Abstract
The objective of this study was to examine the effects of soil moisture, irrigation pattern, and temperature on gaseous and leaching losses of carbon (C) and nitrogen (N) from soils amended with biogas slurry (BS). Undisturbed soil cores were amended with BS (33 kg N ha−1) and incubated at 13.5°C and 23.5°C under continuous irrigation (2 mm day−1) or cycles of strong irrigation and partial drying (every 6 weeks, 1 week with 12 mm day−1). During the 6 weeks after BS application, on average, 30% and 3.8% of the C and N applied with BS were emitted as carbon dioxide (CO2) and nitrous oxide (N2O), respectively. Across all treatments, a temperature increase of 10°C increased N2O and CO2 emissions by a factor of 3.7 and 1.7, respectively. The irrigation pattern strongly affected the temporal production of CO2 and N2O but had no significant effect on the cumulative production. Nitrogen was predominantly lost in the form of nitrate (NO −3 ). On average, 16% of the N applied was lost as NO −3 . Nitrate leaching was significantly increased at the higher temperature (P < 0.01), while the irrigation pattern had no effect (P = 0.63). Our results show that the C and N turnovers were strongly affected by BS application and soil temperature whereas irrigation pattern had only minor effects. A considerable proportion of the C and N in BS were readily available for soil microorganisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agriculture accounted for 10–12% of total global anthropogenic emissions of greenhouse gases in 2005 (Smith et al. 2007). The most important greenhouse gases released from agricultural land are methane (CH4), nitrous oxide (N2O), and carbon dioxide (CO2) which contribute 6.5%, 5.5%, and 0.1% to total global anthropogenic greenhouse gas emissions, respectively. The net flux of CO2 related to agriculture is estimated to be approximately balanced whereas agriculture contributes about 60% and 50% of the global anthropogenic N2O and CH4 emissions, respectively (Smith et al. 1998, 2007). Due to concerns about the greenhouse effect, renewable energy sources, such as biogas production from energy crops, are becoming increasingly important. As maize has one of the highest CH4 yields per hectare, its use for biogas production is likely to increase in the future (Amon et al. 2007) and therefore the availability of maize-derived biogas slurry (BS) as an organic fertilizer. However, the use of BS from energy crops as fertilizer and the effect of field applications on greenhouse gas emissions have not received much attention yet. Moeller and Stinner (2009) compared the N2O emissions of clover/grass-ley when mulched and incorporated as green manure with the N2O emissions resulting from the field application of BS made from the same clover/grass-ley. The field applications of BS resulted in a large increase in N2O emissions, but it was 38% less than that from the mulched and incorporated clover/grass-ley. Senbayram et al. (2009) reported that soils treated with BS derived from maize emitted more N2O than soils treated with mineral nitrogen (N) fertilizer. In a laboratory study, they found that 2.6% of the applied BS-N was emitted as N2O..
In soil, N2O is produced through denitrification and nitrification, both of which are mediated by microorganisms. While nitrification takes place under aerobic conditions, denitrification predominantly occurs under anaerobic conditions (Dittert and Mühling 2009). Nitrification and denitrification rates have been found to be closely related to the water-filled pore space (WFPS) of a soil. Nitrification rates are generally highest when soil moisture is below field capacity and nearly stops in saturated soils due to lack of oxygen (O2), whereas denitrification rates generally increase with increasing soil moisture content (Davidson 1992; Maag and Vinther 1996). Bateman and Baggs (2005) found that all of the N2O emitted from a silt loam at 70% WFPS was produced during denitrification, while at 35–60% WFPS nitrification was the main process producing N2O. Besides soil moisture content and temperature, the availability of substrates, ammonium (NH +4 ) for nitrification and nitrate (NO −3 ) as well as available carbon (C) for denitrification, affects the rates of N2O emissions (Smith et al. 1998). In general, anaerobic fermentation increases the NH +4 content in the substrate as well as the stability of organic matter but decreases the C to N ratio considerably (Gutser et al. 2005), resulting in a product with a high content of directly available N. However, during anaerobic digestion for CH4 production, volatile fatty acids and other labile organic compounds are formed as intermediates (Cysneiros et al. 2008; Jacobi et al. 2009). These compounds, when still present in the BS, may be readily available C sources for soil microorganisms.
The aim of this study was to investigate the effects of soil moisture, irrigation pattern, and temperature on gaseous emissions (CO2, N2O, and CH4) and leaching losses of C and N compounds from undisturbed soil cores in an incubation experiment.
