Abstract
The objective of the present study was to evaluate the impact of the treatment of slurry liquid fraction (LF) acidified to pH 5.5 (ALF) on nitrification and denitrification processes after soil application. The impact of such treatment was compared with that of untreated LF, LF treated with a nitrification inhibitor (3,4-Dimethylpyrazole phosphate (DMPP)) (LF + DMPP). An incubation was conducted using the denitrification incubation system (DENIS/gas-flow-core technique) at a constant temperature of 20 °C and lasted for 32 days in order to follow nitrogen dynamics and gaseous emissions (N2O, NO, CO2) from soil. Inhibition of ammonium nitrification and nitrate accumulation was evident in both LF + DMPP and ALF at the top soil (0–3.75 cm) and those effects were stronger in the LF + DMPP. Denitrification was the main source of N2O emissions from soils amended with treated and untreated LF. Compared to the untreated LF, the ALF significantly reduced the total N lost as N2O from 0.10% to 0.05% of the applied N whereas the DMPP reduced the total N lost as N2O from 0.10% to 0.07%. Relative to the untreated LF, the ALF reduced the total N lost as NO emissions from 0.03% to 0.02% of the applied N whereas DMPP addition led to a stronger decrease from 0.03% to 0.01%. Both, ALF and LF + DMPP had no impact on CO2 emissions relative to the untreated LF. The ALF reduced CO2 emissions by 19% relative to the LF + DMPP. Our results demonstrate that slurry acidification affect not only nitrification but also the denitrification process. This suggests that slurry acidification is a valid technique to minimize N emissions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Industrialization of animal production and dairy sector has led to an increase in the production of slurry on farms (Merrington et al. 2002). Part of this slurry can be applied to soil as a fertilizer to recycle nutrients (Jensen 2013). However, the agricultural area available for slurry application is limited in some countries and consequently over-application of slurry is frequent (Merrington et al. 2002). Such situation may lead to a surplus of nutrients on farms and consequently, nutrient losses that may have negative implications on the environmental and economic sustainability of farms (Sørensen and Jensen 2013).
Slurry treatment has been proposed as a solution to minimize its environmental impact and increase its agronomic value in terms of the availability of N for crop utilization. Slurry separation is now used in many dairy and pig farms to enhance slurry management, namely the recycling of slurry nutrients on farms (Hjorth et al. 2010). After the application of slurry separation systems, the high dry-matter solid fraction (SF) obtained may be exported out of the farm while the low dry-matter liquid fraction (LF) may be applied on farm instead of the raw slurry. The liquid fraction has a low C/N-ratio which may reduce the potential for N immobilization after soil application (Jensen 2013).
Slurry acidification is considered to be an effective tool for reducing ammonia (NH3) volatilisation from the slurry during its storage and after its application to soil (Fangueiro et al. 2015a). Lowering of slurry pH may affect various chemical (Hjorth et al. 2010) and biological processes in slurry (Ottosen et al. 2009) as well as slurry composition (Fangueiro et al. 2009). In consequence, the fertilizer value of slurry as well as the N dynamics from acidified slurry may differ from patterns already known for non-acidified slurry after soil application (Wenzel and Petersen 2009). Indeed, Fangueiro et al. (2010, 2013) reported a delay in nitrification of ammonium (NH4 +) and subsequent reduction in nitrous oxide (N2O) emissions from soils amended with acidified slurries relative to non-acidified ones. However, such effect of slurry acidification was not observed in some soils with different characteristics (Fangueiro et al. 2016) and more studies are needed to fully assess its impact on N2O and nitric oxide (NO) losses.
Emission of N2O is considered as a major environmental burden associated with slurry application to soil (Sommer et al. 2013). Nitrous oxide is a potent greenhouse gas and also the most important chemical species leading to stratospheric ozone depletion (Ravishankara et al. 2009). Nitric oxide on the other hand contributes to the formation of ozone in the troposphere and is a vital precursor to acid rain (Williams et al. 1992). Both N2O and NO are mainly produced by nitrification and denitrification processes in soils (Firestone and Davidson 1989). Carbon dioxide (CO2), another greenhouse gas, is produced from respiration with soil and vegetation being the main sources from which this gas enters the atmosphere (Smith et al. 2003). Furthermore, significant CO2 emissions may occur after application of slurry to soil due to the availability of C in the slurry which often enhances microbial activities (Pereira et al. 2010).
