Abstract
University of California at Berkeley I or UCB-1 pistachio rootstock is propagated from the cross between Pistacia integerrima male × Pistacia atlantica female. So far, no report has been presented on the proteomic profile of Pistacia genus. In this research, 7-month-old UCB-1 rootstocks that were produced by tissue culture method and grown in pots containing 1/3 perlite, 1/3 clay and 1/3 sand were exposed to the three different concentrations of NaCl including 0, 100 and 200 mM for 30 days in the controlled conditions in the greenhouse. In the first step, under these three different concentrations of NaCl, the content of malondialdehyde and activities of guaiacol peroxidase, superoxide dismutase, catalase and peroxidase were evaluated. Malondialdehyde content increased up to 100 mM NaCl and then decreased. Activities of guaiacol peroxidase, superoxide dismutase and catalase increased with increasing concentration of NaCl, while peroxidase activity reduced. In the second step, 0 and 100 mM NaCl were selected to evaluate changes in the proteomic profile of this rootstock using MALDI-TOF/TOF method. In this study, ribonucleoside-diphosphate reductase small chain, polcalcin Phl p 7-like and golgin subfamily A member 5 were identified for the first time in response to salinity stress and have not been previously reported to be involved in the response of plant under abiotic stresses. Moreover, in this study, five unknown proteins were identified in UCB-1 pistachio under salinity stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the most important members of Anacardiaceae family is pistachio, which has at least 11 species; P. integerrima and P. atlantica are used as rootstocks for P. vera cultivation, while P. vera is solely an economic and cultivated species (Moazzzam Jazi et al. 2017). At the University of California at Berkeley, with a controlled pollination and cross between P. integerrima male × P. atlantica female, a hybrid pistachio rootstock was produced and was named UCB-1(Ahmad et al. 2005). UCB-1 is more resistant to cold (Epstein et al. 2004) and salt stresses (Ahmad et al. 2005; Ferguson et al. 2002; Akbari et al. 2018) and has a higher yield (Ferguson et al. 2002) and greater Verticillium tolerance (Morgan et al. 1992) than other pistachio rootstocks, and for these reasons, UCB-1 is the best rootstock for California climates and likely the other areas in the world. Also, as regards the important role of rootstock in resistance to abiotic stresses, such as salinity in grafted plants, UCB-1 can be the most favorable rootstock for Pistacia genus (Akbari et al. 2018). The United States of America, Iran, and Turkey are the main pistachio breeders in the world, and Iran has a 3000–4000 year history of pistachio agriculture (Moazzzam Jazi et al. 2017). Also, leaf, flower, resin and seeds of pistachio have pharmacological attributes, such as antioxidant, antimicrobial and anti-inflammatory properties (Bozorgi et al. 2013; Tsokou et al. 2007).
Pistachio is classified as a salt-tolerant glycophyte type; however, under moderate and high salinity stresses, its yield is extremely decreased (Hajiboland et al. 2014; Moazzam Jazi et al. 2016). In the last decades, lack of precipitation, global warming and drought in Iran have extremely decreased the forest area of wild pistachio, and consequently increases in soil salt content, which is the most important reason for limitation in the growth and development of rainfed trees, such as pistachio, has happened (Ziya Motalebipour et al. 2016; Moazzam Jazi et al. 2016). Therefore, there is an urgent need for conservation management of this rootstock that warrants fertility, productivity and long growth in this valuable product (Ziya Motalebipour et al. 2016; Moazzam Jazi et al. 2016). Responsive proteins in reaction to salt stress in this study using proteomic analysis can be used in modern breeding programs to produce a more resistant pistachio rootstock under unfavorable conditions of stress.
Agronomical yield, agricultural productivity and plant growth are extremely limited when plants are exposed to soil salinity (Schwarz et al. 2010). Soil salinity severely affects fruit trees; for example, under low concentration of 25 mM NaCl, almond shoot growth is extremely repressed by salt in the soil solution (Zrig et al. 2011). Therefore, researchers and scientists, via selection and breeding of different cultivars, are able to present a stress-resistant cultivar to minimize the effects of abiotic stresses, such as salinity and drought (Cuartero et al. 2006). Also, genetic complexity of resistance to salinity and drought stresses has compelled researchers to use classic methods for improvement of plants tolerance, such as selection of resistant rootstocks in grafted plants (Ashraf and Foolad 2007). It is now more than 50 years that grafting has been used for decreasing destructive effects in plants under abiotic stresses to improve plants tolerance (Schwarz et al. 2010; Lee et al. 2010; Flores et al. 2010). In the recent decade, valuable rootstocks have been widely available and have been used to reclaim destructive effects of salinity stress in woody fruit crops (Massai et al. 2004; Colla et al. 2006). In 2016, a research demonstrated that resistant trees to soil salinity can be extremely recovered by grafting onto resistant rootstocks (Zrig et al. 2016). Resistance to salinity stress is different between different species and severely depends on their root system (Moya et al. 2002). Furthermore, functional relation between rootstock and scion in grafted plants is another ability of woody plants to become more resistant under salinity stress (Moya et al. 2002; Flores et al. 2010; Schwarz et al. 2010).
Resistance to salinity stress is a twisted process including biochemical, physiological and molecular reactions (Mostek et al. 2015). For a better understanding of the plants’ mechanisms in reaction and response to different stresses such as cold, salinity and drought, plant proteomic analysis is quite necessary (Molassiotis et al. 2013). Plant proteomic analysis provides fundamental and important information about the expression of proteins and modifications of post-translation under different stresses (Barkla Bronwyn et al. 2013; Molassiotis et al. 2013).
Also, for identification of proteins that are involved in the plant resistance like VlWRKY3 in grape under different biotic and abiotic stresses (Guo et al. 2018), proline in barley under drought stress (Bandurska et al. 2017), NAM, ATAF, and CUC (NAC) transcription factor in rice under drought and salinity stresses (Hu et al. 2006) and AtHAP5A in Arabidopsis under freezing stress (Shi and Chan 2014), several new techniques are available (Ashoub et al. 2013; Capriotti et al. 2014). Five main essential and important groups of proteins in plants have been identified under salinity stress and are directly related to salt resistance: (I) late embryogenesis abundant proteins (LEA proteins); (II) heat shock proteins (HSPs); (III) proteins involved in carbon metabolism; (IV) enzyme scavengers of ROS; and (V) osmolyte biosynthetic enzymes (Passamani et al. 2017). Also, under salinity stress and in reaction to salt stress, proteins associated with protein synthesis/degradation, proteins associated with ion transportation and proteins associated with signal transduction are also involved in plants such as wheat (Triticum durum L.) (Capriotti et al. 2014), rice (Oryza sativa) (Yan et al. 2005) and sugar beet (Beta vulgaris L.) (Wakeel et al. 2011). Also, Capriotti in 2014 proved that proteins that are identified in these studies can be used as molecular markers for breeding plans in the future (Capriotti et al. 2014).
It is specified that plants can be strongly affected by salinity stress, and considerable information is available about the biochemical, cellular and molecular mechanisms of Pistacia genus under different stresses (Moriana et al. 2018; Akbari et al. 2018; Abbaspour et al. 2012; Chelli-Chaabouni et al. 2010). Analysis of proteome in this study is able to help us to comprehend and identify the reacting mechanisms of UCB-1 pistachio rootstock to salt stress. Also, information of this study about the responsive proteins of pistachio under salinity stress can be used in the modern and classical breeding programs (Agrawal et al. 2012). In the first step, this research was done to evaluate changes in the malondialdehyde (MOD) content and guaiacol peroxidase (GPX), superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD) enzymes activities under three different NaCl concentrations in 7-month-old UCB-1 pistachio rootstock leaves after 30 days of exposure to treatment. In the second step, the proteomic profile of this rootstock under 100 mM NaCl was compared with 0 mM using MALDI TOF/TOF method for better comprehension of mechanisms controlling reactions of UCB-1 rootstock to salinity stress.
Materials and Methods
Plant Materials, Growth Conditions and Induction of Salt Stress
Recently, one of the most important rootstocks for pistachio that has been extremely cultivated in the United States of America is UCB-1, which is severely tolerant to salinity stress (Akbari et al. 2018; Ferguson et al. 2002). UCB-1 was produced by the University of California and is a hybrid cross between Pistacia integerrima male × Pistacia atlantica female (Ferguson et al. 2002). In this research, 6-month-old UCB-1 uniform rootstocks that were produced by tissue culture method were prepared and transplanted into the pots containing 1/3 perlite, 1/3 clay and 1/3 sand. After 4 weeks preculturing UCB-1 rootstocks in pots and irrigating them every 4 days by 1 L of ½ Hoagland’s nutrient solution, three different concentrations of NaCl including 0 as control, 100 and 200 mM were made using Hoagland’s complete nutrient solution and were used to apply salinity stress. Irrigation was performed for 30 days with an every 4 days irrigation frequency (Akbari et al. 2018). To avoid the osmotic stress, different concentrations of salinity stress were gently applied, and 1 L of deionized water was used after every four irrigations to stop NaCl accumulation (Akbari et al. 2018). 28 °C during the day and 18 °C at night, 70% relative humidity, and 16/8 h light/dark (450–550 lmol m−2 s−1 flux density) were the green house conditions (Akbari et al. 2018). 8-month-old UCB-1 rootstock leaves were collected and immediately put in an aluminum paper and frozen in liquid nitrogen and stored at − 80 °C for all analyses.
MDA Content
Thiobarbituric acid or TBA reaction was used to determine lipid peroxidation in UCB-1 rootstock under salinity stress (Heath and Packer 1968). Frozen samples were homogenized with two volumes of ice-cold 0.1% (w/v) TCA and centrifuged for 20 min at 16,000×g. Assay admixture containing 1 ml of the supernatant and 2 ml of 0.6% (w/v) TBA in 20% (w/v) TCA was heated at 92 °C for 36 min and then quickly cooled in an ice bath. After centrifugation (12,000×g for 15 min at 3 °C), the absorbance of the supernatant was read at 532 nm, and the quantities corresponding to nonspecific sorption (600 nm) were reduced. Lipid peroxidation crops were calculated as the quantity of TBA-reactive materials. The content of malondialdehyde was measured according to the molar extinction coefficient of 155/(mM cm).
Enzymes Extraction
UCB-1 fresh leaf samples were submersed for 3 min in liquid nitrogen, then were kept at − 80 °C for further analyses. 5 ml of extraction buffer, containing 50 mM K-phosphate buffer, 0.1 mM Na2-EDTA with pH 7.6 was used to extract enzymes from 0.5 g of leaf tissue using a mortar and a pestle. The homogenate was centrifuged at 16,000×g for 20 min, and the supernatant was used to assay different enzymes. All stages in the provision of enzyme extracts were done at 3 °C.
GPX Activity
In this study, the total GPX activity was assessed in 2 ml of reaction mixture, including 0.1 µmol L−1 EDTA, 100 mmol L−1 phosphate buffer (pH 7.0), 15.0 mmol L−1 H2O2, 5.0 mmol L−1 guaiacol, and 50 µL enzyme extract. By appending enzyme extract, reaction began, and increase in absorbance was registered at 470 nm for 1 min. The activity of enzyme was quantified using the quantity of tetraguaiacol formed by its molar extinction coefficient (26.6 mmol L−1 cm−1). The outcomes were shown as µmol H2O2 min−1 mg−1 protein, taking into account that 4 mol H2O2 is decreased to generate 1 mol tetraguaiacol (Plewa et al. 1991).
SOD Activity
By using xanthine, cytochrome C and xanthine oxidase, McCord and Fridovich method was used to evaluate the SOD activity (McCord and Fridovich 1969). One unit of SOD was determined as the quantity of enzyme that prevents the speed of Ferricytochrome C reduction by 50%.
CAT Activity
By calculating the reduction at 240 nm for 1 min, due to H2O2 consumption, Aebi method was used to determine the activity of CAT (Aebi 1984).
POD Activity
In this study using pyrogallol as a substrate, Kwak method was applied to assay POD activity (Kwak et al. 1995). One unit of POD activity was determined as the quantity of enzyme essential to take 1 mg of purpurogallin from pyrogallol in 20 s, at 420 nm.
Data Analysis for MDA, GPX, SOD, CAT and POD
Three replicates were considered for each treatment in completely random designs. One-way ANOVA by SPSS package program (version 13.0) was used for the purpose of data analysis. Significant diversities between treatments were evaluated using Duncan’s multiple range tests (P < 0.05). The results of this study were reported as mean values ± standard deviation (SD).
Protein Extraction and 2-Dimensional Gel Electrophoresis (2D) Analysis
Three biological replicates were considered for this study. After 30 days of NaCl treatment, trichloroacetic acid (TCA)-acetone method, modified from Hurkman and Tanaka (Hurkman and Tanaka 1986), was used to extract UCB-1 leaf proteins, and for this purpose 500 mg of fresh leaves in liquid nitrogen were homogenized by a mortar and a pestle. The powder was put in a 1.5-mL microtube, and 1 mL of cold extraction buffer that contains 1 mM ethylenebis (oxyethylenenitrilo) tetraacetic acid (EGTA), 20 mM Tris–HCl (pH 7.5), 1 mM phenylmethyl sulfonyl fluoride (PMSF) and 1 mM dithiothreitol (DTT) was added to the tube. For 90 min at 4 °C, the microtubes were incubated and then centrifuged at 20,000×g for 45 min. After centrifuging, the outcoming supernatant was carried over to a microtube, and with four volumes of cold acetone including 0.08% β-mercaptoethanol and 12% TCA at − 10 °C for 15 h was precipitated. The microtube again at 20,000×g for 45 min was centrifuged, and then the pellet was washed seven times using cold acetone including 0.08% β-mercaptoethanol at − 10 °C for 4 h. The protein was kept for 24 h to air-dry, and then by using a rehydration buffer containing 2 M thiourea, 7 M urea, 4% 3-[(3-cholanidopropyl) dimethylammonio]-1- propanesulfonic acid (w/v), 0.01% (w/v) bromophenol blue, 40 mM DTT and 0.5% (v/v) immobilized pH gradient (IPG) buffer 4–7 was rehydrated. Bradford method (Bradford 1976) was used to quantify the concentrations of protein, and for drawing the standard curve bovine serum albumin (BSA) was applied.
