Abstract
Seabirds can shunt nutrients and contaminants from marine to terrestrial ecosystems by forming dense breeding colonies and releasing wastes to these sites. A large colony of seabirds at Cape Vera (Devon Island, High Arctic Canada) has resulted in eutrophic conditions and potentially toxic concentrations of sedimentary metals in several freshwater ponds that drain their nesting sites. Here, we investigated the effects of elevated nutrient and sedimentary metal concentrations on the distribution of subfossil chironomids in surface sediments from 21 ponds that span a gradient of seabird influence. Although many ponds registered high nutrient concentrations (e.g., mean TP = 45 μg l −1), eutrophic taxa typical of temperate waters were not common, with most assemblages being dominated by morphotypes of Psectrocladius and Tanytarsina, as well as Corynoneura arctica-type, and Metriocnemus hygropetricus-type. Although the ponds within and outside the area influenced by seabirds contained largely similar taxa, variations did exist in the relative abundances of the different species. Lakewater pH was the only measured environmental variable that explained statistically significant amounts of variation in the chironomid assemblages. Although direct effects of pH on chironomids cannot be ruled out, pH is likely tracking production-related changes driven by limnetic dissolved inorganic carbon dynamics. Sediment cores collected from seabird-affected and seabird-free ponds showed a greater number of chironomid taxa and higher head capsule abundances in the pond receiving seabird inputs. Chironomid assemblages in both cores recorded increased abundances in recent decades, likely in response to warmer conditions and lengthened growing seasons.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The application of chironomid paleoecology in high latitude regions has increased in recent years, propelled largely by the ability of chironomids to provide reasonably accurate inferences of past temperatures in these climatically sensitive regions (e.g., Walker and Macdonald 1995; Langdon et al. 2008; Rolland et al. 2008; Axford et al. 2009; Kurek et al. 2009a, b). Chironomid community structure also tracks changes in lake trophic status (Brodersen and Quinlan 2006), acidification (Brodin 1990), catchment disturbances (Eggermont 1999; Maloney and Feminella 2006) and contaminants such as metals (Ilyashuk et al. 2003; Mousavi et al. 2003). The utility of chironomids as paleoenvironmental indicators makes them aptly poised to document the response of Arctic freshwaters to the multitude of stressors that are impinging upon these remote ecosystems (ACIA 2005).
Surface sediment training sets describing the relationship between modern chironomid assemblages and environmental variables provide the foundation for quantitative paleo-inferences. Although such studies are increasing in the Canadian Arctic (Walker and MacDonald 1995; Gajewski et al. 2005; Barley et al. 2006; Porinchu et al. 2009), their geographic coverage still lags behind those of other aquatic paleoindicators such as diatoms (Lim et al. 2001a, b; Michelutti et al. 2003a, 2006, 2007a; Antoniades et al. 2008). As noted by Walker and Cwynar (2006), to have confidence in error statistics associated with chironomid-based quantitative reconstructions, data sets incorporating long gradients in climate and other environmental variables are required. In this study, we extend the environmental gradient of high latitude, chironomid-based training sets by documenting chironomid assemblages in a series of ponds that have atypical limnological characteristics for Arctic freshwaters. Specifically, our study sites have greatly elevated nutrient and contaminant levels due to ornithogenic inputs from a nearby seabird colony.
Our study region, located near Cape Vera on Devon Island, High Arctic Canada (Fig. 1a, b), is the nesting site of a large seabird colony whose waste products (e.g., guano, carcasses, regurgitated stomach contents) have caused the ponds draining their nesting sites to become hypereutrophic (Brimble et al. 2009a; Keatley et al. 2009), with potentially toxic concentrations of sedimentary metals (Brimble et al. 2009b). Seabirds feed in the ocean but breed on land, and thus they transport and focus marine-derived nutrients to their terrestrial nesting grounds. Seabirds have a protein-rich diet and, as a consequence, produce excrement rich in nitrogen and phosphorus, which in most Arctic regions are limiting nutrients in terrestrial and freshwater ecosystems (Rigler 1974). At Cape Vera, the nutrient pulse delivered by the seabird wastes creates a hotspot of production and biodiversity that is unparalleled in other Arctic regions not affected by similar processes. An unfortunate irony is that this avian biological transport pathway also concentrates contaminants through biomagnification and bioaccumulation in the marine foodweb, thereby threatening the very ecosystem it supports and sustains (Blais et al. 2005; Brimble et al. 2009b).
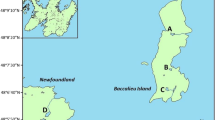
Map (a) showing the location of our study region near Cape Vera on Devon Island (star), as well as the Haughton Crater on Devon Island (circle), Cape Herschel on Ellesmere Island (square), the location of Meretta Lake near Resolute Bay on Cornwallis Island (diamond), and Cambridge Bay on Victoria Island (triangle). Also, map (b) showing the location of our study sites near Cape Vera on Devon Island. The shaded region represents the approximate area of the current northern fulmar colony
The biotic response to nutrient fertilization in Arctic freshwaters has been documented in only a few sites, with most research conducted on a single lake (Meretta Lake, Cornwallis Is., Arctic Canada; Fig. 1a) during the International Biological Programme in the late 1960s to the early 1970s (Rigler 1974). Although Arctic lakes show predictable responses to nutrient enrichment, such as increased biomass of phytoplankton (Kalff and Welch 1974), and greater respiration (Welch 1974), there are several key differences when compared with the response of temperate lakes. For example, in eutrophied Arctic lakes, there is a notable absence of large cyanobacterial blooms (Schindler et al. 1974), and a subdued, often delayed, diatom response (Douglas and Smol 2000; Michelutti et al. 2007b). The data so far suggest that prolonged ice cover and cold temperatures largely override the influence of nutrients on lakes in these climatic extremes (Smol and Douglas 2007a). However, most of the research on eutrophication at high latitudes has focused on the response of primary producers, and no studies exist documenting the response of chironomids. In addition, most of the research on chironomid responses to nutrient amendments has focused on temperate freshwaters (e.g., Brooks et al. 2001; Brodersen and Quinlan 2006). The nutrient-rich seabird inputs draining into our Cape Vera study ponds (n = 21) allow us to examine the distributions and patterns of chironomid assemblages across a large gradient of nutrient concentrations in a High Arctic setting.
Bennike et al. (2004) noted that a bias in many chironomid training sets is the strong correlation among environmental variables that can influence chironomid distributions, citing a typical pattern of cold lakes also being deep and unproductive whilst warm lakes are shallow and productive. The shallow nature of the Cape Vera study ponds (Z max < 2 m for all sites) minimizes some of these traditional biases, as the nutrient gradient in this training depends largely on the distance to the nesting cliffs and will have no correlation to depth. This may allow for a clearer picture of how chironomids respond to nutrient enrichment in the Arctic.
