 %). Nitrogen leaching contributed 2.3 ± 0.8 % in NF and 1.9 ± 0.5 % in NADS, while N discharged with floodwater drainage only contributed 0.67 ± 0.12 % in NF and 0.70 ± 0.15 % in NADS. Soil retention and N2 emission of applied N were estimated to account for 53.0 ± 9.1 % in NF and 39.4 ± 8.4 % in NADS. Compared to urea application, the rice plant N recovery efficiency, ammonia volatilization, and N2O emission were higher in the paddy fields fertilized with ADS. ADS application impacts the environment mainly through gaseous losses rather than water losses. ADS application had a positive impact on rice grain yield and also reduced chemical fertilizer use leading to higher economic returns. Considering the wide distribution of paddy fields and the ever increasing quantities of available ADS, application of ADS to paddy fields appears to provide an environmental acceptable means for ADS disposal and rice cultivation, although mitigating NH3 and N2O gaseous losses requires further investigation.
References
Abraham ER, Ramachandran S, Ramalingam V (2007) Biogas: can it be an important source of energy? Environ Sci Pollut Res Int 14:67–71. doi:10.1065/espr2006.12.370
Abubaker J (2012) Effects of fertilisation with biogas residues on crop yield, soil microbiology and greenhouse gas emissions. Dissertation, Swedish University of Agricultural Sciences
Amon B, Kryvoruchko V, Amon T (2006) Methane, nitrous oxide and ammonia emissions during storage and after application of dairy cattle slurry and influence of slurry treatment. Agri Ecosys Environ 112:153–162. doi:10.1016/j.agee.2005.08.030
Arthurson V (2009) Closing the global energy and nutrient cycles through application of biogas residue to agricultural land—potential benefits and drawbacks. Energies 2:226–242. doi:10.3390/en20200226
Breitenbeck GA, Blackmer AM, Bremner JM (1980) Effects of different nitrogen fertilizers on emission of nitrous oxide from soil. Geophys Res Lett 7:85–88. doi:10.1029/GL007i001p00085
Cai ZC, Xing GX, Yan XY, Xu H, Tsuruta H, Kazuyuki Y, Katsuyuki M (1997) Methane and nitrous oxide emissions from rice paddy fields as affected by nitrogen fertilisers and water management. Plant Soil 196:7–14. doi:10.1023/A:1004263405020
Chantigny MH, Angers DA, Bélanger G, Rochette P, Eriksen-Hamel N, Bittman S, Buckley K, Massé D, Gasser M (2008) Yield and nutrient export of grain corn fertilized with raw and treated liquid swine manure. Agron J 100:1303–1309. doi:10.2134/agronj2007.0361
Chen HYH, Shrestha BM (2012) Stand age, fire and clearcutting affect soil organic carbon and aggregation of mineral soils in boreal forests. Soil Biol Biochem 50:149–157. doi:10.1016/j.soilbio.2012.03.014
Cho JC, Cho HB, Kim SJ (2000) Heavy contamination of a subsurface aquifer and a stream by livestock wastewater in a stock farming area, Wonju, Korea. Environ Pollut 109:137–146. doi:10.1016/S0269-7491(99)00230-4
Ding WX, Yu HY, Cai ZC (2011) Impact of urease and nitrification inhibitors on nitrous oxide emissions from fluvo-aquic soil in the North China Plain. Biol Fertil Soils 47:91–99. doi:10.1007/s00374-010-0504-6
Fan XH, Song YS, Lin DX, Yang LZ, Luo JF (2006) Ammonia volatilization losses and 15 N balance from urea applied to rice on paddy soil. J Environ Sci 18:299–303
Ferree MA, Shannon RD (2001) Evaluation of a second derivative UV/visible spectroscopy technique for nitrate and total nitrogen analysis of wastewater samples. Water Res 35:327–332. doi:10.1016/S0043-1354(00)00222-0
Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, Asner GP, Cleveland C, Green P, Holland E, Karl DM, Michaels AF, Porter JH, Townsend A, Vörösmarty C (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70:153–226. doi:10.1007/s10533-004-0370-0
Ghoneim AM (2008) Nitrogen dynamics and fertilizer use efficiency in rice using the nitrogen-15 isotope techniques. World Appl Sci J 3:869–874. doi:10.3923/ijss.2008.11.19
Ghoneim AM, Ueno H, Asagi N, Watanabe T (2012) Indirect 15N isotope techniques for estimating N dynamics and N uptake by rice from poultry manure and sewage sludge. Asian J Earth Sci. doi:10.8928fajes.2012
Ghoneim AM, Ueno H, Ebid A, Asagi N, AI Ed (2008) Analysis of nitrogen dynamics and fertilizer use efficiency in rice using the nitrogen-15 isotope dilution method following the application of biogas slurry or chemical fertilizer. Int J Soil Sci 3:11–19. doi:doi:10.3923/ijss.2008.11.19
Giles J (2005) Nitrogen study fertilizes fears of pollution. Nature 433:791. doi:10.1038/433791a
Granli T, Bøckman OC (1994) Nitrous oxide from agriculture. Nor J Agric Sci 12:48–53
Hou H, Zhou S, Hosomi M, Toyota K, Yosimura K, Mutou Y, Nisimura T, Takayanagi M, Motobayashi T (2007) Ammonia emissions from anaerobically-digested slurry and chemical fertilizer applied to flooded forage rice. Water Air Soil Pollut 183:37–48. doi:10.1007/s11270-007-9353-9
Li S, Li H, Liang XQ, Chen YX, Cao ZH, Xu ZH (2009) Rural wastewater irrigation and nitrogen removal by the paddy wetland system in the Tai Lake region of China. J Soil Sediment 9:433–442. doi:10.1007/s11368-009-0095-8
Lu J, Jiang LN, Chen DJ, Toyota K, Strong PJ, Wang HL (2012) Decontamination of anaerobically digested slurry in a paddy field ecosystem in Jiaxing region of China. Agri Ecosys Environ 146:13–22. doi:10.1016/j.agee.2011.10.011
Meijide A, DiezM JA, Sanchez-Martin L, Lopez-Femandez S, Vakkejo A (2007) Nitrogen oxide emissions from an irrigated maize crop amended with treated pig slurries and composts in a Mediterranean climate. Agri Ecosys Environ 121:383–394. doi:10.1016/j.agee.2006.11.020
Moeller K, Stinner W (2009) Effects of different manuring systems with and without biogas digestion on soil mineral nitrogen content and on gaseous nitrogen losses (ammonia, nitrous oxides). Eur J Agron 30:1–16. doi:10.1016/j.eja.2008.06.003
Nannipieri P, Ascher J, Ceccherini MT, Landi L, Pietramellara G, Renella G (2003) Microbial diversity and soil functions. Eur J Soil Sci 54:655–670. doi:10.1046/j.1365-2389.2003.00556.x
Nelson DW, Sommers LE (1982) Total carbon, organic carbon and organic matter. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. Part 2, 2nd ed. Agronomy, vol. 9. American Society of Agronomy, Madison, pp 539–577
Nguyen MD, Brandt KK, Sørensen J, Hung NN, Hach CV, Tan PS, Dalsgaard T (2012) Effects of alternating wetting and drying versus continuous flooding on fertilizer nitrogen fate in rice fields in the Mekong Delta, Vietnam. Soil Biol Biochem 47:166–174. doi:10.1016/j.soilbio.2011.12.028
Palm O (2008) The quality of liquid and solid digestate from biogas plants and its application in agriculture. ENC/ORBIT e.V. Workshop 2008. The future for anaerobic digestion of organic waste in Europe. Pres Nr 20:1–2