For the first-dimensional, 17 cm IPG strips (Bio-Rad, USA) were applied to perform isoelectric focusing with pH 4–7. Then, 400 µL of protein, containing 850 µg protein, was loaded to IPG strips for 14–18 h at 24 °C for identification of proteins, and then at 20 ◦C, IEF was done on an Ettan IPG-phor system (GE Healthcare, USA) using the following position: 100 V for 1 h, 200 V for 2 h, 4000 V for 3 h, 1000 V for 2 h, 4000 V for 1 h, and a gradient of 9000 V for 2 h (50 µA per strip) (Yuan et al. 2016).
2D balance buffer [2% sodium dodecyl sulfate (SDS), 6 M urea, 30% glycerol (v/v), 50 mM Tris–HCl, pH 8.8] including 1% DTT for 20 min and again identical 2D balance buffer including 2.5% iodoacetamide for 20 min were used to balance IEF strips. 13% polyacrylamide-SDS gels were prepared, and balance strips were put on them and sealed using 0.6% agarose dilution including bromophenol blue. Also, the EttanDaltSix electrophoresis system (GE Healthcare, USA) was used to perform second dimensional SDS–polyacrylamide gel electrophoresis (SDSPAGE), and Coomassie brilliant blue gel (CBB) R-250 (CBB; Sigma, USA) was used for visualization of spots.
Image and Data Analysis
Three replicates were considered for each treatment in completely random designs. The image scanner III (GE Healthcare, USA) was applied to visualize the image of gels and the gels were analyzed with version 7 Melani software. Also, the measurement of percentage volume (vol%) was used to evaluate plenty of each protein spot. The percentage volume of each protein spot in 0 and 100 mM NaCl conditions was considered to assess the significant difference of each protein by t test method using SPSS 13.0 software (Student’s t test, P < 0.05). Protein spots were normalized as the proportion of the content of a single spot to the whole set of spots available in the gel. To measure the fold change of each protein spot, protein percentage volume data were transferred to an Excel file, and protein spots with considerable level (Duncan’s test at significant P < 0.05) and 1.5-fold change were applied for mass spectrometry. The level of fold change for each protein was calculated based on increasing or decreasing the percentage volume of each protein spot in 100 mM NaCl compared to 0 mM. The results of the protein percentage volume were reported as mean values and percentage coefficients of variations (%CV) for each protein spot (in three replicates).
Protein Identification
Desirable protein spots were cut off from the gel, and wash solution including 50% acetonitrile/50 mM ammonium bicarbonate (NH4CHO3) was used to destain spots for 1 h at 28 °C. After alkylation of protein, solution was taken, and spots at room temperature were left to dry. After drying the spots, trypsin solution was applied to digest proteins at 37 °C for one night. After digestion of proteins, 50% acetonitrile and 0.1% TFA in water were used for washing the peptides and were then put onto the MALDI target plate to dry. In the final step, peptides were handled for MALDI-TOF/TOF at University of York (England) by MALDITOF/TOF analyzer (Applied Biosystems, USA). Version 2.2 MASCOT software (Matrix Science, London, UK) was applied for analysis of the mass spectrometry data using the NCBI (http://www.ncbi.nlm.nih.gov/) and NCBI protein databases (https://www.ncbi.nlm.nih.gov/refseq/about/nonredundantproteins/). Following criteria were considered in the databases for spectra search: trypsin as enzyme, carbamidomethylation of cysteine as fixed modification, MS/MS tolerance (0.8 Da), peptide tolerance (200 ppm) and variable modifications (oxidation (M)) (Fatehi et al. 2012).
Results
MDA Content
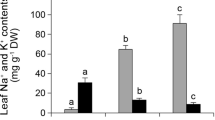
Lipid peroxidation in the leaves of UCB-1 pistachio rootstock was measured as MDA content and is shown in Fig. 1. Increasing salinity from 0 to 100 mM NaCl led to an increase in MDA content, but this amount of MDA decreased at 200 mM NaCl compared to the 100 mM (Fig. 1). Also, no significant difference was observed between 0 and 200 mM NaCl. 0.54, 0.84 and 0.58 µmol/g fr wt% were measured under 0, 100 and 200 mM NaCl, respectively (Fig. 1).
GPX Activity
In order to determine the response of GPX to salinity stress in UCB-1 pistachio rootstock, its activity was measured. With increasing NaCl concentrations, the activity of GPX increased. 0.012, 0.029 and 0.034 µmol H2O2 min−1 mg−1 protein were calculated for GPX activity in UCB-1 pistachio rootstock leaf under 0, 100 and 200 mM NaCl, respectively (Fig. 2).
SOD Activity
As shown in Fig. 3, 100 and 200 mM NaCl treatments increased the activity of SOD in UCB-1 pistachio rootstock leaf compared to control. SOD activity was calculated 2.87, 3.2 and 3.77 units/mg of protein under 0, 100 and 200 mM sodium chloride in UCB-1 rootstocks leaf, respectively (Fig. 3).
CAT Activity
CAT activity enhanced with increasing NaCl concentrations. Under 0, 100 and 200 mM sodium chloride, 0.015, 0.019 and 0.022 units/mg protein were calculated in leaf of UCB-1 pistachio rootstocks (Fig. 4).
POD Activity
The POD activity significantly decreased in the UCB-1 rootstocks leaf at all stress concentrations. In UCB-1 rootstock leaf, the amounts of 33.81, 28.92 and 26.41 units/mg of protein were estimated for POD activity under 0, 100 and 200 mM NaCl, respectively (Fig. 5).
Analysis of Two-Dimensional Gel Electrophoresis
The proteome maps of UCB-1 pistachio leaf in different NaCl conditions were produced from two-dimensional gel electrophoresis (2-DE), as shown in Fig. 6. The protein spots were spread from pH 4–7, and their molecular masses ranged from 10 to 100 kDa. More than 406 protein spots were detected on gels. Of these spots, 25 protein spots had 1.5-fold variations between 100 and 0 mM NaCl environments and these spots were selected for MALDI TOF/TOF analysis (Table 1 and Supplementary Table 1). Of these 25 spots, 15 spots were up-regulated, and 10 spots were down-regulated by the salinity stress (Fig. 6, Table 1, Supplementary Table 1).
MALDI-TOF/TOF Identification and Classification of Responsive Proteins
Twenty-five salinity-responsive proteins which had 1.5-fold variations between 100 and 0 mM NaCl environments were selected for MALDI-TOF/TOF mass spectrometry identification. Mass data were analyzed by Mascot schedule and NCBI non-redundant protein database. All identified salinity responsive proteins by MALDI TOF–TOF are listed in Table 1 and supplementary data for these 25 responsive proteins are listed in Supplementary Table 1. Generally, these responsive spots represented 20 unique proteins, which were classified into nine functional categories: photosynthesis (20%), enzymatic antioxidant defense system (12%), replication and repair of DN (4%), energy pathway (12%), non-enzymatic antioxidant defense system (4%), transportation of material (16%), calcium-binding proteins (4%), transcription factor (4%) and chaperone (4%), as shown in Fig. 7 and Table 1. Also, 5 unknown function proteins (20%) were determined under salinity stress in UCB-1 pistachio (Fig. 7, Table 1). The photosynthetic related proteins consist of the 50S ribosomal protein L13 chloroplastic (spot 337), ribulose-1.5-bisphosphate carboxylase/oxygenase large subunit (spot 446), ribulose bisphosphate carboxylase small chain (spot 229), ribulose bisphosphate carboxylase/oxygenase activase 1 (spot 66) and PREDICTED: phosphoribulokinase chloroplastic (spot 161) (Table 1). The enzymatic antioxidant defense system-related proteins consist of superoxide dismutase [Cu–Zn] isoform X1 and X2 (spots 261 and 81) and Catalase 2 (spot 326) (Table 1). Ribonucleoside-diphosphate reductase small chain A was recognized as the only protein involved in the replication and repair of DNA (spot 76) (Table 1). As shown in Table 1, proteins involved in the energy pathway included ATP synthase CF1 epsilon subunit plastid (spot 64), external NADH-ubiquinone oxidoreductase 2 mitochondrial-like protein (spot 243) and ADP-glucose pyrophosphorylase (AGPase) small subunit (spot 198). Phytoene synthase 2 was detected as the only protein involved in the non-enzymatic antioxidant defense system (spot 243) (Table 1). As shown in Table 1, the transportation of material related proteins consisted of the Vesicle-associated membrane protein (VAMP) 722- and 724-like (spots 96 and 188), Golgin subfamily A member 5 (spot 303) and PREDICTED: ABC transporter C family member 12-like (spot 104). The protein involved in calcium-binding proteins was recognized as polcalcin Phl p 7-like (spot 336) (Table 1). One protein was determined as transcription factor entitled PREDICTED: nascent polypeptide-associated complex subunit alpha, muscle-specific form-like isoform X2 (spot 112) (Table 1). Stromal 70 kDa heat shock-related protein, chloroplastic (spot 30), was determined as chaperon (Table 1). 5 unknown function proteins were identified under salinity stress including uncharacterized protein LOC107620721, PREDICTED: uncharacterized protein LOC105974023, PREDICTED: uncharacterized protein LOC109219975 partial, PREDICTED: uncharacterized protein LOC109178037 and hypothetical protein CISIN_1g0462972 mg partial (spots 596, 201, 335, 216 and 206), respectively (Table 1).
Discussion
One of the most important abiotic stresses is soil salinity, which reduces water potential and causes nutrients to be unbalanced in plants, and they adversely affect plant development and growth (Mansour and Salama 2004; GENC et al. 2007). One of the actions that can be performed to decline the destructive effects of different stresses is usage of rootstock and grafting in plants. Selection of a tolerant rootstock is mandatory in most plants and hence, usage of grafting method to choose a salt resistant root system as rootstock may enhance salt resistance in plants (Zhang et al. 2018). In grafted plants, rootstock root system is responsible for resistance in response to salinity stress. In this procedure, if the rootstock is effectively chosen, it will strengthen water and nutrient absorption (Kumar et al. 2018), enhance nitrogen and carbon metabolism (Shahid et al. 2018) and consequently enhance salt resistance in plants. Grafting technique not only enhances the tolerance of plants in different stress conditions, but also preserves good strains of seeds and stabilizes heterosis (Penella et al. 2014). For example, grafted tomato decreased transportation of Cl− and Na+ by roots to aerial sections under salinity stress (Estan et al. 2005) and precipitated absorption of K+ by roots to adjust itself to unfavorable conditions of salinity stress (Fan et al. 2011). In this example, a grafted tomato enhanced photosynthetic ability and improved salt resistance by enhancement of the antioxidant enzymes activity (He et al. 2009). In leaves of grafted cucumber, the level of photoinhibition was significantly declined under salinity stress (Huang et al. 2011), and metabolism ability of nitrogen was elevated (Liu et al. 2013). Also, it is specified that grafted seedlings absorbed more phosphorous and nitrogen under salinity stress in watermelon (Uygur and Yetisir 2009). Accordingly, understanding the molecular basis of salt stress resistance mechanisms in rootstocks is necessary for genetic engineering and breeding programs of salt resistance in plants. In spite of intensive studies on plants’ response to salt stress, there have not been any studies on the proteomic profile of Pistacia genus or Anacardiaceae family with proteomic method. In this study, the 8-month-old UCB-1 rootstocks that were exposed to 0, 100 and 200 mM NaCl for 30 days were used as experimental samples. All analyses were performed using the 8-month-old UCB-1 rootstock leaves. The MDA content and GPX, CAT, SOD and POD activities were calculated under these three different stress. Also, under 100 mM NaCl, changes in the leaf proteomic profile of this rootstock were compared to 0. The results of this study showed that MDA increases under salinity stress in leaves of UCB-1 pistachio rootstock up to 100 mM NaCl, and then decreases. In citrus plants, MDA increased under salinity stress (Tanou et al. 2009). In a research in 2008, Yaser and coworkers showed that in green bean, MDA content increases under salinity stress, significantly (Yasar et al. 2008). Also, another study in cowpeas showed that under salinity stress plants’ growth reduces, while MDA content increases (Cavalcanti et al. 2004). ROS generation under salinity stress is unavoidable (Abogadallah 2010), and ROS causes membrane lipid peroxidation and membrane fluidity reduction. Lipid peroxidation is measured as MDA, which is a product of lipid peroxidation (Dhindsa and Matowe 1981; Wise and Naylor 1987). The content of MDA is a significant pattern in evaluating the resistance of plants under stress situations. The results of a study showed that salt-tolerance genotypes have less lipid peroxidation (Taïbi et al. 2016), and this result is quite similar to our result under 200 mM NaCl. In this study, increasing MDA content under 100 mM NaCl compared to 0 mM is an oxidative damage of salinity on UCB-1 pistachio rootstock, while decease in the content of MDA under 200 mM NaCl compared to 100 mM may indicate the adaptive reaction of this rootstock under salinity stress. In this study, with increasing the concentrations of NaCl, GPX, CAT, and SOD activities increased in all samples. Several previous studies showed that GPX enzyme activity in soybean under drought stress (Moloi et al. 2016) and in pearl millet under salinity application at reproductive and vegetative stages (Heidari and Jamshidi 2011), CAT activity in pearl millet at reproductive and vegetative stages under salinity stress (Heidari and Jamshidi 2011), and SOD activity in salt-sensitive and salt-tolerance genotypes of colza (Brassica napus L.) under sodium chloride stress (Jalali-e-Emam et al. 2011) increase, significantly. Also, Ahmad et al. in several studies showed that CAT and SOD enzymes activity along with the content of MDA increases under salinity stress in different plant species, significantly (Ahmad et al. 2016; Ahmad et al. 2017; Ahmad et al. 2018; Ahmad et al. 2019). It is proved that different abiotic stresses produce reactive nitrogen species (RNS) and reactive oxygen species (ROS), resulting in nitrosative and oxidative stress in plants (Molassiotis and Fotopoulos 2011), but on the other hand, Molassiotis et al. concluded that RNS and ROS are able to coordinately adjust plant stress responses to harmful environmental conditions (Molassiotis et al. 2016). The concentration of alkyl hydroperoxides or H2O2 and other reactive oxygen species or ROSs, such as hydroxyl radical (OH·), superoxide radical (O2·−), and singlet oxygen (1O2) can be enhanced either by their decreased activity of the defense system or enhanced production (Foyer et al. 1997; Bela et al. 2015). ROSs may hurt intracellular biomacromolecule, resulting in enzyme deactivation, membrane lipid peroxidation, and DNA damage (Abogadallah 2010). Also, stability and synthesis of protein can be affected by ROSs (Abogadallah 2010). Under oxidative stress conditions, the activities of antioxidant enzymes such as catalase (CAT), superoxide dismutase (SOD), and guaiacol peroxidase (GPX) are commonly increased in plants. For example, SOD and POD enzymes activity increased in the leaves and stems of apple rootstock under NaCl stress (Molassiotis et al. 2006). Also, the activity of the aforementioned enzymes generally has a correlation with enhanced resistance (Foyer et al. 1997). The functions of GPX, SOD, and CAT are not completely understood. One of the most important peroxidase enzymes is guaiacol peroxidase located in the cytosol, apoplast, vacuole, and cell wall (Uarrota et al. 2016). It is proved that under salinity stress, guaiacol peroxidases enzyme is involved in the detoxification of induced ROS in plants (Uarrota et al. 2016). GPXs protect cells from stress induced by oxidative damage and are involved in plant development and growth. Detoxification of organic hydroperoxides and H2O2 is the main role of GPX isoenzymes in plants. Under salinity stress in UCB-1 rootstock pistachio, GPX may increase to protect cells from oxidative damage and enhance plant development and growth. Superoxide dismutases or SOD, which is an antioxidant enzyme, has the main role in preventing oxidative stress caused by extreme superoxide anion or O−2 (Smirnoff 1993). This enzyme catalyzes the dismutation of O−2 with excellent performance, resulting in the generation of O2 and H2O2 (Smirnoff 1993). Increasing SOD under salinity stress in our study may enhance the ability of UCB-1 rootstock to scavenge ROS under this stress and lead to normal growth. CAT, which is an antioxidant enzyme in various plant tissues (Chawla et al. 2013), reacts with H2O2 straightly to form oxygen and water (Smirnoff 1993). Also, CAT is mostly related to an enhanced resistance to salinity stress (Mittova et al. 2004). In this study, CAT may increase to reduce the destructive effects of salinity, including ROSs, and to enhance the resistance of this rootstock under NaCl stress. In this research, POD activity decreased with increasing the concentration of NaCl in UCB-1 pistachio rootstock leaf. Previous studies reported the reduction of POD activity in carrot (Bano et al. 2014) and salt-tolerant variety Pokkali (Oryza sativa L.) (Dionisio-Sese and Tobita 1998) under salinity stress. The produced hydrogen peroxide is scavenged by a variety of peroxidases (Dionisio-Sese and Tobita 1998), and this enzyme catalyzes H2O2 by oxidation of co-substrates, such as antioxidants and/or phenolic compounds (Dionisio-Sese and Tobita 1998). Plants use multiple isoforms of antioxidant enzymes to scavenge the produced ROS by salinity stress (Kim et al. 2005b), and it is specified that NaCl stress can either inhibit or stimulate the expression of the isoforms of many antioxidant enzymes (Kim et al. 2005b). For example, in potato, new SOD and POD isoenzymes emerged in response to salinity stress (Rahnama and Ebrahimzadeh 2005). In a previous study, POD enzyme activity decreased in the Broussonetia papyrifera leaves under 100 and 150 mM NaC1 treatment compared to the normal condition, while POD isoenzyme activity increased in the leaves of this plant (Zhang et al. 2013) and this matter can be the main reason of POD enzyme activity decreasing in the leaves of this rootstock. Also, since the metabolic responses to the salinity stress are a complex process, under salinity stress, many processes such as growth, energy metabolism, accumulation of compatible osmolytes, ion partitioning, and carbon metabolism are modified (Bohnert and Sheveleva 1998). At the cellular level, it is specified that salinity stress affects the cell wall by the increment in the polymerization of monolignols of the root (Cruz et al. 1992) and reduction in cell expansion (Iraki et al. 1989). Also in plants, peroxidases are involved in the biosynthesis of cell wall (Negrel and Lherminier 1987), including suberization and lignification (Polle et al. 1994; Espelie et al. 1986). In several plant systems, a reverse relationship was reported between POD enzyme activity and growth rate (Lee and Lin 1995; Gardiner and Cleland 1974; Carpita and Gibeaut 1993; Chen and Kao 1995), and in this study, decreasing POD enzyme activity can be reversely related to the growth rate of UCB-1 pistachio rootstock under salinity stress.