Materials and methods
Soil and biogas slurry
Soil was sampled in March 2009 at the long-term experimental site “Hohes Feld” near Göttingen in Lower Saxony, Germany. The preceding crop was winter wheat, harvested in July 2008. In August 2008, the field was tilled with a rotary harrow. During winter, the field lay fallow. The mean annual precipitation at the site is 645 mm, and the mean annual temperature is 8.7°C (Deutscher Wetterdienst 2009). The soil type is a Haplic Luvisol (FAO 1998) derived from loess (Ehlers et al. 2000). The soil sampled had sand, silt, and clay contents of 15%, 67%, and 18%, respectively, a bulk density of 1.42 g cm−3, and a pH of 6.9. The total carbon (Ct) content gradually decreased with depth in the plow layer (26 cm) from 13.3 g kg−1 in the top 9 cm to 8.2 g kg−1 dry soil in the lowest 9 cm of the profile. The C to N ratio ranged from 9.5 to 10.0 and was not affected by depth. When the incubation started (see below), the microbial biomass C was 328 mg kg−1 in the top 9 cm of the profile and 125 mg kg−1 in the lower part of the plow layer (the methods used are described below).
Undisturbed soil cores were sampled using plexiglass cylinders with an inner diameter of 15 cm. The cylinders were inserted into the soil to a depth of 26 cm and dug out with a spade. The soil cores remained in the cylinders for the duration of the incubation.
BS was obtained from the Institute for Anaerobic Technology at the University of Applied Sciences of Giessen. The BS is a by-product of the anaerobic digestion (4 months at 55°C) of corn silage for CH4 production in a laboratory-scale reactor. The BS had a dry matter content of 8.4% and a pH of 8.9. The C and N contents in the dry matter were 41% and 5.1%, respectively, resulting in a C to N ratio of 8. About 24% of the N was in the form of NH +4 , while no NO −3 was detectable.
Incubation
The study was carried out in a climate chamber equipped with an automated microcosm system (Hantschel et al. 1994). The cores were placed on a ceramic plate with a 1-μm pore diameter to which a constant suction of 100 hPa was applied. Prior to the incubation, the cores were conditioned at 13.5°C for 6 months and irrigated with 2 mm day−1 of a 0.01 M CaCl2 solution adjusted to a pH of 5.5. After the conditioning phase, the cores were randomly assigned to one of the treatments (see below). All cores were incubated for 30 weeks. During the first 16 weeks of the experiment, the temperature was maintained at 13.5°C, while it was increased to 23.5°C for weeks 17 to 30. Three factors were investigated, namely (1) BS application (cores amended with BS and unamended controls), (2) temperature (13.5°C and 23.5°C), and (3) irrigation treatment. Three irrigation treatments were included:
-
1.
Continuous irrigation (2 mm day−1),
-
2.
Cycles of strong irrigation and partial drying (1 week with an irrigation rate of 12 mm day−1 was followed by 5 weeks without irrigation) with BS application immediately before a period of strong irrigation, and
-
3.
Cycles of strong irrigation and partial drying with BS application 1 week after the end of a period of strong irrigation.
In all treatments, the cumulative irrigation over any 6-week period was 84 mm. Separate cores were used for BS applications at the two temperatures. To the cores fertilized at 13.5°C, BS was applied during week 5 when applied before the period of strong irrigation or week 8 when applied after the period of strong irrigation. To the cores fertilized after the temperature rise, BS was applied during week 17 or 20. Four cores per treatment were incubated. BS was applied at a rate of 33 kg total N per hectare. For this, a 3–4 cm-thick layer of the topsoil was removed, the slurry, suspended in 50 mL of 0.01 M CaCl2 solution, added to the cores, and the topsoil placed back on top. The control cores were treated identically, except that 50 mL of 0.01 M CaCl2 solution without BS was added. The irrigation was reduced by the amount of liquid added in the fertilized cores. During the incubation, the soil cores were hermetically sealed, and fresh air flow through the headspace was 20 ml min−1.
Leachates were collected weekly, weighed, and analyzed for concentrations of NO −3 , NH +4 , total organic C (TOC), and total dissolved N (TDN). Every 3.7 h, a gas sample was analyzed for CO2, CH4, and N2O. Soil moisture content was monitored in two cores per treatment with soil moisture probes (ECH2O EC-5; Decagon Devises, Inc., Pullman, WA, USA). Measurements were taken every 30 min. The soil moisture probes were placed at a depth of 5–6 cm. During week 18 and at the end of the incubation, soil moisture content in the topsoil of all cores was determined using a ThetaProbe Soil Moisture Sensor-ML2x (Delta-T Devices Ltd., Cambridge, UK). To calibrate the soil moisture probes, gravimetric water content was determined in the top 5 cm of each core at the end of the experiment by drying a sample at 105°C for 24 h.