The Cape Vera training set also presents the opportunity to investigate the influence of seabird-derived metals on chironomid assemblages. The issue of metal-contaminated sediments has direct relevance to chironomids, which are predominantly benthic. Moreover, chironomids are key mediaries of energy, and potentially contaminant, transfer to higher trophic levels in both freshwater and terrestrial foodwebs. Previous research has demonstrated spatial patterns in chironomid assemblages consistent with species-specific tolerances to heavy metals (Mousavi et al. 2003), as well as downcore shifts in chironomid assemblages in response to metal pollution (Ilyashuk et al. 2003). In an investigation of nine Cape Vera ponds that spanned a gradient of seabird inputs, Brimble et al. (2009b) showed that fluxes of certain metals such as Cd, K, Zn and As were highest in the ponds closest to the colony and declined exponentially with distance. Sediments in several Cape Vera ponds had concentrations of As, Cd and Zn that exceeded Canadian standards for the protection of aquatic life (Brimble et al. 2009b). Here, we compare the composition of chironomid species in the same ponds analysed by Brimble et al. (2009b) to determine whether differences in sedimentary metals had any influence on chironomid distributions.
In this study, we take advantage of the unique ecosystem created by the seabird colony at Cape Vera to investigate the influence of elevated nutrient and metal concentrations on chironomid assemblages in a High Arctic setting. We ask the following questions: (1) What are the dominant chironomid taxa in the Cape Vera ponds? (2) Of the measured limnological variables, which ones best explain variation in chironomid assemblages? (3) How do chironomid assemblages in the seabird-affected sites at Cape Vera compare to those elsewhere in the Canadian Arctic? (4) Do chironomid distributions vary across an ornithogenic gradient? We also provide some historical context by comparing trajectories in chironomid composition and abundance in sediment cores spanning the last few centuries, from a seabird-affected and seabird-free pond. The data from our study will expand our knowledge of chironomid ecology in High Arctic regions potentially leading to refined chironomid-based inference models, as well as address fundamental questions regarding the response of secondary consumers to nutrient enrichment and contaminants in Arctic freshwaters.
Materials and methods
Site description
Cape Vera (76°15′N, 89°15′W), located on Devon Island in the Canadian High Arctic (Fig. 1a, b), is the breeding site for a seabird colony of approximately 10,000 breeding pairs of northern fulmars (Fulmarus glacialis), a medium-sized petrel common to the North Atlantic. Steep-sided dolostone/limestone cliffs that rise about 300 m above sea level provide ideal nesting habitat for fulmars, which breed at this site between May and September each year (Gaston et al. 2006). Our study ponds are located along a small coastal foreland that is bound between the fulmar nesting cliffs and the Arctic Ocean (Fig. 1b). The ponds beneath the nesting cliffs receive anomalously high inputs of nutrients and contaminants due to waste products released from the fulmar colony, including excrement, carcasses, regurgitated stomach contents and eggshells. The study sites span a gradient of seabird influence, depending largely on their proximity to the cliffs, but also local features of hydrology and topography. In general, the ponds nearest to the fulmar colony record the highest nutrient levels (Brimble et al. 2009a; Keatley et al. 2009), as well as the greatest sedimentary concentrations of bioaccumulated contaminants including PCBs (Michelutti et al. 2009a) and metals such as Hg, As, Cd and Zn (Blais et al. 2005; Brimble et al. 2009b).
Field and laboratory methods
Twenty-four ponds located across a gradient of ornithogenic influence were sampled over a 3-year period during the first 2 weeks of July 2004–2006. Surface sediments were collected by hand from the upper-most ~1 cm of sediments and placed into Whirlpak® bags and kept cool and dark until analysis. All ponds were given an unofficial designation beginning with the prefix “CV” followed by a number. The study sites were divided into three general categories according to their degree of seabird influence, which was based mainly on their proximity to the nesting cliffs. These included the following: (1) No seabird inputs (n = 6; CV16-18, 22, 23, 24), (2) Moderate seabird inputs (n = 5; CV1-4, 12) and (3) High seabird inputs (n = 10; CV5-7, 9, 9A, 10, 14, 15, 20, 31). Ponds categorized as having “high seabird inputs” were located <1,000 m from the nesting cliffs, whereas ponds categorized as having “moderate seabird inputs” were located >1,000 m from the cliffs but still received drainage from the nesting site. Ponds categorized as having “no seabird inputs” received no inputs from the Cape Vera nesting cliffs. The ponds in this study were the same ones analysed by Keatley et al. (2009) for water chemistry and modern diatom assemblages. Also, Brimble et al. (2009b) measured trace element concentrations in sediment cores collected from a subset of nine of these ponds. It is these same nine ponds analysed by Brimble et al. (2009b) that we used to explore the influence of sedimentary metals on chironomid distributions.
Sediment cores from two ponds were obtained to provide a long-term perspective (past ~200 years) on chironomid community changes from Cape Vera. Cores were recovered from a pond heavily affected by seabird inputs (CV9) and a ‘control’ site that is currently outside the influence of the fulmar colony (CV22). The cores were obtained using a plexiglass tube pushed directly into the sediments; a technique designed specifically for the collection of an undisturbed sediment-water interface. Cores were sectioned on-site in continuous 0.5-cm intervals using a close-interval extruder (Glew et al. 2001). A low flux of excess 210Pb at Cape Vera prevented the establishment of reliable 210Pb chronologies for the study cores (Michelutti et al. 2008), a situation not uncommon in High Arctic lakes (Wolfe et al. 2004). However, 137Cs was measured simultaneously with 210Pb and had an activity peak at 662 keV. The artificial nuclide 137Cs is associated with above ground testing of nuclear weapons and has a peak activity at 1963, the time of its maximum atmospheric fallout (Appleby 2001).
Sediment processing for chironomid analysis followed standard procedures outlined by Walker (2001). For each sediment sample, ~2 g of wet material was deflocculated in 5% KOH and heated to 50°C for 30 min. Sediments were then sieved through a 100-μm mesh, with the retained sediments rinsed into glass scintillation vials using distilled water and preserved with ethanol. Chironomid head capsules from the sieved sediment slurry were hand-picked using forceps in a Bogorov counting tray under a dissecting microscope at 50× magnification. Chironomid head capsules were transferred to microscope cover slips and permanently mounted onto slides using Entellen. Chironomid identifications were made at 400× or 1,000× magnification and primarily followed the taxonomy of Walker (1988), Larocque and Rolland (2006) and Brooks et al. (2007). At least 45 head capsules (mean = 72) were enumerated and identified from the surface sediments of the 24 study ponds. Ponds CV8, 11 and 13 were removed from the study due to low head capsule recovery (<20). Sediment samples from ponds CV2 and CV22 only contained 26 and 21 head capsules, respectively. These ponds were still included in our dataset, but were not used in the creation of any inference models where minimum head capsule numbers of 40–50 are recommended (Quinlan and Smol 2001). No less than 66 head capsules (mean = 112) were identified at any one interval in the CV9 sediment core. However, the CV22 sediment core had few head capsules, ranging from a low of 1.5 to a maximum of 23. As a result of the low head capsule counts in CV22, the sediment core data are presented as absolute abundances (head capsules per gram dry mass; HC g−1 dm), as interpretations of community change based on relative abundances could be misleading. Measurements of percent water content on subsamples of all sediment intervals from both cores were used to convert sediment mass from wet to dry.