Sänger A, Geisseler D, Ludwig B (2010) Effects of rainfall pattern on carbon and nitrogen dynamics in soil amended with biogas slurry and composted cattle manure. J Plant Nutr Soil Sci 173:692–698. doi:10.1002/jpln.200900254
Sänger A, Geisseler D, Ludwig B (2011) Effects of moisture and temperature on greenhouse gas emissions and C and N leaching losses in soil treated with biogas slurry. Biol Fertil Soils 47:249–259. doi:10.1007/s00374-010-0528-y
Sasada Y, Win KT, Nonaka R, Win AT, Toyota K, Motobayashi T, Hosomi M, Chen DJ, Lu J (2011) Methane and N2O emissions, nitrate concentrations of drainage water, and zinc and copper uptake by rice fertilized with anaerobically digested cattle or pig slurry. Biol Fertil Soils 48:1–8. doi:10.1007/s00374-011-0601-1
Sawamoto T, Yoshida R, Abe K, Matsunaka T (2010) No significant difference in N2O emission, fertilizer-induced N2O emission factor and CH4 absorption between anaerobically digested cattle slurry and chemical fertilizer applied timothy (Phleum pratense L.) sward in central Hokkaido. Soil Sci Plant Nutr 56:492–502. doi:10.1111/j.1747-0765.2010.00465
Senbayram M, Chen RR, Muhling KH, Dittert K (2009) Contribution of nitrification and denitrification to nitrous oxide emissions from soils after application of biogas waste and other fertilizers. Rapid Commun Mass Spectrom 23:2489–2498. doi:10.1002/rcm.4067
Shi YF, Jiao XY, Luo YF, Guo SY (2010) Synchronous estimating crop coefficients and daily seepage using a water balance model for paddy fields. Sciencepaper Online of China, http://www.paper.edu.cn/index.php/default/releasepaper/content/201105-404. Accessed 18 May 2011
Sommer SG, Hutchings NJ (2001) Ammonia emission from field applied manure and its reduction—invited paper. Eur J Agron 15:1–15. doi:10.1016/S1161-0301(01)00112-5
Sunaga K, Yoshimura N, Hou H, Win KT, Tanaka H, Yoshikawa M, Watanabe H, Motobayashi T, Kato M, Nishimura T, Toyota K, Hosomi M (2009) Impacts of heavy application of anaerobically digested slurry to whole crop rice cultivation in paddy environment on water, air and soil qualities. Jpn J Soil Sci Plant Nutr 80:596–605 (in Japanese with English summary)
Wang ZH, Liu XJ, Ju XT, Zhang FS, Malhi SS (2004) Ammonia volatilization loss from surface-broadcast urea: comparison of vented- and closed-chamber methods and loss in winter wheat–summer maize rotation in North China Plain. Commun Soil Sci Plant 35:2917–2939. doi:10.1081/CSS-200036499
Win KT, Nonaka R, Toyota K, Motobayashi T, Hosomi M (2010) Effects of option mitigating ammonia volatilization on CH4 and N2O emissions from a paddy field fertilized with anaerobically digested cattle slurry. Biol Fertil Soils 46:589–595. doi:10.1007/s00374-010-0465-9
Win KT, Toyota K, Motobayashi T, Hosomi M (2009) Suppression of ammonia volatilization from a paddy soil fertilized with anaerobically digested cattle slurry by wood vinegar application and floodwater management. Soil Sci Plant Nutr 55:190–202. doi:10.1111/j.1747-0765.2008.00337.x
Xing GX, Shi SL, Shen GY, Du LJ, Xiong ZQ (2002) Nitrous oxide emissions from paddy soil in three rice-based cropping systems in China. Nutr Cycl Agroecosys 64:135–143. doi:10.1023/A:1021131722165
Xing GX, Zhu ZL (2000) An assessment of N loss from agricultural fields to the environment in China. Nutr Cycl Agroecosys 57:67–73. doi:10.1023/A:1009717603427