The addition of nitrification inhibitors (NIs) such as 3,4-Dimethylpyrazole phosphate (DMPP) to N fertilizers is a common practice to reduce N losses from arable soils (Ruser and Schulz 2015). A delay in nitrification and reductions in N2O and NO emissions from slurry amended soils is possible when NIs are applied (Ruser and Schulz 2015), whereas for CO2 emissions contrasting results have been reported (Ruser and Schulz 2015). Earlier studies on the impact of NIs on gaseous emissions from organic fertilizers have mainly been conducted using cattle or pig whole slurry (Hatch et al. 2005; Menéndez et al. 2009; Fangueiro et al. 2010) and have rarely considered the effect of NIs addition to the fractions obtained after slurry separation on gaseous emissions (Pereira et al. 2010).
Our hypotheses were that: 1) lower nitrification will occur in soil amended with acidified LF (ALF) than with raw LF; 2) the effect of acidification of the LF on nitrification after soil application is comparable to the effect of DMPP and 3) lower N2O is emitted from soil amended with ALF or slurry treated with DMPP relative to raw LF amended soil. The objectives of the present study were to evaluate the impact of two mitigation strategies: (a) LF acidification to pH 5.5 or (b) LF treatment by a NI (DMPP) addition prior application to a clay loam soil, on N speciation in soil (organic N, NH4 +, NO3 −) and on gaseous emissions (N2O, NO, CO2).
Materials and methods
Soil preparation and analysis
Soil samples were collected (0–15 cm) in October 2013 from an experimental grassland site (Rowden Moor) at Rothamsted Research Station, in the southwest of the United Kingdom. The dominant species of the grassland were Agrostis stolonifera and Juncus effuses. The field had not been grazed for over 20 years and not received any N input over that time period. Soil samples were taken from the top 0–15 cm layer in a W-shape across the field. At sampling, the gravimetric moisture content was between 58 and 65%. Due to the high clay content, the soil was air-dried at room temperature to 30% gravimetric moisture content to enable its handling. The grass remains were removed before breaking the soil up and sieving (< 2 mm). The soil was then stored at 4 °C until packing of cores. Total N was determined after air-drying and grinding using Kjeldahl analysis and total C using an elemental analyser (Carlo-Erba). Soils were analysed for exchangeable NH4 + and nitrate (NO3 −), both before, and after the incubation by extraction in 2 M KCl (1:5 w/v) by molecular absorption spectrophotometry in a segmented flow analyser (SanPlus, Skala, Breda) using the Berthelot and sulphanilamide methods for NH4 + and NO3 − (Houba et al. 1989).
The main soil physical-chemical characteristics were: pH water [1:2.5], 5.6; exchangeable NH4 +-N, 12.0 mg N kg−1 dry soil; NO3 −-N, 79.0 mg N kg−1 dry soil; total N, 5.4 g N kg−1 dry soil; organic matter, 117.0 g kg−1 dry soil; bulk density, 0.8 g cm−3.
Slurry preparation and analysis
The liquid fraction of slurry was sampled from a slurry pit receiving effluent from a mechanical slurry separator from a dairy farm in Devon, United Kingdom. Dairy cows were fed mainly with grass and maize silage. The main characteristics of the effluent (Table 1) were analysed both before and after its application to soil. Slurry organic matter, electrical conductivity, phosphorus, total N and soluble organic N were determined according to the methods described by Fangueiro et al. (2015b). Potassium was determined using the segmented flow analyser and flame photometer after sulphuric acid digestion. A description of the methods used to analyse the rest of the physical-chemical properties of the slurry can be found in Fangueiro et al. (2013).
Slurry treatments were prepared as follows: approximately 1.2 L of the raw liquid fraction was collected and separated into three containers (each containing 0.4 L of the effluent). Part of the liquid fraction (LF) was left with no additive. The second part (ALF) was acidified to a pH of 5.5 by adding 0.001 L of concentrated H2SO4. The third part (LF + DMPP) was amended with the nitrification inhibitor DMPP by adding 0.015 g of DMPP (equivalent to the commercial application rate of 37.5 g of DMPP per tonne of slurry) directly to the raw LF.
Experimental set up
Main experiment
The treatments were: raw liquid fraction (LF), acidified liquid fraction (ALF), liquid fraction + DMPP (LF + DMPP) and an unfertilized soil (control). Slurry treatments were applied at an application rate of 0.3 g N per vessel or 0.12 L of slurry per vessel (equivalent to an application rate of 140 kg N ha−1 or 76 m3 ha−1). The control treatment received 0.12 L of water.