In recent decades, proteomics method has emerged as a valuable tool to identify potential biomarkers in plants in response to different environmental conditions (Molassiotis et al. 2013). For example, in a research and using proteomics method, Tanou et al. (Tanou et al. 2009) discovered 85 leaf proteins under salinity stress in Citrus aurantium, most of the researches in plants have been done on this family using proteomics method (Tanou et al. 2010). For the most part, changing environmental conditions causes rapid changes in the structure, level and composition of different protein and RNA molecules, and these changes lead to the stress acclimation or signal transduction events in plants (Beckers and Conrath 2007). For example, Molassiotis and colleagues showed that ROS (in the form of H2O2), which is the production of salinity stress in plants (Tanou et al. 2012) and acts as the key component of redox homeostasis (Lounifi et al. 2013), in the first step is able to change many genes expression levels and proteins abundance and consequently is able to alter photosynthesis pattern in plants (Molassiotis et al. 2016). For these reasons, in the present research, comparative proteomics was conducted to investigate changes in the proteomic profile of UCB-1 pistachio rootstock under NaCl stress to recognize the main proteins involved in response to salinity stress. According to the results of this study, 25 proteins and their families that are listed below were identified in response to salinity stress in this rootstock.
Proteins Involved in the Photosynthesis
In response to 100 mM NaCl stress, some fundamental proteins of the photosynthesis were detected in the UCB-1 pistachio rootstock compared to the normal condition, including 50S ribosomal protein L13, chloroplastic (spot 337), ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) large subunit (spots 446), ribulose bisphosphate carboxylase small chain (RBCS, spots 229), ribulose bisphosphate carboxylase/oxygenase activase 1 (RCA 1, spot 66) and phosphoribulokinase, chloroplastic (spot 161). In two previous studies in whitetop (Lepidium draba L.) and Aegilops tauschii and in response to exogenous glucose and moisture stress, it was determined that 22 and 28.5% of changes in the proteomic profile of these two plants belonged to the photosynthesis-related proteins, respectively (Rezaee et al. 2018; Nazari et al. 2018). Tanou et al. proved that the largest category (44.7%) of the NaCl-responsive proteins was associated with photosynthesis (Tanou et al. 2009).
50S Ribosomal Protein L13, Chloroplastic
11.24-fold downregulation was observed for this protein under salinity stress in UCB-1 rootstock. Under short term of salinity in soybean, a research showed that 50S ribosomal protein L12-3 decreases significantly (Sobhanian et al. 2010), but another study reported that this protein increases under long-term salinity stress in barley (Fatehi et al. 2012). Ribosomal proteins (RPs), which have large and small subunits, have the main role in sustaining the durability of the ribosomal complex and in mediating synthesis of protein (Moin et al. 2016). In this study, decreasing 50S ribosomal protein L13 demonstrates the inhibitory effect of sodium chloride on UCB-1 protein biosynthesis and likely leads to a reduction in plant development.
Ribulose-1.5-bisphosphate Carboxylase/Oxygenase (RuBisCO) Large Subunit
Our results showed that RuBisCO large subunit was down-regulated 2.75-fold under 100 mM NaCl, while in Sorghum under 100 mM NaCl, RuBisCO large subunit increased 110%, which was reported by Ngara et al. (2012). Zhang and colleagues reported that in cotton diploid wild species, eight RuBisCO subunits down-regulated significantly (Zhang et al. 2016). One of the main enzymes that has an essential role in the assimilation of photosynthetic carbon is RuBisCO, and plants can produce better yield by increasing the assimilation of photosynthetic carbon (Raines 2011). RuBisCO plays the main role in photorespiratory and photosynthetic response paths, and RuBisCO affinity for CO2 in different plants on the proportion of their carboxylase to oxygenase activities is accessible (Yeoh et al. 1980). Therefore, under abiotic stress such as salinity, plants can improve their tolerance along with better yield by higher amount of RuBisCO. Most likely, in this study, RuBisCO downregulation occurs because of the light response inactivation or CO2 influx reduction. Also, it can be concluded that reduction in RuBisCO activity may be clarified by loss of RuBisCO protein when the rootstock was under salinity stress (Parry et al. 2002).
Ribulose Bisphosphate Carboxylase Small Chain
In this study, RuBisCO small chain was up-regulated 2.73-fold under salinity stress in UCB-1 rootstock. A previous study showed that this protein up-regulated 1.14-fold under late salinity stress in rice (Lakra et al. 2018). One of the main proteins in the stroma of chloroplasts is RuBisCO, which exists in plants as a complex of 8 small and 8 large subunits. Ribulose bisphosphate carboxylase is a small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase (Pichersky et al. 1986). It is specified that RuBisCO subunits are sensitive to fragmentation under moisture stress situations. This event leads to producing the isoforms of various isoelectric points and molecular weights, and enhancing in the number of rubisco subunit (Salekdeh et al. 2002; Guo et al. 2016; Budak et al. 2013).
Ribulose Bisphosphate Carboxylase/Oxygenase (RuBisCO) Activase 1
RuBisCO activase 1 was down-regulated 1.60-fold in response to 100 mM NaCl. Increasing 1.7- and 2.5-fold of RuBisCO activase was reported by Parker in rice under short- and long-term salinity, respectively (Parker et al. 2006), while the proteomic analysis of diploid and tetraploid black locust under salinity stress showed that in 4X plants, RuBisCO activase content decreased by 20% (Wang et al. 2013). One of the members of AAA+ family is RuBisCO activase, which has various chaperone-like functions (Bhat et al. 2017). Preservation of the catalytic activity of RuBisCO is the principal role of the activase. Activase, through the removal of inhibitory sugars from the energetic position of carbamylated RuBisCO and uncarbamylated, preserves the catalytic activity of RuBisCO (Portis 2003). Direct decrease in stomatal conduction followed by low CO2 concentrations may be induced under salinity stress, and subsequently increasing activase activity may happen (Parker et al. 2006). In this study, RuBisCO activase content downregulation may cause a reduction in Calvin-Benson cycle activity. This would hold a suitable assimilation valency of photosynthetic CO2 by lightening energy consumption to intensify salinity resistance.
Predicted: Phosphoribulokinase, Chloroplastic
In this study, a significant 2.75-fold downregulation of phosphoribulokinase protein was observed under 100 mM NaCl in UCB-1 pistachio rootstock. Also, Srivastava and colleagues observed that phosphoribulokinase decreases after 1 and 24 h of 150 mM NaCl treatment (Srivastava et al. 2008). Furthermore, under salinity stress in Arabidopsis, Seki et al. (2002) reported that phosphoribulokinase decreases significantly (Seki et al. 2002). A necessary photosynthetic enzyme that catalyzes the ATP-dependent phosphorylation of ribulose 5-phosphate into ribulose 1,5-bisphosphate is phosphoribulokinase, its principal function is regeneration of ribulose 1,5-bisphosphate, which is the primary substrate and CO2-acceptor molecule of the Calvin Cycle (Miziorko 2000). Under NaCl stress, downregulation of phosphoribulokinase points toward inhibition of the Calvin cycle and pentose phosphate pathway, thereby explaining the decline in NADPH content (Srivastava et al. 2008).
Proteins Involved in the Enzymatic Antioxidant Defense System
Superoxide Dismutase [Cu–Zn] Isoform X1 and X2
[Cu–Zn] superoxide dismutase (SOD) isoforms X1 and X2 were illustrated to decrease after 30 days growing under 100 mM NaCl. Cu–Zn SOD isoforms X1 and X2 decreased 119.61- and 78.45-fold, respectively. Parker and coworkers in 2006 showed that chloroplast [Cu–Zn] SOD in rice increased 1.7-fold after 7 days of treatment by 50 mM NaCl (Parker et al. 2006). Also, Hernandez and coworkers illustrated that in NaCl-susceptible variety, Cu–Zn SOD activity reduced 35%, while in the NaCl-resistant plants, Cu–Zn SOD remained fixed (Hernández et al. 2001). In Vigna unguiculata L., and under 100 mM NaCL, mitochondrial and cytosolic Cu–Zn SOD I and protoplasts Cu–Zn SOD II decreased remarkably (HernÁNdez et al. 1994). Also, the transcripts of copper-zinc SOD in underground stems’ organs of Polygonum sibiricum were significantly down-regulated after 3% NaHCO3 stress (Qu et al. 2010). Reactive oxygen species (ROSs) are produced as a result of abiotic stresses, such as salinity and drought. The first line of cell advocacy versus ROSs is SODs family. Copper-zinc SOD (Cu–Zn SOD), iron-SOD (Fe-SOD), and manganese-SOD (Mn-SOD) are three different kinds of SODs, which are placed in various plant sub-cellular organelles (Grene 2002; Hernandez and Almansa 2002). A section of an enzymatic detoxification system is engendered by SOD to scavenge ROSs (Asada 1999; Parker et al. 2006). When the concentration of CO2 decreases in the stroma, SOD [Cu–Zn] activity increases to maintain electron flux in the thylakoids. In this study, downregulation of Cu–Zn SOD isoforms X1 and X2 may be related to the results of H2O2-products of Cu–Zn SOD.
Catalase 2
Catalase 2 was up-regulated 2.28-fold in response to 100 mM NaCl. Under salinity stress, and in bread wheat, catalase was up-regulated significantly, which was reported by Peng in 2009 (Peng et al. 2009). Also, Molassiotis et al. showed that enzymatic and non-enzymatic antioxidant activities as well as contents of H2O2 increased in leaves and stems of apple rootstock under different abiotic stresses (Molassiotis et al. 2006). In cells, H2O2 detoxification is done with glutathione peroxidase, ascorbate peroxidase, thioredoxin peroxidase, and catalase (Mehlhorn et al. 1996). Under stress situation, a remarkable enhancement in CAT activity in leaves can preserve chloroplasts and give a sustained electron flow (Foyer and Shigeoka 2011). When plants are exposed to water deficiency of salinity stress, increasing CAT activity of leaves allows the removal of photorespiratory H2O2, which is produced under violent abiotic stresses (Sofo et al. 2015).