Data analysis
Detrended correspondence analysis (DCA) was used to determine whether unimodal or linear ordination techniques should be applied, based on the gradient length expressed by the distribution of the chironomid taxa. The results of the DCA indicated linear techniques were most appropriate (Birks 1998), and so redundancy analysis (RDA) was used to explore relationships between chironomid taxa and the measured environmental variables. For ponds that were sampled more than once for water chemistry, mean values were taken for all limnological variables. Each measured environmental variable was run separately in a series of singly constrained RDAs, where each variable was constrained to the first axis, in order to evaluate the explanatory power of each variable individually. RDA with forward selection was then run using the subset of variables identified as significant (P ≤ 0.05) in the singly constrained RDAs. Only those variables identified as explaining significant (P ≤ 0.05) and independent amounts of variation in the chironomid dataset were included in the final ordination. Principal components analysis (PCA), an indirect ordination technique that models species as linear responses along synthetic variables (axes) with no a priori ecological information, thereby revealing the main direction of variation in species assemblages, was used to compare the similarities/differences of Cape Vera sites relative to one another and to assess how chironomid distributions vary across a gradient of sedimentary metal concentrations. All ordinations were performed with the entire species dataset using the program CANOCO version 4.0 (ter Braak and Šmilauer 2002).
Taxonomic notes
A total of 23 taxa were identified in the 21 ponds from Cape Vera (surface sediments and cores), 10 of which were found in relative abundances greater than 5%. Overall, identification to species-group level was possible, but some genera proved difficult to split. For example, several morphotypes of Psectrocladius were commonly identified in the Cape Vera sediments, namely P. subgroup Psectrocladius, with lesser numbers of P. sordidellus-type, and P. septentrionalis-type. These morphotypes could often be split based largely upon differences in the shape of their mental teeth (Brooks et al. 2007); however, obvious differences were not always evident especially on specimens with half mentums and worn median teeth. Thus, the Psectrocladius morphotypes were grouped together. Also, problematic were Corynocera oliveri-type and Tanytarsus lugens-type, which can usually be distinguished based on the wider spacing of the lateral teeth in T. lugens-type (Brooks et al. 2007). However, in the Cape Vera samples, there appeared to be a gradation in the degree of overlap among lateral teeth, and thus the two taxa were grouped. We also grouped Hydrobaenus and Oliveridia. Although it is possible to distinguish the two based on whether the median tooth is single (Oliveridia) or paired (Hydrobaenus), we mostly encountered half mentums that were usually worn, which made it difficult to discern (Wiederholm 1983).
Results and discussion
What are the dominant chironomid taxa in the Cape Vera ponds?
We identified 18 taxa in the 21 surface sediment samples from Cape Vera. The average number of taxa at each site was nine, with a maximum of 16 (CV3) and a minimum of five (CV18). Table 1 lists the number of occurrences, minimum and maximum percent abundances and Hill’s N2 diversity values for all surface sediment taxa. The relative abundances of the most common taxa (defined here as taxa that occurred in >50% of the study sites) are plotted in Fig. 2, with taxa arranged in order of increasing PCA axis 1 site score. The most common taxa included Psectrocladius, Corynoneura arctica-type, Metriocnemus hygropetricus-type and Tanytarsina undifferentiated, which were found in at least 18 or more of the 21 study ponds. Moderately common were Chironomus plumosus-type, Hydrobaenus/Oliveridia, Corynocera oliveri-type/Tanytarsus lugens-type, Eukieferiella, Diamesa and Metriocnemus terrester-type, which were present in at least half of the study ponds (Table 1; Fig. 2). The rarer taxa, which were found in less than five sites and did not exceed 5% relative abundance, included Abiskomyia, Chironomus anthracinus-type, Cladotanytarsus mancus group, Limnophyes, Micropsectra atrofasciata radialis group, Rheotanytarsus and two unknown Orthocladiinae taxa (Table 1).
The ponds closest to the fulmar colony are extreme outliers with respect to water chemistry when compared with Arctic ponds not influenced by seabird activities (Table 2). For example, when compared to the control (or seabird-free) sites at Cape Vera, or to similar-sized ponds at nearby Haughton Crater on Devon Island (Fig. 1a), the seabird-affected sites show elevated production-related variables including chlorophyll a, pH, DOC, as well as greater concentrations of nutrients such as total phosphorus (TP) and nitrogen (TN; Table 2). Online Resource 1 lists the full suite of water chemistry variables for the study ponds.
Given the highly productive ponds created by the nutrient-rich seabird wastes, we would predict to find chironomid taxa that thrive under eutrophic conditions. One such taxon is Chironomus plumosus-type, which, although common in lakes across a gradient of trophic states, tends to dominate in highly productive waters (Brodersen and Lindegaard 1999; Brodersen and Quinlan 2006). At Cape Vera, C. plumosus-type is indeed common, occurring in 15 of the 21 sites; however, it reaches its highest relative abundances in three of the seabird-free sites (Fig. 2). The genus Psectrocladius, although relatively common and abundant in many lake types, is more common in lakes with higher TP in the Canadian Arctic (Gajewski et al. 2005). Overall, our data are consistent with this finding as Psectrocladius, while present in ponds across a range of trophic states, is generally most abundant in the seabird-affected (i.e., nutrient-rich) sites compared with the seabird-free ponds (Fig. 2).
Nutrient inputs from the fulmar colony have also boosted terrestrial production in the region beneath the nesting sites. The abundant mosses in the catchments and peripheries of the Cape Vera ponds can also influence chironomid assemblages. For example, Metriocnemus is often associated with plants, and some species are known to be semi-terrestrial living in damp moss, while others are hygropetric (Cranston 1982; Cranston et al. 1983). Morphotypes of the genus Metriocnemus, namely M. hygropetricus-type and M. terrester-type, were common and abundant in our study sites; however, they attained their highest percent abundances in the seabird-free sites (Fig. 2), which support very little moss habitat relative to the sites immediately beneath the nesting cliffs. Terrestrial vegetation can also have a profound impact on water chemistry and thus affect chironomid assemblages indirectly. Most notably, dissolved organic carbon (DOC) is known to increase in concentration in heavily vegetated catchments. In their survey of 50 lakes across the Canadian Arctic islands, Gajewski et al. (2005) determined that T. lugens-type/C. oliveri-type were more abundant in lakes with high DOC concentrations. This finding is consistent with our data, which show T. lugens-type/C. oliveri-type to be most common and abundant in seabird-affected sites (Fig. 2), which record greater DOC concentrations than the seabird-free sites (Table 2).