Chemical analyses
Two soil cores were used for determination of soil characteristics after the conditioning phase. Soil moisture content was determined by drying at 105°C for 24 h. The pH was measured in a 0.01 M CaCl2 solution (2.5 ml solution per gram of fresh soil) and particle size distribution with the pipet method according to DIN ISO 11277 (2002). Soil microbial biomass was determined with the chloroform fumigation extraction method (Vance et al. 1987). Total contents of C and N were determined by dry combustion on a CN Elemental Analyzer (Elementar Vario El, Heraeus, Hanau, Germany). The same methods were used for BS analyses, except that total N was analyzed by the Kjeldahl method on a Büchi 323 (Büchi Labortechnik, Essen, Germany). Ammonium and NO −3 were extracted from the BS with 0.5 M K2SO4 (20 ml per gram of fresh BS) and determined on a continuous flow analyzer (Evolution II auto-analyzer, Alliance Instruments, Salzburg, Austria).
Gaseous emissions of CO2, N2O, and CH4 were measured by a gas chromatograph (Shimadzu GC-14, Shimadzu Scientific Instruments, Columbia, USA). Nitrate and NH +4 concentrations in the leachates were determined on a continuous flow analyzer. TDN and TOC in the leachates were analyzed using a DIMATOC® 2000 (Dimatec Analysentechnik, Essen, Germany). Several times during the incubation, subsamples of the leachate were filtered through a 0.45-μm polyamide filter before analysis to determine the dissolved organic C content.
Statistical analysis
Statistical analyses were performed using SPSS 14.0.1 (SPSS 2005). The data were analyzed as a three-way ANOVA with temperature, fertilizer treatment, and irrigation pattern as factors. Mean comparisons were performed using the Tukey test. Effects were considered significant for P < 0.05. In order to avoid confounding effects due to differences in irrigation water application rates, comparisons of gaseous emissions from the different treatments were made for the 6-week period following the application of BS, as the amount of irrigation water applied was the same for all soil cores during any 6-week period. Effects of BS application on leaching losses became first evident after 4 weeks. Leaching losses from the different treatment were therefore compared for the 6-week period between weeks 4 and 10 after BS applications.
Results
Soil moisture
The gravimetric moisture content in the upper 6 cm of the cores with continuous irrigation averaged 27.5%, which corresponds to an average WFPS of 84% in the soil core (data not shown). In the treatments with cycles of strong irrigation and partial drying, the gravimetric moisture content in the upper 6 cm reached 29.5% during periods of strong irrigation and dropped to 26.5% during the 5 weeks without irrigation. These gravimetric moisture contents correspond to 90% and 81% WFPS in the soil core during periods of strong irrigation and partial drying, respectively. As the topsoil was loosened to incorporate the BS, the WFPS may have been lower in the topsoil after the BS application than in the rest of the core. Neither the increase in temperature after 16 weeks nor the application of BS had a significant effect on the gravimetric moisture content (data not shown).
Carbon dioxide production
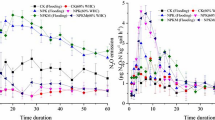
At 13.5°C, CO2 emission rates from the continuously irrigated control soils averaged 20 mg CO2-C m−2 h−1 and decreased slightly over time (Fig. 1a). The temperature rise resulted in a steep and immediate increase in CO2-C production rates up to 49 mg m−2 h−1 for 1 week, and average emission rates at 23.5°C were increased to 33 mg CO2-C m−2 h−1 (Fig. 1d). Therefore, the Q 10 factor for CO2 emissions was 1.7. The application of BS increased CO2 emission rates at both temperatures for a few weeks. However, after 3 weeks (when BS was applied at 13.5°C) to 7 weeks (at 23.5°C), the CO2 emissions had dropped to the level of the control. The amount of applied C, which evolved as CO2 during the 6 weeks following BS application, was 13% and 34% at 13.5°C and 23.5°C, respectively (Fig. 2).
Average CO2 emissions from soil cores amended with BS and unfertilized cores (control). The treatments included continuous irrigation (a, d), cycles of strong irrigation and partial drying with BS application before a period of strong irrigation (b, e), and cycles of strong irrigation and partial drying with BS application after a period of strong irrigation (c, f). The undisturbed soil cores were incubated at 13.5°C and 23.5°C. Arrows indicate the time of BS application. Each line represents the average of four cores. The standard error of the mean (n = 4) is shown for the first measurement each week
Proportion of BS C and N lost from soil cores incubated under continuous irrigation, and cycles of strong irrigation and partial drying with slurry applied before (periodic strong irrigation-1) and after a period of strong irrigation (periodic strong irrigation-2). The undisturbed soil cores were incubated at 13.5°C and 23.5°C. Gaseous losses include CO2 and N2O, and losses in leachate include TOC, NO −3 -N, and Norg. The difference between TDN and NO −3 -N corresponded to Norg. Negative values for Norg indicate that the losses in the BS-amended cores were lower than in the control
At 13.5°C, the average CO2 production of control soils in the treatments with cycles of strong irrigation and partial drying was 23 mg CO2-C m−2 h−1, with emissions averaging 25 mg CO2-C m−2 h−1 during partial drying periods and 14 mg CO2-C m−2 h−1 during wetter periods (Fig. 1b–c). Similar to the soils under continuous irrigation, the temperature rise led to an increase in average CO2 production rates to 34 mg m−2 h−1 (Fig. 1e–f), corresponding to a Q 10 factor of 1.5.