Proteins Involved in the Replication and Repair of DNA
Ribonucleoside-Diphosphate Reductase Small Chain A
In this research, ribonucleoside-diphosphate (RDP) reductase decreased 166.12-fold under salinity stress. No report about the RDP reductase under abiotic stresses is available. RDP reductase, which brings the ribonucleotide diphosphate reduction to deoxy-ribonucleotide, is an enzyme containing Fe and is a major enzyme in DNA synthesis. RDP reductase or ribonucleotide reductase (RDR) is a main target of the DNA damage checkpoint pathways in mammals, higher plants, and yeasts (Huang et al. 1998). The reduction of all four ribonucleotide diphosphates (NDPs) into their corresponding deoxyribonucleosides (dNDPs) is done by RDP reductase (Kolberg et al. 2004). RDP reductase contains two large subunits (R1) and two small subunits (R2) (Wang and Liu 2006). The R1 subunit is the aim of feedback adjustment, which warrants that dNTPs are not overproduced in cells, and ensures that enough NDPs are left for synthesis of RNA (Wang and Liu 2006). The R2 subunit is necessary for the reduction of NDP to dNDP (Wang and Liu 2006). In mammal and yeast, it is indicated that damaged RDP reductase frequently leads to p53-dependent apoptosis, growth delay, and cell cycle arrest. Also, higher mutation rate happens when RDP reductase activity increases (Wang and Liu 2006). Forasmuch as increasing RDP reductase activity increases mutation rate, most likely in this study, RDP reductase small chain decreases for decreasing RDP reductase activity to reduce mutation rate in UCB-1 rootstock under salinity stress.
Proteins Involved in the Energy Pathway
ATP Synthase CF1 Epsilon Subunit (Plastid)
In this study, ATP synthase CF1 epsilon subunit (plastid) was up-regulated 3.11-fold under 100 mM sodium chloride. ATP synthase CF1 increased in cotton under drought stress (Deeba et al. 2012), in rice (Kim et al. 2005a), and in the roots and shoots of maize under salinity stress (Zuther et al. 2004), significantly. One of the main enzymes in energy transduction in mitochondria and chloroplasts is ATP synthase, which decreases the destructive effect of stress in plants. Water stresses such as salinity and drought reduce CO2 assimilation by reduction of the net ATP value (Tezara et al. 1999). Also, ATP synthesis induction assists plants to enhance their resistance to abiotic stresses (Zhang et al. 2008). Furthermore, when ATP synthase was overexpressed in Arabidopsis, greater resistance was observed under drought stress (Zhang et al. 2008). In this study, similarly, this protein was up-regulated to enhance the resistance of UCB-1 pistachio rootstock under salinity stress by increasing energy transduction.
External NADH-Ubiquinone Oxidoreductase 2, Mitochondrial-Like Protein
Decreasing 142.84-fold was calculated for external NADH-ubiquinone oxidoreductase 2, mitochondrial-like protein in UCB-1 pistachio rootstock under salinity stress. Also, Witzel and coworkers showed that in barley and under salinity stress, (NADH)-ubiquinone oxidoreductase decreased significantly (Witzel et al. 2014). Complex I or NADH-ubiquinone oxidoreductase is the first enzyme in the electron transition chain (Walker 1992), and is associated with the inner membrane of mitochondria with their NADH reaction position facing either internal alternative NADH-ubiquinone oxidoreductases (the mitochondrial matrix) or external alternative NADH-ubiquinone oxidoreductases (the cytoplasm). Little information is available about the external NADH-ubiquinone oxidoreductases. The internal mitochondria membranes have three multi-subunit enzyme complexes that act to transmit electrons from NADH to oxygen (Walker 1992). Ripening-induced proteins and abscisic acid are involved in abiotic stress responses (Zhang et al. 2015), and these proteins increase under abiotic stresses such as drought and salinity (Zhang et al. 2015). Ripening-induced proteins are able to bind to a wide number of respiratory electron transport chain genes and affect decreasing NADH-ubiquinone oxidoreductase.
ADP-Glucose Pyrophosphorylase (AGPase) Small Subunit
The result of our research showed that under salinity stress, this protein was up-regulated 1.82-fold in UCB-1 pistachio rootstock. Yin and colleagues reported that AGPase increases during the early development stages under salinity concentration (160 mM NaCl) in tomato (Yin et al. 2010). One of the most important regulatory enzymes in starch biosynthesis is AGPase, which catalyzes the transformation of glucose-1-phosphate and ATP to ADP-glucose. AGPase, which is a heterotetramer, has two B and two S subunits (Li et al. 2002). It can be concluded that most likely, AGPase small subunit increases in order to increase AGPase for adjustment of starch synthesis under salinity stress.
Protein Involved in the Non-enzymatic Antioxidant Defense System
Phytoene Synthase 2
In our study, the expression of phytoene synthase (PSY) 2 decreased 90.96-fold under salinity stress. A previous study reported that PSY2 is significantly induced under ABA and salinity stresses in Daucus carota (Simpson et al. 2018). Phytoene synthase is the first enzyme and one of the most important points of adjustment in carotenoid biosynthesis path (Simpson et al. 2018). Carotenoids synthesis can be adjusted by sequestration and reposition in various kinds of plastids (Deruere et al. 1994; Vishnevetsky et al. 1999) at the epigenetic surface and even at the post-translational area (Cazzonelli et al. 2009). Carotenoids, which in plants intercede abiotic stress resistance responses, are abscisic acid precursors (Simpson et al. 2018). The synthesis of abscisic acid precursors occurs through induction of PSY expression (Simpson et al. 2018). Previous studies showed that salt stress induces the PSY expression in the root of A. thaliana (Meier et al. 2011; Ruiz-Sola et al. 2014), and the expression of PSY3 in the roots of maize and rice, whereas PSY1 and PSY2 are only induced in the leaves of maize and rice by light (Welsch et al. 2008; Li et al. 2009). Also, a previous study on carrot showed that PSY1 is expressed in leaves, while PSY2 expression is higher during root development (Fuentes et al. 2012). Another research showed that Daucus carota PSY2 gene expression is induced by ABA in roots of carrot, which is associated with the attendance of ABREs in its promoter. ABA-responsive element binding proteins, which are induced by ABA and abiotic stresses such as salinity and drought, are the mediators of PSY2 induction under salinity stress through ABA in Daucus carota (Simpson et al. 2018). The same research showed that three ABREs are necessary for the ABA response, and most likely, Daucus carota AREB3 is the mediator of Daucus carota PSY2 induction under salt stress through ABA. Carotenoids function as collectors of light energy for photosynthesis and also act as quenchers of triplet O2 and chlorophyll (Demmig-Adams and Adams 2002). In addition, carotenoids dissipate additional energy through the xanthophyll cycle and can function as the strong chloroplast membrane stabilizers, which are divided between the lipid phase of thylakoid membranes and light-harvesting complexes, decreasing susceptibility to lipid peroxidation and membrane fluidity (Demmig-Adams and Adams 2002). Therefore, decrease in carotenoid content under salt stress shows that the conservation by carotenoid is not an important mechanism in plants (Taïbi et al. 2016).
Proteins Involved in the Transportation of Materials (Endocytosis and Exocytosis)
Vesicle-Associated Membrane Protein (VAMP) 722- and 724-Like (Exocytosis)
VAMPs 722- and 724-like were up- and down-regulated 5.56- and 30.36-fold under 100 mM NaCl, respectively. A previous study reported that VAMP 722 is down-regulated under abscisic acid application in Arabidopsis (Yi et al. 2013). Also, they observed more growth inhibition in Arabidopsis by abscisic acid, which is an abiotic stress hormone and the plant growth barrier (Yi et al. 2013). Another study reported that VAMP 722 is up-regulated at 4 and/or 24 h of salt treatment in cotton (Peng et al. 2014). Also, VAMP 722 may become activated in response to pathogen attack (Sup Yun et al. 2013). A strong upregulation was recognized under salinity stress for VAMP 724 in Ginkgo biloba by Mohanta et al. (2012). VAMP 721/722 are involved in plant development, immunity, and growth (Yi et al. 2013), and specifically have a main role in plant development and growth because the VAMP 721/722-silenced plants are dwarf and the vamp 721/722 plants are lethal (Zhang et al. 2011; Kwon et al. 2008). Because of the smaller cell size in VAMP 721/722-silenced plants (Kwon et al. 2008), it is proved that VAMP 721/722 vesicles are employed in the default secretory pathway (Yi et al. 2013), which transports essential materials required for cell development and acts as a plant advocacy response (Yi et al. 2013). It is proved that ABA increases under salinity stress (Jia et al. 2002), and ABA suppresses plant growth by reducing the VAMP 722 abundance (Yi et al. 2013). Most likely, UCB-1 pistachio rootstock increases VAMP 722 level to enhance plant development and growth under salinity stress via transportation of molecules related to the plant growth and development such as lipids (Ichikawa et al. 2015) in the plasma membrane and cell wall (Yi et al. 2013). VAMP 724 forms a complex that is known as soluble N-ethylmaleimide-sensitive-factor attachment protein receptor or SNARE, which plays a main role in vesicle trafficking to vacuoles and transfers molecules to their goals. The main role carried out by VAMP 724 is to move ROS from endosomes to vacuoles. Suppression of vesicle VAMP 724 expression in Arabidopsis plant inhibits assimilation of H2O2 containing vesicles with vacuoles (Leshem et al. 2006; Mohanta et al. 2012). Downregulation of this protein could be justified by the inability of this rootstock to move produced ROSs under salinity stress from endosomes to vacuoles.
Golgin Subfamily A Member 5 (Endocytosis and Exocytosis)
This protein was up-regulated 2.73-fold under salinity stress. In this study, this protein is reported for the first time in response to abiotic stresses, such as salinity stress. In the secretory pathway, Golgi apparatus partakes in glycosylation and transportation of lipids and proteins (Gillingham et al. 2002). In plant cells, protein N-glycosylation in the endoplasmic reticulumin and in Golgi apparatus is a necessary process (Kang et al. 2008). Also, N-glycosylation pathway in the endoplasmic reticulumin regulates protein quality control, cellulose biosynthesis, and salt tolerance (Kang et al. 2008). Interplays between the microtubules and Golgi are significant for the reorganization of the Golgi during mitosis (Gillingham et al. 2002). Golgins, which are long coiled-coil proteins and are well conserved in evolution, are present on the Golgi (Munro 2011). Golgins have roles in Golgi structure and membrane traffic, but their accurate function is unknown in most instances (Munro 2011). Golgins are goals of modification during apoptosis and mitosis, which are two operations involved in Golgi fragmentation (Munro 2011). According to the aforementioned issues, it can be concluded that most likely, Golgin subfamily A member 5 increases in response to salinity stress to increase glycosylation and transportation of lipids and proteins.
PREDICTED: ABC Transporter C Family Member 12-Like (Endocytosis)
Our results showed enhancement of 2.18-fold for this protein under salinity stress. In transgenic tobacco under salt and osmotic stresses, ABC transporter C family is up-regulated (Singh et al. 2016), and under 200 mM NaCl in cotton roots, this protein increased, significantly (Li et al. 2015). Terpenoids, alkaloids, quinines, and polyphenols, which are stress-related secondary metabolites, are transported by ABC transporters (Theodoulou 2000). Lee and coworkers showed that ABC transporter affected Na+/K+ homeostasis in Arabidopsis and elicited a salt stress reaction (Lee et al. 2004). Upregulation of an ABC transporter C family member 12-like in UCB-1 pistachio rootstock suggests that it may have a main role in salt stress responses. Probably, expression of this protein under salinity stress surges to increase transportation of stress-related secondary metabolites.
Calcium-Binding Proteins
Polcalcin Phl p 7-Like
In this study, polcalcin Phl p 7-like protein was up-regulated 2.87-fold under salinity stress. No report is available from the response of this protein under abiotic stresses. Polcalcins, which have two ‘‘EF-hand’’ motifs and range from 77 to 84 residues in length, are small Ca2+-binding proteins (Ledesma et al. 1998; Rozwadowski et al. 1999; Suphioglu et al. 1997). The characteristic structural base of the greatest type of intracellular Ca2+-binding proteins is EF-hand (Grabarek 2006; Kretsinger and Wasserman 1980; Gifford et al. 2007). Calcium has a main role in tube growth and pollen germination (MalhÓ et al. 2000). Polcalcin may act as a calcium reservoir and is also thought to be involved in intracellular signaling (Grote et al. 2008). Many studies proved that Ca2+ is able to directly reduce Na+ toxicity and also indirectly improve K-alleviation of Na+ toxicity (Shabala and Cuin 2008; Cramer 2002; Demidchik and Tester 2002). Many mechanisms in plants are controlled by sodium-calcium interactions (changes in the balance of sodium-calcium ions), such as photosynthesis, growth, mineral nutrition, ionization, and watering (Cramer 2002). Under salinity stress in two different pistachio cultivars, Ca2+ decrement was recorded by Rahneshan et al. (2018). According to the discussions above and because of the decrease in amount of Ca2+ under NaCl stress, most likely, UCB-1 pistachio rootstock increases polcalcins to reduce the destructive effects of Na+ in response to salinity stress.
Transcription Factors
Predicted: Nascent Polypeptide-Associated Complex (NAC) Subunit Alpha, Muscle-Specific Form-Like Isoform X2
Our results showed that this protein was up-regulated twofold under 100 mM NaCl. Yan and colleagues in 2005 reported that in response to salinity stress in rice root, α-NAC was down-regulated significantly (Yan et al. 2005), while other studies showed that in response to salinity, NAC subunit alpha-like 3 in barley (Fatehi et al. 2012) and NAC in tomato (Chen et al. 2009) up-regulated significantly. NAC or nascent polypeptide-associated complex, which is involved in protein translocation and sorting (Yan et al. 2005; Chen et al. 2009), attaches to eukaryotic ribosomes and is the first cytosolic protein to contact nascent polypeptide fetters appearing from ribosome (Rospert et al. 2002). It is specified that NAC prevents mistargeting of nascent polypeptide fetters to the endoplasmic reticulum (Rospert et al. 2002). NAC is a heterodimeric complex of β chain and α chain (Yan et al. 2005). α-NAC acts as a transcriptional coactivator (Moreau et al. 1998; Yotov et al. 1998). If α-NAC protein rate decreases, it probably affects the NAC function; changes in NAC function affect the processes of protein translation and gene transcription and ultimately lead to an erratic metabolism (Yan et al. 2005; Chen et al. 2009). Therefore, plants probably enhance the α-NAC protein concentration to reduce their own metabolism disorder under salinity stress.