The incubation experiment was conducted as a completely randomized design with four treatments and three replicates each, making a total of 12 experimental units. We used the denitrification incubation system (DENIS) described by Cárdenas et al. (2003) and improved according to Loick et al. (2016). Briefly, the system consists of 12 enclosed vessels allowing gas fluxes to be automatically analysed under a nitrogen free atmosphere. This is achieved by first removing atmospheric N2 from the soil, headspace and gas lines using a mixture of He (80%) and O2 (20%), directed through the bottom of the incubation vessels. The flow is then directed over the top of the soil core within each vessel for N2O, NO, and CO2 emissions from the soil surface to be transported by the carrier gas stream. Effluent gases from each of the 12 chambers passes through an outlet in the lid to an actuated selection valve, either for analyses by chromatography (GC) or chemiluminescence, or vented to the atmosphere. The incubation device was kept constant at 20 °C and the incubation lasted for 32 days.
Twelve soil cores (14.2 cm diameter) were packed with fresh soil equivalent to 1305 g to a height of 7.5 cm and a bulk density of 0.8 g cm−3. The moisture content was adjusted to a final WFPS of 60%, taking the later addition of the amendments into consideration; the cores were placed into a tray, watered and left overnight loosely covered with plastic bags to prevent evaporation. Water contents were finally adjusted the following day by adding water (~10 ml) to the top of the core (by weight).
After packing, the cores were placed into the incubation system (DENIS system). To remove gaseous nitrogen from soil pores, a gas mixture of helium (80%) and oxygen (20%) (He/O2 mixture) was passed from the bottom of the vessels and through the soil core (as described above) at a rate of 30 ml min−1 for 6 days in order to remove any N2 present in the soil atmosphere, headspace, and all gas lines before gas measurement.
Amendments were added through a secondary vessel (amendment vessel) fitted to the centre of the lid of each of the incubation vessels. Normally the amendment vessel is flushed with He to avoid the entrance of atmospheric N2 into the incubation vessel when applying amendment. In order to prevent NH3 losses while flushing, each amendment vessel was instead covered with polyurethane which fitted exactly on the surface of the amendments to minimize the entrance of N2 into the system during application.
The flow of the He/O2 mixture was then reduced to 12 ml min−1 and directed to flow over the top of the vessels (flow-over mode), so that emissions of N2O and NO from the headspace were transported by the carrier gas stream.
Gas analyses
Fluxes of N2O and CO2 were measured with a Perkin Elmer Clarus 500 gas chromatograph (Perkin Elmer Instruments, Beaconsfield, UK) with an electron capture detector for N2O and a flame ionization detector and methanizer (to convert CO2 to CH4) for CO2 (Phillips) Loick et al. (2016). The NO was measured through chemiluminescence using an NO-analyser (Sievers NOA280i, GE Instruments, Colorado, USA) (Loick et al. 2016). Measurements were done every 2 h for each vessel. The detection limits of N2O and CO2 was 1ppmv and that for NO was 1ppbv (Cárdenas et al. 2003). All gas concentrations were corrected for flow rate; fluxes were expressed as mg N or g C m−2 day−1 basis. Cumulative gas losses were calculated assuming a mean flux rate between two successive sampling dates and multiplying the difference by the time considered time interval (Pereira et al. 2010). The percentage of N lost from the total N applied was estimated by dividing the cumulative N emissions by the total N applied and multiplied by 100.
Parallel incubation
A parallel incubation was set up for destructive soil sampling. Soil cores with the same height but 1/10 of the surface area (4.5 cm diameter, 7.5 cm height, containing 131 g dry soil) were packed to the same characteristics as for the main experiment; i.e. bulk density of 0.8 g cm−3, WFPS of 60%. All cores were placed into a sealed chamber and to keep conditions equivalent to those of the main experiment, the atmosphere inside the chamber was replaced with a gas mixture of He/O2 flowing through at a rate of 12 ml min−1, and the temperature was kept at 20 °C.
The same 4 treatments described in the main experiment were used, here with 4 replicates each for 8 sampling periods making a total of 128 experimental units. Amendments were added to the top of each core. Treatments were applied at the same amendment-to-surface area ratio as in the main experiment with all slurry treatments receiving 0.03 g of N per core (equivalent to 0.012 L of slurry). The control treatment received 0.012 L of water equivalent to the volume applied via the slurry in the other treatments.