When BS was applied directly before a week of stronger irrigation, the CO2-C emissions slightly decreased during the period of stronger irrigation but increased considerably afterwards compared to the control (Fig. 1b, e). During subsequent periods of stronger irrigation, fertilization had a positive effect on CO2 emissions only at the higher temperature (Fig. 1e). The amount of applied C which evolved as CO2 within 6 weeks after BS application was 17% and 58% at 13.5°C and 23.5°C, respectively, when BS was applied directly before a week of stronger irrigation. BS applications 1 week after a period of strong irrigation also increased CO2 emission rates during periods without irrigation and had little effect during periods of stronger irrigation (Fig. 1c, f). The CO2, which evolved within 6 weeks after BS application, accounted for 21% and 38% of the C applied with the BS at the lower and higher temperature, respectively (Fig. 2).
Across all treatments, differences due to BS application and temperature were highly significant (P < 0.01 for both factors), while no significant differences were induced by the irrigation treatments (P = 0.34; Table 2).
Nitrous oxide emissions
On average, control soils under continuous irrigation emitted 22 μg N2O-N m−2 h−1 at 13.5°C (Fig. 3a). With the rise in temperature, N2O production reached a peak of 408 μg m−2 h−1 within a day. The average emission rate at the higher temperature was 194 μg N2O-N m−2 h−1 (Fig. 3d), which resulted in a Q 10 factor of 8.8. BS application at the lower temperature in the treatment with continuous irrigation resulted in an instant increase in N2O emissions, followed by a decrease over the next 3 weeks to the level of the control (Fig. 3a). When soils were fertilized at the higher temperature, maximum N2O emissions of 1,250 μg N2O-N m−2 h−1 were measured, and the emission rates remained above the level of the control for about 6 weeks (Fig. 3d). During the 6 weeks following the BS application, 2.2% and 6.8% of the N applied were lost in the form of N2O at 13.5°C and 23.5°C, respectively (Fig. 2).
Average N2O emissions from soil cores amended with BS and unfertilized cores (control). The treatments included continuous irrigation (a, d), cycles of strong irrigation and partial drying with BS application before a period of strong irrigation (b, e), and cycles of strong irrigation and partial drying with BS application after a period of strong irrigation (c, f). The undisturbed soil cores were incubated at 13.5°C and 23.5°C. Arrows refer to time of BS application. Each line represents the average of four cores. The standard error of the mean (n = 4) is shown for the first measurement each week
In the treatments with cycles of strong irrigation and partial drying, N2O emissions were highest 1 or 2 days after the beginning of the period of stronger irrigation. A second smaller peak was observed 1 day after the end of the period of stronger irrigation. This trend was independent of temperature and BS application time. During drier periods, N2O production was generally low, except directly after the application of BS (Fig. 3b–c, e–f). Most of the BS-related N2O emissions took place within a few weeks after the BS application. However, during periods of stronger irrigation, increased N2O emissions could still be observed after several irrigation cycles. The increase in temperature had little effect on the proportion of the N applied lost as N2O because N2O emissions increased in the BS-amended and in the control soil. When BS was applied before a period of stronger irrigation, 3.5% and 3.7% of the applied N were lost as N2O at 13.5°C and 23.5°C, respectively. When the application took place during the drier period, 3.9% and 3.0% of the N applied were lost at 13.5°C and 23.5°C, respectively (Fig. 2).
As with CO2, no significant differences were induced by irrigation pattern (P = 0.71), as higher N2O emissions during periods of stronger irrigation were compensated for by lower emissions during drier periods in treatments with cycles of strong irrigation and partial drying (Table 2). In contrast, the increase in temperature resulted in significantly higher N2O emissions (P < 0.01), with the Q 10 factor across all treatments reaching 3.7. Slurry application also significantly increased N2O emissions compared to the unamended control (P < 0.01).