Chaperon
Stromal 70 kDa Heat Shock-Related Protein, Chloroplastic
Under salinity stress in UCB-1 rootstock leaf, this protein was up-regulated 2.53-fold. Many reports are available in response to this protein under different situations, such as exposure to cold, UV light, biotic stresses, wound healing or tissue remodeling (Boston et al. 1996; Lindquist and Craig 1988; Vierling 1991). Also, Wang in 2004 showed that this protein is a key protein in response to abiotic stresses (Wang et al. 2004). Furthermore, the role of heat shock proteins under abiotic stresses using proteomic method was proved by Timperio et al. (2008). HSPs or heat shock proteins are proteins found in animal and plant cells. Heat shock proteins respond to a broad diversity of stresses (Park and Seo 2015). In eukaryotic and prokaryotic cells, HSPs are essential parts contributing to cellular homeostasis under detrimental and optimal growth situations (Wang et al. 2004; Lindquist and Craig 1988). Also, HSPs have the main role in protein folding, translocation, assembly, and depression during normal cellular development and growth (Lindquist and Craig 1988; Wang et al. 2004). Furthermore, under stress conditions, HSPs play a role in proteins’ stabilization and assist refolding of proteins (Hüttner and Strasser 2012; Sitia and Braakman 2003). In animals and plants, there are five main families of HSPs, such as small HSP (sHSP), HSP60, HSP70, HSP90, and HSP100 (Wang et al. 2004; Gupta et al. 2010; Kotak et al. 2007). 70 kDa heat shock proteins or Hsp70 s function as molecular chaperones involved in fundamental cellular processes, such as protein transportation and folding (Latijnhouwers et al. 2010). Also, Hsp70s play a role in the cell’s reaction to a diverse range of stress situations (Latijnhouwers et al. 2010). In plants, the protein involved in chaperone is represented by Stromal 70 kDa heat shock-related protein (Latijnhouwers et al. 2010), and one mechanism to obtain stress resistance is to synthesize Hsp70 as a response to a stressor factor (Lyytinen et al. 2012; Yocum 2001).
Unknown Function Proteins
Unknown (Hypothetical) Proteins
5 proteins in UCB-1 pistachio rootstock were found without any function by our results. Uncharacterized protein LOC107620721, PREDICTED: uncharacterized protein LOC105974023, PREDICTED: uncharacterized protein LOC109219975 partial, PREDICTED: uncharacterized protein LOC109178037, and hypothetical protein CISIN_1g0462972 mg were up-regulated 1.85-, 2.95-, 2.29-, 1.85-, and 6.05-fold, respectively.
Conclusion
According to our results, MDA content increased at 100 mM NaCl compared to normal condition, while it decreased at 200 mM in comparison with the 100 mM. Decreasing content of MOD at 200 mM NaCl compared to the 100 mM in UCB-1 pistachio rootstock can indicate its adaptation ability in response to salinity stress. Based on our results, GPX, SOD, and CAT enzymes activity increased with increasing concentrations of salinity stress in all samples. Likely, increasing activity of GPX, SOD, and CAT enzymes occurred to scavenge produced ROS under salinity stress, and most likely, these enzymes have a fundamental role in protecting the UCB-1 pistachio rootstock under unfavorable conditions of salinity stress. Proteins play a main role in the response of plant stress which leads to stress-adapted reactions. Differential expression of 25 salt stress responsive proteins in this study reveals the considerable effect of salt stress on the leaf proteome of tolerant UCB-1 pistachio rootstock. In the tolerant UCB-1 pistachio rootstock, proteins involved in photosynthesis including 50S ribosomal protein L13, ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) large subunit, ribulose bisphosphate carboxylase small chain, ribulose bisphosphate carboxylase/oxygenase activase 1 and phosphoribulokinase assigned themselves the highest amount of protein expression variations in response to 100 mM NaCl. These results show that proteins involved in the photosynthesis can be considered as biomarkers in the breeding programs to increase the tolerance of other pistachio rootstocks to salinity stress. Also, these proteins are probably of particular significance in the adaptation of UCB-1 pistachio rootstock to salinity stress conditions. In addition to these results, the changes in proteomic profile observed in UCB-1 pistachio rootstock induced by 100 mM NaCl suggest the presence of a sophisticated antioxidant defense system that fine-tunes and regulates the activities of different enzymes to retain ROS homeostasis. Ribonucleoside-diphosphate reductase small chain, polcalcin Phl p 7-like and Golgin subfamily A member 5 are three proteins that were detected for the first time in response to abiotic stresses, and most likely, they are the key proteins in response of this rootstock to salinity and likely participate in plant resistance to NaCl stress. Based on our results, 5 unknown (hypothetical) proteins were found in the UCB-1 pistachio rootstock, which would be very valuable proteins in response to salinity stress. These proteins should be further studied and other different bioinformatics surveys are needed to specify the function of these unknown proteins. The proteins identified in this research provide new information regarding the tolerance of UCB-1 pistachio rootstock to salinity stress.
References
Abbaspour H, Saeidi-Sar S, Afshari H, Abdel-Wahhab MA (2012) Tolerance of Mycorrhiza infected Pistachio (Pistacia vera L.) seedling to drought stress under glasshouse conditions. J Plant Physiol 169(7):704–709. https://doi.org/10.1016/j.jplph.2012.01.014
Abogadallah GM (2010) Insights into the significance of antioxidative defense under salt stress. Plant Signal Behav 5(4):369–374. https://doi.org/10.4161/psb.5.4.10873
Aebi H (1984) Catalase in vitro. In: Methods in enzymology, vol 105. Elsevier, New York, pp 121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Agrawal GK, Pedreschi R, Barkla BJ, Bindschedler LV, Cramer R, Sarkar A, Renaut J, Job D, Rakwal R (2012) Translational plant proteomics: A perspective. J Proteomics 75(15):4588–4601. https://doi.org/10.1016/j.jprot.2012.03.055
Ahmad R, Ferguson L, Southwick SM (2005) Molecular marker analyses of pistachio rootstocks by simple sequence repeats and sequence-related amplified polymorphisms. J Hortic Sci Biotechnol 80(3):382–386. https://doi.org/10.1080/14620316.2005.11511948
Ahmad P, Abdel Latef AA, Hashem A, Abd Allah EF, Gucel S, Tran L-SP (2016) Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front Plant Sci 7:347–347. https://doi.org/10.3389/fpls.2016.00347
Ahmad P, Ahanger MA, Alyemeni MN, Wijaya L, Egamberdieva D, Bhardwaj R, Ashraf M (2017) Zinc application mitigates the adverse effects of NaCl stress on mustard [Brassica juncea (L.) Czern & Coss] through modulating compatible organic solutes, antioxidant enzymes, and flavonoid content. J Plant Interact 12(1):429–437. https://doi.org/10.1080/17429145.2017.1385867
Ahmad P, Abass Ahanger M, Nasser Alyemeni M, Wijaya L, Alam P, Ashraf M (2018) Mitigation of sodium chloride toxicity in Solanum lycopersicum L. by supplementation of jasmonic acid and nitric oxide. J Plant Interact 13(1):64–72. https://doi.org/10.1080/17429145.2017.1420830
Ahmad P, Ahanger MA, Alam P, Alyemeni MN, Wijaya L, Ali S, Ashraf M (2019) Silicon (Si) supplementation alleviates NaCl toxicity in Mung Bean [Vigna radiata (L.) Wilczek] through the modifications of physio-biochemical attributes and key antioxidant enzymes. J Plant Growth Regul 38(1):70–82. https://doi.org/10.1007/s00344-018-9810-2
Akbari M, Mahna N, Ramesh K, Bandehagh A, Mazzuca S (2018) Ion homeostasis, osmoregulation, and physiological changes in the roots and leaves of pistachio rootstocks in response to salinity. Protoplasma. https://doi.org/10.1007/s00709-018-1235-z
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639. https://doi.org/10.1146/annurev.arplant.50.1.601
Ashoub A, Beckhaus T, Berberich T, Karas M, Bruggemann W (2013) Comparative analysis of barley leaf proteome as affected by drought stress. Planta 237(3):771–781. https://doi.org/10.1007/s00425-012-1798-4
Ashraf M, Foolad M (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59(2):206–216. https://doi.org/10.1016/j.envexpbot.2005.12.006
Bandurska H, Niedziela J, Pietrowska-Borek M, Nuc K, Chadzinikolau T, Radzikowska D (2017) Regulation of proline biosynthesis and resistance to drought stress in two barley (Hordeum vulgare L.) genotypes of different origin. Plant Physiol Biochem (PPB) 118:427–437. https://doi.org/10.1016/j.plaphy.2017.07.006
Bano S, Ashraf M, Akram NA (2014) Salt stress regulates enzymatic and nonenzymatic antioxidative defense system in the edible part of carrot (Daucus carota L.). J Plant Interact 9(1):324–329. https://doi.org/10.1080/17429145.2013.832426
Barkla Bronwyn J, Vera-Estrella R, Pantoja O (2013) Progress and challenges for abiotic stress proteomics of crop plants. Proteomics 13(12–13):1801–1815. https://doi.org/10.1002/pmic.201200401
Beckers GJM, Conrath U (2007) Priming for stress resistance: from the lab to the field. Curr Opin Plant Biol 10(4):425–431. https://doi.org/10.1016/j.pbi.2007.06.002
Bela K, Horváth E, Gallé Á, Szabados L, Tari I, Csiszár J (2015) Plant glutathione peroxidases: emerging role of the antioxidant enzymes in plant development and stress responses. J Plant Physiol 176:192–201. https://doi.org/10.1016/j.jplph.2014.12.014
Bhat JY, Miličić G, Thieulin-Pardo G, Bracher A, Maxwell A, Ciniawsky S, Mueller-Cajar O, Engen JR, Hartl FU, Wendler P (2017) Mechanism of enzyme repair by the AAA + chaperone Rubisco activase. Mol Cell 67(5):744–756.e746. https://doi.org/10.1016/j.molcel.2017.07.004
Bohnert HJ, Sheveleva E (1998) Plant stress adaptations–making metabolism move. Curr Opin Plant Biol 1(3):267–274. https://doi.org/10.1016/S1369-5266(98)80115-5
Boston RS, Viitanen PV, Vierling E (1996) Molecular chaperones and protein folding in plants. Plant Mol Biol 32(1):191–222. https://doi.org/10.1007/BF00039383
Bozorgi M, Memariani Z, Mobli M, Salehi Surmaghi MH, Shams-Ardekani MR, Rahimi R (2013) Five Pistacia species (P. vera, P. atlantica, P. terebinthus, P. khinjuk, and P. lentiscus): a review of their traditional uses, phytochemistry, and pharmacology. Sci World J 2013:219815. https://doi.org/10.1155/2013/219815
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Budak H, Akpinar BA, Unver T, Turktas M (2013) Proteome changes in wild and modern wheat leaves upon drought stress by two-dimensional electrophoresis and nanoLC-ESI-MS/MS. Plant Mol Biol 83(1–2):89–103. https://doi.org/10.1007/s11103-013-0024-5
Capriotti AL, Borrelli GM, Colapicchioni V, Papa R, Piovesana S, Samperi R, Stampachiacchiere S, Lagana A (2014) Proteomic study of a tolerant genotype of durum wheat under salt-stress conditions. Anal Bioanal Chem 406(5):1423–1435. https://doi.org/10.1007/s00216-013-7549-y
Carpita NC, Gibeaut DM (1993) Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J 3(1):1–30. https://doi.org/10.1111/j.1365-313X.1993.tb00007.x
Cavalcanti FR, Oliveira JTA, Martins-Miranda AS, Viégas RA, Silveira JAG (2004) Superoxide dismutase, catalase and peroxidase activities do not confer protection against oxidative damage in salt-stressed cowpea leaves. New Phytol 163(3):563–571. https://doi.org/10.1111/j.1469-8137.2004.01139.x
Cazzonelli CI, Cuttriss AJ, Cossetto SB, Pye W, Crisp P, Whelan J, Finnegan EJ, Turnbull C, Pogson BJ (2009) Regulation of carotenoid composition and shoot branching in Arabidopsis by a chromatin modifying histone methyltransferase, SDG8. Plant Cell 21(1):39–53. https://doi.org/10.1105/tpc.108.063131
Chawla S, Jain S, Jain V (2013) Salinity induced oxidative stress and antioxidant system in salt-tolerant and salt-sensitive cultivars of rice (Oryza sativa L.). J Plant Biochem Biotechnol 22(1):27–34. https://doi.org/10.1007/s13562-012-0107-4
Chelli-Chaabouni A, Mosbah AB, Maalej M, Gargouri K, Gargouri-Bouzid R, Drira N (2010) In vitro salinity tolerance of two pistachio rootstocks: Pistacia vera L. and P. atlantica Desf. Environ Exp Bot 69(3):302–312. https://doi.org/10.1016/j.envexpbot.2010.05.010
Chen SL, Kao CH (1995) Cd induced changes in proline level and peroxidase activity in roots of rice seedlings. Plant Growth Regul 17(1):67–71. https://doi.org/10.1007/BF00024497
Chen S, Gollop N, Heuer B (2009) Proteomic analysis of salt-stressed tomato (Solanum lycopersicum) seedlings: effect of genotype and exogenous application of glycinebetaine. J Exp Bot 60(7):2005–2019. https://doi.org/10.1093/jxb/erp075