Destructive soil sampling after 0.25, 1, 2, 5, 7, 12, 15 and 32 days, were times coinciding with those of emission peaks occurring during the main experiment. During each sampling period, 4 soil cores from each treatment in the incubation chamber were randomly sampled. In order to determine the impact of treatments on NO3 −, soil cores were separated into two equal halves by removing the top 3.75 cm and the bottom 3.75 cm layers; the two halves were put into plastic bags and stored at 4 °C until analysis. The parallel incubation lasted for 32 days.
Statistical analysis
The emissions and soils data were subjected to analysis of variance (ANOVA) using the statistical program STATISTIX (version 7.0). Bonferroni test was used for multiple comparisons among means. Significant differences among means with a P-value less than 0.05 were considered significant.
Results
Soil N dynamics
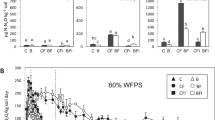
The exchangeable NH4 + (Fig. 1a) concentrations (top soil layer) during the parallel incubation were similar in all slurry treatments within the first 5 days of incubation. After 5 days, the exchangeable NH4 + concentration in the LF declined (P < 0.05) from approximately 200 mg N kg−1 dry soil, to values comparable to the control until the end of the experiment. In contrast, the ALF and the LF + DMPP treatments maintained significantly higher (P < 0.05) exchangeable NH4 + concentrations after day 5 until the end of the experiment compared to the control and the LF. Although similar exchangeable NH4 + evolution was found between the ALF and the LF + DMPP during the experiment, a significantly higher (P < 0.05) exchangeable NH4 + concentration was found in the LF + DMPP on day 15 relative to the ALF.
The NO3 − concentrations (Fig. 1b) in the top soil layer showed no significant differences between treatments within the first 5 days of application. The NO3 − in the LF increased (P < 0.05) sharply at day 7 and maintained significantly higher (P < 0.05) values relative to the ALF and the LF + DMPP treatments until the end of the experiment. On day 7, 12 and 15, the NO3 − in the LF + DMPP was significantly lower (P < 0.05) than in the ALF and similar to the control treatment. There were no significant differences in the NO3 − between the ALF, LF + DMPP and control on day 32. In general, the NO3 − in the control showed an increasing trend within the first 15 days of application indicating potential N mineralization in treatments after application.
There were no significant differences in the exchangeable NH4 + concentration at the bottom layer (Fig. 1c) of soil cores in all slurry treatments within the first 2 days of incubation. Subsequently, a decline (P < 0.05) in exchangeable NH4 + was observed between day 5 and 15 in all treatments although no significant differences were observed between treatments. Similarly, no significant differences in exchangeable NH4 + between slurry treatments were found on day 32. However, exchangeable NH4 + in the LF + DMPP treatment was significantly higher (P < 0.05) than the control.
There were no significant differences between treatments relative to the NO3 − at the bottom layer (Fig. 1d) within the first 5 days of incubation. There was a significant increase (P < 0.05) in the NO3 − at day 7 in all treatments. The NO3 − in the LF treatment at day 7 was comparable to the ALF and control but significantly higher (P < 0.05) than the LF + DMPP. There were no significant differences in NO3 − in all treatments on day 12 and 15. On day 32, the NO3 − in the LF + DMPP was comparable to the ALF and control but significantly lower (P < 0.05) than the LF. The NO3 − in the ALF on day 32 was not significantly different from the LF.
Nitrous oxide emissions
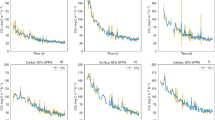
The cumulative N2O emissions (Table 2) in the LF (14.11 mg N m−2) were not statistically different from the LF + DMPP (9.82 mg N m−2) but were higher (P < 0.05) than the ALF (6.84 mg N m−2) and control (4.71 mg N m−2). Relative to the N2O fluxes, the first peak (Fig. 2a) was observed in the ALF treatment (6 mg N m−2 d−1) after 8.2 h (0.34 days) followed by a peak in the LF + DMPP treatment (9 mg N m−2 d−1) at 9.8 h (0.41 days) whereas the N2O peak in the LF treatment (10 mg N m−2 d−1) peaked at 0.9 days. It is noteworthy that the maximum flux in the LF and the LF + DMPP treatments were not significantly different but both treatments (LF and LF + DMPP) had peak values significantly higher (P < 0.05) than that of the ALF treatment. All peaks of treatments were significantly higher (P < 0.05) than that of the control.