Methane consumption
Methane emission rates were constantly negative in all treatments, indicating that net CH4 consumption took place (data not shown). The average CH4 consumption in the treatments with continuous irrigation was 4 μg CH4-C m−2 h−1, which corresponds to 0.13 mg CH4 m−2 day−1. The same rate was measured during periods of stronger irrigation in the treatments with cycles of strong irrigation and partial drying, while CH4 consumption reached 8 μg CH4-C m−2 h−1 during drier periods (Table 1). No significant differences in cumulative CH4 consumption were caused by BS application (P = 0.96) or temperature (P = 0.33; Table 2). In contrast, CH4 consumption was significantly increased in the treatments with cycles of strong irrigation and partial drying compared to the continuously irrigated cores (P < 0.01).
Leaching losses
Nitrate leaching from the cores under continuous irrigation without BS application was relatively constant averaging 37 mg NO −3 -N m−2 day−1 at 13.5°C and of 51 mg NO −3 -N m−2 day−1 at 23.5°C, which corresponds to a Q 10 factor of 1.38 (Fig. 4). Between 60% and 70% of the NO −3 -N leached from the control soils of the treatments with cycles of strong irrigation and partial drying was lost during periods of stronger irrigation. While only 22 to 28 mg NO −3 -N m−2 day−1 was lost during drier periods, the average losses reached 257 to 368 mg NO −3 -N m−2 day−1 during periods of stronger irrigation, with the higher rates occurring at the higher temperature. Across all treatments, BS application and temperature both had highly significant effects on NO −3 leaching (P < 0.01), while the irrigation treatments had no significant effect (P = 0.63). Effects of BS application on NO −3 leaching were most pronounced between weeks 4 and 10 after the application date. During this 6-week period, cumulative NO −3 -N losses across all irrigation treatments corresponded to 18% and 14% of the N applied in the BS at 13.5°C and 23.5°C, respectively.
Average leaching losses of NO −3 -N from soil cores amended with BS and unfertilized cores (control). The treatments included continuous irrigation (a, d), cycles of strong irrigation and partial drying with BS application before a period of strong irrigation (b, e), and cycles of strong irrigation and partial drying with BS application after a period of strong irrigation (c, f). The undisturbed soil cores were incubated at 13.5°C and 23.5°C. Arrows refer to time of BS application. Symbols represent means (n = 4) with standard error of the mean
Losses of TDN closely followed NO −3 -N losses. In fact, about 80% of the N in TDN was generally in the form of NO −3 , while the concentration of NH +4 in the leachate was always below the detection limit (data not shown). Thus, the difference between TDN and NO −3 -N corresponded to organic N (Norg). In contrast to NO −3 , the application of BS before a period of stronger irrigation decreased leaching of organic N compared to the unamended control (Fig. 2).
Leaching losses of TOC were significantly increased at the higher temperature (P < 0.01; Table 2), with losses averaging 24 and 36 mg C m−2 day−1 at the lower and higher temperature, respectively. In contrast, the application of BS had no significant effect on TOC leaching (P = 0.73). However, TOC losses were significantly higher under continuous irrigation than under cycles of strong irrigation and partial drying. Dissolved organic C (<0.45 μm) accounted for about 94% of the TOC leached.
Discussion
Under the conditions of our experiment, C and N turnovers in undisturbed soil cores were strongly affected by BS application and soil temperature. In contrast, the irrigation pattern and time of BS application had minor effects. In addition, only a few weak interactions were observed between these factors (Table 2).
Biogas slurry application
With the application of BS, CO2 production increased immediately. Within 6 weeks, on average, 30% of the C applied with BS was respired as CO2. These results indicate that a considerable proportion of the BS was readily available. In some microsites, O2 demand likely exceeded supply by diffusion, resulting in anaerobic conditions favorable for denitrification to occur.
Across all treatments, 3.8% of the N applied with the BS was lost as N2O during the 6 weeks following its application. In a study carried out at 13.5°C with a similar soil type and BS from the same fermenter, Sänger et al. (2010) found that about 1.2% of the added N was lost as N2O. Surprisingly, this proportion is lower than the average of 3.2% found in the present study at the lower temperature even though the application rate in the present study was three times lower. This suggests that the application rate had only a minor effect on the proportion of N lost as N2O. In fact, in a pot experiment over 35 days with soil held at 85% WFPS, Senbayram et al. (2009) found that about 1.7% and 1.5% of the applied BS were lost as N2O with application rates of 90 and 360 kg N ha−1, respectively. When applied to grassland at a rate of 480 kg N ha−1, less than 0.5% of the BS-N was lost in the form of N2O (Senbayram et al. 2009).
In a review of the literature, de Klein et al. (2001) found that the N2O emissions reported for mineral fertilizers generally ranged from 0.1% to about 2% of the N applied; however, a few short-term studies using nitrate-based fertilizers reported emissions of up to 12%. For a wide variety of animal manures, emissions ranged from 0% to about 5% of the manure N applied (de Klein et al. 2001). This comparison suggests that the N2O emissions from BS in our study are similar to those reported for animal waste and slightly higher than emissions from mineral fertilizers. However, in the studies reviewed by de Klein et al. (2001), N fertilizer was applied to crops in the field. The fact that no N uptake by plants took place in our study most likely resulted in higher N2O emissions compared to field studies.