Xu H, Xing GX, Cai ZC, Tsuruta H (1997) Nitrous oxide emissions from three rice paddy fields in China. Nutr Cycl Agroecosys 49:23–28. doi:10.1023/A:1009763023828
Xu X, Boeckx P, Wang Y, Huang Y, Zheng X, Hu F, Cleemput OV (2002) Nitrous oxide and methane emissions during rice growth and through rice plants: effect of dicyandiamide and hydroquinone. Biol Fertil Soils 36:53–58. doi:10.1007/s00374-002-0503-3
Xue LH, Yu YL, Yang LZ (2011) Nitrogen balance and environmental impact of paddy field under different N management methods in Taihu Lake region. Environ Sci 32:1133–1138 (in Chinese)
Yan XY, Cai ZC, Yang R, Ti CP, Xia YQ, Li FY, Wang JQ, Ma AJ (2011) Nitrogen budget and riverine nitrogen output in a rice paddy dominated agricultural watershed in eastern China. Biogeochemistry 106:489–501. doi:10.1007/s10533-010-9528-0
Yu FB, Luo XP, Song CF, Zhang MX, Shan SD (2010) Concentrated biogas slurry enhanced soil fertility and tomato quality. Acta Agr Scand B-S P 60:262–268. doi:10.1080/09064710902893385
Zhang QW, Yang ZL, Zhang H, Yi J (2012) Recovery efficiency and loss of 15N-labelled urea in a rice-soil system in the upper reaches of the Yellow River basin. Agri Ecosys Environ 158:118–126. doi:10.1016/j.agee.2012.06.003
Zhao X, Xie YX, Xiong ZQ, Yan XY, Xing GX, Zhu ZL (2009) Nitrogen fate and environmental consequence in paddy soil under rice-wheat rotation in the Taihu Lake region, China. Plant Soil 319:225–234. doi:10.1007/s11104-008-9865-0
Zhao X, Zhou Y, Wang SQ, Xing GX, Shi WM, Xu RK, Zhu ZL (2012) Nitrogen balance in a highly fertilized rice–wheat double-cropping system in the Taihu Lake Region, China. Soil Sci Soc Am J 76:1068–1078. doi:10.2136/sssaj2011.0236
Zhou S, Nishiyama K, Watanabe Y, Hosomi M (2009) Nitrogen budget and ammonia volatilization in paddy fields fertilized with liquid cattle waste. Water Air Soil Pollut 201:135–147. doi:10.1007/s11270-008-9933-3
Zhou S, Sakiyama Y, Riya S, Song XF, Terada A, Hosomi M (2012) Assessing nitrification and denitrification in a paddy soil with different water dynamics and applied liquid cattle waste using the 15 N isotopic technique. Sci Total Environ 430:93–100. doi:10.1016/j.scitotenv.2012.04.056
Zhou S, Sugawara S, Riya S, Sagehashi M, Toyota K, Terada A, Hosomi M (2011) Effect of infiltration rate on nitrogen dynamics in paddy soil after high-load nitrogen application containing 15 N tracer. Ecol Eng 37:685–692. doi:10.1016/j.ecoleng.2010.04.032
Zhu JG, Liu G, Han Y, Zhang YL, Xing GX (2003) Nitrate distribution and denitrification in the saturated zone of paddy field under rice/wheat rotation. Chemosphere 50:725–732. doi:10.1016/S0045-6535(02)00212-6
Acknowledgments
This work was supported by the National Natural Science Foundation of China (nos. 41001120 and 40821140540), the National Key Technology R&D Program of China (no. 2012BAC17B01), Priority Themes of Science and Technology in Zhejiang Province of China (grant no. 2008C13049), and Japan Science and Technology (JST). We thank Zhejiang Nanhu Agricultural Bureau and Environmental Protection Bureau for in-kind support to the field work and providing relevant monitoring data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, D., Jiang, L., Huang, H. et al. Nitrogen dynamics of anaerobically digested slurry used to fertilize paddy fields. Biol Fertil Soils 49, 647–659 (2013). https://doi.org/10.1007/s00374-012-0752-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-012-0752-8