Colla G, Rouphael Y, Cardarelli M, Temperini O, Rea E, Salerno A, Pierandrei F (2006) Influence of grafting on yield and fruit quality of pepper (Capsicum annuum L.) grown under greenhouse conditions. In: IV International symposium on seed, transplant and stand establishment of horticultural crops; translating seed and seedling, vol 782, pp 359–364. https://doi.org/10.17660/actahortic.2008.782.45
Cramer GR (2002) Sodium-calcium interactions under salinity stress. In: Läuchli A, Lüttge U (eds) Salinity: environment—plants—molecules. Springer, Dordrecht, pp 205–227. https://doi.org/10.1007/0-306-48155-3_10
Cruz RT, Jordan WR, Drew MC (1992) Structural changes and associated reduction of hydraulic conductance in roots of Sorghum bicolor L. following exposure to water deficit. Plant Physiol 99(1):203–212. https://doi.org/10.1104/pp.99.1.203
Cuartero J, Bolarin MC, Asins MJ, Moreno V (2006) Increasing salt tolerance in the tomato. J Exp Bot 57(5):1045–1058. https://doi.org/10.1093/jxb/erj102
Deeba F, Pandey AK, Ranjan S, Mishra A, Singh R, Sharma YK, Shirke PA, Pandey V (2012) Physiological and proteomic responses of cotton (Gossypium herbaceum L.) to drought stress. Plant Physiol Biochem (PPB) 53:6–18. https://doi.org/10.1016/j.plaphy.2012.01.002
Demidchik V, Tester M (2002) Sodium fluxes through nonselective cation channels in the plasma membrane of protoplasts from Arabidopsis roots. Plant Physiol 128(2):379–387. https://doi.org/10.1104/pp.010524
Demmig-Adams B, Adams WW 3rd (2002) Antioxidants in photosynthesis and human nutrition. Science 298(5601):2149–2153. https://doi.org/10.1126/science.1078002
Deruere J, Romer S, d’Harlingue A, Backhaus RA, Kuntz M, Camara B (1994) Fibril assembly and carotenoid overaccumulation in chromoplasts: a model for supramolecular lipoprotein structures. Plant Cell 6(1):119–133. https://doi.org/10.1105/tpc.6.1.119
Dhindsa RS, Matowe W (1981) Drought tolerance in two mosses: correlated with enzymatic defence against lipid peroxidation. J Exp Bot 32(1):79–91. https://doi.org/10.1093/jxb/32.1.79
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135(1):1–9. https://doi.org/10.1016/S0168-9452(98)00025-9
Epstein L, Beede R, Kaur S, Ferguson L (2004) Rootstock effects on pistachio trees grown in Verticillium dahliae-infested soil. Phytopathology 94(4):388–395. https://doi.org/10.1094/phyto.2004.94.4.388
Espelie KE, Franceschi VR, Kolattukudy PE (1986) Immunocytochemical localization and time course of appearance of an anionic peroxidase associated with suberization in wound-healing potato tuber tissue. Plant Physiol 81(2):487–492
Estan MT, Martinez-Rodriguez MM, Perez-Alfocea F, Flowers TJ, Bolarin MC (2005) Grafting raises the salt tolerance of tomato through limiting the transport of sodium and chloride to the shoot. J Exp Bot 56(412):703–712. https://doi.org/10.1093/jxb/eri027
Fan M, Bie Z, Krumbein A, Schwarz D (2011) Salinity stress in tomatoes can be alleviated by grafting and potassium depending on the rootstock and K-concentration employed. Sci Hortic 130(3):615–623. https://doi.org/10.1016/j.scienta.2011.08.018
Fatehi F, Hosseinzadeh A, Alizadeh H, Brimavandi T, Struik PC (2012) The proteome response of salt-resistant and salt-sensitive barley genotypes to long-term salinity stress. Mol Biol Rep 39(5):6387–6397. https://doi.org/10.1007/s11033-012-1460-z
Ferguson L, Poss J, Grattan S, Grieve C, Wang D, Wilson C, Donovan T, Chao C-T (2002) Pistachio rootstocks influence scion growth and ion relations under salinity and boron stress. J Am Soc Hortic Sci 127(2):194–199
Flores FB, Sanchez-Bel P, Estañ MT, Martinez-Rodriguez MM, Moyano E, Morales B, Campos JF, Garcia-Abellán JO, Egea MI, Fernández-Garcia N, Romojaro F, Bolarín MC (2010) The effectiveness of grafting to improve tomato fruit quality. Sci Hortic 125(3):211–217. https://doi.org/10.1016/j.scienta.2010.03.026
Foyer CH, Shigeoka S (2011) Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol 155(1):93. https://doi.org/10.1104/pp.110.166181
Foyer CH, Lopez-Delgado H, Dat JF, Scott IM (1997) Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiol Plant 100(2):241–254. https://doi.org/10.1111/j.1399-3054.1997.tb04780.x
Fuentes P, Pizarro L, Moreno JC, Handford M, Rodriguez-Concepcion M, Stange C (2012) Light-dependent changes in plastid differentiation influence carotenoid gene expression and accumulation in carrot roots. Plant Mol Biol 79(1–2):47–59. https://doi.org/10.1007/s11103-012-9893-2
Gardiner MG, Cleland R (1974) Peroxidase changes during the cessation of elongation in Pisum sativum stems. Phytochemistry. https://doi.org/10.1016/0031-9422(74)80081-6
Genc Y, Mcdonald GK, Tester M (2007) Reassessment of tissue Na+ concentration as a criterion for salinity tolerance in bread wheat. Plant Cell Environ 30(11):1486–1498. https://doi.org/10.1111/j.1365-3040.2007.01726.x
Gifford JL, Walsh MP, Vogel HJ (2007) Structures and metal-ion-binding properties of the Ca2+ -binding helix-loop-helix EF-hand motifs. Biochem J 405(2):199–221. https://doi.org/10.1042/bj20070255
Gillingham AK, Pfeifer AC, Munro S, Drubin D (2002) CASP, the alternatively spliced product of the gene encoding the CCAAT-displacement protein transcription factor, is a golgi membrane protein related to giantin. Mol Biol Cell 13(11):3761–3774. https://doi.org/10.1091/mbc.e02-06-0349
Grabarek Z (2006) Structural basis for diversity of the EF-hand calcium-binding proteins. J Mol Biol 359(3):509–525. https://doi.org/10.1016/j.jmb.2006.03.066
Grene R (2002) Oxidative stress and acclimation mechanisms in plants. Arabidopsis Book/American Society of Plant Biologists 1:e0036. https://doi.org/10.1199/tab.0036.1
Grote M, Westritschnig K, Valenta R (2008) Immunogold electron microscopic localization of the 2 EF-hand calcium-binding pollen allergen Phl p 7 and its homologues in pollens of grasses, weeds and trees. Int Arch Allergy Immunol 146(2):113–121. https://doi.org/10.1159/000113514
Guo B, Luan H, Lin S, Lv C, Zhang X, Xu R (2016) Comparative proteomic analysis of two barley cultivars (Hordeum vulgare L.) with contrasting grain protein content. Front Plant Sci. https://doi.org/10.3389/fpls.2016.00542
Guo R, Qiao H, Zhao J, Wang X, Tu M, Guo C, Wan R, Li Z, Wang X (2018) The grape VlWRKY3 gene promotes abiotic and biotic stress tolerance in transgenic Arabidopsis thaliana. Front Plant Sci 9:545–545. https://doi.org/10.3389/fpls.2018.00545
Gupta SC, Sharma A, Mishra M, Mishra RK, Chowdhuri DK (2010) Heat shock proteins in toxicology: how close and how far? Life Sci 86(11–12):377–384. https://doi.org/10.1016/j.lfs.2009.12.015
Hajiboland R, Norouzi F, Poschenrieder C (2014) Growth, physiological, biochemical and ionic responses of pistachio seedlings to mild and high salinity. Trees 28(4):1065–1078. https://doi.org/10.1007/s00468-014-1018-x
He Y, Zhu Z, Yang J, Ni X, Zhu B (2009) Grafting increases the salt tolerance of tomato by improvement of photosynthesis and enhancement of antioxidant enzymes activity. Environ Exp Bot 66(2):270–278. https://doi.org/10.1016/j.envexpbot.2009.02.007
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125(1):189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Heidari M, Jamshidi P (2011) Effects of salinity and potassium application on antioxidant enzyme activities and physiological parameters in pearl millet. Agric Sci China 10(2):228–237. https://doi.org/10.1016/S1671-2927(09)60309-6
Hernandez JA, Almansa MS (2002) Short-term effects of salt stress on antioxidant systems and leaf water relations of pea leaves. Physiol Plant 115(2):251–257. https://doi.org/10.1034/j.1399-3054.2002.1150211.x
HernÁNdez JA, Del RÍO LA, Sevilla F (1994) Salt stress-induced changes in superoxide dismutase isozymes in leaves and mesophyll protoplasts from Vigna unguiculata (L.) Walp. N Phytol 126(1):37–44. https://doi.org/10.1111/j.1469-8137.1994.tb07527.x
Hernández JA, Jiménez A, Mullineaux P, Sevilia F (2001) Tolerance of pea (Pisum sativum L.) to long-term salt stress is associated with induction of antioxidant defences. Plant Cell Environ 23(8):853–862. https://doi.org/10.1046/j.1365-3040.2000.00602.x
Hu H, Dai M, Yao J, Xiao B, Li X, Zhang Q, Xiong L (2006) Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc Natl Acad Sci 103(35):12987. https://doi.org/10.1073/pnas.0604882103
Huang M, Zhou Z, Elledge SJ (1998) The DNA replication and damage checkpoint pathways induce transcription by inhibition of the Crt1 repressor. Cell 94(5):595–605. https://doi.org/10.1016/S0092-8674(00)81601-3
Huang Y, Bie ZL, Liu ZX, Zhen A, Jiao XR (2011) Improving cucumber photosynthetic capacity under NaCl stress by grafting onto two salt-tolerant pumpkin rootstocks. Biol Plant 55(2):285–290. https://doi.org/10.1007/s10535-011-0040-8
Hurkman WJ, Tanaka CK (1986) Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol 81(3):802–806. https://doi.org/10.1104/pp.81.3.802
Hüttner S, Strasser R (2012) Endoplasmic reticulum-associated degradation of glycoproteins in plants. Front Plant Sci 3:67. https://doi.org/10.3389/fpls.2012.00067
Ichikawa M, Nakai Y, Arima K, Nishiyama S, Hirano T, Sato MH (2015) A VAMP-associated protein, PVA31 is involved in leaf senescence in Arabidopsis. Plant Signal Behav 10(3):e990847–e990847. https://doi.org/10.4161/15592324.2014.990847
Iraki NM, Bressan RA, Hasegawa PM, Carpita NC (1989) Alteration of the physical and chemical structure of the primary cell wall of growth-limited plant cells adapted to osmotic stress. Plant Physiol 91(1):39–47
Jalali-e-Emam SMS, Alizadeh B, Zaefizadeh M, Zakarya RA, Khayatnezhad M (2011) Superoxide dismutase (SOD) activity in NaCl stress in salt-sensitive and salt-tolerance genotypes of Colza (Brassica napus L.). Middle East J Sci Res 7:7–11
Jia W, Wang Y, Zhang S, Zhang J (2002) Salt-stress-induced ABA accumulation is more sensitively triggered in roots than in shoots. J Exp Bot 53(378):2201–2206. https://doi.org/10.1093/jxb/erf079
Kang JS, Frank J, Kang CH, Kajiura H, Vikram M, Ueda A, Kim S, Bahk JD, Triplett B, Fujiyama K, Lee SY, von Schaewen A, Koiwa H (2008) Salt tolerance of Arabidopsis thaliana requires maturation of N-glycosylated proteins in the Golgi apparatus. Proc Natl Acad Sci USA 105(15):5933–5938. https://doi.org/10.1073/pnas.0800237105
Kim DW, Rakwal R, Agrawal GK, Jung YH, Shibato J, Jwa NS, Iwahashi Y, Iwahashi H, Kim DH, Shim Ie S, Usui K (2005a) A hydroponic rice seedling culture model system for investigating proteome of salt stress in rice leaf. Electrophoresis 26(23):4521–4539. https://doi.org/10.1002/elps.200500334
Kim SY, Lim JH, Park MR, Kim YJ, Park TI, Seo YW, Choi KG, Yun SJ (2005b) Enhanced antioxidant enzymes are associated with reduced hydrogen peroxide in barley roots under saline stress. J Biochem Mol Biol 38(2):218–224
Kolberg M, Strand KR, Graff P, Andersson KK (2004) Structure, function, and mechanism of ribonucleotide reductases. Biochem Biophys Acta 1699(1–2):1–34. https://doi.org/10.1016/j.bbapap.2004.02.007
Kotak S, Larkindale J, Lee U, von Koskull-Döring P, Vierling E, Scharf K-D (2007) Complexity of the heat stress response in plants. Curr Opin Plant Biol 10(3):310–316. https://doi.org/10.1016/j.pbi.2007.04.011
Kretsinger RH, Wasserman RH (1980) Structure and evolution of calcium-modulated protein. Crit Rev Biochem 8(2):119–174. https://doi.org/10.3109/10409238009105467
Kumar S, Awasthi OP, Dubey AK, Pandey R, Sharma VK, Mishra AK, Sharma RM (2018) Root morphology and the effect of rootstocks on leaf nutrient acquisition of Kinnow mandarin (Citrus nobilis Loureiro × Citrus reticulata Blanco). J Hortic Sci Biotechnol 93(1):100–106. https://doi.org/10.1080/14620316.2017.1345333
Kwak S-S, Kim S-K, Lee M-S, Jung K-H, Park I-H, Liu J-R (1995) Acidic peroxidases from suspension-cultures of sweet potato. Phytochemistry 39(5):981–984. https://doi.org/10.1016/0031-9422(95)00098-R
Kwon C, Neu C, Pajonk S, Yun HS, Lipka U, Humphry M, Bau S, Straus M, Kwaaitaal M, Rampelt H (2008) Co-option of a default secretory pathway for plant immune responses. Nature 451(7180):835. https://doi.org/10.1038/nature06545
Lakra N, Kaur C, Anwar K, Singla-Pareek SL, Pareek A (2018) Proteomics of contrasting rice genotypes: Identification of potential targets for raising crops for saline environment. Plant Cell Environ 41(5):947–969. https://doi.org/10.1111/pce.12946
Latijnhouwers M, Xu X-M, Møller SG (2010) Arabidopsis stromal 70-kDa heat shock proteins are essential for chloroplast development. Planta 232(3):567–578. https://doi.org/10.1007/s00425-010-1192-z
Ledesma A, Villalba M, Batanero E, Rodriguez R (1998) Molecular cloning and expression of active Ole e 3, a major allergen from olive-tree pollen and member of a novel family of Ca2 + -binding proteins (polcalcins) involved in allergy. Eur J Biochem 258(2):454–459. https://doi.org/10.1046/j.1432-1327.1998.2580454.x