The application of BS increased leaching losses of NO −3 but not of TOC, indicating that the microorganisms were limited by the availability of C while N availability exceeded demand. This result is not surprising given the narrow C to N ratio of BS, which was 8, and the high content of NH +4 . On average, about 16% of the N applied with the BS was leached from the cores between weeks 5 and 10 after application.
In summary, a large proportion of the C and N were readily available to soil microorganisms. The excess N, which leached as NO −3 in our study, could have been taken up by crops in a planted field. In contrast, as with slurry from animal husbandry, the application of BS to fallow fields may result in high NO −3 leaching losses.
Soil moisture effects
In general, soil moisture has a strong effect on the decomposition of organic material. While at low moisture water is the limiting factor, excess moisture inhibits aerobic metabolism because of O2 diffusion limitations. In an incubation study with different soils, Franzluebbers (1999) found that C mineralization was highest when the WFPS was between 53% and 66%. In the present study, WFPS ranged between 80% and 90%, which was likely at the upper limit for optimal aerobic respiration. This is evident from the fact that CO2 evolution strongly decreased during rewetting periods. However, while the irrigation treatment affected the temporal pattern of CO2 evolution, it had no significant effect on the cumulative CO2 production. This is likely due to the fact that the variation in soil moisture observed in our study was relatively small.
While soil moisture had a strong effect on the temporal pattern of N2O emissions, there was no significant effect of the different irrigation treatments on cumulative N2O emissions. These results were unexpected and differ from Clayton et al. (1997) and Smith et al. (1998) who found, in a field study, that N2O emissions depend particularly on WFPS and rainfall at the time of fertilization. However, the WFPS in these two studies ranged from 40% to 90%, while the fluctuations in WFPS in our study were much smaller. This is most likely the reason for the small effects the irrigation treatments had in our study.
Uptake of atmospheric CH4 through biological oxidation has been reported in a variety of upland soil ecosystems. Methane oxidation generally occurs in well-aerated soils, although an anaerobic pathway for CH4 oxidation has also been described (Segers 1998). Boeckx and van Cleemput (2001) summarized experimentally determined CH4 uptake rates from different ecosystems and from different parts of the world. For arable soils, the calculated average of the minimum and maximum values from these data was 0.25–0.83 mg CH4 m−2 day−1. With 0.13 to 0.26 mg CH4 m−2 day−1, the CH4 consumption measured in our study was therefore at the lower end of this range. In contrast to N2O, soil moisture significantly affected CH4 consumption. Methane consumption rates were increased during drier periods which may be due to the fact that CH4 consumption in aerated soils is mainly controlled by soil moisture, as CH4 diffusion is much slower in water than in air (Dörr et al. 1993). However, as soils contain both methanotrophic and methanogenic soil microorganisms, it is not possible to determine with the data available whether this difference was due to increased CH4 consumption or decreased CH4 production.
Temperature
While the irrigation treatment and BS application time had only minor effects on the proportion of BS-N lost in the form of N2O, the effect of temperature was much stronger. Across all treatments, the 10°C increase in temperature increased N2O losses by a factor of 3.7, which is more than twice as high as the factor for CO2 (1.7). The Q 10 factor for CO2 found in our study is within the range of values reported in a number of laboratory studies summarized by von Lützow and Kögel-Knabner (2009). High Q 10 factors for N2O have been reported in several studies. Abdalla et al. (2009) found Q 10 factors ranging from 4.4 to 6.2 for a temperature range of 10–25°C in an anaerobic laboratory incubation with pasture soil amended with mineral fertilizer. In an incubation with soil cores of an imperfectly drained Gleysol fertilized with NH4NO3, Dobbie and Smith (2001) reported Q 10 factors for an arable soil of 8.9 for the 12–18°C interval. These results indicate that N2O emissions are very sensitive to increasing temperature.
Aerobic and anaerobic processes
The high N2O emission rates indicate that anaerobic conditions were dominant in the soil cores, especially after the addition of BS and at the higher temperature. However, the high CO2 production rate, CH4 consumption, and nitrification suggest aerobic conditions.