Nitric oxide emissions
The LF treatment showed a significantly higher (P < 0.05) cumulative NO emissions relative to the rest of the treatments (Table 2). There were no significant differences between the cumulative emissions observed in the LF + DMPP and control. The cumulative emissions in the ALF were significantly higher (P < 0.05) than the LF + DMPP and control. Relative to NO fluxes (Fig. 2b), there was an immediate increase in all slurry peaking at day 1. The highest peaks were observed for the LF + DMPP (0.14 mg N m−2 d−1) and LF (0.12 mg N m−2 d−1) treatments whose values were not significantly different but were significantly higher (P < 0.05) than the value of the ALF (0.05 mg N m−2 d−1) treatment. The second NO peak occurred after 6 days and it was significantly higher (P < 0.05) in the LF than the peaks observed in the rest of the treatments. A third NO peak was observed on day 15 in the LF treatment reaching a value of 0.25 mg N m−2 d−1. The NO emissions from the ALF increased steadily from day 7.7 until the end of the experiment reaching a final value of 0.12 mg N m−2 d−1. The LF + DMPP treatment maintained lower fluxes similar to those of the control after the first peak until the end of the experiment.
Carbon dioxide
As expected, significantly higher (P < 0.05) cumulative emissions were observed in all slurry treatments relative to the control (Table 2). There were no significant differences in cumulative emissions between LF and the other two amended treatments (Table 2). Relative to CO2 fluxes (Fig. 2c), an initial peak was observed after raw and treated LF applications. The highest CO2 emission rate was observed in the LF + DMPP treatment but it was not significantly different from the ALF value. There was a decline in CO2 fluxes in all slurry treatments and in the ALF, CO2 emission rates reached the value of 2.5 g C m−2 d−1 after 0.9 days while the same value was reached in the LF and LF + DMPP treatments 2.0 days after the first peak. The ALF showed a second peak after 1.7 days reaching a value of 5.0 g C m−2 d−1. All slurry treatments showed a peak around day 6 and there were no significant differences between them. These emission rates declined after the peak at day 6 and maintained similar fluxes as the control until the end of the experiment.
Molecular NO/N2O
The NO/N2O (Fig. 2d) in all treatments remained close to zero within the first 3 days and showed an initial peak around day 6 with values below 0.5. The LF showed three additional peaks around 15.6, 22.8 and 30.0 days and the NO/N2O during these peak periods ranged between 0.9 and 0.8. In the case of the ALF, the NO/N2O showed an increasing trend after the initial peak until the end of the experiment reaching a value of 1.0. The NO/N2O in the LF + DMPP and control remained below 0.4 after the initial peak until the end of the experiment.
Discussion
Mechanism responsible for N2O emissions
Evolution of N2O, which mainly occurred within the first 2 days, depended on denitrification and not nitrification for the following reasons:
-
(i)
Emissions of N2O are not expected to occur in the LF + DMPP treatment if nitrification is responsible for the initial N2O peaks due to the effect of DMPP in delaying the oxidation of NH4 + to NO3 − in soils (Ruser and Schulz 2015). Indeed, studies (Maienza et al. 2014; Shi et al. 2016) have shown that DMPP slows nitrification by inhibiting the growth of microorganisms such as ammonia oxidizing bacteria (AOB). The initial N2O peaks of slurry treatments are most likely a result of denitrification of NO3 − initially present (79 mg N kg−1 dry soil), as observed by Fangueiro et al. (2015c) within the first day of slurry application. The denitrification might have been stimulated by the availability of C in the slurry treatments (Rochette et al. 2000).
-
(ii)
The NO/N2O ratio may be used to determine the dominant process (nitrification or denitrification) responsible for NO or N2O emissions (Fangueiro et al. 2012; Cheng et al. 2014); values close to 0.01 indicated that denitrification was the dominant process whereas values >1 indicated that nitrification was dominant. The NO/N2O ratio within the first 2 days of treatments being close to 0.01 (Fig. 2d) agrees with denitrification as the dominant source of N2O emissions during that period.