Lee T-M, Lin Y-H (1995) Changes in soluble and cell wall-bound peroxidase activities with growth in anoxia-treated rice (Oryza sativa L.) coleoptiles and roots. Plant Sci 106(1):1–7. https://doi.org/10.1016/0168-9452(94)04053-J
Lee EK, Kwon M, Ko JH, Yi H, Hwang MG, Chang S, Cho MH (2004) Binding of sulfonylurea by AtMRP5, an Arabidopsis multidrug resistance-related protein that functions in salt tolerance. Plant Physiol 134(1):528–538. https://doi.org/10.1104/pp.103.027045
Lee SJ, Kang JY, Park HJ, Kim MD, Bae MS, Choi HI, Kim SY (2010) DREB2C interacts with ABF2, a bZIP protein regulating abscisic acid-responsive gene expression, and its overexpression affects abscisic acid sensitivity. Plant Physiol 153(2):716–727. https://doi.org/10.1104/pp.110.154617
Leshem Y, Melamed-Book N, Cagnac O, Ronen G, Nishri Y, Solomon M, Cohen G, Levine A (2006) Suppression of Arabidopsis vesicle-SNARE expression inhibited fusion of H2O2-containing vesicles with tonoplast and increased salt tolerance. Proc Natl Acad Sci USA 103(47):18008–18013
Li X, Xing J, Gianfagna TJ, Janes HW (2002) Sucrose regulation of ADP-glucose pyrophosphorylase subunit genes transcript levels in leaves and fruits. Plant Sci 162(2):239–244. https://doi.org/10.1016/S0168-9452(01)00565-9
Li F, Tsfadia O, Wurtzel ET (2009) The phytoene synthase gene family in the grasses: subfunctionalization provides tissue-specific control of carotenogenesis. Plant Signal Behav 4(3):208–211. https://doi.org/10.4161/psb.4.3.7798
Li W, Zhao F, Fang W, Xie D, Hou J, Yang X, Zhao Y, Tang Z, Nie L, Lv S (2015) Identification of early salt stress responsive proteins in seedling roots of upland cotton (Gossypium hirsutum L.) employing iTRAQ-based proteomic technique. Front Plant Sci 6:732. https://doi.org/10.3389/fpls.2015.00732
Lindquist S, Craig E (1988) The heat-shock proteins. Annu Rev Genet 22(1):631–677. https://doi.org/10.1146/annurev.ge.22.120188.003215
Liu Z, Bie Z, Huang Y, Zhen A, Niu M, Lei B (2013) Rootstocks improve cucumber photosynthesis through nitrogen metabolism regulation under salt stress. Acta Physiol Plant 35(7):2259–2267. https://doi.org/10.1007/s11738-013-1262-5
Lounifi I, Arc E, Molassiotis A, Job D, Rajjou L, Tanou G (2013) Interplay between protein carbonylation and nitrosylation in plants. Proteomics 13(3–4):568–578. https://doi.org/10.1002/pmic.201200304
Lyytinen A, Mappes J, Lindström L (2012) Variation in Hsp70 levels after cold shock: signs of evolutionary responses to thermal selection among Leptinotarsa decemlineata populations. PLoS ONE 7(2):e31446. https://doi.org/10.1371/journal.pone.0031446
MalhÓ R, Camacho L, Moutinho A (2000) Signalling pathways in pollen tube growth and reorientation. Ann Bot 85:59–68. https://doi.org/10.1006/anbo.1999.0991
Mansour MMF, Salama KHA (2004) Cellular basis of salinity tolerance in plants. Environ Exp Bot 52(2):113–122. https://doi.org/10.1016/j.envexpbot.2004.01.009
Massai R, Remorini D, Tattini M (2004) Gas exchange, water relations and osmotic adjustment in two scion/rootstock combinations of Prunus under various salinity concentrations. Plant Soil 259(1):153–162. https://doi.org/10.1023/B:PLSO.0000020954.71828.13
McCord JM, Fridovich I (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244(22):6049–6055
Mehlhorn H, Lelandais M, Korth HG, Foyer CH (1996) Ascorbate is the natural substrate for plant peroxidases. FEBS Lett 378(3):203–206. https://doi.org/10.1016/0014-5793(95)01448-9
Meier S, Tzfadia O, Vallabhaneni R, Gehring C, Wurtzel ET (2011) A transcriptional analysis of carotenoid, chlorophyll and plastidial isoprenoid biosynthesis genes during development and osmotic stress responses in Arabidopsis thaliana. BMC Syst Biol 5:77. https://doi.org/10.1186/1752-0509-5-77
Mittova V, Guy M, Tal M, Volokita M (2004) Salinity up-regulates the antioxidative system in root mitochondria and peroxisomes of the wild salt-tolerant tomato species Lycopersicon pennellii. J Exp Bot 55(399):1105–1113. https://doi.org/10.1093/jxb/erh113
Miziorko HM (2000) Phosphoribulokinase: current perspectives on the structure/function basis for regulation and catalysis. Adv Enzymol Relat Areas Mol Biol 74:95–127. https://doi.org/10.1002/9780470123201.ch3
Moazzam Jazi M, Ghadirzadeh Khorzoghi E, Botanga C, Seyedi SM (2016) Identification of reference genes for quantitative gene expression studies in a non-model tree pistachio (Pistacia vera L.). PloS ONE 11(6):e0157467. https://doi.org/10.1371/journal.pone.0157467
Moazzzam Jazi M, Seyedi SM, Ebrahimie E, Ebrahimi M, De Moro G, Botanga C (2017) A genome-wide transcriptome map of pistachio (Pistacia vera L.) provides novel insights into salinity-related genes and marker discovery. BMC Genomics 18(1):627. https://doi.org/10.1186/s12864-017-3989-7
Mohanta TK, Occhipinti A, Zebelo SA, Foti M, Fliegmann J, Bossi S, Maffei ME, Bertea CM (2012) Ginkgo biloba responds to herbivory by activating early signaling and direct defenses. PLoS ONE 7(3):e32822. https://doi.org/10.1371/journal.pone.0032822
Moin M, Bakshi A, Saha A, Dutta M, Madhav SM, Kirti PB (2016) Rice ribosomal protein large subunit genes and their spatio-temporal and stress regulation. Front Plant Sci 7:1284. https://doi.org/10.3389/fpls.2016.01284
Molassiotis A, Fotopoulos V (2011) Oxidative and nitrosative signaling in plants: two branches in the same tree? Plant Signal Behav 6(2):210–214. https://doi.org/10.4161/psb.6.2.14878
Molassiotis AN, Sotiropoulos T, Tanou G, Kofidis G, Diamantidis G, Therios E (2006) Antioxidant and anatomical responses in shoot culture of the apple rootstock MM 106 treated with NaCl, KCl, mannitol or sorbitol. Biol Plant 50(1):61–68. https://doi.org/10.1007/s10535-005-0075-9
Molassiotis A, Tanou G, Filippou P, Fotopoulos V (2013) Proteomics in the fruit tree science arena: new insights into fruit defense, development, and ripening. Proteomics 13(12–13):1871–1884. https://doi.org/10.1002/pmic.201200428
Molassiotis A, Job D, Ziogas V, Tanou G (2016) Citrus plants: a model system for unlocking the secrets of NO and ROS-inspired priming against salinity and drought. Front Plant Sci. https://doi.org/10.3389/fpls.2016.00229
Moloi MJ, Mwenye OJ, Van der Merwe R (2016) Differential involvement of ascorbate and guaiacol peroxidases in soybean drought resistance. South Afr J Sci 112(9–10):1–4. https://doi.org/10.17159/sajs.2016/20160028
Moreau A, Yotov WV, Glorieux FH, St-Arnaud R (1998) Bone-specific expression of the alpha chain of the nascent polypeptide-associated complex, a coactivator potentiating c-Jun-mediated transcription. Mol Cell Biol 18(3):1312–1321. https://doi.org/10.1128/mcb.18.3.1312
Morgan D, Epstein L, Ferguson L (1992) Verticillium wilt resistance in pistachio rootstock cultivars: assays and an assessment of two interspecific hybrids. Plant Dis 76(3):310–313. https://doi.org/10.1094/PD-76-0310
Moriana A, Memmi H, Centeno A, Martín-Palomo MJ, Corell M, Torrecillas A, Pérez-López D (2018) Influence of rootstock on pistachio (Pistacia vera L. cv Kerman) water relations. Agric Water Manag 202:263–270. https://doi.org/10.1016/j.agwat.2017.12.026
Mostek A, Börner A, Badowiec A, Weidner S (2015) Alterations in root proteome of salt-sensitive and tolerant barley lines under salt stress conditions. J Plant Physiol 174:166–176. https://doi.org/10.1016/j.jplph.2014.08.020
Moya JL, Primo-Millo E, Talon M (2002) Morphological factors determining salt tolerance in citrus seedlings: the shoot to root ratio modulates passive root uptake of chloride ions and their accumulation in leaves. Plant Cell Environ 22(11):1425–1433. https://doi.org/10.1046/j.1365-3040.1999.00495.x
Munro S (2011) The golgin coiled-coil proteins of the Golgi apparatus. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a005256
Nazari M, Moosavi SS, Maleki M (2018) Morpho-physiological and proteomic responses of Aegilops tauschii to imposed moisture stress. Plant Physiol Biochem 132:445–452
Negrel J, Lherminier J (1987) Peroxidase-mediated integration of tyramine into xylem cell walls of tobacco leaves. Planta 172(4):494–501. https://doi.org/10.1007/BF00393865
Ngara R, Ndimba R, Borch-Jensen J, Jensen ON, Ndimba B (2012) Identification and profiling of salinity stress-responsive proteins in Sorghum bicolor seedlings. J Proteomics 75(13):4139–4150. https://doi.org/10.1016/j.jprot.2012.05.038
Park C-J, Seo Y-S (2015) Heat shock proteins: a review of the molecular chaperones for plant immunity. Plant Pathol J 31(4):323–333. https://doi.org/10.5423/PPJ.RW.08.2015.0150
Parker R, Flowers TJ, Moore AL, Harpham NV (2006) An accurate and reproducible method for proteome profiling of the effects of salt stress in the rice leaf lamina. J Exp Bot 57(5):1109–1118. https://doi.org/10.1093/jxb/erj134
Parry MAJ, Andralojc PJ, Khan S, Lea PJ, Keys AJ (2002) Rubisco activity: effects of drought stress. Ann Bot 89(7):833–839. https://doi.org/10.1093/aob/mcf103
Passamani LZ, Barbosa RR, Reis RS, Heringer AS, Rangel PL, Santa-Catarina C, Grativol C, Veiga CFM, Souza-Filho GA, Silveira V (2017) Salt stress induces changes in the proteomic profile of micropropagated sugarcane shoots. PLoS ONE 12(4):e0176076. https://doi.org/10.1371/journal.pone.0176076
Penella C, Nebauer SG, Bautista AS, Lopez-Galarza S, Calatayud A (2014) Rootstock alleviates PEG-induced water stress in grafted pepper seedlings: physiological responses. J Plant Physiol 171(10):842–851. https://doi.org/10.1016/j.jplph.2014.01.013
Peng Z, Wang M, Li F, Lv H, Li C, Xia G (2009) A proteomic study of the response to salinity and drought stress in an introgression strain of bread wheat. Mol Cell Proteomics (MCP) 8(12):2676–2686. https://doi.org/10.1074/mcp.M900052-MCP200
Peng Z, He S, Gong W, Sun J, Pan Z, Xu F, Lu Y, Du X (2014) Comprehensive analysis of differentially expressed genes and transcriptional regulation induced by salt stress in two contrasting cotton genotypes. BMC Genomics 15(1):760. https://doi.org/10.1186/1471-2164-15-760
Pichersky E, Bernatzky R, Tanksley SD, Cashmore AR (1986) Evidence for selection as a mechanism in the concerted evolution of Lycopersicon esculentum (tomato) genes encoding the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase. Proc Natl Acad Sci USA 83(11):3880–3884. https://doi.org/10.1073/pnas.83.11.3880
Plewa MJ, Smith SR, Wagner ED (1991) Diethyldithiocarbamate suppresses the plant activation of aromatic amines into mutagens by inhibiting tobacco cell peroxidase. Mutat Res/Fund Mol Mech Mutagen 247(1):57–64. https://doi.org/10.1016/0027-5107(91)90033-K
Polle A, Otter T, Seifert F (1994) Apoplastic peroxidases and lignification in needles of Norway spruce (Picea abies L.). Plant Physiol 106(1):53–60. https://doi.org/10.1104/pp.106.1.53
Portis AR Jr (2003) Rubisco activase—Rubisco’s catalytic chaperone. Photosynth Res 75(1):11–27. https://doi.org/10.1023/a:1022458108678
Qu C-P, Xu Z-R, Liu G-J, Liu C, Li Y, Wei Z-G, Liu G-F (2010) Differential expression of copper-zinc superoxide dismutase gene of Polygonum sibiricum leaves, stems and underground stems, subjected to high-salt stress. Int J Mol Sci 11(12):5234–5245. https://doi.org/10.3390/ijms11125234
Rahnama H, Ebrahimzadeh H (2005) The effect of NaCl on antioxidant enzyme activities in potato seedlings. Biol Plant 49(1):93–97. https://doi.org/10.1007/s10535-005-3097-4
Rahneshan Z, Nasibi F, Moghadam AA (2018) Effects of salinity stress on some growth, physiological, biochemical parameters and nutrients in two pistachio (Pistacia vera L.) rootstocks. J Plant Interact 13(1):73–82. https://doi.org/10.1080/17429145.2018.1424355
Raines CA (2011) Increasing photosynthetic carbon assimilation in C < sub > 3</sub > plants to improve crop yield: current and future strategies. Plant Physiol 155(1):36. https://doi.org/10.1104/pp.110.168559
Rezaee F, Lahouti M, Maleki M, Ganjeali A (2018) Comparative proteomics analysis of whitetop (Lepidium draba L.) seedlings in response to exogenous glucose. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2018.09.016
Rospert S, Dubaquié Y, Gautschi M (2002) Nascent-polypeptide-associated complex. Cell Mol Life Sci CMLS 59(10):1632–1639. https://doi.org/10.1007/PL00012490
Rozwadowski K, Zhao R, Jackman L, Huebert T, Burkhart WE, Hemmingsen SM, Greenwood J, Rothstein SJ (1999) Characterization and immunolocalization of a cytosolic calcium-binding protein from Brassica napus and Arabidopsis pollen. Plant Physiol 120(3):787–798. https://doi.org/10.1104/pp.120.3.787
Ruiz-Sola MÁ, Arbona V, Gómez-Cadenas A, Rodríguez-Concepción M, Rodríguez-Villalón A (2014) A root specific induction of carotenoid biosynthesis contributes to ABA production upon salt stress in arabidopsis. PLoS ONE 9(3):e90765. https://doi.org/10.1371/journal.pone.0090765