The greatly increased N2O emissions observed after the application of BS suggest that microorganisms used NO −3 as an alternative electron acceptor when O2 became limiting. As the BS contained almost no NO −3 , denitrifying bacteria must have either used NO −3 from the soil solution or NH +4 from the BS must have been nitrified in aerobic microsites and diffused to anaerobic sites where denitrification took place. However, nitrification may also result in the production of N2O. In a summary of published data, Mathieu et al. (2006) found that the proportion of nitrified N lost as N2O generally ranged between 0.05% and 0.5% for well-aerated soils while it may increase to values higher than 1% under O2 limiting conditions. In the present study, about 37 and 51 mg NO −3 -N m−2 day−1 were leached from the unamended cores under continuous irrigation at 13.5°C and 23.5°C, respectively. Under the assumption that most of the NO −3 produced was leached during the course of the experiment and that between 0.05% and 1% of the N was lost as N2O during nitrification, the production of the NO −3 leached would have caused N2O-N emissions of 19 to 371 μg m−2 day−1 at 13.5°C and of 26 to 510 μg m−2 day−1 at 23.5°C. Therefore, at the lower temperature, between 4% and 76% of the N2O emissions may have been produced through nitrification, while the contribution of nitrification was below 11% at the higher temperature. Even though these calculations are rough estimates, they indicate that nitrification may have considerably contributed to the N2O emissions even though the soils were relatively moist. However, denitrification was most likely the predominant source of N2O in our study, especially at the higher temperature. Applying the same calculations for the other treatments, some general patterns emerge. The contribution of nitrification was likely higher at the lower temperature than at the higher temperature. In addition, nitrification likely contributed less to N2O emissions in the BS-amended soils compared to the control. In both cases, these findings can be explained by the increased O2 demand at the higher temperature and in BS-amended soil, which resulted in more anaerobic microsites.
Our finding that net CH4 consumption took place throughout the experiment in all treatments also indicates that aerobic microsites existed, as aerobic CH4 oxidation requires both oxygen and CH4. Anaerobic methane oxidation is also possible; however, little is known about this process in the soil environment. Its contribution to CH4 consumption seems low (Segers 1998). These results highlight the complexity of biochemical reactions in soil, which is a heterogeneous environment. During the incubation, aerobic and anaerobic microsites likely existed on a temporal and on a spatial scale. The application of an organic C and N source, such as BS, may strongly affect the O2 balance in the soil and, with it, the rates of the biochemical processes taking place.
Conclusion
The use of maize and other crops for CH4 production is likely to increase in the future, and increasing quantities of maize-derived BS will become available as fertilizer. So far, only a few studies have dealt with the effects of BS applications on C and N turnovers in soil.
Our results indicate that N2O emissions may be relatively large when BS is applied to moist soil. However, the proportion of N lost as N2O is within the range reported for slurries from animal husbandry. The small effect of soil moisture at the time of application was at least partly due to the high initial moisture contents and subsequent small fluctuations in soil moisture content observed in our study and cannot be generalized.
Our results also indicate that a relatively large proportion of the N is mineralized and nitrified during the first few weeks after BS application and could be susceptible to N leaching in fallow fields. However, our study was carried out under controlled conditions, and there is still little known about the proportion of N which becomes available during the cropping season. In addition, we used BS from only one fermenter in our study, and the properties of BS from other fermenters and production processes may be quite variable.
References
Abdalla M, Jones M, Smith P, Williams M (2009) Nitrous oxide fluxes and denitrification sensitivity to temperature in Irish pasture soils. Soil Use Manage 25:376–388
Amon T, Amon B, Kryvoruchko V, Machmuller A, Hopfner-Sixt K, Bodiroza V, Hrbek R, Friedel J, Potsch E, Wagentristl H, Schreiner M, Zollitsch W (2007) Methane production through anaerobic digestion of various energy crops grown in sustainable crop rotations. Bioresour Technol 98:3204–3212
Bateman EJ, Baggs EM (2005) Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol Fertil Soils 41:379–388
Boeckx P, Van Cleemput O (2001) Estimates of N2O and CH4 fluxes from agricultural lands in various regions in Europe. Nutr Cycl Agroecosyst 60:35–47
Clayton H, McTaggart IP, Parker J, Swan L, Smith KA (1997) Nitrous oxide emissions from fertilised grassland: A 2-year study of the effects of N fertiliser form and environmental conditions. Biol Fertil Soils 25:252–260