Mechanism responsible for NO emissions
Evolution of NO depended on both nitrification and denitrification for the following reasons:
-
(i)
If nitrification was the main source of the first NO peak around day 1, the LF + DMPP treatment should not lead to an increase in NO emission rates due to inhibition of nitrification by DMPP (Ruser and Schulz 2015). In addition, the NO/N2O ratio within the first 2 days of slurry treatments were closer to 0.01 (Fig. 2d) which agrees with denitrification as the dominant process responsible for the first NO peak.
-
(ii)
The second NO peak at day 6 coincided with the last CO2 peak suggesting that organic C was still available for some microbial activity. However, the decline in exchangeable NH4 + contents (Fig. 1a) in all treatments within the first 6 days suggest that nitrification also occurred and thus the NO peak produced by slurry treatments was due to nitrification at 6 days. Indeed, the NO/N2O ratio around day 6 was below 0.4 in all treatments (Fig. 2d) indicating that probably both processes were responsible for NO emissions. Indeed, both nitrification and denitrification processes may occur at 60% WFPS (Merino et al. 2001).
-
(iii)
The third NO peak in the LF treatment on day 15 corresponded with an increase in the NO3 − soil content (Fig. 1b) which was particular evident on the top soil layer. This indicates that soil was under aerobic conditions and the peak was due to nitrification. In addition, the NO/N2O ratio in the LF treatment on day 15 being close to 1 (Fig. 2d) confirms nitrification as the process responsible for the NO peak in the LF treatment.
Impact of acidification on N evolution
Inhibition of nitrification was observed in the ALF treatment after day 5 until the end of the experiment relative to the untreated LF and this may account for the lower (P < 0.05) cumulative NO emissions in the ALF relative to the untreated LF. Such inhibitory effects on nitrification have been observed in previous studies (Fangueiro et al. 2010, 2013, 2016) and were attributed to a decrease in soil pH induced by acidified slurry application which decreases microbial biomass (Fangueiro et al. 2013). Indeed, Fangueiro et al. (2013) reported immediate pH increase in the untreated slurry from 5.5 to 7.2–8.3 within the first 9 days of application whereas in soils amended with acidified slurry, pH remained close to 6 within the first 9 days. Similar findings were made by Gandhapudi et al. (2006), who reported that at a pH lower than 6, nitrification is strongly reduced due to a lower activity of the bacterial nitrifiers. The potential decline in biomass or microbial activity due to the effect of LFA on soil pH after application may have decreased nitrification and consequently led to lower (P < 0.05) cumulative N2O emissions relative to the untreated LF. Since N2O emissions were mainly produced from denitrification, it can be hypothesised that slurry acidification inhibits not only nitrification (Fangueiro et al. 2010, 2013, 2016) but also denitrification. These findings support the hypothesis that a lower nitrification will occur in soils amended with acidified LF than the untreated LF.
Impact of DMPP on N evolution
A delay in NH4 + nitrification was observed in the LF + DMPP after day 5 until the end of the experiment relative to the untreated LF. This is due to the already mentioned effect of DMPP in delaying NH4 + oxidation (Ruser and Schulz 2015). This effect may account for the lower (P < 0.05) NO emissions in the LF + DMPP treatment relative to the untreated LF. The LF + DMPP treatment showed a stronger inhibitory effect on nitrification relative to the ALF and that led to a higher (P < 0.05) reduction in NO emissions in the LF + DMPP relative to the ALF. Thus, the hypothesis that the effect of acidification of the LF on nitrification after soil application is comparable to the effect of DMPP is not supported. The DMPP was not effective in reducing N2O emissions relative to the untreated LF since N2O was produced from denitrification and not nitrification. Thus, the hypothesis that lower N2O will be emitted from soil amended with ALF or slurry treated with DMPP relative to the untreated LF is partly supported from the present study.
Conclusion
There was a clear inhibition of ammonium oxidation by LF + DMPP and by ALF although the inhibition effect was stronger for LF + DMPP. Nitrate accumulation was therefore inhibited for LF + DMPP and ALF with a stronger effect for LF + DMPP. The facts above were clearer in the top (0–3.75 cm) than deep (3.75–7.5 cm) soil layer. The N2O was inhibited during 24 h after the application of ALF when compared to LF + DMPP and LF. The LF + DMPP markedly reduced NO emissions whereas the ALF reduced NO emissions only for the first 20 days. The LF + DMPP and ALF had no clear effect on CO2 emissions. Overall, the ALF can be used as an alternative to LF + DMPP to mitigate N2O emissions but in the case of NO emissions, the ALF was not as beneficial as the LF + DMPP. Nevertheless, the results obtained here need to be confirmed at field scale and similar studies using different soils and manure might also contribute to a better understanding of our results.