Salekdeh GH, Siopongco J, Wade LJ, Ghareyazie B, Bennett J (2002) Proteomic analysis of rice leaves during drought stress and recovery. Proteomics 2(9):1131–1145. https://doi.org/10.1002/1615-9861(200209)2:9%3c1131:AID-PROT1131%3e3.0.CO;2-1
Schwarz D, Rouphael Y, Colla G, Venema JH (2010) Grafting as a tool to improve tolerance of vegetables to abiotic stresses: thermal stress, water stress and organic pollutants. Sci Hortic 127(2):162–171. https://doi.org/10.1016/j.scienta.2010.09.016
Seki M, Ishida J, Narusaka M, Fujita M, Nanjo T, Umezawa T, Kamiya A, Nakajima M, Enju A, Sakurai T, Satou M, Akiyama K, Yamaguchi-Shinozaki K, Carninci P, Kawai J, Hayashizaki Y, Shinozaki K (2002) Monitoring the expression pattern of around 7,000 Arabidopsis genes under ABA treatments using a full-length cDNA microarray. Funct Integr Genomics 2(6):282–291. https://doi.org/10.1007/s10142-002-0070-6
Shabala S, Cuin TA (2008) Potassium transport and plant salt tolerance. Physiol Plant 133(4):651–669. https://doi.org/10.1111/j.1399-3054.2007.01008.x
Shahid MA, Balal RM, Khan N, Zotarelli L, Liu G, Ghazanfar MU, Rathinasabapathi B, Mattson NS, Martínez-Nicolas JJ, Garcia-Sanchez F (2018) Ploidy level of citrus rootstocks affects the carbon and nitrogen metabolism in the leaves of Chromium-stressed Kinnow mandarin plants. Environ Exp Bot 149:70–80. https://doi.org/10.1016/j.envexpbot.2018.02.010
Shi H, Chan Z-L (2014) AtHAP5A modulates freezing stress resistance in Arabidopsis independent of the CBF pathway. Plant Signal Behav 9(7):e29109–e29109. https://doi.org/10.4161/psb.29109
Simpson K, Fuentes P, Quiroz-Iturra LF, Flores-Ortiz C, Contreras R, Handford M, Stange C (2018) Unraveling the induction of phytoene synthase 2 expression by salt stress and abscisic acid in Daucus carota. J Exp Bot 69(16):4113–4126. https://doi.org/10.1093/jxb/ery207
Singh VK, Mishra A, Haque I, Jha B (2016) A novel transcription factor-like gene SbSDR1 acts as a molecular switch and confers salt and osmotic endurance to transgenic tobacco. Sci Rep 6:31686. https://doi.org/10.1038/srep31686
Sitia R, Braakman I (2003) Quality control in the endoplasmic reticulum protein factory. Nature 426:891. https://doi.org/10.1038/nature02262
Smirnoff N (1993) The role of active oxygen in the response of plants to water deficit and desiccation. N Phytol 125(1):27–58. https://doi.org/10.1111/j.1469-8137.1993.tb03863.x
Sobhanian H, Razavizadeh R, Nanjo Y, Ehsanpour AA, Jazii FR, Motamed N, Komatsu S (2010) Proteome analysis of soybean leaves, hypocotyls and roots under salt stress. Proteome Sci 8:19–19. https://doi.org/10.1186/1477-5956-8-19
Sofo A, Scopa A, Nuzzaci M, Vitti A (2015) Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int J Mol Sci 16(6):13561–13578. https://doi.org/10.3390/ijms160613561
Srivastava AK, Bhargava P, Thapar R, Rai LC (2008) Salinity-induced physiological and proteomic changes in Anabaena doliolum. Environ Exp Bot 64(1):49–57. https://doi.org/10.1016/j.envexpbot.2007.12.012
Sup Yun H, Yi C, Kwon H, Kwon C (2013) Model for regulation of VAMP721/722-mediated secretion: growth vs. stress responses. Plant Signal Behav 8(11):e27116. https://doi.org/10.4161/psb.27116
Suphioglu C, Ferreira F, Knox RB (1997) Molecular cloning and immunological characterisation of Cyn d 7, a novel calcium-binding allergen from Bermuda grass pollen. FEBS Lett 402(2–3):167–172. https://doi.org/10.1016/S0014-5793(96)01520-7
Taïbi K, Taïbi F, Ait Abderrahim L, Ennajah A, Belkhodja M, Mulet JM (2016) Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. South Afr J Bot 105:306–312. https://doi.org/10.1016/j.sajb.2016.03.011
Tanou G, Job C, Rajjou L, Arc E, Belghazi M, Diamantidis G, Molassiotis A, Job D (2009) Proteomics reveals the overlapping roles of hydrogen peroxide and nitric oxide in the acclimation of citrus plants to salinity. Plant J 60(5):795–804. https://doi.org/10.1111/j.1365-313X.2009.04000.x
Tanou G, Job C, Belghazi M, Molassiotis A, Diamantidis G, Job D (2010) Proteomic signatures uncover hydrogen peroxide and nitric oxide cross-talk signaling network in citrus plants. J Proteome Res 9(11):5994–6006. https://doi.org/10.1021/pr100782h
Tanou G, Filippou P, Belghazi M, Job D, Diamantidis G, Fotopoulos V, Molassiotis A (2012) Oxidative and nitrosative-based signaling and associated post-translational modifications orchestrate the acclimation of citrus plants to salinity stress. Plant J 72(4):585–599. https://doi.org/10.1111/j.1365-313X.2012.05100.x
Tezara W, Mitchell V, Driscoll S, Lawlor D (1999 ) Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature 401(6756):914
Theodoulou FL (2000) Plant ABC transporters. Biochim Biophys Acta (BBA)—Biomembr 1465(1):79–103. https://doi.org/10.1016/S0005-2736(00)00132-2
Timperio AM, Egidi MG, Zolla L (2008) Proteomics applied on plant abiotic stresses: role of heat shock proteins (HSP). J Proteomics 71(4):391–411. https://doi.org/10.1016/j.jprot.2008.07.005
Tsokou A, Georgopoulou K, Melliou E, Magiatis P, Tsitsa E (2007) Composition and enantiomeric analysis of the essential oil of the fruits and the leaves of Pistacia vera from Greece. Molecules 12(6):1233–1239. https://doi.org/10.3390/12061233
Uarrota VG, Moresco R, Schmidt EC, Bouzon ZL, Nunes Eda C, Neubert Ede O, Peruch LA, Rocha M, Maraschin M (2016) The role of ascorbate peroxidase, guaiacol peroxidase, and polysaccharides in cassava (Manihot esculenta Crantz) roots under postharvest physiological deterioration. Food Chem 197(Pt A):737–746. https://doi.org/10.1016/j.foodchem.2015.11.025
Uygur V, Yetisir H (2009) Effects of rootstocks on some growth parameters, phosphorous and nitrogen uptake watermelon under salt stress. J Plant Nutr 32(4):629–643. https://doi.org/10.1080/01904160802715448
Vierling E (1991) The roles of heat shock proteins in plants. Annu Rev Plant Biol 42(1):579–620. https://doi.org/10.1146/annurev.pp.42.060191.003051
Vishnevetsky M, Ovadis M, Vainstein A (1999) Carotenoid sequestration in plants: the role of carotenoid-associated proteins. Trends Plant Sci 4(6):232–235. https://doi.org/10.1016/S1360-1385(99)01414-4
Wakeel A, Asif AR, Pitann B, Schubert S (2011) Proteome analysis of sugar beet (Beta vulgaris L.) elucidates constitutive adaptation during the first phase of salt stress. J Plant Physiol 168(6):519–526. https://doi.org/10.1016/j.jplph.2010.08.016
Walker JE (1992) The NADH: ubiquinone oxidoreductase (complex I) of respiratory chains. Q Rev Biophys 25(3):253–324. https://doi.org/10.1017/S003358350000425X
Wang C, Liu Z (2006) Arabidopsis ribonucleotide reductases are critical for cell cycle progression, DNA damage repair, and plant development. Plant Cell 18(2):350–365. https://doi.org/10.1105/tpc.105.037044
Wang W, Vinocur B, Shoseyov O, Altman A (2004) Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci 9(5):244–252. https://doi.org/10.1016/j.tplants.2004.03.006
Wang Z, Wang M, Liu L, Meng F (2013) Physiological and proteomic responses of diploid and tetraploid black locust (Robinia pseudoacacia L.) subjected to salt stress. Int J Mol Sci 14(10):20299–20325. https://doi.org/10.3390/ijms141020299
Welsch R, Wust F, Bar C, Al-Babili S, Beyer P (2008) A third phytoene synthase is devoted to abiotic stress-induced abscisic acid formation in rice and defines functional diversification of phytoene synthase genes. Plant Physiol 147(1):367–380. https://doi.org/10.1104/pp.108.117028
Wise RR, Naylor AW (1987) Chilling-enhanced photooxidation: evidence for the role of singlet oxygen and superoxide in the breakdown of pigments and endogenous antioxidants. Plant Physiol 83(2):278–282
Witzel K, Matros A, Strickert M, Kaspar S, Peukert M, Mühling KH, Börner A, Mock H-P (2014) Salinity stress in roots of contrasting barley genotypes reveals time-distinct and genotype-specific patterns for defined proteins. Mol Plant 7(2):336–355. https://doi.org/10.1093/mp/sst063
Yan S, Tang Z, Su W, Sun W (2005) Proteomic analysis of salt stress-responsive proteins in rice root. Proteomics 5(1):235–244. https://doi.org/10.1002/pmic.200400853
Yasar F, Ellialtioglu S, Yildiz K (2008) Effect of salt stress on antioxidant defense systems, lipid peroxidation, and chlorophyll content in green bean. Russ J Plant Physiol 55(6):782. https://doi.org/10.1134/S1021443708060071
Yeoh HH, Badger MR, Watson L (1980) Variations in K(m)(CO(2)) of ribulose-1,5-bisphosphate carboxylase among grasses. Plant Physiol 66(6):1110–1112
Yi C, Park S, Yun HS, Kwon C (2013) Vesicle-associated membrane proteins 721 and 722 are required for unimpeded growth of Arabidopsis under ABA application. J Plant Physiol 170(5):529–533. https://doi.org/10.1016/j.jplph.2012.11.001
Yin Y-G, Kobayashi Y, Sanuki A, Kondo S, Fukuda N, Ezura H, Sugaya S, Matsukura C (2010) Salinity induces carbohydrate accumulation and sugar-regulated starch biosynthetic genes in tomato (Solanum lycopersicum L. cv. ‘Micro-Tom’) fruits in an ABA- and osmotic stress-independent manner. J Exp Bot 61(2):563–574. https://doi.org/10.1093/jxb/erp333
Yocum G (2001) Differential expression of two HSP70 transcripts in response to cold shock, thermoperiod, and adult diapause in the Colorado potato beetle. J Insect Physiol 47(10):1139–1145. https://doi.org/10.1016/S0022-1910(01)00095-6
Yotov WV, Moreau A, St-Arnaud R (1998) The alpha chain of the nascent polypeptide-associated complex functions as a transcriptional coactivator. Mol Cell Biol 18(3):1303–1311. https://doi.org/10.1128/mcb.18.3.1303
Yuan Y, Zhong M, Shu S, Du N, Sun J, Guo S (2016) Proteomic and physiological analyses reveal putrescine responses in roots of cucumber stressed by NaCl. Front Plant Sci 7:1035. https://doi.org/10.3389/fpls.2016.01035
Zhang X, Liu S, Takano T (2008) Overexpression of a mitochondrial ATP synthase small subunit gene (AtMtATP6) confers tolerance to several abiotic stresses in Saccharomyces cerevisiae and Arabidopsis thaliana. Biotech Lett 30(7):1289–1294. https://doi.org/10.1007/s10529-008-9685-6
Zhang L, Zhang H, Liu P, Hao H, Jin JB, Lin J (2011) Arabidopsis R-SNARE proteins VAMP721 and VAMP722 are required for cell plate formation. PLoS ONE 6(10):e26129. https://doi.org/10.1371/journal.pone.0026129
Zhang M, Fang Y, Ji Y, Jiang Z, Wang L (2013) Effects of salt stress on ion content, antioxidant enzymes and protein profile in different tissues of Broussonetia papyrifera. South Afr J Bot 85:1–9. https://doi.org/10.1016/j.sajb.2012.11.005
Zhang L, Hu W, Wang Y, Feng R, Zhang Y, Liu J, Jia C, Miao H, Zhang J, Xu B, Jin Z (2015) The MaASR gene as a crucial component in multiple drought stress response pathways in Arabidopsis. Funct Integr Genomics 15(2):247–260. https://doi.org/10.1007/s10142-014-0415-y
Zhang F, Zhu G, Du L, Shang X, Cheng C, Yang B, Hu Y, Cai C, Guo W (2016) Genetic regulation of salt stress tolerance revealed by RNA-Seq in cotton diploid wild species, Gossypium davidsonii. Sci Rep 6:20582. https://doi.org/10.1038/srep20582
Zhang H, Li X, Zhang S, Yin Z, Zhu W, Li J, Meng L, Zhong H, Xu N, Wu Y, Sun GY (2018) Rootstock alleviates salt stress in grafted mulberry seedlings: physiological and PSII function responses. Front Plant Sci. https://doi.org/10.3389/fpls.2018.01806
Ziya Motalebipour E, Kafkas S, Khodaeiaminjan M, Çoban N, Gözel H (2016) Genome survey of pistachio (Pistacia vera L.) by next generation sequencing: development of novel SSR markers and genetic diversity in Pistacia species. BMC Genomics 17(1):998. https://doi.org/10.1186/s12864-016-3359-x
Zrig A, Tounekti T, Vadel AM, Ben Mohamed H, Valero D, Serrano M, Chtara C, Khemira H (2011) Possible involvement of polyphenols and polyamines in salt tolerance of almond rootstocks. Plant Physiol Biochem (PPB) 49(11):1313–1322. https://doi.org/10.1016/j.plaphy.2011.08.009
Zrig A, Ben Mohamed H, Tounekti T, Khemira H, Serrano M, Valero D, Vadel AM (2016) Effect of rootstock on salinity tolerance of sweet almond (cv. Mazzetto). South Afr J Bot 102:50–59. https://doi.org/10.1016/j.sajb.2015.09.001
Zuther E, Huang S, Jelenska J, Eilenberg H, Arnold EM, Su X, Sirikhachornkit A, Podkowinski J, Zilberstein A, Haselkorn R, Gornicki P (2004) Complex nested promoters control tissue-specific expression of acetyl-CoA carboxylase genes in wheat. Proc Natl Acad Sci USA 101(5):1403–1408. https://doi.org/10.1073/pnas.0307846100
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jamshidi Goharrizi, K., Baghizadeh, A., Kalantar, M. et al. Assessment of Changes in Some Biochemical Traits and Proteomic Profile of UCB-1 Pistachio Rootstock Leaf under Salinity Stress. J Plant Growth Regul 39, 608–630 (2020). https://doi.org/10.1007/s00344-019-10004-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-019-10004-3