Cysneiros D, Banks CJ, Heaven S (2008) Anaerobic digestion of maize in coupled leach-bed and anaerobic filter reactors. Wat Sci Tech 58:1505–1511
Davidson EA (1992) Sources of nitric oxide and nitrous oxide following wetting of dry soil. Soil Sci Soc Am J 56:95–102
de Klein CAM, Sherlock RR, Cameron KC, van der Weerden TJ (2001) Nitrous oxide emissions from agricultural soils in New Zealand—a review of current knowledge and directions for future research. J Roy Soc New Zeal 31:543–574
Deutscher Wetterdienst (2009) Mittelwerte der Temperatur für den Zeitraum 1961–1990. http://www.dwd.de/. Accessed 25 Aug 2009
DIN ISO 11277 (2002) Bodenbeschaffenheit—Bestimmung der Partikelgrößenverteilung in Mineralböden—Verfahren mittels Siebung und Sedimentation, ISO 11277: 1998/Cor.1:2002. Beuth Verlag Berlin, Germany
Dittert K, Mühling KH (2009) Emission klimarelevanter Spurengase in der intensiven Pflanzenproduktion. J Verbr Lebensm 4:207–211
Dobbie KE, Smith KA (2001) The effects of temperature, water-filled pore space and land use on N2O emissions from an imperfectly drained gleysol. Eur J Soil Sci 52:667–673
Dörr H, Katruff L, Levin I (1993) Soil texture parameterization of the methane uptake in aerated soils. Chemosphere 26:697–713
Ehlers W, Werner D, Mähner T (2000) Wirkung mechanischer belastung auf gefüge und ertragsleistung einer löss-parabraunerde mit zwei bearbeitungssystemen. J Plant Nutr Soil Sci 163:321–333
FAO (1998) World reference base for soil resources. FAO, Rome
Franzluebbers AJ (1999) Microbial activity in response to water-filled pore space of variably eroded southern piedmont soils. Appl Soil Ecol 11:91–101
Gutser R, Ebertseder T, Weber A, Schraml M, Schmidhalter U (2005) Short-term and residual availability of nitrogen after long-term application of organic fertilizers on arable land. J Plant Nutr Soil Sci 168:439–446
Hantschel RE, Flessa H, Beese F (1994) An automated microcosm system for studying soil ecological processes. Soil Sci Soc Am J 58:401–404
Jacobi HF, Moschner CR, Hartung E (2009) Use of near infrared spectroscopy in monitoring of volatile fatty acids in anaerobic digestion. Wat Sci Tech 60:339–346
Maag M, Vinther FP (1996) Nitrous oxide emission by nitrification and denitrification in different soil types and at different soil moisture contents and temperatures. Appl Soil Ecol 4:5–14
Mathieu O, Henault C, Leveque J, Baujard E, Milloux MJ, Andreux F (2006) Quantifying the contribution of nitrification and denitrification to the nitrous oxide flux using N-15 tracers. Environ Pollut 144:933–940
Moeller K, Stinner W (2009) Effects of different manuring systems with and without biogas digestion on soil mineral nitrogen content and on gaseous nitrogen losses (ammonia, nitrous oxides). Eur J Agron 30:1–16
Sänger A, Geisseler D, Ludwig B (2010) Effects of rainfall pattern on carbon and nitrogen dynamics in soil amended with biogas slurry and composted cattle manure. J Plant Nutr Soil Sci 173:692–698
Segers R (1998) Methane production and methane consumption: a review of processes underlying wetland methane fluxes. Biogeochemistry 41:23–51
Senbayram M, Chen RR, Muhling KH, Dittert K (2009) Contribution of nitrification and denitrification to nitrous oxide emissions from soils after application of biogas waste and other fertilizers. Rapid Commun Mass Spectrom 23:2489–2498
Smith KA, Thomson PE, Clayton H, McTaggart IP, Conen F (1998) Effects of temperature, water content and nitrogen fertilisation on emissions of nitrous oxide by soils. Atmos Environ 32:3301–3309
Smith P, Martino D, Cai Z, Gwary D, Janzen H, Kumar P, McCarl B, Ogle S, O’Mara F, Rice C, Scholes B, Sirotenko O (2007) Agriculture. Climate change 2007: mitigation. Contribution of working group III to the fourth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
SPSS (2005) SPSS version 14.0.1 [computer software]. SPSS Inc., Chicago
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass-C. Soil Biol Biochem 19:703–707
Von Lützow M, Kögel-Knabner I (2009) Temperature sensitivity of soil organic matter decomposition—what do we know? Boil Fertil Soils 46:1–15
Acknowledgments
We would like to thank Anja Sawallisch and Margit Rode for the technical assistance and two anonymous reviewers and the editor-in-chief for their valuable comments on the manuscript as well as the Institute for Anaerobic Technology from the University of Applied Sciences of Giessen for the BS. This project is financed by the Deutsche Forschungsgemeinschaft (DFG-Research Training Group 1397 “Regulation of soil organic matter and nutrient turnover in organic agriculture”).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sänger, A., Geisseler, D. & Ludwig, B. Effects of moisture and temperature on greenhouse gas emissions and C and N leaching losses in soil treated with biogas slurry. Biol Fertil Soils 47, 249–259 (2011). https://doi.org/10.1007/s00374-010-0528-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-010-0528-y