As would be expected in a High Arctic region such as Cape Vera, a number of cold stenotherms were present including Hydrobaenus/Oliveridia, Diamesa, Tanytarsus lugens-type/Corynocera oliveri-type and Corynoneura arctica-type, although this taxon also occurs in warmer water (Brooks et al. 2007). Also, one of the morphotypes of Psectrocladius found at Cape Vera, namely P. (monopsectrocladius) septentrionalis-type, occurs towards the cold end of the temperature gradient (Brooks et al. 2007).
Of potential interest are high relative abundances of the genera Eukieferiella/Tvetenia, which approach or exceed 20% in several sites (Fig. 2). Although frequently documented in lake sediment samples (Cranston et al. 1983; Brooks et al. 2007), Eukieferiella/Tvetenia are rarely common in the abundances recorded at Cape Vera (e.g., Gajewski et al. 2005). In a review of Arctic/Subarctic chironomid taxa, Bennike et al. (2004) rank Eukieferiella/Tvetenia as having amongst the lowest temperature optima, and thus its high abundances in some sites may be a reflection of the cool temperatures at Cape Vera.
Which of the measured limnological variables best explain variation in chironomid assemblages?
A series of singly constrained RDAs identified pH, conductivity, TN, DOC, Ca, Mg, Al, As and Cd as explaining significant amounts of variation in the chironomid dataset. Of these variables, Mg was removed due to a high variance inflation factor (VIF), and all remaining variables had VIFs <10. The remaining eight predictor variables explained 47.6% of the variance in the chironomid dataset, and the eigenvalues of axes 1 and 2 were 0.299 and 0.095, respectively. An RDA with forward selection using the eight predictor variables identified only pH and Al as explaining statistically significant (P < 0.05) amounts of the overall variance in the dataset. An RDA constrained to these two variables accounted for 29.2% of the variance captured by aforementioned eight predictor variables, with pH accounting for 2/3 (19.4%) of that variance. Although Al was identified as significant, this was driven by an atypically high Al concentration in one site, pond CV7. The Al concentration in pond CV7 was 202 μg l−1, whereas the mean Al value in all other sites was 20.4 ± 18.8 μg l−1. With pond CV7 removed from the analysis, Al fails to be a significant predictor.
The identification of lakewater pH as the lone predictor variable is not surprising, as the Cape Vera ponds record a large pH gradient (3 pH units, Table 2), and previous studies in the Canadian Arctic have identified lakewater pH as explaining statistically significant amounts of variation in chironomids assemblages (Gajewski et al. 2005; Barley et al. 2006; Porinchu et al. 2009). We anticipated that nutrient concentrations would be significant predictor variables as chironomids are well known as indicators of lake production and eutrophication (e.g., Brodersen and Quinlan 2006; Langdon et al. 2006; Woodward and Shulmeister 2006), and the Cape Vera ponds recorded large gradients in TP, TN and other production-related variables (Table 2). However, lakewater pH and nutrient concentrations are often closely linked via production-related changes driven by limnetic dissolved inorganic carbon (DIC) dynamics (Wolfe 2002). For example, increased nutrient levels enhance algal production, which increases photosynthetic drawdown of limnetic CO2 and shifts the relative proportion of DIC species towards HCO3 −, thereby increasing pH. In fact, lakewater pH is significantly (P < 0.05) correlated with both TP and TN in the Cape Vera ponds (Keatley 2007).
Stable isotopes of carbon (δ13C) measured in the Cape Vera pond sediments provide further support of increased photosynthetic drawdown of limnetic CO2, and subsequent pH shifts. For example, as seabird-derived nutrients increase algal production, the supply of limnetic DIC is decreased, which in turn decreases algal discrimination in favour of the lighter 12C isotope, resulting in an enrichment of δ13C values in the sediment (Brenner et al. 1999). Sedimentary δ13C measured in a subset of Cape Vera ponds that spanned a gradient of seabird inputs shows, in general, that the ponds with the greatest seabird inputs (e.g., CV9, CV9A) recorded higher δ13C values compared with ponds with moderate seabird inputs, and the control site (CV22), completely outside the influence of the fulmar colony, recorded the lowest value (Michelutti et al. 2009a). Thus, although direct effects of pH on chironomid assemblages at Cape Vera cannot be ruled out, it may be that pH is tracking production-related changes that are also important in governing their distributions.
How do chironomid assemblages in seabird-affected sites at Cape Vera compare to elsewhere in the Canadian Arctic?
There are large differences between chironomid assemblages documented at Cape Vera and those described in lakes elsewhere in the Arctic. For example, a study of chironomid distributions in recent sediments from 50 lakes throughout the Canadian Arctic archipelago revealed that assemblages were dominated mainly by Micropsectra-type, Paracladius, Sergentia, Abiskomyia, Pseudodiamesa, Heterotrissocladius and Cricotopus/Orthocladius (Gajewski et al. 2005). In contrast, the Cape Vera sediments were dominated by Corynoneura arctica-type, Tanytarsina undiff., Psectrocladius, Eukieferiella, Hydrobaenus/Oliveridia, Metriocnemus and Corynocera oliveri-type/Tanytarsus lugens-type (Fig. 2). However, these differences may be less related to nutrient levels than to difference in the size and depth of the waterbodies, as all of the study sites at Cape Vera are classified as ponds (i.e., Z max < 2 m), whereas all but two of the sites in the Gajewski et al. study were lakes (i.e., Z max > 2 m). Thus, we restrict our comparisons of Cape Vera chironomid assemblages to other studies that have examined chironomid assemblages in High Arctic ponds.
Although the majority of High Arctic chironomid paleoecology has focused on lakes (Gajewski et al. 2005), a few studies have examined chironomids in pond sediments. Quinlan et al. (2005) examined subfossil chironomid assemblages in sediment cores from three ponds located at Cape Herschel, Ellesmere Island, Nunavut (Fig. 1a), a region with well-documented ecological responses to recent warming (Douglas et al. 1994; Smol and Douglas 2007a, b). There were some similarities in chironomid species found in the ponds at Cape Herschel and Cape Vera, including Chironomus, Psectrocladius, Tanytarsina, Corynocera oliveri-type and Eukieferiella. However, there were also several taxa that, although common in lakes elsewhere in the Arctic, were not recorded at Cape Vera including Cricotopus/Orthocladius, Parakieferiella sp. B, Sergentia and Procladius. Meanwhile, Porinchu et al. (2009) completed a chironomid training set of the central Canadian Arctic, which included several shallow lakes and ponds in the northern mainland and mid-Arctic tundra of Victoria Island northward from Cambridge Bay (Fig. 1a). Although differences between lakes and ponds were not examined explicitly, the chironomid distributions appeared to be more strongly governed by biogeographical differences, as the study spanned large gradients in climate and vegetation. The most northern sites in the Porinchu et al. (2009) study most closely resembled the chironomid distributions at Cape Vera with chironomid communities dominated by Tanytarsus spp., Psectrocladius-sordidellus-type, Corynocera oliveri-type and Chironomus anthracinus-type. The one exception was Dicrotendipes, a thermophilous taxon, which was not observed at Cape Vera.