References
Cárdenas LM, Hawkins JMB, Chadwick D, Scholefield D (2003) Biogenic gas emissions from soils measured using a new automated laboratory incubation system. Soil Biol Biochem 35:867–870
Cheng Y, Wang J, Wang SQ, Zhang JB, Cai ZC (2014) Effects of soil moisture on gross N transformations and N2O emission in acid subtropical forest soils. Biol Fertil Soils 50:1099–1108
Fangueiro D, Ribeiro H, Vasconcelos E, Coutinho J, Cabral F (2009) Treatment by acidification followed by solid–liquid separation affects slurry and slurry fractions composition and their potential of N mineralization. Bioresour Technol 100:4914–4917
Fangueiro D, Ribeiro H, Coutinho J, Cardenas L, Trindade H, Cunha-Queda C, Vasconcelos E, Cabral F (2010) Nitrogen mineralization and CO2 and N2O emissions in a sandy soil amended with original or acidified pig slurries or with the relative fractions. Biol Fertil Soils 46:383–391
Fangueiro D, Coutinho J, Cabral F, Fidalgo P, Bol R, Trindade H (2012) Nitric oxide and greenhouse gases emissions following the application of significantly different cattle slurry particle size fractions to soil. Atmos Environ 47:373–380
Fangueiro D, Surgy S, Coutinho J, Vasconcelos E (2013) Impact of cattle slurry acidification on carbon and nitrogen dynamics during storage and after soil incorporation. J Plant Nutr Soil Sci 176:540–550
Fangueiro D, Hjorth M, Gioelli F (2015a) Acidification of animal slurry–a review. J Environ Qual 149:46–56
Fangueiro D, Surgy S, Fraga I, Cabral F, Coutinho J (2015b) Band application of treated cattle slurry as an alternative to slurry injection: implications for gaseous emissions, soil quality, and plant growth. Agric Ecosyst Environ 211:102–111
Fangueiro D, Pereira J, Bichana A, Surgy S, Cabral F, Coutinho J (2015c) Effects of cattle-slurry treatment by acidification and separation on nitrogen dynamics and global warming potential after surface application to an acidic soil. J Environ Manag 162:1–8
Fangueiro D, Surgy S, Fraga I, Monteiro FG, Cabral F, Coutinho J (2016) Acidification of animal slurry affects the nitrogen dynamics after soil application. Geoderma 281:30–38
Firestone MK, Davidson EA (1989) Microbial basis of NO and N2O production and consumption in soil. In: Andreae MO, Schimel DS (eds) Exchange of trace gases between terrestrial ecosystems and the atmosphere. Wiley, New York
Gandhapudi SK, Coyne MS, D’Angelo E.M, Matocha C (2006) Potential nitrification in alum-treated soil slurries amended with poultry manure. Bioresour Technol 97:664–670.
Hatch D, Trindade H, Cardenas L, Carneiro J, Hawkins J, Scholefield D, Chadwick D (2005) Laboratory study of the effects of two nitrification inhibitors on greenhouse gas emissions from a slurry-treated arable soil: impact of diurnal temperature cycle. Biol Fertil Soils 41:225–232
Hjorth M, Christensen KV, Christensen ML, Sommer SG (2010) Solid–liquid separation of animal slurry in theory and practice. A review Agron Sustainable Dev 30:153–180
Houba VJG, van der Lee JJ, Novozamsky I, Walling I (1989) Soil and plant analysis, part 5, soil analysis procedures. Wageningen Agricultural University, Wageningen, The Netherlands
Jensen LS (2013) Animal manure fertiliser value, crop utilisation and soil quality impacts. In: Sommer SG, Christensen ML, Schmidt T, Jensen LS (eds) Animal manure recycling: treatment and management, 1st edn. Wiley, United Kingdom, pp 295–328
Loick N, Dixon ER, Abalos D, Vallejo A, Matthews GP, McGeough KL, Well R, Watson CJ, Laughlin RJ, Cardenas LM (2016) Denitrification as a source of nitric oxide emissions from incubated soil cores from a UK grassland soil. Soil Biol Biochem 95:1–7
Maienza A, Bååth E, Stazi SR, Benedetti A, Grego S, Dell’Abate MT (2014) Microbial dynamics after adding bovine manure effluent together with a nitrification inhibitor (3, 4 DMPP) in a microcosm experiment. Biol Fertil Soils 50:869–877