Do chironomid distributions at Cape Vera vary across an ornithogenic gradient?
Despite its small geographic extent (Fig. 1b), the Cape Vera ponds record amongst the largest limnological gradients in any chironomid- (or diatom-) based calibration set. These large gradients are due to nutrient-rich seabird inputs that have had a marked impact on aquatic production, with the ponds closest to the nesting cliffs registering the highest concentrations of nutrients (Keatley et al. 2009). For example, TP concentrations range from ultraoligotrophic (4 μg l−1 in CV2) to hypereutrophic (177 μg l−1 in CV8), with a mean value of 45.2 μg l−1 in the seabird-affected sites and 14.9 μg l−1 in the seabird-free sites (Table 2). Similarly, large gradients exist for TN and other production-related variables such as pH, DOC and chlorophyll a (Table 2).
Although there was considerable variation in chironomid distributions among some ponds, particularly with respect to variation in the percent abundances of specific taxa, there were no distinct differences in chironomid assemblages between seabird-affected and seabird-free sites (Fig. 2). The gradient length recorded along axis 1 in a DCA was <2 standard deviation units, indicating minimal compositional turnover among all sites. Visual inspection of the chironomid profiles at Cape Vera reveals no apparent differences in chironomid distributions between ponds within and outside the area affected by the fulmar colony. This is also borne out in the PCA ordination, as sites with no seabird inputs ordinate alongside sites with high seabird inputs (Fig. 3). These results are similar to Keatley et al. (2009) who showed that the distribution of dominant diatom taxa in these same Cape Vera ponds was not easily explained by nutrients, even across such a large gradient. Likewise, the chironomid data show that secondary consumers also do not respond to nutrient enrichment in a straightforward manner in High Arctic regions. The lack of any major chironomid species differences between seabird-free and seabird-affected ponds lends further support to the notion that the harsh climate at high latitudes largely overrides the influence of nutrients (Douglas and Smol 2000; Michelutti et al. 2007b).
In addition to nutrients, seabirds can also act as potent biovectors of contaminants, which bioaccumulate and biomagnify through the marine foodweb (Blais et al. 2005; Michelutti et al. 2009a, 2010). Brimble et al. (2009b) measured sedimentary metal concentrations from a subset of nine of the same ponds analysed in this present study, and determined that several ponds had metal concentrations that exceeded the Canadian Sediment Quality Guidelines for the Protection of Aquatic Life. In particular, ponds CV9, CV9A, CV10 and CV1 had Cd levels greater than the interim sediment quality guideline (ISQG) of 0.6 mg kg−1, defined as the level above which adverse biological effects occasionally occur. Likewise, ponds CV9, CV9A, CV20 and CV10 exceeded the ISQG for As (5.9 mg kg−1). Pond CV9 recorded sedimentary Zn concentrations above the probable effects level (PEL) for Zn (315 mg kg−1), defined as the level above which adverse biological effects frequently occur. Several ponds (CV5, CV6, CV12 and CV22) recorded values below any set guidelines for the protection of aquatic life. A PCA biplot summarizing the main patterns of variation between the nine Cape Vera sites and select sedimentary metals is shown in Fig. 4a.
a, b Principal components analysis (PCA) biplots showing main patterns of variation between ponds with varying distances to the nesting cliffs and (a) sedimentary metal concentrations, and (b) chironomid species assemblages. The numbers in parentheses denote the distances (in meters) of each pond from the nesting cliffs. Sites CV1, CV9, CV9A, CV10 contain sedimentary Cd levels greater than the interim sediment quality guideline (ISQG) set by the Canadian Sediment Quality Guidelines for the Protection of Aquatic Life. Sites CV9, CV9A, CV20 and CV10 exceed the ISQG for sedimentary As, and CV9 exceeds the ‘probable effects level’ for Zn. Ponds CV5, CV6, CV12 and CV22 do not contain any concentrations of sedimentary metals above the Canadian guidelines
The subfossil chironomid assemblages from the Brimble et al. (2009b) sites do not show any apparent species-specific differences along the sedimentary metals gradient (Fig. 4b). None of the metals identified as exceeding ISQG or PEL concentrations (i.e., As, Cd, Zn) explained any significant (P < 0.05) variation in the chironomid distributions in a canonical correspondence analysis (CCA) with forward selection. The PCA species biplot (Fig. 4b) shows no obvious clustering of sites with elevated vs. low sedimentary metal concentrations, suggesting that factors other than sedimentary metals may be more important in structuring chironomid assemblages at these sites although our sample sizes were small in this analysis. Of course, the occurrence of adverse biological effects cannot be predicted exclusively from concentration data alone because site-specific factors that influence the bioavailability of the metals are important. These include a variety of physico-chemical variables (e.g., pH, redox potential, particle size), biological factors (e.g., feeding behaviour and uptake rates) and differences in geochemistry (e.g., organic matter, sulphide levels; Environment Canada 1997).
Sediment core assemblages
The surface sediment data showed that there were no major differences in chironomid assemblages along an ornithogenic gradient, suggesting that climate is more important than trophic status in governing changes in chironomid distributions at these high latitudes. Paleolimnological studies from throughout the Canadian High Arctic have shown that climate warming of the twentieth century has resulted in marked limnological changes (Smol and Douglas 2007a, b) as well as regime shifts in biota including diatoms (Smol et al. 2005) and chironomids (Quinlan et al. 2005). Below we examine chironomid distributions in two sediment cores from Cape Vera to explore how chironomid assemblages have changed over the past two centuries. We examine sediment cores from CV9, a pond located immediately beneath the nesting cliffs and thus heavily affected by fulmar activity, and CV22, a pond currently outside the influence of seabird activities (Fig. 1b). Aside from the differences in ornithogenic inputs, ponds CV22 and CV9 are of comparable size and depth, occupy the same geological setting and experience similar climate. This comparative analysis will allow us to explore the response of chironomids to recent warming in both a seabird-affected and a seabird-free site.
The lower half of the CV9 core is dominated by Tanytarsina taxa, Psectrocladius, Hydrobaenus/Oliveridia, with lesser amounts of Cornynoneura arctica-type (Fig. 5). This assemblage remains relatively constant until about 7.25 cm depth when Hydrobaenus/Oliveridia declines to negligible abundances, with concomitant increases in Corynocera oliveri-type/Tanytarsus lugens-type. Tanytarsina (undiff.) increases in abundance near 5.25 cm depth, which corresponds to an overall increase in total chironomid head capsules (Fig. 5).
The assemblages in the profile from CV9 do not reflect the current eutrophic condition of this pond (e.g., TP = 37.9 μg l−1). For example, there are few eutrophic taxa, with the possible exception of low abundances of Chironomus plumosus-type and Cladotanytarsus mancus group. In addition, the most prominent species shift in the CV9 core is the replacement of one apparent cold stenothermous group for another, namely Hydrobaenus/Oliveridia for Corynocera oliveri-type/Tanytarsus lugens-type.
The lack of eutrophic taxa in CV9, despite elevated nutrient levels, is consistent with the suggestion that climate is more important than trophic status in governing changes in chironomid assemblages at high latitudes. Sediment core data from Meretta Lake (Fig. 1), a High Arctic lake that eutrophied in 1949 in response to sewage inputs, show that chironomids were absent or near-absent until after ~1973 (Antoniades et al. in press). The greatest change in chironomid assemblages occurred in the most recent sediments after Meretta Lake had already recovered from cultural eutrophication, due to the cessation of sewage inputs in 1998, and nutrient levels had returned to pre-impact conditions (Douglas and Smol 2000). The increased abundances of chironomid taxa in the uppermost sediments of Meretta Lake coincided with the timing of climate-driven limnological change in this region (Michelutti et al. 2003b) and the accompanying longer growing seasons, rather than the trophic changes of the lake (Antoniades et al. in press).
Similar to the changes recorded in Meretta Lake (Antoniades et al. on press), both CV9 and CV22 show marked increases in chironomid abundances in recent sediments (Fig. 5) that cannot be explained by differences in seabird-derived nutrients. The Canadian High Arctic has experienced dramatic warming in recent decades, with marked limnological changes (Smol and Douglas 2007a, b), and impacts on chironomid assemblages have been recorded at ponds near Cape Vera. For example, sediment records from three High Arctic ponds at Cape Herschel on Ellesmere Island, Nunavut (Fig. 1a) show rapid increases in chironomid abundance and diversity beginning ~1850 AD (Quinlan et al. 2005), the timing of which corresponds to well-documented limnological responses to recent warming in this region (Smol and Douglas 2007a, b). Thus, the increased chironomid abundances in the recent sediment of CV9 and CV22 appear to be primarily climate-driven.
The surface sediment data from Cape Vera, similar to the sediment core data from Meretta Lake (Antoniades et al. in press), show that the chironomid response to nutrient enrichment is not as straightforward as often recorded in temperate regions (e.g., Clerk et al. 2000), and that the cool climate and short growing seasons likely override the influence of nutrients. However, when comparing the chironomid profiles from the seabird-affected pond (CV9) and the seabird-free site (CV22), it appears as if the ornithogenic inputs do have some effect on chironomids.
The chironomid assemblage recorded in the most recent sediments from CV22 is composed of identical taxa (excepting Eukieferiella/Tvetenia) to those in CV9, although CV22 records lower numbers of total head capsules (Fig. 5). Indeed, the chironomid profile from CV22 shows a depauperate assemblage in both total number of taxa and overall abundance, until the uppermost 1.25 cm of the core (Fig. 5). In contrast, the stratigraphy from CV9 records higher abundances and a greater number of taxa throughout its entire history (Fig. 5). Sedimentary δ15N levels, a proxy for seabird abundance, measured in the CV9 core indicate that seabirds have been present at CV9 for at least the time period captured by the sediment core (Michelutti et al. 2009b). Thus, although both CV9 and CV22 endure the same short growing season, seabird-enhanced production levels in CV9 appear to have allowed for greater numbers and higher abundances of chironomid taxa to become established.
Conclusions
The seabird-affected ponds at Cape Vera were not dominated by eutrophic chironomid taxa, at least not ones commonly reported in temperate freshwaters. In fact, the assemblages at Cape Vera contained largely similar taxa to the control sites that were outside the influence of the fulmar colony. Many species were cold stenotherms (e.g., Hydrobaenus/Oliveridia, Diamesa, Tanytarsus lugens-type/Corynocera oliveri-type and Corynoneura arctica-type), which are common to oligotrophic ponds elsewhere in the Canadian Arctic (Quinlan et al. 2005; Porinchu et al. 2009). Although the surface sediment chironomid assemblages did not show marked species differences along nutrient gradients or between seabird-affected and seabird-free ponds, lakewater pH was identified as explaining a significant amount of their distribution, and it seems likely that pH is influenced by production-related changes via DIC dynamics. The sediment core studies may have provided the most insight into the influence of ornithogenic inputs on chironomids. The seabird-affected site (CV9) recorded greater a number of taxa and higher head capsule abundances throughout most of its history, while the seabird-free site (CV22) was historically depauperate in chironomids. The largely similar chironomid taxa present in the sediment cores from CV9 and CV22 indicate that the seabird nutrients allowed the chironomid taxa already present to attain greater population numbers than otherwise possible without nutrient amendments.
References
ACIA (2005) Arctic climate impact assessment. Cambridge University Press, Cambridge
Antoniades D, Hamilton PB, Douglas MSV, Smol JP (2008) The freshwater floras of Prince Patrick, Ellef Ringnes, and Northern Ellesmere Islands from the Canadian Arctic Archipelago. Iconographica Diatomologica 17:1–649
Antoniades D, Michelutti N, Quinlan R, Blais JM, Bonilla S, Douglas MSV, Pienitz R, Smol JP, Vincent W (in press) Cultural eutrophication, anoxia, and ecosystem recovery in Meretta Lake, high Arctic Canada. Limnol Oceaonogr
Appleby PG (2001) Chronostratigraphic techniques in recent sediments. In: Last WM, Smol JP (eds) Tracking environmental change using lake sediments, vol 1: basin analysis, coring, and chronological techniques. Kluwer Acadamic Publishers, Dordrecht, The Netherlands, pp 172–203
Axford Y et al (2009) Recent changes in a remote Arctic lake are unique within the past 200,000 years. Proc Nat Acad Sci. doi:10.1073/pnas.0907094106
Barley EM, Walker IR, Kurek J, Cwynar LC, Mathewes RW, Gajewski K, Finney BP (2006) A northwest North American training set: distribution of freshwater midges in relation to air temperature and lake depth. J Paleolimnol 36:295–314
Bennike O, Brodersen KP, Jeppesen E, Walker IR (2004) Aquatic invertebrates and high latitude paleolimnology. In: Pientiz RP, Douglas MSV, Smol JP (eds) Long-term environmental change in Arctic and Antarctic lakes, developments in Paleoenvironmental research, vol 8. Springer, Dordrecht, pp 159–186