Menéndez S, Merino P, Pinto M, González-Murua C, Estavillo JM (2009) Effect of N-(−butyl) Thiophosphoric Triamide and 3, 4 dimethylpyrazole phosphate on gaseous emissions from grasslands under significantly different soil water contents. J Environ Qual 38:27–35
Merino P, Estavillo JM, Besga G, Pinto M, González-Murua C (2001) Nitrification and denitrification derived N2O production from a grassland soil under application of DCD and Actilith F2. Nutri Cycl Agroecosyst 60:9–14
Merrington G, Nfa LW, Parkinson R, Redman M, Winder L (2002) Agricultural pollution: environmental problems and practical solutions. Spon Press, New York
Ottosen LD, Poulsen HV, Nielsen DA, Finster K, Nielsen LP, Revsbech NP (2009) Observations on microbial activity in acidified pig slurry. Biosyst Eng 102:291–297
Pereira J, Fangueiro D, Chadwick DR, Misselbrook TH, Coutinho J, Trindade H (2010) Effect of cattle slurry pre-treatment by separation and addition of nitrification inhibitors on gaseous emissions and N dynamics: a laboratory study. Chemosphere 79:620–627
Ravishankara AR, Daniel JS, Portmann RW (2009) Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the twenty-first century. Science 326:123–125
Rochette P, Angers DA, Coté D (2000) Soil carbon and nitrogen dynamics following application of pig slurry for the 19th consecutive year: carbon dioxide fluxes and microbial biomass carbon. Soil Sci Soc Am J 64:1389–1395
Ruser R, Schulz R (2015) The effect of nitrification inhibitors on the nitrous oxide (N2O) release from agricultural soils—a review. J Plant Nutr Soil Sci 178:171–188
Shi X, Hu H, He J, Chen D, Suter HC (2016) Effects of 3,4-dimethylpyrazole phosphate (DMPP) on nitrification and the abundance and community composition of soil ammonia oxidizers in three land uses. Biol Fertil Soils 52:927–939
Smith KA, Ball T, Conen F, Dobbie KE, Massheder J, Rey A (2003) Exchange of greenhouse gases between soil and atmosphere: interactions of soil physical factors and biological processes. Eur J Soil Sci 54:779–791
Sommer SG, Clough TJ, Chadwick D, Petersen SO (2013) Greenhouse gas emissions from animal manures and technologies for their reduction. In: Sommer SG, Christensen ML, Schmidt T, Jensen LS (eds) Animal manure recycling: treatment and management, 1st edn. Wiley, United Kingdom pp, pp 177–194
Sørensen P, Jensen LS (2013) Nutrient leaching and runoff from land application of animal manure and measures for reduction. In: Sommer SG, Christensen ML, Schmidt T, Jensen LS (eds) Animal manure recycling: treatment and management, 1st edn. Wiley, United Kingdom pp, pp 195–210
Wenzel H, Petersen BM (2009) Life cycle assessment of slurry management technologies. Environmental Project No. 1298. Danish Ministry of the Environment, Environmental Protection Agency, Copenhagen, Denmark
Williams EJ, Hutchinson GL, Fehsenfeld FC (1992) NOx and N2O emissions from soil. Glob Biogeochem Cycles 6:351–388
Acknowledgements
The research leading to these results has received funding from the People Programme (Marie Curie Actions) of the European Union’s Seventh Framework Programme FP7/2007-2013/ under REA grant agreement n° [289887] and FCT (PTDC/AGR-PRO/119428/2010), UID/AGR/04033/2013 and POCI-01-0145-FEDER-006958. Rothamsted Research receives strategic funding by the Biotechnology and Biological Sciences Research Council (BBSRC, Grant number BB/J004286/1). This study was also part funded by BBSRC project grant BB/K001051/1. The findings of this study reflect only the author’s view and that the Union is not liable for any use that may be made of the information contained therein.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Owusu-Twum, M., Loick, N., Cardenas, L.M. et al. Nitrogen dynamics in soils amended with slurry treated by acid or DMPP addition. Biol Fertil Soils 53, 339–347 (2017). https://doi.org/10.1007/s00374-017-1178-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-017-1178-0