Birks HJB (1998) Numerical tools in palaeolimnology—progress, potentialities, and problems. J Paleolim 20:307–332
Blais JM, Kimpe LE, McMahon D, Keatley BE, Mallory ML, Douglas MSV, Smol JP (2005) Arctic seabirds transport marine-derived contaminants. Science 309:445
Brenner M, Whitmore TJ, Curtis JH, Hodell DA, Schelske CL (1999) Stable isotope (δ13C and δ15N) signatures of sedimented organic matter as indicators of historic lake trophic state. J Paleolimnol 22:205–221
Brimble SK, Blais JM, Kimpe LE, Mallory ML, Keatley BE, Douglas MSV, Smol JP (2009a) Bioenrichment of trace elements in a series of ponds near a northern fulmar (Fulmarus glacialis) colony at Cape Vera, Devon Island. Can J Fish Aquat Sci 66:949–958
Brimble SK et al (2009b) High Arctic ponds receiving biotransported nutrients from a nearby seabird colony are also subject to potentially toxic loadings of arsenic, cadmium, and zinc. Environ Toxicol Chem 28:2426–2433
Brodersen KP, Lindegaard C (1999) Classification, assessment and trophic reconstruction of Danish lakes using chironomids. Freshwat Biol 42:143–157
Brodersen KP, Quinlan R (2006) Midges as palaeoindicators of lake productivity, eutrophication and hypolimnetic oxygen. Quat Sci Rev 25:1995–2012
Brodin YW (1990) Midge fauna development in acidified lakes in Northern Europe. Philos Trans R Soc B 327:295–298
Brooks SJ, Bennion H, Birks HJB (2001) Tracing lake eutrophic history with a chironomid-total phosphorus inference model. Freshw Biol 46:513–533
Brooks SJ, Langdon PG, Heiri O (2007) The identification and use of palaeoarctic chironomidae larvae in palaeoecology. Quaternary Research Association, London
Clerk S, Hall R, Quinlan R, Smol JP (2000) Quantitative inferences of past hypolimnetic anoxia and nutrient levels from a Canadian Precambrian Shield lake. J Paleolimnol 23:319–336
Cranston PS (1982) A key to the larvae of the British Orthocladiinae (Chironomidae). Freshw Biol Assoc Sci 45:1–152
Cranston PS, Oliver DR, Saether OA (1983) Keys and diagnoses of the larvae of the subfamily Orthocladiinae (Diptera, Chironomidae) of the Holarctic Region. Entomologica Scandinavica Supplement 19:149–291
Douglas MSV, Smol JP (2000) Eutrophication and recovery in the High Arctic: Meretta Lake (Cornwallis Island, Nunavut, Canada) revisited. Hydrobiol 431:193–204
Douglas MSV, Smol JP, Blake W Jr (1994) Marked post-18th century environmental change in high-arctic ecosystems. Science 266:416–419
Eggermont H (1999) Impact of soil erosion in Burundi and western Tanzania on the larval chironomid fauna of river deltas in Lake Tanganyika, East Africa. [in Dutch: De invloed van bodemerosie in Burundi en westelijkTanzanie op de benthische biodiversiteit van LakeTanganyika, Oost-Afrika]. Licentiate in Zoology thesis, Ghent University, Ghent
Environment Canada (1997) Canadian sediment quality guidelines for cadmium: supporting document. Environmental Conservation Service, Ecosystem Science Directorate, Science Policy and Environmental Quality Branch, Guidelines and Standards Division, Ottawa
Gajewski K, Bouchard G, Wilson SE, Kurek J, Cwynar LC (2005) Distribution of Chironomidae (Insecta: Diptera) head capsules in recent sediments of Canadian Arctic lakes. Hydrobiol 549:131–143
Gaston AJ, Mallory ML, Gilchrist HG, O’Donovan K (2006) Status, trends and attendance patterns of the Northern Fulmar Fulmarus glacialis in Nunavut, Canada. Arctic 59:165–178
Glew JR, Smol JP, Last WM (2001) Sediment core collection and extrusion. In: Last WM, Smol JP (eds) Tracking environmental change using lake sediments: zoological indicator, vol 1: basin analysis, coring, and chronological techniques. Kluwer Academic Publishers, Dordrecht, pp 73–105
Hirvenoja M, Hirvenoja E (1988) Corynoneura brundini spec. nov. Ein Beitrag zur Systematik der Gattung Corynoneura (Diptera, Chironomidae). Spix Suppl 14:213–238
Hofmann W (1971) Zur Taxonomie und Palökologie subfossiler Chironomiden (Dipt.) in Seesedimenten. Arch Hydrobiol Beih 6:1–50
Ilyashuk B, Ilyashuk E, Dauvalter V (2003) Chironomid responses to long-term metal contamination: a paleolimnological study in two bays of Lake Imandra, Kola Peninsula, northern Russia. J Paleolimnol 30:217–230
Kalff J, Welch HE (1974) Phytoplankton production in Char lake, a natural polar lake, and in Meretta Lake, a polluted polar lake, Cornwallis Island, Northwest Territories. J Fish Res Bd Can 31:621–636
Keatley BE (2007) Limnological and paleolimnological investigations of environmental change in three distinct ecosystem types, Canadian High Arctic. Dissertation, Queen’s University at Kingston
Keatley BE, Douglas MSV, Blais JM, Mallory ML, Smol JP (2009) Impacts of seabird-derived nutrients on water quality and diatom assemblages from Cape Vera, Devon Island, Canadian High Arctic. Hydrobiol 621:191–205
Kurek J, Cwynar LC (2009a) The potential of site-specific and local chironomid-based inference models for reconstructing past lake levels. J Paleolmnol 42:37–50
Kurek J, Cwynar LC (2009b) Effects of within-lake gradients on the distribution of fossil chironomids from maar lakes in western Alaska: implications for environmental reconstructions. Hydrobiol 623:37–52
Langdon PG, Ruiz Z, Brodersen KP, Foster IDL (2006) Assessing lake eutrophication using chironomids: understanding the nature of community response in different lake types. Freshw Biol 51:562–577
Langdon PG, Holmes N, Caseldine CJ (2008) Environmental controls on modern chironomid faunas from NW Iceland and implications for reconstructing climate change. J Paleolimnol 40:273–293
Larocque I, Rolland N (2006) A visual guide to sub-fossil chironomids from Quebec to Ellesmere Island. Rapport R-900. Institut National de la Recherche Scientifique, Quebec
Lim DSS, Kwan C, Douglas MSV (2001a) Periphytic diatom assemblages from Bathurst Island, Nunavut, Canadian High Arctic: an examination of community relationships and habitat preferences. J Phycol 37:379–392
Lim DSS, Douglas MSV, Smol JP (2001b) Diatoms and their relationship to environmental variables from lakes and ponds on Bathurst Island, Nunavut, Canadian High Arctic. Hydrobiol 450:215–230
Maloney KO, Feminella JW (2006) Evaluation of single- and multi-metric benthic macroinvertebrate indicators of catchment disturbance over time at the Fort Benning Military Installation, Georgia, USA. Ecol Indic 6:469–484
Michelutti N, Holtham AJ, Douglas MSV, Smol JP (2003a) Periphytic diatom assemblages from ultra-oligotrophic and UV transparent lakes and ponds on Victoria Island, and comparisons to other diatom surveys in the Canadian Arctic. J Phycol 39:465–480
Michelutti N, Douglas MSV, Smol JP (2003b) Diatom response to recent climatic change in a high arctic lake (Char Lake, Cornwallis Island, Nunavut). Glob Plan Change 38:257–271
Michelutti N, Smol JP, Douglas MSV (2006) Ecological characteristics of modern diatom assemblages from Axel Heiberg Island (High Arctic Canada) and their application to paleolimnological inference models. Can J Bot 84:1695–1713
Michelutti N, Douglas MSV, Smol JP (2007a) Evaluating diatom community composition in the absence of marked limnological gradients in the high Arctic: a surface sediment calibration set from Cornwallis Island (Nunavut, Canada). Polar Biol 30:1459–1473
Michelutti N, Hermanson MH, Smol JP, Dillon PJ, Douglas MSV (2007b) Delayed response of diatom assemblages to sewage inputs in an Arctic lake. Aquat Sci 69:523–533
Michelutti N, Blais JM, Liu H, Keatley BE, Douglas MSV, Mallory ML, Smol JP (2008) A test of the possible influence of seabird activity on the 210Pb flux in high Arctic ponds at Cape Vera, Devon Island, Nunavut: implications for radiochronology. J Paleolimnol 40:783–791
Michelutti N, Liu H, Smol JP, Kimpe LE, Keatley BE, Mallory M, Macdonald RW, Douglas MSV, Blais JM (2009a) Accelerated delivery of polychlorinated biphenyls (PCBs) in recent sediments near a large seabird colony in Arctic Canada. Environ Pollut 157:2769–2775
Michelutti N, Keatley BE, Brimble S, Blais JM, Liu H, Douglas MSV, Mallory ML, Macdonald RW, Smol JP (2009b) Seabird-driven shifts in Arctic pond ecosystems. Proc R Soc B 276:591–596. doi:10.1098/rspb.2008.1103
Michelutti N, Blais JM, Mallory M, Brash J, Thienpont J, Kimpe LE, Douglas MSV, Smol JP (2010) Trophic position influences the efficacy of seabirds as metal biovectors. Proc Nat Acad Sci. doi:10.1073/pnas.1001333107
Mousavi SK, Primicerio R, Amundsen P-A (2003) Diversity and structure of Chironomidae (Diptera) communities along a gradient of heavy metal contamination in a subarctic watercourse. Sci Total Environ 307:93–110
Porinchu D, Rolland N, Moser K (2009) Development of a chironomid-based air temperature inference model for the central Canadian Arctic. J Paleolimnol 41:349–368
Quinlan R, Smol J (2001) Setting minimum head capsule abundance and taxa deletion criteria in chironomid-based inference models. J Paleolimnol 26:327–342
Quinlan R, Douglas MSV, Smol JP (2005) Food web changes in arctic ecosystems related to climate warming. Glob Change Biol 11:1381–1386
Rigler FH (1974) Char lake project PF-2, final report 1974. Canadian Committee for the International Biological Programme, Toronto, Canada
Rolland N, Larocque I, Francus P, Pienitz R, Laperrière L (2008) Holocene climate inferred from biological (Diptera: Chironomidae) analyses in a Southampton Island (Nunavut, Canada) lake. Holocene 18:229–241
Schindler DW, Welch HE, Kalff J, Brunskill GJ, Kritsch N (1974) Physical and chemical limnology of Char Lake (75° N lat.). J Fish Res Bd Can 31:585–607
Smol JP, Douglas MSV (2007a) From controversy to consensus: making the case for recent climate change in the Arctic using lake sediments. Front Ecol Environ 5:466–474. doi:10.1890/060162
Smol JP, Douglas MSV (2007b) Crossing the final ecological threshold in high Arctic ponds. Proc Nat Acad Sci 104:12395–12397. doi:10.1073/pnas.0702777104
Smol JP, Wolfe AP, Birks HJB, Douglas MSV, Jones VJ, Korhola A, Pienitz R, Rühland K, Sorvari S, Antoniades D, Brooks SJ, Fallu M-Á, Hughes M, Keatley B, Laing T, Michelutti N, Nazarova L, Nyman M, Paterson AM, Perren B, Quinlan R, Rautio M, Saulneir-Talbot É, Siitonen S, Solovieva N, Weckström J (2005) Climate-driven regime shifts in the biological communities of arctic lakes. Proc Nat Acad Sci 102:4397–4402
ter Braak CJF, Šmilauer P (2002) CANOCO reference manual and CanoDraw for Windows user’s guide: software for canonical community ordination (version 4.5). Microcomputer Power, Ithaca
Walker IR (1988) Late-Quaternary Paleoecology of Chironomidae (Diptera: Insecta) from Lake Sediments in British Columbia. Dissertation, Simon Fraser University, Burnaby, Canada
Walker IR (2001) Midges: chironomidae and related diptera. In: Smol JP, Birks JB, Last WM (eds) Tracking environmental change using lake sediments: zoological indicators. Kluwer Academic Publishers, Dordrecht, pp 43–66
Walker IR, Cwynar LC (2006) Midges and palaeotemperature reconstruction—the North American experience. Quat Sci Rev 25:1911–1925
Walker IR, MacDonald GM (1995) Distributions of Chironomidae (Insecta, Diptera) and other fresh-water midges with respect to treeline, northwest-territories, Canada. Arct Alp Res 27:258–263
Welch HE (1974) Metabolic rates of arctic lakes. Limnol Oceanogr 19:65–73
Wiederholm T (1983) Chironomidae of the Holarctic region. Keys and diagnoses. Part 1—larvae. Entomologica Scandinavica 19(Suppl):1–457
Wolfe AP (2002) Climate modulates the acidity of arctic lakes on millennial time scales. Geology 30:215–218
Wolfe AP, Miller GH, Olsen CA, Forman SL, Doran PT, Holmgren SU (2004) Geochronology of high latitude lake sediments. In: Pienitz R, Douglas MSV, Smol JP (eds) Long-term environmental change in Arctic and Antarctic Lakes. Springer, The Netherlands, pp 19–52
Woodward CA, Shulmeister J (2006) New Zealand chironomids as proxies for human-induced and natural environmental change: transfer functions for temperature and lake production (chlorophyll a). J Paleolimnol 36:407–429
Acknowledgments
This project was funded by Natural Science and Engineering Research Council (NSERC) awards to JPS, MSVD and JMB. We are grateful to the Indian and Northern Affairs Canada (NSTP), Natural Resources Canada (PCSP) and Environment Canada (CWS) for financial and logistical support pertaining to fieldwork. Chironomid identifications were greatly aided by Roberto Quinlan, Andrew Medeiros, Yarrow Axford, Elizabeth Thomas and Joshua Kurek. Konrad Gajewski generously provided chironomid data that we used for comparison with our dataset. Helpful comments on the manuscript were provided by Joshua Kurek and Bronwyn Keatley. This project is PCSP/EPCP no. 00910.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Michelutti, N., Mallory, M.L., Blais, J.M. et al. Chironomid assemblages from seabird-affected High Arctic ponds. Polar Biol 34, 799–812 (2011). https://doi.org/10.1007/s00300-010-0934-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-010-0934-5